Abstract
We describe a simple one-pot mist copolymerization process to fabricate superhydrophobic cotton fabrics. A mixture solution consisting of a free radical initiator, tert-butyl peroxybenzoate (TBPB), and three monomers, lauryl methacrylate (LMA), 2-isocyanatoethyl methacrylate (IEM), and ethylene glycol dimethacrylate (EGD), is atomized to one side of a cotton fabric and polymerized on the surface. SEM images indicate that the copolymer layer on the cotton fiber surface has a randomly wrinkled morphology exhibiting nanoscale roughness. Wetting tests demonstrate that the modified surface possesses a remarkable superhydrophobicity with multiple healing functionalities. A simple ironing treatment at about 200 °C can recover the degraded superhydrophobicity of the modified cotton fabric suffered from 60 cycles of laundries or 2000 cycles of Martindale abrasion. Notably, the mist copolymerization process has no significant impact on the cotton advantages, such as flexibility, water absorptivity, and vapor permeability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Superhydrophobic surfaces have drawn considerable attention because of their potential applications such as self-cleaning, water–oil separation, anti-fogging, anti-corrosion, drag reduction, anti-bioadhesion, and highly protective clothes (Chu et al. 2015; Wang et al. 2015a; Wen et al. 2015; Wolfs et al. 2013; Dyett et al. 2014). According to the basic principle that the combination of low surface energy material and appropriate hierarchically nanostructures produces superhydrophobicity (Wenzel 1936), various superhydrophobic surfaces have been successfully prepared (Zhang et al. 2012; Wang et al. 2015b; Shirtcliffe et al. 2011). Fluorinated chemicals and polysiloxane polymers were the materials most frequently utilized to lower surface energy (Zhou et al. 2012; Zou et al. 2013; Hua et al. 2013; Wang et al. 2013; Shateri-Khalilabad and Yazdanshenas 2013). On the other hand, various methodologies, for example, dip coating (Nguyen et al. 2012), sol-gel coating (Periolatto et al. 2012), soft-lithography (Kim et al. 2015; Liu et al. 2006; Yao et al. 2009), electrochemical oxidation (Lee et al. 2010; Darmanin et al. 2013; Xu et al. 2011; Ottone et al. 2014), chemical vapor deposition (Liu et al. 2004; Crick et al. 2012), spray deposition (Hwang et al. 2011; Sparks et al. 2013), chemical etching (Qian and Shen 2005; Dong et al. 2011), surface polymerization (Nystrom et al. 2006; Miao et al. 2010; Yu et al. 2013; Xue et al. 2015), and laser microfabrication (Chen et al. 2013), have been tried to produce such rough surfaces with well-designed nanostructuration. In recent years, beyond the fabrication of superhydrophobic surfaces, more attention has been paid to the functional integration of superhydrophobic surfaces towards practical applications (Zhang et al. 2012; Liu et al. 2014).
Cotton fabrics are soft, comfortable, breathable, skin-friendly, and have an excellent water and moisture absorption ability. Recently, many attempts have been made to increase the functionalities of conventional cotton textiles, such as water repellence (Zou et al. 2013; Abbas et al. 2014; Shin et al. 2012), antimicrobial activity (Dastjerdi and Montazer 2010; Ali et al. 2014; Xi et al. 2015), and flame retardance (Abou-Okeil et al. 2013; Chen et al. 2015). Despite the successful advances in the modification of cotton fabrics reported to date, there are several shortcomings from the perspective of textile engineering. For example, (1) most of the fluorine-containing chemicals and organosilicon compounds are toxic and problematic for the environment (Conder et al. 2008; Isquith et al. 1988); (2) the coating films often affect the original cotton properties such as softness and wearing comfort; (3) the abrasion stability and laundering durability of the superhydrophobic coatings are hardly acceptable in practical applications.
In our previous works (Wang et al. 2014; Wan et al. 2014), we reported a "mist polymerization" technique developed from the gas-phase-assisted surface polymerization methodology (Yang et al. 2012; Andou et al. 2009a, b) to modify cotton fabric surfaces. The "mist polymerization" process can apply to a wider range of monomers and can build nanostructures simply on various substrates. Furthermore, the damage to the original cotton properties can be reduced to a very low level. However, the cumbersome introduction of the radical initiator to the fabric surface, and the unsatisfactory abrasion resistance and laundering durability of the modified cotton fabrics are the obvious disadvantages.
In this work, we report an improved one-pot mist copolymerization technique to generate robust superhydrophobic surfaces having abrasion-durable and healable behaviors. The mixture solution of lauryl methacrylate (LMA), 2-isocyanatoethyl methacrylate (IEM), ethylene glycol dimethacrylate (EGD), and a free radical initiator in cyclohexane are atomized to and heated on the cotton surface. The resulting layer possesses superhydrophobicity with remarkable healing functionality by heating. Notably, the original cotton properties such as water absorbency and moisture transmissibility are still found in the modified cotton fabrics.
