Abstract
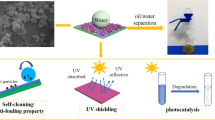
Robust superhydrophobic cotton textiles exhibiting photocatalytic self-cleaning ability under UV light were successfully achieved by surface functionalization with anatase TiO2 sol and mercapto silanes, and hydrophobization with vinyl-terminated polydimethylsiloxane (V-PDMS) via thiol-ene click reaction. The modified cotton textiles not only showed outstanding water repellency with a water contact angle of 154.2°, but exhibited desirable photodegradation of oil red O by photocatalysis under UV irradiation. Moreover, the modified textiles exhibited excellent durability and stability after exposure to different severe conditions, such as acid and base solutions, organic solvents, laundering and UV exposure. The durably coated textiles manifested desirable separation performance in oil–water mixtures, and the separation efficiency was about 99.0% even after 20 times use. Cotton textiles with multi-functionality of superhydrophobicity, photocatalysis and oil-water separation are hopefully applied in a diverse range of practical applications in self-cleaning and oil-removal fields.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Frequent oil spills, as well as the oily wastewater, have caused severe damage to the natural environment and human society [1, 2]. Therefore, interest in developing facile, economical, efficient oil–water separation methods has grown. So far, many traditional approaches have been adopted for the application of oil–water separation, such as centrifugation, chemical oxidation, gravity separation, and bioremediation [3, 4]. However, these traditional methods still have the limitations including time-consuming, high cost, low separation efficiency and secondary pollution [5, 6]. Thus, there is still an urgent demand for developing a facile, rapid, inexpensive and effective approach to separate oil–water mixtures.
In recent years, superhydrophobic materials with high water contact angles (WCAs > 150o) have attracted worldwide attention, which can be especially used for self-cleaning and oil–water separation [7,8,9]. To remove and collect oil from oil–water mixtures, much effort has been focused on creating superhydrophobic surface by the combination of the proper hierarchical structures and low surface energy on various materials, such as meshes, films, membranes, porous media and other substrates [10,11,12,13]. Among these above materials, cotton textiles possessing superhydrophobicity are broadly considered as suitable candidates mainly due to their high flexibility, biodegradability, low cost, easy handling and high efficiency [14,15,16,17]. Numerous approaches have been adopted for the preparation of superhydrophobic materials, such as sol–gel [18, 19], chemical etching [20, 21], chemical vapor deposition [22, 23], dip-coating [24, 25], thiol-ene click chemistry [26, 27] and other methods [28, 29]. Among all these methods, the thiol-ene click chemistry is a promising way to prepare superhydrophobic materials owing to its versatility, high efficiency, inexpensive and mild reaction conditions [30, 31].
Generally, both surface roughness and chemical composition play roles in constructing superhydrophobic surfaces by the combination of different nanoparticles including SiO2, Fe3O4, TiO2 and ZrO2 and low-surface-energy materials [32,33,34]. In most instances, the superhydrophobicity can be easily achieved by the usage of fluorinated chemicals, particularly long-chain perfluoroalkylsilanes owing to its ultra-low surface energy [35,36,37]. Although these obtained superhydrophobic textiles can separate oil–water mixtures efficiently and exhibit outstanding self-cleaning ability, fluorinated chemicals are environmentally and economically undesirable which have such characteristics as non-biodegradability, high cost and relatively desirable stability [38, 39]. Thus, using fluorine-free chemical modification is of vital importance in fabricating cotton textiles with superhydrophobicity and superoleophilicity for self-cleaning and oil–water separation. Gao et al. [40] successfully prepared robust superhydrophobic fabric for oil–water separation via a hot-pressing method by the combination of n-octyltriethoxysilane (OTES) and tetrabutyl titanate (TBOT) precursor. Li et al. [41] developed superhydrophobic fabrics by surface modification of MnO2 nanoparticles and stearic acid (STA), which exhibited high oil–water separation efficiency. Bano et al. [42] reported durable superhydrophobic fabric and mesh by titania nanoparticles and polydimethylsiloxane (PDMS), which could separate four types of oil–water mixtures in various harsh conditions. Moreover, these works were mainly concentrated on the superhydrophobic self-cleaning property, which could remove water-insoluble pollutants by the flow of water due to the superhydrophobicity commonly known as the lotus effect [43]. However, these superhydrophobic self-cleaning textiles are easily polluted by organic stains or oil.
