Abstract
Cotton fabrics were modified with difunctional polysiloxanes to impart biocidal and hydrophobic properties. The modification was performed in two steps, using tetraethoxysilane in the first step, and then polysiloxanes having in their structure both alkoxy groups and long-chain quaternary ammonium salts in the second step. The modified fabrics were tested for the action of a mixture of five mold species that most often cause decomposition of cellulose. The hydrophobicity was determined by measuring the water contact angle. Moreover, samples were evaluated by SEM, FTIR Spectra and elemental analysis. Results showed that multifunctional fabrics were obtained with both biocidal and hydrophobic properties that are resistant to washing. All modified samples showed protection against mold growth on level 2 and a water contact angle up to 140°. The modification does not cause any apparent changes such as stiffening, color changes, or decrease in mechanical properties.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The growing interest in the ecological solutions to the problems associated with the application of renewable raw materials such as fibers and natural fabrics forces manufacturers to search for innovations aimed at increasing the use of the above materials. The lack of resistance to microorganisms in natural textiles allows them to be a favorable medium for the development of microorganisms, e.g. fungi and bacteria that weakens the textiles and are hazardous to human health. Furthermore, their high absorption of moisture supports microbial development.
To avoid the above drawbacks, natural fibers are subjected to some surface modifications that should result in the formation of a biocidal and/or hydrophobic barrier. One of the ways of modification of fibers and natural fabrics is the immobilization of agents imparting functionality to the silica sol formed as a result of hydrolysis and condensation of silica precursors (Brinker and Scherer 1989; Perliolatto et al. 2013; Mahltig et al. 2005; Przybylak et al. 2016a, b, c). The application of this method makes it possible to form on the surface of fibers a siloxane layer into which incorporated are modifying agents and this improves its mechanical properties and creates a barrier on the surface. Many papers on the modification of fabrics by the sol–gel method have been published by Textor and Böttcher, who performed numerous optimizations aimed at obtaining desired properties (Böttcher 2000; Mahltig et al. 2009; Böttcher et al. 1999; Textor 2009; Textor and Mahltig 2010; Textor et al. 2010). The following agents have been applied to impart biocidal properties to natural fibers: quaternary ammonium salts (Chen et al. 2013; Lin et al. 2014; Qin et al. 2015; Hou and Shi 2009), quaternary phosphonium salts (Gao et al.2013; Cai et al. 2013), gold and silver nanoparticles (Scholz et al. 2005; Tomšič et al. 2009; Textor et al. 2010; Fouda et al. 2013), triclosan (Ibrahim et al. 2015; Mahbubul and Sunderland 2015; Chen et al. 2010), N-halamines (Liu et al. 2013; Jiang et al. 2014; Ren et al. 2008a, b; Cheng et al. 2015; Wu et al. 2014; Chen et al. 2012), chitosan and its derivatives (Shin et al. 2013; Wang et al. 2012; Xiao et al. 2013). In our earlier study we have applied silica sol (prepared from tetraethoxysilane) in which triclosan and quaternary ammonium salt were immobilized, followed by using this product for the modification of cotton fabrics. As a result of the those processes, fabrics with excellent fungicidal and hydrophobic properties (as well as resistance to washing) were obtained (Foksowicz-Flaczyk et al. 2016).
Modification of natural textiles can be carried out using organosilicon compounds containing at the same time reactive groups, e.g. alkoxy groups, which are susceptible to hydrolysis and condensation with hydroxyl groups (present on fiber surfaces) and functional groups imparting desired properties to surfaces subjected to the modification (Xie et al. 2010). Our research group produced hydrophobic natural fabrics using difunctional polysiloxanes and silsesquioxanes containing alkoxy groups and long fluoroalkyl chains. As a result of the performed impregnation experiments, highly hydrophobic and superhydrophobic fabrics were obtained that were fully resistant to washing (Przybylak et al. 2016a, b, c). The combination of our experience in the modification of fabrics with difunctional polysiloxanes and in the modification with silica sol containing quaternary ammonium salts was an idea for a new way of obtaining difunctional textiles with biocidal and hydrophobic properties.
These studies were aimed at synthesizing new difunctional polysiloxanes with biocidal activity and applying them onto the surface of cotton fabrics. Difunctional polysiloxanes were not employed so far to impart biocidal and hydrophobic properties to cotton fabrics.
In the present work we have employed difunctional polysiloxanes, the structures of which contain alkoxy groups capable of forming bonds with hydroxyl groups of cellulose and long-chain quaternary ammonium salts responsible for the biocidal activity. The effect of the modification with tetraethoxysilane in the sol–gel process on the effectiveness of the processes was also studied. The samples were subjected to microbiological tests, whereas hydrophobicity was evaluated by measuring water contact angle.