Experimental section
Materials
The cotton fabrics used in this work were purchased from a local fabric store (60 ends cm−1, 30 picks cm−1, 0.42 mm thickness, 120 g m−2 weight, 35.2 m2 g−1 specific surface area). Before chemical modification, the cotton samples (15 mm × 15 mm) were cleaned by ultrasonic washing in ethanol (2 h) and deionized water (30 min × 3 times). LMA, IEM, ethylene glycol dimethacrylate (EGD), tert-butyl peroxybenzoate (TBPB), and cyclohexane were purchased from Shanghai Crystal Pure Industrial Co., Ltd. (China). All the chemicals were used as received without further purification. Deionized water with a resistivity of 18.2 MΩ cm was used in all experiments.
Typical procedures of the one-pot mist copolymerization
A mixture solution of LMA (monomer 1, 3.562 g, 14.000 mmol), IEM (monomer 2, 0.261 g, 1.680 mmol), EGD (monomer 3, 0.056 g, 0.280 mmol), and TBPB (initiator, 0.068 g, 0.350 mmol) in cyclohexane (16 ml) was atomized using an air compression-type atomizer (DongHan DH-M01, China), fed (0.360 ml min−1) to a cotton fabric sample (15 mm × 15 mm) for 1 min, heated at 80 °C for 1 h and at 110 °C for 4 h, washed by cyclohexane (50 ml × 3 times) to remove the unreacted monomer, and dried under vacuum to obtain the asymmetrically super-hydrophobic cotton fabric. Other modified cotton fabric samples were obtained by adjusting the monomer concentrations and the mist stream feeding time as listed in every Figure or Table. Fully modified cotton fabrics were prepared via a similar process in which the mist feeding is replaced by a dipping treatment in a mixed solution of LMA (0.700 mol l−1), IEM (0.084 mol l−1), EGD (0.014 mol l−1), and TBPB (0.018 mol l−1).
Characterizations
Static contact angles were measured using deionized water droplets (4 μl) with a Kruss contact angle instrument (DSA 100, Germany) at 25 °C. The water contact angle (WCA) value was recorded when the exposure time of the water droplet on the cotton fabric surface was 5 s. Average contact angle values were obtained by measuring three different positions on the same sample. Surface morphology was investigated by a JSM-6700F field emission scanning electron microscope (FE-SEM, JEOL, Japan) after gold coating (approximately 10 nm thickness). X-ray photoelectron spectroscopy (XPS) analysis was performed by an AXIS multifunctional X-ray photoelectron spectrometer (ULTRA DLD, Shimadzu Ltd., Japan) at a power of 450 W. FTIR spectra were collected from a Nicolet Fourier Transform spectrophotometer (AVATAR 5700, US) with an attenuated total reflection (ATR) accessory.
Laundering durability was evaluated by monitoring WCA on the cotton surface periodically after every stringent washing process. The cotton fabrics (15 mm × 15 mm) were washed by 50 ml of an aqueous solution of sodium dodecane sulfonate (2.0 %, w/w) in a beaker (diameter, 50 mm) with stirring (300 rpm, magnetic stirrer, 9 mm × 25 mm) at 25 °C for 10 min, rinsed with deionized water (35 °C, 10 ml × 4 times), and dried at 60 °C. The wearing durability tests were carried out on a Martindale-type fabric abrader (HZ-8029A, China). The abrasive material was pristine cotton fabric, and the loading pressure was 12 kPa. The abrasion rotation rate of the top pristine cotton fabric (D = 120 mm) against the modified fabric surface (D = 20 mm) was 50 rpm. The number of rotation cycles required for exposing the cellular structure of the fabric was noted as the index of abrasion durability. After every 200 (or more) abrasive cycles, the modified cotton surface was ironed by an electric iron (iron surface temperature was about 200 °C) for 1 min.
Water absorptivity was examined by weighing the weight increment of the cotton sample after soaking in plenty of deionized water for 10 min and hanging it out for another 10 min. It is expressed as the weight ratio of adsorbed water to the cotton sample. Water vapor permeability was evaluated using the ASTM E-96 (open cup test) method. The test fabric sample was placed tightly over a shallow dish containing distilled water. The weight loss of the test assembly over 24 h was measured, and the vapor transmission rate (g m−2 d−1) was calculated as water vapor permeability. Flexibility was determined by the flat loop method (IS 7016 Part 11). Fabric samples were cut from warp and weft directions (40 mm × 160 mm). A loop was made and placed on a horizontal plane. The height of the loop was measured as an idea of the flexibility of the fabric. The lower the height of the loop, the greater is the flexibility.