Besides the superhydrophobicity, the photocatalysis is another way to realize self-cleaning property in which the organic pollutants can be decomposed into small molecules, such as water and CO2, when exposed to light [44, 45]. Recently, titanium dioxide (TiO2), as a photocatalyst, has been extensively utilized to develop self-cleaning textiles with photocatalytic activity [46, 47]. Wang et al. [48] presented a facile and environmentally friendly method to prepare TiO2—immobilized fabrics for oil–water separation, which exhibited self-cleaning property and could decompose the grease. Zhang et al. [49] successfully fabricated polybenzoxazine (PBZ)/TiO2-modified fabrics via a facile dip-coating method, which were efficient for oil–water separation and showed wonderful self-cleaning ability of the removal of oil contaminations. Based on these, it is infinitely preferable to achieve superhydrophobic and photocatalytic self-cleaning textiles for oil–water separation by the synergistic effect of anatase TiO2 nanoparticles and the superhydrophobicity.
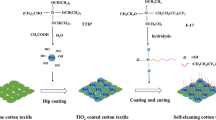
In this work, a simple and inexpensive approach was proposed in constructing robust fluorine-free and self-cleaning cotton textiles for oil–water separation, which were surface modified with anatase TiO2 sol and mercapto silanes, and hydrophobizated with vinyl-terminated polydimethylsiloxane (V-PDMS) via thiol-ene click reaction. The as-obtained textiles showed excellent superhydrophobicity with a WCA of 154.2o. Moreover, the superhydrophobic textiles were resistant to acidic and alkaline solutions, organic solvents and UV exposure. The superhydrophobic and photocatalytic self-cleaning properties of the coated textiles were also investigated. And the superhydrophobic textiles exhibited desirable oil–water separation performance.
Experimental
Materials
Titanium tetraisopropoxide (TTIP), acetic acid, N, N-dimethylformamide (DMF), and tetrahydrofuran (THF), and 2,2-dimethoxy-2-phenylacetophenone (DMPA) were purchased from Aladdin reagent Co., Ltd. China. Vinyl-terminated polydimethylsiloxane (V-PDMS, average Mw = 17200) was supplied by lota Silicone Oil (Anhui) Co., Ltd. China. Anhydrous ethanol (EtOH), acetone, dichloromethane, hexane, 3-mercaptopropyltriethoxysilane (MPTS), oil red O (OR) and methylene blue (MB) were all obtained from Sinopharm Chemical Reagent Co., Ltd. China. Commercial cotton textiles (plain weave, weight 110 g/m2) were bought from a local textile store. The pristine textiles were washed by EtOH and distilled water three times before experiments. The other chemical reagents were used as received in the experiment without further purification.
Preparation of TiO2-modified textile (TiO2@textile)
The TiO2@textile was fabricated by the following preparation process. The anatase TiO2 sol was synthesized by a facile sol–gel method at ambient condition based on a published work [50]. Typically, 8.0 g of TTIP was slowly added to 200.0 g of deionized water with the existence of 22.0 g of acetic acid and stirred vigorously at room temperature (25 °C) for 24 h. Subsequently, the anatase TiO2 sol was obtained after storage without stirring for 2 weeks at room temperature. Then, the cleaned textiles were immersed in the as-obtained TiO2 sol for 30 min and afterward dried at 80 °C for 1 h in an oven. The TiO2-modified textile was named as TiO2@textile.
Preparation of MPTS-modified TiO2@textile (TiO2@textile-SH)
The MPTS-modified TiO2@textile was prepared by immersing the TiO2@fabric in 0.2 mol/L MPTS ethanol solution for 2 h at room temperature and subsequently heated at 80 °C, named as TiO2@textile-SH.
Preparation of the superhydrophobic textile (ST) via thiol-ene click reaction
The TiO2@textile-SH was placed in a conical flask containing DMF (20.0 g), V-PDMS (2.0 g) and DMPA (0.02 g), and then irradiated under 365 nm UV light (20 mW cm−2) for 1 h. After click reaction, the resultant samples were washed by DMF and dried at 80 °C for 1 h to obtain the superhydrophobic textile (denoted as ST).