Experimental
Materials
100% cotton fabric with 145 g/m2 was used for this study. Before the modification process, the fabric was bleached in a hydrogen peroxide bath.
Mold strains of Aspergillus niger van Tieghem, Chaetomium globosum Kunze, Aureobasidium pullulans (de Bary) Arnaud, Paecilomyces variotii Bainier and Penicillium ochrochloron Biourge were purchased from Pure Culture Collection of the Institute of Fermentation Technology and Microbiology, Technical University of Łódź, Poland.
Tetraethoxysilane was obtained from “Unisil” (Tarnów, Poland), poly(dimethyl, hydrogen methyl)siloxane 50/25 was provided by Wacker, and allyl chloride, vinyltriethoxysilane, trialkylamines and other reagents and solvents were purchased from Aldrich.
Synthesis of difunctional polysiloxanes
The synthesis of this type of biocidal polysiloxanes has been developed by research group and has not been published previously. Synthesis of difunctional polysiloxanes was carried in two steps. In the first step, polysiloxane containing 10 trimethoxysilyl groups was obtained by hydrosilylation, while in the next step the reaction of quaternization was employed to substitute chloropropyl groups (present in the intermediate product) with tertiary dimethylalkylamines having alkyl chains of different length (12, 14, 16 carbon atoms). The course of the process is presented in Scheme 1.
Polysiloxane with quaternary ammonium salt
In a three-neck round-bottom flask of 100 ml capacity, equipped with a reflux condenser, a magnetic stirrer and a thermometer, were placed 34 g of poly(dimethyl, hydrogen methyl)siloxane 50/25, followed by adding 9 g of vinyltrimethoxysilane and 5.7 μl of Karstedt’s catalyst (10−5 mol Pt per mol SiH). The mixture was heated at 100–105 °C for about 1 h with continuous stirring. The reaction was monitored by FT-IR spectroscopy (a partial disappearance of Si–H band). Then 9 g of allyl chloride (30% excess) and 8.56 μl of Karstedt’s catalyst were added, followed by heating at 110–115 °C for another 1 h with continuous stirring. Monitoring of the reaction by FT-IR spectroscopy was conducted until the complete disappearance of Si–H band. The excess of allyl chloride was removed by evaporation under reduced pressure. The product was obtained with the yield of 96%.
In the next step of the reaction, in the flask were placed the obtained polysiloxane and a relevant N,N-dimethylalkylamine at the mole ratio of 1:15. The mixture was heated at 70 °C for 24 h. The product, in the form of a dense liquid, was obtained with the yield of 95%.
Spectroscopic characterization of polysiloxane with substituted N,N-dimethyldodecylamine (P12)
1H NMR (CDCl3, 298 K, 300 MHz) (ppm): 0.22 (m, 393H, SiCH3); 0.71 (m, 70H, SiCH2); 0.99 (t, 45H, CH3); 1.30 (m, 270H, -CH2-); 1.37 (m, 30H, CH2CH3); 1.77 (m, 30H, CH2); 3.31 (s, 90H, NCH3); 3.41 (t, 60H, NCH2); 3.59 (s, 90H, OCH3).
13C NMR (CDCl3, 298 K, 75.5 MHz) (ppm): − 3.84 (SiCH3); − 1.35 (SiCH2); 0.49 (Si(CH3)2); 0.99 (Si(CH3)3); 7.80 (SiCH2); 8.97 (CH2Si); 14.02–31.65 (-CH2-); 50.73 (OCH3); 51.94 (NCH3); 65.21 (NCH2); 65.47 (NCH2).
29Si NMR (CDCl3, 298 K, 59.6 MHz) (ppm): 7.40 (Si(CH3)3); 19.4 (Si(CH3)2); 22 (CH3SiCH2); − 45.90 (Si(OCH3)3).
Spectroscopic characterization of polysiloxane with substituted N,N-dimethyltetradecylamine (P14)
1H NMR (CDCl3, 298 K, 300 MHz) (ppm): 0.22 (m, 393H, SiCH3); 0.71 (m, 70H, SiCH2); 0.99 (t, 45H, CH3); 1.30 (m, 330H, -CH2-); 1.37 (m, 30H, CH2CH3); 1.77 (m, 30H, CH2); 3.31 (s, 90H, NCH3); 3.41 (t, 60H, NCH2); 3.59 (s, 90H, OCH3).
13C NMR (CDCl3, 298 K, 75.5 MHz) (ppm): − 3.84 (SiCH3); − 1.35 (SiCH2); 0.49 (Si(CH3)2); 0.99 (Si(CH3)3); 7.80 (SiCH2); 8.97 (CH2Si); 14.02–31.65 (-CH2-); 50.73 (OCH3); 51.94 (NCH3); 65.21 (NCH2); 65.47 (NCH2).