Results and discussion
Fabrication of the superhydrophobic cotton surfaces
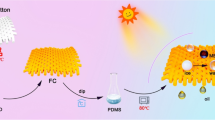
As shown in Scheme 1, the superhydrophobic surface was obtained through a one-pot mist copolymerization procedure. The mixture solution, consisting of a free radical initiator (TBPB) and three monomers (LMA, IEM, and EGD), was atomized and fed to the cotton surface. The liquid droplets in the mist stream are small (diameter, 150–500 nm), and only a few percent of them can be fixed on the cotton surface (Wang et al. 2014; Wan et al. 2014). Distinguished from other spray-coating processes (Hwang et al. 2011; Sparks et al. 2013) by these features, mist polymerization is beneficial to fabricating a very thin layer with complex morphology on the substrate surface. The three monomers have their respective roles in the surface modification. LMA, a methacrylate monomer having a long aliphatic chain, is chosen to lower the surface energy. EGD, a difunctional monomer, is used as a crosslinker to improve porous structures. IEM, a methacrylate monomer having an isocyanate group, is designed to react with the hydroxyl groups of cellulose. The copolymerization on the cotton fabric surface was monitored using ATR-FTIR. Figure 1 shows the ATR-FTIR spectra of the pristine cotton fabric sample and the modified samples with different monomer proportions. The three characteristic peaks at 2927, 2856, and 1728 cm−1, which are attributable to C–H2 and C=O bonds, respectively, are found in all the spectra of the modified cotton fabrics. The peak at 2268 cm−1, which is assigned to the isocyanate group, is not observed in the spectrum of the unmodified fabric. The intensities of the peaks in the spectra of the modified cotton fabrics decrease with decreasing IEM concentration, indicating that there are excessive isocyanate groups remaining in the coating layer after the copolymerization process.
XPS spectroscopy was employed to better understand the interfacial chemical composition of the modified samples. As shown in Fig. 2, the modified fabric sample exhibits C1, N1, and O1 s peaks (Fig. 2a), but the pristine cotton fabric sample only displays two typical binding energy peaks for C1 and O1 s at 284 and 532 eV (Fig. 2c). Furthermore, the deconvoluted C1 s spectrum (Fig. 2b) of the modified fabric sample is different from that of the pristine cotton (Fig. 2d). The peaks at 285.9 and 288.7 eV, which are assigned to C=O and C–N bonds, respectively, arise only in the modified cotton sample. Compared to Fig. 2d, the decrescence of the C–OH peak and appearance of the C–N peak in Fig. 2b suggest that the isocyanate reacts with the hydroxyl groups at the interface of the cotton fiber.
The surface morphologies of the modified cotton surfaces were investigated using FE-SEM. Figure 3 shows the low- and high-magnification SEM images for the modified fabrics with a variety of IEM dosages. Low magnification SEM images of the original and modified cotton fibers, shown respectively in Fig. 3a, b, exhibit no significant morphological difference on their fabric surface. The statistic diameters calculated from the SEM images are 14.7 ± 1.6 and 15.0 ± 1.0 μm for the pristine and modified cotton fibers, respectively, meaning that the copolymer layer is very thin. High-magnification SEM images of the modified fabrics (Fig. 3c–f) display the presence of wrinkled structures (300–600 nm) on the fiber surface. The surface roughness of the modified fibers increase with increasing IEM dosage, suggesting that the wrinkle structures may be caused by the combination of the precipitation of the networked polymer and reaction between the isocyanate and hydroxyl groups of cotton. Undoubtedly, the fine structures are helpful for improving the surface superhydrophobicity.
Low magnification SEM images of a pristine and b modified cotton fabrics (IEM/LMA = 12/100); high magnification SEM images of the modified cotton fabrics with different IEM/LMA ratios: c 4/100, d 8/100, e 12/100, and f 16/100. Modification conditions: LMA, 0.700 mol l−1; EGD, 0.014 mol l−1; mist feeding, 1 min
The surface wetting behavior of the modified fabrics was evaluated by measuring the static water contact angle. Figure 4a shows the relationship between the water contact angle and mist feeding time for each of the IEM loading levels. For the mist solution containing 0.028 mol l−1 IEM (IEM/LMA = 4 mol%), the static WCA increased steadily upon increasing the mist feeding time, trending to a constant value of 151°. At other IEM loadings, the WCA increased first and then slowed down to being almost constant, and their maximum WCA values were obtained within 30–60 s. The best superhydrophobicity, WCA of 157°, was obtained at the optimized condition, IEM/LMA = 12 mol%, 1 min of the mist feeding time. For original cotton fabrics, the capillary action generally drives the water droplets to move through the fabric from one face to the other side. In contrast, the modified fabrics show stable superhydrophobicity as shown in Fig. 4b. The WCA values decreased by <7 % over 30 min. This result indicates that the inherent capillary action of the original cotton fabrics is weakened by the mist copolymerization.