Characterization
The surface morphology of the pristine textile, TiO2@textile, TiO2@textile-SH and ST was performed on a SEM (Hitachi-S4800). The elemental analysis was performed by energy-dispersive spectroscopy (EDS) attached to SEM equipment. X-ray photoelectron spectra (XPS, Escalab 250Xi, Thermo Scientific) were obtained using X-ray photoelectron spectroscopy with an Al kα X-ray source, and the binding energies were normalized to C 1s at 284.8 eV. Fourier-transform infrared-attenuated total reflectance (FTIR-ATR, TENSOR 27, Bruker Co., Germany) spectroscopy was used to analyze the surface composition of the pristine textile and ST. The crystal structure of the TiO2 nanoparticles was conducted by an X-ray diffractometer (XRD, X’Pert-Pro MRD, Philips) with Cu kα radiation (λ = 0.154 nm). The average WCA value of the ST was measured at least five different positions using a contact angle goniometer (Shanghai Zhongchen, China) with 4 μL droplets of deionized water. Aqueous solutions of different pH values and various organic solvents were employed to investigate the chemical stability of the ST. The samples were treated in the above conditions for 72 h. Then, the samples were thoroughly rinsed with distilled water and cured at 80 °C for WCA measurement. Additionally, the ST was subjected to a laundering test according to a previous published work [51]. The STs were placed in a 150 mL aqueous solution with the presence of 0.15 wt% aqueous detergent (DIAO brand detergent, provided by Nice group in China) and laundered at 300 rpm for 30 cycles (15 min for one cycle) at 50 °C. Then, the WCAs after different laundering cycles were investigated.
Results and discussion
Preparation and characterization of the STs
In this study, the preparation process of the STs is presented in Fig. 1. Firstly, the pristine textiles were treated with anatase TiO2 sol, which was prepared via a mild and green sol–gel method. Additionally, to identify the crystal structure of the obtained TiO2 nanoparticles, XRD analysis was further performed. Figure 2 shows that all diffraction peaks at 25.3o, 38.0o, 47.8o, 54.0o and 62.5o of TiO2 nanoparticles can be attributed to the anatase phase [52, 53]. This result suggested that the resultant TiO2 nanoparticles exhibited desirable photocatalytic ability [54]. Secondly, the TiO2@textile-SH was fabricated by MPTS modification to form the reactive thiol layers on fiber surface. Lastly, the superhydrophobic textiles were prepared via a mild click reaction using V-PDMS with ene moieties. More importantly, in our work V-PDMS possessing low surface energy could substitute for the long-chain perfluoroalkylsilanes, which were commonly very expensive and environmentally hazardous.
The surface morphology of the pristine textile, TiO2@textile, TiO2@textile-SH and ST was investigated by SEM. As shown in Fig. 3a, a’, the original textile surface was relatively smooth. When coating with anatase TiO2 sol, a compact layer was formed on each fiber surface as shown in Fig. 3b, b’, which generated the primary roughness at nanoscale. Figure 3c, c’ shows that no obvious changes were observed after modification with MPTS. Also importantly, it was found that the ST surface was similar to the TiO2@textile and TiO2@textile-SH surface, as displayed in Fig. 3d, d’. Consequently, it was significant to maintain the nanoscale roughness of TiO2@textile, resulting in proper roughness for desirable superhydrophobicity of the as-obtained coated textiles. Furthermore, the element distribution of the ST was measured by EDS. As shown in Fig. 3e and f, the primary elements (C, O, Ti, Si and S) were distributed on the ST surface uniformly, indicating the successful preparation of the ST.
The chemical composition of the pristine textile, TiO2@textile, TiO2@textile-SH and ST was further evaluated by XPS and FTIR-ATR, and the corresponding spectra are presented in Figs. 4 and 5. Figure 4a verifies that the original textile only contained C and O elements, while a new element of Ti was clearly found in the spectrum of the TiO2@textile. After modified with MPTS, the TiO2@textile-SH surface dominated new Si and S signals, demonstrating the successful coating of MPTS on its surface. After the TiO2@textile-SH was coupled with V-PDMS, the presence of the strong Si peak confirmed that V-PDMS had been successfully coated on the fiber surface. According to the XPS data (Table 1), the atomic ratios of C/O/Ti/Si/S of the pristine textile and ST were calculated to be 44.6:55.4:0:0:0 and 25.0:45.6:19.3:8.8:1.3, respectively. Figure 4b shows the curved fitted Ti 2p spectrum of the ST, which consisted of two distinct peaks at 459.1 and 464.8 eV corresponding to Ti 2p3/2 and 2p1/2, respectively. In addition, the spin-orbital gap of the Ti 2p was about 5.7 eV, which was consistent with previous work [40]. This result indicated that the introduction of anatase TiO2 could endow the coated textile with photocatalytic activity [55]. As shown in Fig. 4c, the C 1s peak of the pristine textile showed three different peaks at 284.6 eV, 286.2 eV and 288.5 eV, which were assigned to the C–C, C–O and C=O bonds, respectively [56]. Moreover, a new peak C–Si (284.1 eV) was obviously detected in Fig. 4d, demonstrating that V-PDMS had been successfully attached to the fiber surface.