29Si NMR (CDCl3, 298 K, 59.6 MHz) (ppm): 7.40 (Si(CH3)3); 19.4 (Si(CH3)2); 22 (CH3SiCH2); − 45.90 (Si(OCH3)3).
Spectroscopic characterization of polysiloxane with substituted N,N-dimethylhexadecylamine (P16)
1H NMR (CDCl3, 298 K, 300 MHz) (ppm): 0.22 (m, 393H, SiCH3); 0.71 (m, 70H, SiCH2); 0.99 (t, 45H, CH3); 1.30 (m, 390H, -CH2-); 1.37 (m, 30H, CH2CH3); 1.77 (m, 30H, CH2); 3.31 (s, 90H, NCH3); 3.41 (t, 60H, NCH2); 3.59 (s, 90H, OCH3).
13C NMR (CDCl3, 298 K, 75.5 MHz) (ppm): − 3.84 (SiCH3); − 1.35 (SiCH2); 0.49 (Si(CH3)2); 0.99 (Si(CH3)3); 7.80 (SiCH2); 8.97 (CH2Si); 14.02–31.65 (-CH2-); 50.73 (OCH3); 51.94 (NCH3); 65.21 (NCH2); 65.47 (NCH2).
29Si NMR (CDCl3, 298 K, 59.6 MHz) (ppm): 7.40 (Si(CH3)3); 19.4 (Si(CH3)2); 22 (CH3SiCH2); − 45.90 (Si(OCH3)3).
Modification of cotton fabrics
The modification of the cotton fabrics was carried out either in a one-step or a two-step process. All samples were subjected to chemical modification in polysiloxane (P12, P14, P16) solutions, however, a part of them was additionally modified in the first step with silica sol. The durability of hydrophobic and biocidal properties of the studied fabrics was determined after the modification and after one and five washings at 40 °C for 1 h.
-
(a)
Modification with silica sol (S)
Tetraethoxysilane (10 vol.%), distilled water (0.9 vol.%), dibutyltin diacetate (0.9 vol.%) and isopropanol (88.2%) were placed in a flask equipped with a magnetic stirrer and a reflux condenser. A 15-min hydrolysis process was conducted at room temperature and after that time the solution was transferred into trays in which the cotton fabrics were impregnated for 2 min. Then the excess of the modifier solution was removed by squeezing followed by drying the samples at 80 °C for 2 h.
-
(b)
Chemical modification with polysiloxanes (P12, P14, P16)
In a round-bottom flask equipped with a reflux condenser and a magnetic stirrer were placed 5 vol.% of isopropanolic solution of polysiloxane and 5 vol.% of water to conduct hydrolysis for 15 min at room temperature. The obtained solution was transferred into trays and the bleached fabrics or tetraethoxysilane-modified samples were impregnated for 15 min. After impregnation, the samples were squeezed followed by drying for 60 min at 80 °C and cured for 3 min at 130 °C.
Characterization of polysiloxanes with quaternary ammonium salt (P12, P14, P16)
Nuclear magnetic resonance spectroscopy (NMR)
Spectra of nuclear magnetic resonance 1H NMR (300 MHz), 13C NMR (75 MHz), 29Si NMR (59 MHz) were obtained on a Varian XL 300 spectrometer at room temperature using CDCl3 as a solvent.
Characterization of modified samples
Determination of hydrophobic properties
The water contact angles (WCA) were measured using an automatic video contact-angle testing apparatus Krüss model DSA 100 Expert. A 10 µL volume of water was applied onto the treated cotton fabrics and the contact angle was determined from the video camera images of the drop in the course of its formation. Each measurement is an average from five drops. The experimental error was ± 2%.
Determination of the amount of modifiers applied on fabrics (add-on)
The total dry solid add-on in cotton fabric samples (A) has been determined by weighing a fabric sample before (Wi) and after (Wf) modification by a given composition and thermal fixing. Analytical balance Ohaus was used for the measurements. The uptake (Table 1) was calculated according to the following equation:
Determination of biocidal properties
The determination of the resistance of tested fabrics to the action of molds was conducted according to the standard EN 14119:2003 “Testing of textiles. Evaluation of the action of microfungi”. An agar medium with pH 6.0–6.5 was used. The modified and unmodified samples of cotton fabric were exposed to the action of the following moulds: A.niger, C. globosum, A. pullulans, P. variotii and P. ochrochloron. The incubation of tested samples was conducted for 4 weeks at the temperature of 29 ± 1 °C and relative air humidity at 90%.