The optical observations in Fig. 5 also show the asymmetrical superhydrophobicity of the modified fabrics. The water droplet (stained by blue ink) quickly disappeared on the unmodified face, whereas another water droplet placed on the modified side kept the spherical shape and exhibited a high contact angle of over 150° (Fig. 5a). A similar superhydrophobic behavior was observed on the modified surface with a variety of water droplets stained by other inks or coffee (Fig. 5b). Figure 5c, d shows the modified cotton fabrics immersed in water. The modified surface remained dry with many air bubbles, but the non-modified fabric was fully wetted. Figure 5e, f compares the wetting results of the modified and unmodified surfaces after a dynamic water repellency test (video is provided in Supporting Information Video S1 and 2). The fabric sample was placed 1.5 cm below the nozzle and at 45° from the horizontal, and liquid coffee was continuously dropped on the fabric for 30 s. After the coffee droplets rolled down to the fabric, almost no dirt was stuck on the modified side (Fig. 5e), but an obvious dirt trace was left on the unmodified side (Fig. 5f).
Optical images of the asymmetrically superhydrophobic cotton fabrics, including stained water droplets on the cotton fabric surfaces with or without modification (a), different droplets (water, coffee, and stained water) on the modified cotton surface (b), the modified (c) and unmodified (d) cotton surfaces in water, and the wetting results after a dynamic water repellency test of the cotton surfaces with (e) or without (f) modification. Modification conditions: LMA, 0.700 mol l−1; IEM, 0.084 mol l−1; EGD, 0.014 mol l−1; mist feeding, 1 min
Wearability, laundering durability, and healability of the superhydrophobic surfaces
A Martindale-type abrasion test was employed to evaluate the durability and the healing ability of the modified fabrics. As shown in Fig. 6a, the WCA of the modified fabric decreases by several degrees every 200 abrasive cycles, but the loss can be regained mostly by the ironing treatment at 200 °C for 1 min. The decreased amplitude of the WCA for each 200 abrasion cycles increases with increasing abrasion cycle numbers; however, the repeated ironing processes made the recovered WCA surpass 150° even up to 1800 cycles. In contrast, the copolymer coating without IEM loading was not durable toward abrasion, as shown in Fig. 6c. The WCA rapidly declined from 154° to 122° at 1000 abrasion cycles, and the WCA loss was not recoverable. Additional results are provided in Fig. 6b to show the effect of the healing frequency on the abrasion durability of the modified cotton fabrics. After 2000 abrasion cycles, the modified fabric healed three times (Fig. 6b), showing a higher WCA compared with the fabric healed ten times (Fig. 6a). This result suggests that the frequent healing treatments may reduce the abrasion durability and even the healing ability of the modified cotton fabrics.
The laundering durability was assessed by a stringent washing process based on AATCC Test Method 61-2003. Figure 7 shows the WCA of the modified cotton fabrics after a number of laundering cycles. Sample 1 was the cotton fabric modified using LMA, EGD, and IEM monomers, while sample 2 was the control without IEM loading. As shown in Fig. 7a, sample 1 kept the WCA almost constant over 30 washing cycles, but the WCA of sample 2 linearly decreased with increasing washing cycle numbers (Fig. 7c). Similar to the previous work (Wang et al. 2014), the improved laundering durability of sample 1 is contributed to the addition of the IEM monomer, which can increase linkages between the grafting polymer and cotton fibers by reactions between the isocyanate groups and the hydroxyl groups in cellulose chains. Although the ironing treatment recovered the WCA of both sample 1 and 2 by several degrees (Fig. 7b, d), it could not make sample 2 superhydrophobic (WCA > 150°).
To investigate the healing mechanism of the superhydrophobicity, FE-SEM and ATR-FTIR were employed to explore the morphological changes and chemical reactions caused by the ironing treatment, respectively.
SEM images show that the pristine cotton fiber has a smooth surface (Fig. 8a), whereas the modified fiber exhibits a nanoscale wrinkled surface (Fig. 8b), which produces a high water repellency (WCA = 156.3°). After 1600 abrasion cycles, the wrinkles were almost removed from the fiber surface as shown in Fig. 8c, but several scale-like fragments, which are guessed to be the disintegrated parts of the copolymer layer, remained. This relatively smooth fiber surface corresponds to the lowered WCA of 143.6°. Remarkably, the smooth fiber surface wrinkles again after the ironing treatment (Fig. 8d), and the WCA was recovered to 152.5°.