Furthermore, Fig. 5 shows the FTIR-ATR spectra of the pristine textile and ST. The absorption peaks at 3336 cm−1, 2900 cm−1 and 1650 cm−1 corresponded to the stretching vibrations of –OH, stretching vibrations of –CH2 and bending vibrations of –OH, respectively. The bands at 1160 cm−1, 1108 cm−1 and 1032 cm−1 were ascribed to stretching vibrations of –C–O. These are characteristic bands of cellulose [24]. After surface modification, bending vibrations and stretching vibrations of Si–C appeared at 1260 cm−1 and 800 cm−1 [13]. This also confirmed that V-PDMS was successfully coated on cotton textile, which was in good agreement with results of the XPS analysis. Thus, the introduction of V-PDMS with low surface energy ultimately imparted excellent superhydrophobic property to the cotton textile.
Hydrophobic property
In order to investigate the influence of the V-PDMS concentration on the WCAs of the acquired textiles, the results are presented in Fig. 6. With a low V-PDMS concentration (4 wt%), the resultant textile exhibited high hydrophobicity with a WCA of 134.4o. Furthermore, the WCA increased from 147.3o to 154.2o with an increase in the V-PDMS concentration from 6 to 10 wt%. However, as the V-PDMS concentration was further increased to 12 and 14 wt%, the WCA decreased to 146.4o and 145.6o, respectively. The main reason is that the V-PDMS created smooth surface which can be identified by SEM, as shown in Fig. 7a–c. Thus, the textiles prepared with the V-PDMS concentration at 10 wt% were chosen in this work.
Durability and stability
In practical applications, it is essential and important to enhance the durability and stability of superhydrophobic surface in complex environments, such as acid and alkali conditions, corrosive solution environment, laundering and strong light exposure. The durability and stability tests of the as-obtained ST had been carried out in detail, and the results are shown in Fig. 8. Figure 8a shows that the ST kept its superhydrophobicity with WCAs above 150o, demonstrating a desirable resistance to different pH values ranging from 1 to 13. Additionally, the ST still maintained its excellent superhydrophobicity after the textile was immersed into various organic solutions for 72 h, such as THF, DMF, acetone and EtOH (Fig. 8b). As shown in Fig. 8c, the ST still remained highly hydrophobicity with a WCA of 146.7o even after 30 cycles of laundering tests, demonstrating wonderful washing resistance. Importantly, UV resistance of the ST was also investigated under UV light irradiation (20 mW cm−2) for different times. As displayed in Fig. 8d, the WCA decreased slightly and the ST still retained its superhydrophobicity with a WCA of 152o after 24-h UV light exposure. This result indicated that V-PDMS owned superior stability and durability against surface destruction caused by UV light irradiation [57, 58]. Thus, the ST exhibits excellent resistance to strong acid and alkali conditions, corrosive solutions, washing and UV exposure, which is advantageous in a diverse range of practical and industrial fields.
Self-cleaning performance
Generally, the superhydrophobicity of materials is greatly affected by surface contamination, which requires self-cleaning performance including superhydrophobic and photocatalytic self-cleaning properties. The superhydrophobic self-cleaning property is measured by estimating the ability of removing the MB powder covered on the ST surface. Figure 9c–d shows the self-cleaning process of the ST. After the self-cleaning test, the ST surface still retained clean and was the same with the newly obtained ST. It was clearly observed that the water droplets immediately took away the MB powder without difficulty and easily rolled off the ST surface. However, for the pristine textile, the water droplets could neither clean the MB powder nor roll off its surface, which was due to its intrinsic hydrophilicity (Fig. 9a–b). In addition, the high-magnified optical images of the pristine textile and ST surface after the self-cleaning process are presented in Fig. 9e–f. The MB powder was almost totally removed by water droplets, compared to the optical image of the pristine textile. Moreover, the superhydrophobic self-cleaning property of the ST is also shown in Movie S1. As mentioned above, a wonderful superhydrophobic self-cleaning property of the ST was confirmed, which could protect the coated textiles from pollution.