After the tests, the evaluation of biocidal properties was performed (according to the standard EN 14119:2003) on the basis of visual assessment consisting in the determination of the growth degree of molds, the size of inhibition zone expressed in mm and the change in breaking force. The rating system for the mold growth was as follows: 0—no visible growth evaluated microscopically, 1—no visible growth evaluated with naked eye but clearly visible microscopically, 2—growth visible with naked eye, covering up to 25% of a tested surface, 3—growth visible with naked eye, covering up to 50% of a tested surface, 4—considerable growth, covering more than 50% of a tested surface, 5—very intense growth, covering all tested surface.
The determination of breaking force of modified fabrics using the strip method was carried out according to the standard PN-EN ISO 13934-1:2013-07 “Part 1: Determination of maximum force and elongation at maximum force using the strip method” The samples were conditioned at 65 ± 2% relative air humidity and at 29 ± 10C for 24 h before the determination of breaking force. The relative loss of breaking force (expressed in %) caused by molds action was calculated from the mean value of six samples, using the formula:
where: A is the arithmetic mean value of breaking force for all samples exposed to the action of molds, B is the arithmetic mean value of breaking force for all samples unexposed to the action of molds.
Studies of surface topography
The microscopic evaluation of surface changes of modified and unmodified samples of the cotton fabric was carried out using a Hitachi S-3400 N scanning electron microscope (SEM), where samples were coated with a thin layer of gold before performing observations. Moreover, scanning electron microscopy (SEM) images were taken using a FEI Quanta 250 FEG instrument equipped with a large field detector (LFD) which records the secondary electrons (SE). In the latter case (i.e. the LFD SE measurements) the samples were not covered by any layer. The microscope was operated at low vacuum mode (70 Pa) and the accelerating voltage was 10 kV.
Analysis of elemental composition of applied coating
The analysis was carried out by employing the SEM-EDS technique to determine ultimate elements (Si, C, O) present in modifying mixtures. A Hitachi S-3500N scanning electron microscope (SEM) equipped with EDS (energy-dispersive X-ray) detector (Ultra Dry Silicon Drift X-ray Detector made by Thermo Scientific) was used in the measurements.
FT-IR analysis
FT-IR spectra of the modifiers and modified fabrics were taken on a Bruker spectrometer, model Tensor 27 with a Specac Golden Gate single reflection diamond ATR accessory.
Results and discussion
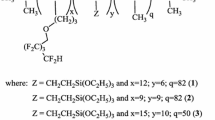
Bleached cotton fabrics were modified with three polysiloxanes (P12, P14, P16) of the general formula 1. The samples were finished with above mentioned chemicals by pad dry cure method. The employed polysiloxanes were substituted with quaternary ammonium salts that are responsible for biocidal activity and hydrophobic effect and alkoxy groups that are capable of forming covalent bonds with hydroxyl groups. The polysiloxanes differed in the length of hydrocarbon chains of quaternary ammonium salt (Fig. 1).
In the first instance, hydrophobicity of the studied polysiloxanes (P12, P14, P16) was determined by measuring water contact angle of unwashed samples and those subjected to one and five washings. The values of water contact angles of the polysiloxane-modified samples are presented in Fig. 2.
The modification of natural fabrics by polysiloxanes with built-in quaternary ammonium salts (P12, P14, P16) resulted in hydrophobization of the fabrics as reflected by values of water contact angles which in most cases were above 110°. After washing the hydrophobicity remained relatively stable, particularly for samples modified by polysiloxane containing built-in quaternary ammonium salt with 16 carbon atoms in its alkyl chain (P16). To increase the hydrophobic effect on the modified fiber surface, we have applied tetraethoxysilane in the sol–gel process in the first step. The modification of fabrics with tetraethoxysilane enables the creation of a siloxane layer with a large number of hydroxyl groups. The formation of siloxane spatial structure with a large number of OH groups can improve binding to polysiloxanes with substituted quaternary ammonium salts and result in their better orientation.
The values of water contact angles on the samples modified in two-steps are shown in Fig. 3.
The application of two-step modification resulted in imparting highly hydrophobic properties to cotton fabrics (water contact angle above 140°, Fig. 4). It is clearly seen that the formation of additional siloxane layer by the sol–gel process substantially increased hydrophobicity due to the creation of an additional barrier protecting from water access. Moreover, as a result of the sol–gel process the number of hydroxyl groups on the fabric surface increased, which improved the effectiveness of binding to polysiloxanes containing quaternary ammonium salts built-in into their structure (P12, P14, P16). It can be seen that the longer hydrocarbon chain in quaternary ammonium salt, the greater the durability of the applied modification. The sample modified with the composition SP16 did not change its contact angle even after multiple washings. On the other hand, the sample modified by polysiloxane containing the quaternary ammonium salt with 12 carbon atoms in its chain (P12) shows less resistance to washing.
All samples obtained in the two-step process have been weighed before and after their modification, as well as after one and five washings. The add-on value was calculated on this basis (Table 1).