SEM images of a pristine cotton fabric, b the modified cotton fabric before the abrasion test, c the modified cotton fabric after 1600 abrasion cycles, and d the modified cotton fabric after 1600 abrasion cycles and an ironing treatment. Modification conditions: LMA, 0.700 mol l−1; IEM, 0.084 mol l−1; EGD, 0.014 mol l−1; mist feeding, 1 min
Figure 9 shows the ATR-FTIR spectra of the four samples for each step in the abrasion-healing process, revealing the interesting result that the peak at 2268 cm−1 disappears after the ironing treatment (Fig. 9d). This suggests that a considerable number of residual isocyanate groups existed in the polymer coating after the abrasion tests, but finally converted to other bonds via some heating reactions. As shown in Scheme 2, isocyanate may reversibly react with the urethane group to form an allophanate (Scheme 2a) or irreversibly react with other isocyanate groups to produce heterocyclic isocyanurate compounds (Scheme 2b) (Delebecq et al. 2013) at an elevated temperature. The first reaction may cause the damaged polymer coatings to wrinkle again, while the last one is likely to consume the reversible groups such as allophanate. In summary, the isocyanate group plays important roles for improving the abrasion durability of the modified cotton fabrics. It not only increases the linkages between the copolymer layer and cotton surface by the reactions with the hydroxyl groups of cellulose, but also endows the modified fabric with healing ability by reactions like in Scheme 2. Besides the chemical reactions, physical realignment of the copolymer chains is another factor involved in the healing behaviors of the modified coating. When the coating was heated (PLMA, Tg < 0 °C) (Chatterjee and Mandal 2006), the long side chains of PLMA could rapidly turn to cover the loss of the aliphatic chains.
IR-ATR spectra of a pristine cotton fabric, b the modified cotton fabric before the abrasion test, c the modified cotton fabric after 1600 abrasion cycles, and d the modified cotton fabric after 1600 abrasion cycles and an ironing treatment. Modification conditions: LMA, 0.700 mol l−1; IEM, 0.084 mol l−1; EGD, 0.014 mol l−1; mist feeding, 1 min
Influences on the intrinsic properties of cotton
The balance between new functionalities and the desired cotton natures is important for finished cotton fabric products. Therefore, the influence on flexibility, water absorptivity, and vapor permeability of the modified fabrics was examined. Water vapor transmission rates were tested at 25 °C and 50 % RH for 1 day. As shown in Fig. 10a, the vapor transmission rate of the fully modified (dipping method) cotton fabrics is 708 ± 30 g m−2 d−1, only about 62 % of that of pristine cotton fabric, 1145 ± 35 g m−2 d−1. Comparatively, the cotton fabrics modified by the mist copolymerization process show acceptable vapor transmission rates above 1000 g m−2 d−1, which is still satisfactory for practical use.
Influence of the surface modification on several cotton natures, including a water vapor transmissibility, b water absorbability, c tensile strength, and d flexibility. In graphs (a) and (b), the bars refer to the pristine cotton fabric (1), the modified cotton fabrics with different mist feeding time (2–5), and the fully modified cotton fabric (6), respectively; in graph (c) and photo (d), the numbers refer to the pristine cotton fabric (1), the modified cotton fabric with mist feeding time of 1 min (2), and the fully modified cotton fabric (3). Modification conditions: LMA, 0.700 mol l−1; IEM, 0.084 mol l−1; EGD, 0.014 mol l−1
Figure 10b shows the water absorptivity of the modified cotton fabrics. The pristine cotton fabrics are of good water absorptivity above 270 %, but the fully modified cotton fabrics are poor at 75 %. In contrast, the cotton fabrics modified by mist polymerization show a medium-level water absorptivity ranging from 260 to 175 %, which is slightly lower than that of the original cotton fabric. These results suggest that the cotton fabrics with a single-sided modified surface keep a large part of the excellent water absorptivity of cotton. For most clothing products, the desired water absorption can lower wetting by sweat drops, thereby being pleasant for the wearer.
The mechanical properties of the cotton fabrics were also studied by measuring the breaking tensile strength. As shown in Fig. 10c, the pristine cotton fabric has a general breaking strength of 17.7 MPa, whereas the modified cotton fabrics are lightly strengthened by the copolymerization process. The test results for the cotton fabrics modified via mist polymerization and the solution dipping method are 18.9 and 22.3 MPa, respectively.
Figure 10d compares the flexibilities of the modified cotton fabrics and pristine cotton fabric. The original cotton fabric exhibited a good flexibility, as the height of the loop less than 10.2 mm, whereas the fully modified cotton fabric revealed a large loop height of more than 17.0 mm. The fabric modified by the mist polymerization showed a small loop height of 10.8 mm, which is similar to that of the original cotton fabric.