However, superhydrophobic self-cleaning property has no effect on such surfaces which were polluted by organic contaminations or oil. Therefore, the incorporation of anatase TiO2 nanoparticles which can decompose organic pollutions and induce a photocatalytic self-cleaning surface will deal with this challenge. As illustrated in Fig. 10, the photocatalytic self-cleaning property was qualitatively detected by observing the degradation of OR as model organic stain. After exposure to UV light, the OR stain on pristine textile still retained intact. In contrast, it could be found that tremendous degradation of the OR stain occurred in TiO2@textile and ST. The OR stains on TiO2@textile were remarkably degraded after 2 h of UV irradiation, and subsequently a complete discoloration was clearly observed within 4 h, indicating that this phenomenon was attributed to the considerable photocatalytic activity of anatase TiO2. However, the OR stain on the ST was almost discolored after 12 h of UV irradiation, suggesting that the photodegradation degree of TiO2@textile was superior to that of the ST under the same UV condition. The reason for this phenomenon is that chemical surface modification can reduce the photocatalytic activity of the anatase TiO2 to a certain degree [59]. More importantly, the ST surface still showed excellent superhydrophobicity with a WCA of 153o after complete discoloration of OR. These results proved that the ST not merely showed a good superhydrophobic self-cleaning ability but also possessed excellent photocatalytic activity, which has promising applications in the fields of self-cleaning.
Oil–water separation
Owing to the outstanding superhydrophobicity and superoleophilicity, the ST was considered as a promising material for the separation of oil and water. As shown in Fig. 11a, the ST could quickly absorb the oil without any water uptake, regardless of light or heavy oil (such as hexane or dichloromethane). The oil–water separation experiment was conducted by a facile homemade setup, as shown in Fig. 11b. A mixture of oil (hexane) and water (weight ratio = 1:1) was poured onto the ST, and the oil was dyed with OR to make this procedure more distinct. During the separation process, the oil quickly passed through the ST under the gravity, while the water was completely kept above the ST surface owing to its outstanding superhydrophobicity. The separation efficiency was further calculated according to the weight ratio between the collected water and the original water. Additionally, the ST could be easily cleaned and reused. As shown in Fig. 11c, the ST retained the high separation efficiency (99.0%) after 20 separation cycles. Figure 11d shows that ST maintained its high hydrophobicity with a WCA of 151o after 20 separation cycles, displaying its stable wettability. Consequently, the ST has a broad scope in the fields of oil–water separation.
Conclusion
We have successfully prepared robust fluorine-free superhydrophobic cotton textiles, which were achieved by introducing anatase TiO2 and mercapto silanes on the textile, followed by hydrophobization with V-PDMS. The coated textiles not merely showed a WCA of 154.2o, but exhibited considerable photodegradation of oil red O under UV light irradiation. In addition, the modified textiles were highly resistant to acid and alkali conditions, various organic solvents, laundering and UV irradiation. Importantly, the coated textiles can be utilized for oil–water separation with high separation efficiency and remained with outstanding superhydrophobicity after 20 separation cycles. This study provides a facile, inexpensive and environmentally friendly method for constructing robust fluorine-free multifunctional cotton textiles, which have great potential applications for self-cleaning and oil removal.