The add-on values are comparable for all the samples. Even washing five times has not reduced the add-on value, except for the sample modified with polysiloxane substituted with quaternary ammonium salt containing twelve-carbon atom chain (P12).
The modification of fibers with tetraethoxysilane in the sol–gel process causes the formation of a siloxane layer on their surface. Tetraethoxysilane hydrolyzes in this process to give silanols that undergo condensation among themselves as well as condensation with hydroxyl groups present on the surface of cellulose fibers. The formed siloxane layer abounds in free hydroxyl groups on its surface, therefore its modification (in the second step) by functionalized polysiloxane (P12, P14, P16) enables a durable (covalent) bonding of polysiloxane via condensation of alkoxy groups (present in functionalized polysiloxane) with hydroxyl groups present in the siloxane layer that covers the fibers (as a result of the sol–gel process). This can be seen in SEM images of unmodified fabric (Fig. 5a) and modified fabric denoted as SP16 (Fig. 5b).
In the images (b,c,d) one can see a homogeneous polysiloxane tightly surrounding each of the fibers. No agglomerates or cracks in the polysiloxane layer are visible in the image.
To estimate the thickness of the modifier layer we have made cross-sections of fibers by means of freeze cutting, followed by taking SEM images of the cross-sections. (Figure 6).
Due to high flexibility and strength of polysiloxanes, a precise cutting of polysiloxane layer to be flush with fiber cross-section was impossible. In spite of the application of freeze-cutting technique, the modifier layer was breaking apart and extended beyond the plane of cutting. In the image (Fig. 6 in the revised manuscript) visible are thin layers of the modifier at fibers ends that extend beyond the cutting area. The analysis has shown that the modifier layer thickness is of order of one tenth of micrometer; however, precise measuring was not possible. This is why we employed another method of layer thickness evaluation, using the following eq. (Chen et al. 2017):
where h is the thickness of the modifier layer, d is the average diameter of pristine cotton fiber, W1 and W0 are weights of the cotton fiber weight after and before the impregnation procedure, respectively; ρco and ρqp are densities of cotton and polysiloxane, respectively.
In our study we have found that results obtained by SEM measurements and weight calculations (using the quoted formula) are identical, giving a thickness of the formed layer of 177 nm.
The process of the modification and formation of modifier layers on the fiber surface is presented in Scheme 2.
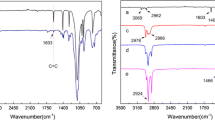
To confirm binding of modifiers to the fabric, FT-IR spectra were taken of modified samples and unmodified fabric (Fig. 7).
In the spectra visible are differences resulting from the attachment of organosilicon compound to cellulose hydroxyl groups. The band at 3330 cm−1, that is characteristic of free OH groups present on the fiber surface, is slightly smaller which indicates bonding between fibers and alkoxysilyl groups. Furthermore, the spectra of the modified samples contain bands at about 800 and 1260 cm−1, originating from Si–O–Si symmetric stretching vibrations and Si–O–C stretching vibration shoulder, respectively. The band expected at 1018 cm−1 (Si–O–Si) is overlapped with a broad band between 950 and 1100 cm−1, attributed to characteristic peaks of cellulose. Moreover, a band is visible that confirms the presence of quaternary ammonium salts in the structure of the modifiers, namely at 2900 cm−1 which originates from stretching vibrations of C–H in CH2 groups present in long-chain quaternary ammonium salts.
The fabrics were also subjected to surface microanalysis to determine elemental composition of samples obtained as a result of modification. The percentages of elements in the modified samples before washing and after five washings is given in Table 2.
The modification with all polysiloxanes, containing built-in quaternary ammonium salts (P12, P14, P16), resulted in changes in the elemental composition of investigated samples. The results of elemental analysis confirm that the modification was successful as evidenced by 7% silicon in impregnated natural fabrics. The introduction of quaternary ammonium salts into polysiloxane structure has also been proved by the presence of chlorine in the samples. Also, silicon and chlorine contents in five-times washed fabrics remained at roughly the same levels as those before washing, which testifies to the resistance to leaching of the waterproofing agent. The only sample showing some reduction in silicon and chlorine contents as a result of washing was SP12.
Biocidal activity of the modifications studied was determined by exposing fabric samples to the actions of five strains of molds. The fungicidal activity was assessed on the five-degree scale (Experimental, 2.6.) Results of fungicidal activity tests, carried out in accordance with the standard EN 14119:2003, are presented in Table 3.
All applied modifiers protected against mold growth on level 2 (up to 25% mold-covered surface) and slight discolorations appeared on sample edges. There are differences between samples washed one and five times. The least resistant to washing were fabrics impregnated by polysiloxane with built-in quaternary ammonium salt having twelve carbon atom-containing alkyl substituent (P12). The whole surface of the aforementioned sample, subjected to five times washing, was totally infected by mold and a considerable reduction in breaking force occurred as well. After a single washing, up to 50% of the sample was covered by mold and a few percent decrease in breaking force has been observed (Fig. 8).