Conclusion
We have demonstrated a one-pot mist copolymerization technique that enables fabrication of healable and asymmetrically superhydrophobic cotton fabrics. With sufficient IEM loading, a randomly wrinkled morphology exhibiting nanoscale roughness is formed on the cotton fiber surface as shown by SEM. The combination of the hydrophobic composition of PLMA and the fine wrinkles endows the modified surface with superhydrophobicity as the water contact angles are greater than 150°. The superhydrophobic copolymer layers are abrasion resistant, laundering-durable, and healable by a simple ironing treatment. Notably, the one-pot mist copolymerization process does not significantly affect the original cotton characteristics such as flexibility, water absorptivity, and vapor permeability. Considering the excellent balance of the new functionalities with the intrinsic natures of cotton fabrics, we believe the process has potential for fabricating superhydrophobic surfaces for textiles and other industrial fabrics.
References
Abbas R, Khereby MA, Sadik WA, El Demerdash AGM (2014) Fabrication of durable and cost effective superhydrophobic cotton textiles via simple one step process. Cellulose 22:887–896. doi:10.1007/s10570-014-0514-x
Abou-Okeil A, El-Sawy SM, Abdel-Mohdy FA (2013) Flame retardant cotton fabrics treated with organophosphorus polymer. Carbohyd Polym 92:2293–2298. doi:10.1016/j.carbpol.2012.12.008
Ali SW, Purwar R, Joshi M, Rajendran S (2014) Antibacterial properties of aloe vera gel-finished cotton fabric. Cellulose 21:2063–2072. doi:10.1007/s10570-014-0175-9
Andou Y, Jeong J-M, Hiki S, Nishida H, Endo T (2009a) Design of nanocomposites by vapor-phase assisted surface polymerization. Macromolecules 42:768–772. doi:10.1021/ma802453n
Andou Y, Jeong J-M, Nishida H, Endo T (2009b) Simple procedure for polystyrene-based nanocomposite preparation by vapor-phase-assisted surface polymerization. Macromolecules 42:7930–7935. doi:10.1021/ma901357p
Chatterjee DP, Mandal BM (2006) Triblock Thermoplastic elastomers with poly(lauryl methacrylate) as the center block and poly(methyl methacrylate) or poly(tert-butyl methacrylate) as end blocks. Morphology and thermomechanical properties. Macromolecules 39:9192–9200. doi:10.1021/ma061391q
Chen F, Zhang D, Yang Q et al (2013) Bioinspired wetting surface via laser microfabrication. ACS Appl Mater Interfaces 5:6777–6792. doi:10.1021/am401677z
Chen S, Li X, Li Y, Sun J (2015) Intumescent flame-retardant and self-healing superhydrophobic coatings on cotton fabric. ACS Nano 9:4070–4076. doi:10.1021/acsnano.5b00121
Chu Z, Feng Y, Seeger S (2015) Oil/water separation with selective superantiwetting/superwetting surface materials. Angew Chem Int Ed 54:2328–2338. doi:10.1002/anie.201405785
Conder JM, Hoke RA, Wolf Wd, Russell MH, Buck RC (2008) Are PFCAs bioaccumulative? A critical review and comparison with regulatory criteria and persistent lipophilic compounds. Environ Sci Technol 42:995–1003. doi:10.1021/es070895g
Crick CR, Bear JC, Kafizas A, Parkin IP (2012) Superhydrophobic photocatalytic surfaces through direct incorporation of titania nanoparticles into a polymer matrix by aerosol assisted chemical vapor deposition. Adv Mater 24:3505–3508. doi:10.1002/adma.201201239
Darmanin T, De Givenchy ET, Amigoni S, Guittard F (2013) Superhydrophobic surfaces by electrochemical processes. Adv Mater 25:1378–1394. doi:10.1002/adma.201204300
Dastjerdi R, Montazer M (2010) A review on the application of inorganic nano-structured materials in the modification of textiles: focus on anti-microbial properties. Colloid Surf B 79:5–18. doi:10.1016/j.colsurfb.2010.03.029
Delebecq E, Pascault J-P, Boutevin B, Ganachaud F (2013) On the versatility of urethane/urea bonds: reversibility, blocked isocyanate, and non-isocyanate polyurethane. Chem Rev 113:80–118. doi:10.1021/cr300195n
Dong C, Gu Y, Zhong M, Li L, Sezer K, Ma M, Liu W (2011) Fabrication of superhydrophobic Cu surfaces with tunable regular micro and random nano-scale structures by hybrid laser texture and chemical etching. J Mater Process Technol 211:1234–1240. doi:10.1016/j.jmatprotec.2011.02.007