References
Xiang Y, Pang Y, Jiang X, Huang J, Xi F, Liu J (2018) One-step fabrication of novel superhydrophobic and superoleophilic sponge with outstanding absorbency and flame-retardancy for the selective removal of oily organic solvent from water. Appl Surf Sci 428:338–347
Panda A, Varshney P, Mohapatra SS, Kumar A (2018) Development of liquid repellent coating on cotton fabric by simple binary silanization with excellent self-cleaning and oil–water separation properties. Carbohydr Polym 181:1052–1060
Gupta RK, Dunderdale GJ, England MW, Hozumi A (2017) Oil/water separation techniques: a review of recent progresses and future directions. J Mater Chem A 5(31):16025–16058
Xue ZX, Cao YZ, Liu N, Feng L, Jiang L (2014) Special wettable materials for oil/water separation. J Mater Chem A 2(8):2445–2460
Ma Q, Cheng H, Fane AG, Wang R, Zhang H (2016) Recent development of advanced materials with special wettability for selective oil/water separation. Small 12(16):2186–2202
Varshney P, Nanda D, Satapathy M, Mohapatra SS, Kumar A (2017) A facile modification of steel mesh for oil–water separation. N J Chem 41(15):7463–7471
Pan Z, Cheng F, Zhao B (2017) Bio-inspired polymeric structures with special wettability and their applications: an overview. Polymers 9(12):725
Li SH, Huang JY, Chen Z, Chen GQ, Lai YK (2017) A review on special wettability textiles: theoretical models, fabrication technologies and multifunctional applications. J Mater Chem A 5(1):31–55
Shah SM, Zulfiqar U, Hussain SZ, Ahmad I, Habib ur R, Hussain I, Subhani T (2017) A durable superhydrophobic coating for the protection of wood materials. Mater Lett 203:17–20
Tran VT, Lee BK (2017) Novel fabrication of a robust superhydrophobic PU@ZnO@Fe3O4@SA sponge and its application in oil–water separations. Sci Rep 7(1):17520
Lei S, Shi Z, Ou J, Wang F, Xue M, Li W, Qiao G, Guan X, Zhang J (2017) Durable superhydrophobic cotton fabric for oil/water separation. Colloids Surf Physicochem Eng Asp 533:249–254
Jiang B, Zhang H, Sun Y, Zhang L, Xu L, Hao L, Yang H (2017) Covalent layer-by-layer grafting (LBLG) functionalized superhydrophobic stainless steel mesh for oil/water separation. Appl Surf Sci 406:150–160
Zulfiqar U, Hussain SZ, Subhani T, Hussain I, Habib ur R (2018) Mechanically robust superhydrophobic coating from sawdust particles and carbon soot for oil/water separation, colloids surf. Physicochem Eng Asp 539:391–398
Zhu T, Li S, Huang J, Mihailiasa M, Lai Y (2017) Rational design of multi-layered superhydrophobic coating on cotton fabrics for UV shielding, self-cleaning and oil–water separation. Mater Des 134:342–351
Wang J, Han F, Liang B, Geng G (2017) Hydrothermal fabrication of robustly superhydrophobic cotton fibers for efficient separation of oil/water mixtures and oil-in-water emulsions. J Ind Eng Chem 54:174–183
Ge B, Zhang ZZ, Zhu XT, Men XH, Zhou XY, Xue QJ (2017) A graphene coated cotton for oil/water separation. Compos Sci Technol 102:100–105
Chen JY, Zhong XM, Lin J, Wyman I, Zhang GW, Yang H, Wang JB, Wu JZ, Wu X (2016) The facile preparation of self-cleaning fabrics. Compos Sci Technol 122:1–9
Su X, Li H, Lai X, Zhang L, Wang J, Liao X, Zeng X (2017) Vapor-liquid sol–gel approach to fabricating highly durable and robust superhydrophobic polydimethylsiloxane@silica surface on polyester textile for oil–water separation. ACS Appl Mater Interfaces 9(33):28089–28099
Shi Y, Wang Y, Feng X, Yue G, Yang W (2012) Fabrication of superhydrophobicity on cotton fabric by sol–gel. Appl Surf Sci 258(20):8134–8138
Choi D, Yoo J, Sang MP, Dong SK (2017) Facile and cost-effective fabrication of patternable superhydrophobic surfaces via salt dissolution assisted etching. Appl Surf Sci 393:449–456
He Y, Jiang C, Yin H, Chen J, Yuan W (2011) Superhydrophobic silicon surfaces with micro-nano hierarchical structures via deep reactive ion etching and galvanic etching. J Colloid Interface Sci 364(1):219–229