In the case of the fabric modified by polysiloxane with built-in quaternary ammonium chloride having 14 carbon atoms in its alkyl substituent (P14), a moderate mold growth (up to 50% of tested surface), a few percent decrease in breaking force and clearly marked discolorations were observed after five washings. On the other hand, a single washing did not deteriorate fungicidal activity compared to unwashed sample (Fig. 9). However, neither reduction in breaking force nor damage of elementary cotton fibers were not found. In SEM image, single fungal spores have been observed, particularly well visible on fibers of the fabric subjected to five washings (Fig. 10).
The fabric modified by polysiloxane with built-in quaternary ammonium chloride containing 16 carbon atoms in alkyl chain (P16) showed a very good antifungal activity. No reduction in the antifungal effect (compared to unwashed fabric) has been observed both in the case of one and five-times washed fabrics. Only at the sample edges a mold growth occurred, covering up to 25% of sample area, however, it did not cause discolorations (Fig. 11). Neither the modification process nor mold growth resulted in a reduction in fabric strength nor damage of elementary cotton fibers. Slight post-impregnation residues and single clusters of fungi are visible in SEM images (Fig. 12).
The results indicate that all polysiloxanes with built-in quaternary ammonium salts show a strong biocidal effect, irrespective of the length of alkyl chain on the amino group. This observation is in agreement with the literature data that quaternary ammonium salts with alkyl chains containing twelve or more carbon atoms are the most effective from the point of view of biocidal activity (Kaur and Liu 2016; Sun 2016). Long hydrocarbon chains show a greater ease of perforating cell membranes of microorganisms thus resulting in their death. Moreover, the length of alkyl chain of quaternary ammonium salts also had an effect on the durability of the performed modifications. One can notice that the longer hydrocarbon chain the greater durability. Silanes and polysiloxanes form covalent bonds with cellulose hydroxyl groups via alkoxy groups. The created layer forms an additional barrier protecting fabrics and fibers from water access.
Summary
The modification of cotton fabric with difunctional polysiloxanes containing built-in long-chain quaternary ammonium salts resulted in multifunctional fabrics showing both hydrophobic and biocidal activities. The presence of alkoxy groups enables formation of covalent bonds on the modified surface, whereas quaternary ammonium salts impart biocidal properties. Moreover, the created polysiloxane layer exerts a positive effect on hydrophobocity. It has been observed that the longer the hydrocarbon chains in quaternary ammonium salts built-in into the polysiloxane, the greater the durability of the performed modifications and their resistance to washing.
All samples denoted as SP16, irrespective of the number of washings, exhibited a high biocidal activity and strong hydrophobicity, which means that the above modification is totally resistant to washing. No decrease in breaking force under the influence of fungi was observed in the samples modified with the aforementioned composition. SEM images confirmed that the modifying compositions covered fibers and proved the absence of damage caused by fungi in the fiber structure. The presented research is the first study on the application of difunctional polysiloxanes with alkoxy groups and quaternary ammonium salts to the preparation of fabrics with biocidal and hydrophobic propeties. The polysiloxanes employed for the modification of fabrics permit to cover fibers by a homogeneous coating that is waterproof and at the same time protects against fungi.