Dyett BP, Wu AH, Lamb RN (2014) Mechanical stability of surface architecture-consequences for superhydrophobicity. ACS Appl Mater Interfaces 6:18380–18394. doi:10.1021/am505487r
Hua Z, Yang J, Wang T, Liu G, Zhang G (2013) Transparent surface with reversibly switchable wettability between superhydrophobicity and superhydrophilicity. Langmuir 29:10307–10312. doi:10.1021/la402584v
Hwang HS, Kim NH, Lee SG, Lee DY, Cho K, Park I (2011) Facile fabrication of transparent superhydrophobic surfaces by spray deposition. ACS Appl Mater Interfaces 3:2179–2183. doi:10.1021/am2004575
Isquith A, Slesinski R, Matheson D (1988) Genotoxicity studies on selected organosilicon compounds: in vivo assays. Food Chem Toxicol 26:263–266. doi:10.1016/0278-6915(88)90128-7
Kim TH, Ha SH, Jang NS et al (2015) Simple and cost-effective fabrication of highly flexible, transparent superhydrophobic films with hierarchical surface design. ACS Appl Mater Interfaces 7:5289–5295. doi:10.1021/am5086066
Lee W, Park BG, Kim DH, Ahn DJ, Park Y, Lee SH, Lee KB (2010) Nanostructure-dependent water-droplet adhesiveness change in superhydrophobic anodic aluminum oxide surfaces: from highly adhesive to self-cleanable. Langmuir 26:1412–1415. doi:10.1021/la904095x
Liu H, Feng L, Zhai J, Jiang L, Zhu D (2004) Reversible wettability of a chemical vapor deposition prepared ZnO film between superhydrophobicity and superhydrophilicity. Langmuir 20:5659–5661. doi:10.1021/la036280o
Liu B, He Y, Fan Y, Wang X (2006) Fabricating super-hydrophobic lotus-leaf-like surfaces through soft-lithographic imprinting. Macromol Rapid Commun 27:1859–1864. doi:10.1002/marc.200600492
Liu K, Cao M, Fujishima A, Jiang L (2014) Bio-inspired titanium dioxide materials with special wettability and their applications. Chem Rev 114:10044–10094. doi:10.1021/cr4006796
Miao H, Bao F, Cheng L, Shi W (2010) Cotton fabric modification for imparting high water and oil repellency using perfluoroalkyl phosphate acrylate via γ-ray-induced grafting. Radiat Phys Chem 79:786–790. doi:10.1016/j.radphyschem.2010.01.017
Nguyen DD, Tai NH, Lee SB, Kuo WS (2012) Superhydrophobic and superoleophilic properties of graphene-based sponges fabricated using a facile dip coating method. Energy Environ Sci 5:7908–7912. doi:10.1039/c2ee21848h
Nystrom D, Lindqvist J, Ostmark E, Hult A, Malmstrom E (2006) Superhydrophobic bio-fibre surfaces via tailored grafting architecture. Chem Commun 3594–3596. doi:10.1039/b607411a
Ottone C, Lamberti A, Fontana M, Cauda V (2014) Wetting behavior of hierarchical oxide nanostructures: TiO2 nanotubes from anodic oxidation decorated with ZnO nanostructures. J Electrochem Soc 161:D484–D488. doi:10.1149/2.0431410jes
Periolatto M, Ferrero F, Montarsolo A, Mossotti R (2012) Hydrorepellent finishing of cotton fabrics by chemically modified TEOS based nanosol. Cellulose 20:355–364. doi:10.1007/s10570-012-9821-2
Qian B, Shen Z (2005) Fabrication of superhydrophobic surfaces by dislocation-selective chemical etching on aluminum, copper, and zinc substrates. Langmuir 21:9007–9009. doi:10.1021/la051308c
Shateri-Khalilabad M, Yazdanshenas ME (2013) One-pot sonochemical synthesis of superhydrophobic organic–inorganic hybrid coatings on cotton cellulose. Cellulose 20:3039–3051. doi:10.1007/s10570-013-0040-2
Shin B, Lee K-R, Moon MW, Kim HY (2012) Extreme water repellency of nanostructured low-surface-energy non-woven fabrics. Soft Matter 8:1817–1823. doi:10.1039/c1sm06867a
Shirtcliffe NJ, McHale G, Newton MI (2011) The superhydrophobicity of polymer surfaces: recent developments. J Polym Sci Part B Polym Phys 49:1203–1217. doi:10.1002/polb.22286
Sparks BJ, Hoff EF, Xiong L, Goetz JT, Patton DL (2013) Superhydrophobic hybrid inorganic–organic thiol-ene surfaces fabricated via spray-deposition and photopolymerization. ACS Appl Mater Interfaces 5:1811–1817. doi:10.1021/am303165e