Ming Z, Chengheng F, Chunxia W, Weiwei M, Lan C (2009) Superhydrophobic multi-scale ZnO nanostructures fabricated by chemical vapor deposition method. J Nanosci Nanotechnol 9(7):4211–4214
Ma M, Mao Y, Gupta M, And KKG, Rutledge GC (2005) Superhydrophobic fabrics produced by electrospinning and chemical vapor deposition. Macromolecules 38(23):9742–9748
Yang M, Liu W, Jiang C, He S, Xie Y, Wang Z (2018) Fabrication of superhydrophobic cotton fabric with fluorinated TiO2 sol by a green and one-step sol–gel process. Carbohydr Polym 197:75–82
Zhang J, Li B, Wu L, Wang A (2013) Facile preparation of durable and robust superhydrophobic textiles by dip coating in nanocomposite solution of organosilanes. Chem Commun 49(98):11509–11511
Li H, Liang T, Lai X, Su X, Zhang L, Zeng X (2018) Vapor–liquid interfacial reaction to fabricate superhydrophilic and underwater superoleophobic thiol-ene/silica hybrid decorated fabric for oil/water separation. Appl Surf Sci 427:92–101
Hou K, Jin Y, Chen J, Wen X, Xu S, Cheng J, Pi P (2017) Fabrication of superhydrophobic melamine sponges by thiol-ene click chemistry for oil removal. Mater Lett 202:99–102
Xiao X, Cao G, Chen F, Tang Y, Liu X, Xu W (2015) Durable superhydrophobic wool fabrics coating with nanoscale Al2O3 layer by atomic layer deposition. Appl Surf Sci 349:876–879
Liu JF, Xiao XY, Shi YL, Wan CX (2014) Fabrication of a superhydrophobic surface from porous polymer using phase separation. Appl Surf Sci 297:33–39
Wang B, Zhang Y, Zhang L (2017) Selective surface tension induced patterning on flexible textiles via click chemistry. Nanoscale 9(14):4777–4786
Deng S, Huang J, Chen Z, Lai Y (2017) Controllable superhydrophobic coating on cotton fabric by UV induced thiol-ene reaction for wettability patterning and device metallization. Adv Mater Interfaces 4(13):1700268
Hano N, Takafuji M, Ihara H (2017) One-pot preparation of polymer microspheres having wrinkled hard surfaces through self-assembly of silica nanoparticles. Chem Commun 53(65):9147–9150
Yan T, Chen X, Zhang T, Yu J, Jiang X, Hu W, Jiao F (2018) A magnetic pH-induced textile fabric with switchable wettability for intelligent oil/water separation. Chem Eng J 347:52–63
Zhang H, Li Y, Lu Z, Chen L, Huang L, Fan M (2017) A robust superhydrophobic TiO2 NPs coated cellulose sponge for highly efficient oil–water separation. Sci Rep 7(1):9428
Yin X, Sun C, Zhang B, Song Y, Wang N, Zhu L, Zhu B (2017) A facile approach to fabricate superhydrophobic coatings on porous surfaces using cross-linkable fluorinated emulsions. Chem Eng J 330:202–212
Shang Q, Hu L, Hu Y, Liu C, Zhou Y (2017) Fabrication of superhydrophobic fluorinated silica nanoparticles for multifunctional liquid marbles. Appl Phys A 124(1):25
Abbas R, Elkhoshkhany N, Hefnawy A, Ebrahim S, Rahal A (2017) High stability performance of superhydrophobic modified fluorinated graphene films on copper alloy substrates. Adv Mater Sci Eng 2017:1–8
Gao S, Dong X, Huang J, Li S, Li Y, Chen Z, Lai Y (2018) Rational construction of highly transparent superhydrophobic coatings based on a non-particle, fluorine-free and water-rich system for versatile oil–water separation. Chem Eng J 333:621–629
Fu S, Zhou H, Wang H, Ding J, Liu S, Zhao Y, Niu H, Rutledge GC, Lin T (2018) Magnet-responsive, superhydrophobic fabrics from waterborne, fluoride-free coatings. RSC Adv 8(2):717–723
Gao S, Huang J, Li S, Liu H, Li F, Li Y, Chen G, Lai Y (2017) Facile construction of robust fluorine-free superhydrophobic TiO2@fabrics with excellent anti-fouling, water–oil separation and UV-protective properties. Mater Des 128:1–8
Li D, Guo Z (2017) Stable and self-healing superhydrophobic MnO2@fabrics: applications in self-cleaning, oil/water separation and wear resistance. J Colloid Interface Sci 503:124–130