References
Böttcher H (2000) Bioactive so-gel coatings. Adv Synth Catal 342:427–436. https://doi.org/10.1002/1521-3897(200006)342:5%3C427::AID-PRAC427%3E3.0.CO;2-B
Böttcher H, Jagota C, Trepte J, Kallies KH, Haufe H (1999) Sol-gel composite films with controlled release of biocides. J Controll Release 60:57–65. https://doi.org/10.1016/S0168-3659(99)00053-X
Brinker CJ, Scherer GW (1989) Sol–gel science: the physics and chemistry of sol–gel processing. Academic Press, San Diego
Cai X, Zhang JI, Ouyang Y, Ma D, Tan SZ, Peng YI (2013) Bacteria-adsorbed palygorskite stabilizes the quaternary phosphonium salt with specific-targeting capability, long-term antibacterial activity, and lower cytotoxicicity. Langmuir 29:5279–5285. https://doi.org/10.1021/la400824f
Chen Z, Chisholm BJ, Stfslien S, He J, Patel S (2010) Novel, UV-curable coating containing a tethered biocide: synthesis, characterization, and antimicrobial activity. J Biomed Mater Res A 95A:486–494. https://doi.org/10.1002/jbm.a.32876
Chen Y, Zhong X, Zhang Q (2012) Synthesis of CO2-philic polysiloxane with N-halamine side groups for biocidal coating on cotton. Ind Eng Chem Res 51:9260–9265. https://doi.org/10.1021/ie300378b
Chen Y, Niu MQ, Yuan S, Teng HN (2013) Durable antimicrobial finishing of cellulose with QSA silicone by supercritical adsorption. Appl Surf Sci 264:171–175. https://doi.org/10.1016/j.apsusc.2012.09.165
Chen Y, Zhang Q, Han Q, Mi Y, Sun S, Feng C, Xiao H, Yu P, Yang C (2017) Synthesis of polysiloxane with 5,5-dimethylhydantoin-based N-halamine pendants for biocidal functionalization of polyethylene by supercritical impregnation. J Appl Polym Sci 134:17. https://doi.org/10.1002/APP.44721
Cheng X, Li R, Du J, Sheng J, Ma K, Ren X, Huang TS (2015) Antimicrobial activity of hydrophobic cotton coated with N-halamine. Polym Adv Technol 26:99–103. https://doi.org/10.1002/pat.3426
Foksowicz-Flaczyk J, Walentowska J, Przybylak M, Maciejewski H (2016) Multifunctional durable properties of textile materials modified by biocidal agents in the sol-gel process. Surf Coat Technol 304:160–166. https://doi.org/10.1016/j.surfcoat.2016.06.062
Fouda MMG, Abdel-Halim ES, Al-Deyab SS (2013) Antibacterial modification of cotton using nanotechnology. Carbohydr Polym 92:943–954. https://doi.org/10.1016/j.carbpol.2012.09.074
Gao DG, Chen C, Ma JZ, Lu B, Li JX (2013) Preparation, characterization and application of ZnO sol containing quaternary phosphonium salts. J Sol–Gel Sci Technol 65:336–343. https://doi.org/10.1007/s10971-012-2941-1
Hou A, Shi Y (2009) Polymerization and surface active properties of water-soluble amphiphilic polysiloxane copolymers modified with quaternary ammonium salts and long-carbon chain groups. Mater Sci Eng B 163:99–104. https://doi.org/10.1016/j.mseb.2009.05.014
Ibrahim NA, El-Zairy MR, Eid BM, El-Zairy EMR, Emam EM (2015) New finishing possibilities for producing durable multifunctional cotton/wool and viscose/wool blended fabrics. Carbohydr Polym 119:182–193. https://doi.org/10.1016/j.carbpol.2014.11.040
Jiang ZM, Ma KK, Du JM, Li R, Ren XH, Huang TS (2014) Synthesis of novel reactive N-halamine precursors and application in antimicrobial cellulose. Appl Surf Sci 288:518–523. https://doi.org/10.1016/j.apsusc.2013.10.063
Kaur R, Liu S (2016) Antibacterial surface design—contact kill. Prog Surf Sci 91:136–153. https://doi.org/10.1016/j.progsurf.2016.09.001
Lin Y, Liu Q, Cheng L, Lei Y, Zhang A (2014) Synthesis and antimicrobial activities of polysiloxanes-containing quaternary ammonium salts on bacteria and phytopathogenic fungi. React Funct Polym 85:36–44. https://doi.org/10.1016/j.reactfunctpolym.2014.10.002
Liu Y, Ma KK, Li R, Ren XH, Huang TS (2013) Antibacterial cotton treated with N-halamine and quaternary ammonium salts. Cellulose 20:3123–3130. https://doi.org/10.1007/s10570-013-0056-7
Mahbubul HM, Sunderland M (2015) Antimicrobial and insect-resist wool fabrics by coating with microencapsulated antimicrobial and insect-resist agents. Prog Org Coat 85:221–229. https://doi.org/10.1016/j.porgcoat.2015.04.016
Mahltig B, Haufe H, Böttcher H (2005) Functionalisation of textiles by inorganic sol–gel coatings. J Mater Chem 15:4385–4398. https://doi.org/10.1039/B505177K
Mahltig B, Gutmann E, Reinbold M, Meyer DC, Böttcher H (2009) Synthesis of Ag and Ag/SiO2 sols by solvothermal method and their bactericidal activity. J Sol–Gel Sci Technol 51:204–214. https://doi.org/10.1007/s10971-009-1972-8