Wan SJ, Wang L, Xu XJ, Zhao CH, Liu XD (2014) Controllable surface morphology and properties via mist polymerization on a plasma-treated polymethyl methacrylate surface. Soft Matter 10:903–910. doi:10.1039/C3SM52434E
Wang H, Zhou H, Gestos A, Fang J, Lin T (2013) Robust, superamphiphobic fabric with multiple self-healing ability against both physical and chemical damages. ACS Appl Mater Interfaces 5:10221–10226. doi:10.1021/am4029679
Wang L, Xi GH, Wan SJ, Zhao CH, Liu XD (2014) Asymmetrically superhydrophobic cotton fabrics fabricated by mist polymerization of lauryl methacrylate. Cellulose 21:2983–2994. doi:10.1007/s10570-014-0275-6
Wang B, Liang W, Guo Z, Liu W (2015a) Biomimetic super-lyophobic and super-lyophilic materials applied for oil/water separation: a new strategy beyond nature. Chem Soc Rev 44:336–361. doi:10.1039/c4cs00220b
Wang J-N, Zhang Y-L, Liu Y, Zheng W, Lee LP, Sun H-B (2015b) Recent developments in superhydrophobic graphene and graphene-related materials: from preparation to potential applications. Nanoscale 7:7101–7114. doi:10.1039/c5nr00719d
Wen L, Tian Y, Jiang L (2015) Bioinspired super-wettability from fundamental research to practical applications. Angew Chem Int Ed 54:3387–3399. doi:10.1002/anie.201409911
Wenzel RN (1936) Resistance of solid surfaces to wetting by water. Ind Eng Chem 28:988–994. doi:10.1021/ie50320a024
Wolfs M, Darmanin T, Guittard F (2013) Superhydrophobic fibrous polymers. Polym Rev 53:460–505. doi:10.1080/15583724.2013.808666
Xi G, Xiu Y, Wang L, Liu X (2015) Antimicrobial N-halamine coatings synthesized via vapor-phase assisted polymerization. J Appl Polym Sci 132:41824. doi:10.1002/app.41824
Xu W, Song J, Sun J, Lu Y, Yu Z (2011) Rapid fabrication of large-area, corrosion-resistant superhydrophobic Mg alloy surfaces. ACS Appl Mater Interfaces 3:4404–4414. doi:10.1021/am2010527
Xue CH, Guo XJ, Ma JZ, Jia ST (2015) Fabrication of robust and antifouling superhydrophobic surfaces via surface-initiated atom transfer radical polymerization. ACS Appl Mater Interfaces 7:8251–8259. doi:10.1021/acsami.5b01426
Yang R, Asatekin A, Gleason KK (2012) Design of conformal, substrate-independent surface modification for controlled proteinadsorption by chemical vapor deposition (CVD). Soft Matter 8:31–43. doi:10.1039/c1sm06334k
Yao T, Wang C, Lin Q et al (2009) Fabrication of flexible superhydrophobic films by lift-up soft-lithography and decoration with Ag nanoparticles. Nanotechnology 20:065304. doi:10.1088/0957-4484/20/6/065304
Yu M, Wang Z, Liu H et al (2013) Laundering durability of photocatalyzed self-cleaning cotton fabric with TiO(2) nanoparticles covalently immobilized. ACS Appl Mater Interfaces 5:3697–3703. doi:10.1021/am400304s
Zhang YL, Xia H, Kim E, Sun HB (2012) Recent developments in superhydrophobic surfaces with unique structural and functional properties. Soft Matter 8:11217–11231. doi:10.1039/c2sm26517f
Zhou H, Wang H, Niu H, Gestos A, Wang X, Lin T (2012) Fluoroalkyl silane modified silicone rubber/nanoparticle composite: a super durable, robust superhydrophobic fabric coating. Adv Mater 24:2409–2412. doi:10.1002/adma.201200184
Zou H, Lin S, Tu Y et al (2013) Simple approach towards fabrication of highly durable and robust superhydrophobic cotton fabric from functional diblock copolymer. J Mater Chem A 1:11246–11260. doi:10.1039/c3ta12224g
Acknowledgments
This work was financially supported by the Natural Science Foundation of China (51573167), Scientific Research Foundation for the Returned Overseas Chinese Scholars, and State Education Ministry (1101603-C).
Author information
Authors and Affiliations
Corresponding author
Additional information
Guanghui Xi and Jun Wang have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (AVI 6943 kb)
Supplementary material 2 (AVI 6856 kb)
Rights and permissions
About this article
Cite this article
Xi, G., Wang, J., Luo, G. et al. Healable superhydrophobicity of novel cotton fabrics modified via one-pot mist copolymerization. Cellulose 23, 915–927 (2016). https://doi.org/10.1007/s10570-015-0773-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-015-0773-1