Bano S, Zulfiqar U, Zaheer U, Awais M, Ahmad I, Subhani T (2018) Durable and recyclable superhydrophobic fabric and mesh for oil–water separation. Adv Eng Mater 20:1–9
Marmur A (2017) The lotus effect: superhydrophobicity and metastability. Langmuir 20(9):3517–3519
Tung WS, Daoud WA (2011) Self-cleaning fibers via nanotechnology: a virtual reality. J Mater Chem 21(22):7858–7869
Banerjee S, Dionysiou DD, Pillai SC (2015) Self-cleaning applications of TiO2 by photo-induced hydrophilicity and photocatalysis. Appl Catal B-Environ 176:396–428
Gao XY, Guo ZG (2017) Biomimetic superhydrophobic surfaces with transition metals and their oxides: a review. J Bionic Eng 14(3):401–439
Leong S, Razmjou A, Wang K, Hapgood K, Zhang X, Wang H (2014) TiO2 based photocatalytic membranes: a review. J Membr Sci 472:167–184
Wang S, Wu SD, Zhang JZ, Wang T (2017) One-step fabrication of recyclable and robust fluorine/polymer-free superhydrophobic fabrics. RSC Adv 7(39):24374–24381
Zhang W, Lu X, Xin Z, Zhou C (2015) A self-cleaning polybenzoxazine/TiO2 surface with superhydrophobicity and superoleophilicity for oil/water separation. Nanoscale 7(46):19476–19483
Qi K, Xin JH (2010) Room-temperature synthesis of single-phase anatase TiO2 by aging and its self-cleaning properties. ACS Appl Mater Interfaces 2(12):3479–3485
Chen D, Mai Z, Liu X, Ye D, Zhang H, Yin X, Zhou Y, Liu M, Xu W (2018) UV-blocking, superhydrophobic and robust cotton fabrics fabricated using polyvinylsilsesquioxane and nano-TiO2. Cellulose 25(6):3635–3647
Alfieri I, Lorenzi A, Ranzenigo L, Lazzarini L, Predieri G, Lottici PP (2017) Synthesis and characterization of photocatalytic hydrophobic hybrid TiO2–SiO2 coatings for building applications. Build Environ 111:72–79
Xu B, Ding JE, Feng L, Ding YY, Ge FY, Cai ZS (2015) Self-cleaning cotton fabrics via combination of photocatalytic TiO2 and superhydrophobic SiO2. Surf Coat Technol 262:70–76
Liu K, Cao M, Fujishima A, Jiang L (2015) Bio-inspired titanium dioxide materials with special wettability and their applications. Chem Rev 114(19):10044–10094
Pazokifard S, Esfandeh M, Mirabedini SM (2014) Photocatalytic activity of water-based acrylic coatings containing fluorosilane treated TiO2 nanoparticles. Prog Org Coat 77(8):1325–1335
Zou HL, Lin SD, Tu YY, Liu GJ, Hu JW, Li F, Miao L, Zhang GW, Luo HS, Liu F, Hou CM, Hu ML (2013) Simple approach towards fabrication of highly durable and robust superhydrophobic cotton fabric from functional diblock copolymer. J Mater Chem A 1(37):11246–11260
Deng ZY, Wang W, Mao LH, Wang CF, Chen S (2014) Versatile superhydrophobic and photocatalytic films generated from TiO2–SiO2@PDMS and their applications on fabrics. J Mater Chem A 2(12):4178–4184
Zhao Y, Liu Y, Xu Q, Barahman M, Lyons AM (2015) Catalytic, self-cleaning surface with stable superhydrophobic properties: printed polydimethylsiloxane (PDMS) arrays embedded with TiO2 nanoparticles. ACS Appl Mater Interfaces 7(4):2632–2640
Yu M, Wang Z, Liu H, Xie S, Wu J, Jiang H, Zhang J, Li L, Li J (2013) Laundering durability of photocatalyzed self-cleaning cotton fabric with TiO2 nanoparticles covalently immobilized. ACS Appl Mater Interfaces 5(9):3697–3703
Acknowledgments
This work is supported by the Key Laboratory of Cellulose and Lignocellulosics, Guangzhou Institute of Chemistry, Chinese Academy of Sciences, and Provincial Science and technology project of Guangdong Province (No. 2015B090925019).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jiang, C., Liu, W., Yang, M. et al. Robust fabrication of superhydrophobic and photocatalytic self-cleaning cotton textiles for oil–water separation via thiol-ene click reaction. J Mater Sci 54, 7369–7382 (2019). https://doi.org/10.1007/s10853-019-03373-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-019-03373-3