Perliolatto M, Ferrero F, Monarsolo A, Mossoti R (2013) Hydrorepellent finishing of cotton fabrics by chemically modified TEOS based nanosol. Cellulose 20:355–364. https://doi.org/10.1007/s10570-012-9821-2
Przybylak M, Maciejewski H, Dutkiewicz A (2016a) Preparation of highly hydrophobic cotton fabric by modification with bifunctional silsesquioxanes in the sol-gel process. Appl Surf Sci 387:163–174. https://doi.org/10.1016/j.apsusc.2016.06.094
Przybylak M, Maciejewski H, Dutkiewicz A, Dąbek I, Nowicki M (2016b) Fabrication of superhydrophobic cotton fabrics by a simple chemical modification. Cellulose 23:2185–2197. https://doi.org/10.1007/s10570-016-0940-z
Przybylak M, Maciejewski H, Dutkiewicz A, Wesołek D, Władyka-Przybylak M (2016c) Multifunctional, strongly hydrophobic and flame-retarded cotton fabrics modified with flame retardant agents and silicone compounds. Polym Degrad Stab 128:55–64. https://doi.org/10.1016/j.polymdegradstab.2016.03.003
Qin X, Li Y, Zhou F, Ren L, Zhao Y, Yuan X (2015) Polydimethylsiloxane–polymethacrylate block copolymers tethering quaternary ammonium salts groups for antimicrobial coating. Appl Surf Sci 328:183–192. https://doi.org/10.1016/j.apsusc.2014.12.019
Ren XH, Kou L, Kocer HB, Zhu CY, Worley SD, Broughton RM, Huang TS (2008a) Antimicrobial coating of an N-halamine biocidal monomer on cotton fibers via admicellar polymerization. Colloids Surf A Physicochem Eng Asp 317:711–716. https://doi.org/10.1016/j.colsurfa.2007.12.007
Ren XH, Kou L, Liang J, Worley SD, Tzou YM, Huang TS (2008b) Antimicrobial efficacy and light stability of N-halamine siloxane bound to cotton. Cellulose 15:593–598. https://doi.org/10.1007/s10570-008-9205-9
Scholz J, Nocke G, Hollstein F, Weissbach A (2005) Investigations on fabrics coated with precious metals using the magnetron sputter technique with regard to their anti-microbial properties. Surf Coat Technol 192:252–256. https://doi.org/10.1016/j.surfcoat.2004.05.036
Shin HK, Park M, Chung YS, Kim HY, Jin FL, Choi HS, Pork SJ (2013) Preparation and characterization of chlorinated cross-linked chitosan/cotton knit for biomedical applications. Macromol Res 21:1241–1246. https://doi.org/10.1007/s13233-013-1164-9
Sun G (2016) Antimicrobial textiles. Woodhead Publishing, Cambridge
Textor T (2009) Surface modification of textile: modification of textile surfaces using the sol-gel technique. Woodhead Publishing, Cambridge
Textor T, Mahltig B (2010) A sol-gel based surface treatment for preparation of water repellent antistatic textiles. Appl Surf Sci 256:1668–1674. https://doi.org/10.1016/j.apsusc.2009.09.091
Textor T, Fouda MMG, Mahltig B (2010) Deposition of durable thin silver layers onto polyamides employing a heterogeneous Tollens’ reaction. Appl Surf Sci 256:2337–2342. https://doi.org/10.1016/j.apsusc.2009.10.063
Tomšič B, Simončič B, Orel B, Žerjav M, Schroers H, Simončič A, Samardžija Z (2009) Antimicrobial activity of AgCl embedded in a silica matrix on cotton fabric. Carbohydr Polym 75:618–626. https://doi.org/10.1016/j.carbpol.2008.09.013
Wang BI, Wang JI, Li DD, Ren KF, Ji J (2012) Chitosan/poly(vinyl pyrollidone) coatings improve the antibacterial properties of poly(ethylene terephthalate). Appl Surf Sci 258:7801–7808. https://doi.org/10.1016/j.apsusc.2012.03.181
Wu L, Xu Y, Cai L, Zang X, Li Z (2014) Synthesis of a novel multi N-halamines siloxane precursor and its antimicrobial activity on cotton. Appl Surf Sci 314:832–840. https://doi.org/10.1016/j.apsusc.2014.06.190
Xiao W, Xu JB, Liu XY, Hu QL, Huang JG (2013) Antibacterial hybrid materials fabricated by nanocoating of microfibril bundles of cellulose substance with titania/chitosan/silver-nanoparticle composite films. J Mater Chem B 1:3477–3485. https://doi.org/10.1039/C3TB20303D
Xie Y, Hill CAS, Xiao Z, Militz H, Mai C (2010) Silane coupling agents used for natural fiber/polymer composites: a review. Compos Part A 41:806. https://doi.org/10.1016/j.compositesa.2010.03.005
Acknowledgments
The authors gratefully acknowledge financial support from the National Centre for Research and Development (Poland). Project No. 180 480 “Novel organosilicon compounds for upgrading of natural fibers and textiles”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Przybylak, M., Maciejewski, H., Dudkiewicz, A. et al. Development of multifunctional cotton fabrics using difunctional polysiloxanes. Cellulose 25, 1483–1497 (2018). https://doi.org/10.1007/s10570-017-1621-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-017-1621-2