Abstract
Woven cotton fabric was first modified with citric acid by a conventional pad-dry-cure process and then coordinated with Fe(III) ions to prepare a Fe(III)-modified cotton fiber complex. After the characterization by SEM, FTIR, XPS, XRD and DRS, this complex was used as a heterogeneous Fenton catalyst for the degradation of a typical textile dye, Acid Red 88, under visible irradiation. Some factors affecting the modification process, such as the citric acid and NaH2PO4 concentrations as well as the curing temperature, were also investigated with respect to the coordinating performance of the modified fabric and the catalytic activity of its Fe complex. The results indicated that cotton fabric could be esterified with citric acid to impart the carboxylic groups, which successfully reacted with Fe(III) ions to form the complex. Dye degradation was significantly accelerated by the presence of the complex under visible irradiation. Increasing the concentrations of citric acid, NaH2PO4 or the curing temperature enhanced the carboxyl group content of the modified fiber as well as Fe content and catalytic activity of its complex. However, an excessive amount of citric acid and NaH2PO4 or a curing temperature higher than 140 °C reduced the Fe content and catalytic activity of the complex. A higher initial H2O2 concentration promoted the dye degradation. The excellent catalytic and mechanical performance was also found in its reuse processes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, advanced oxidation processes (AOPs) have attracted increasing attention as promising powerful methods to efficiently remove organic dyes in industrial wastewater. Fenton and photo-Fenton processes are of special interest because they offer high reaction yields with low treatment costs and achieve the complete mineralization of the organic pollutants and desired water quality when compared to conventional physically or mechanically based technologies (Blanco et al. 2014; Pignatello et al. 2006). Ferrous salts react with H2O2 to produce hydroxyl radicals during the Fenton reaction, which attack the unsaturated dye molecules, thus degrading the wastewater (Wu et al. 1999). Moreover, it should be stressed that strict control of the pH around 2–3 is required for good catalytic performance in the original homogeneous Fenton reaction, while heterogeneous Fenton processes provide the possibility of working in a wider pH range (Ma et al. 2003). During the last decade, the development of novel heterogeneous Fenton catalysts with high catalytic performance and long-term stability at low cost has become the most important issue in Fenton and photo-Fenton systems (Hartmann et al. 2010; Parra et al. 2004). Apart from granulated and membrane materials, several fibrous materials such as modified polyacrylonitrile fiber (Dong et al. 2010; Han et al. 2011; Ishtchenko et al. 2003a), collagen fiber (Liu et al. 2010) and grafted polytetrafluoroethylene fiber (Li et al. 2014a) have been applied as the supporting materials for immobilizing Fe(III) ions to produce heterogeneous Fenton catalysts with unique performance. However, these catalysts have found little practical application for the treatment of industrial wastewater because of their relatively lower catalytic capacity, mechanical properties, and high manufacturing and operating costs. Moreover, our recent research (Li et al. 2014b) found that the oxygen atom in the carboxyl group from the alginate fiber as the electron donor coordinated with Fe(III) ions to produce the Fe(III)-alginate fiber complex, which showed a number of advantages such as high catalytic activity, good pH and salt insensitivity. However, alginate fiber was too expensive to be an ideal supporting material for the Fenton catalyst in practical applications. Additionally, alginate fiber has a low spinnability for textile processes because of its fragile strength. For future commercial applications, it is necessary to explore an inexpensive supporting material that has good mechanical performance and similar coordinating properties as metal ions, especially Fe(III) ions, to alginate fibers.
Today cotton fiber is the most used textile fiber in the world because of its numerous advantages such as good strength and chemical resistance, easy processing and relatively low cost. Moreover, it is predominantly cellulose polymer (linear β-1,4 glucan), and its chemical reactivity is the same as that of the cellulose polymer (Lewin 2010; Schindler and Hauser 2004). On the other hand, a number of previous works (Chattopadhyay et al. 1999; Karthik et al. 2012; Maulik et al. 2011; Shao et al. 2004; Yang et al. 1997) reported that citric acid as an inexpensive and nontoxic crosslink agent was used to improve the wrinkle resistance of cotton fabric through an esterification between them. Meanwhile, cotton fiber has been easily modified to become carboxylic fiber because of the introduction of several carboxylic groups into its molecular structure (Chattopadhyay et al. 1999; Yang et al. 1997). Therefore, it is reasonable to consider cotton fiber modified with citric acid as a lower priced and strong substitute for alginate fiber for fixing Fe(III) ions by coordination of its carboxylic groups to prepare a novel cellulose fiber-supported heterogeneous Fenton catalyst. Furthermore, other literature reports revealed that citric acid reacted to the cellulose hydroxyls for antimicrobial effectiveness against the some microorganisms (Budimir et al. 2012). Cotton fiber was modified with citric acid to produce a carboxyl cotton chelator (Gong et al. 2007) or non-woven mat (Marshall et al. 2007) for extraction of copper in water. However, little research has been concerned with the synthesis and catalytic activity of the heterogeneous Fenton catalyst with citric acid-modified cotton fiber. Hence, in the present study, cotton fabric was first modified with citric acid to impart carboxylic groups and then was coordinated with Fe(III) ions to produce a heterogeneous photo-Fenton catalyst for dye degradation under visible irradiation. After the characterization of the resulting complex, the factors affecting the modification process, such as the citric acid and NaH2PO4 concentrations as well as curing temperature, were also investigated with respect to the coordinating performance of the modified cotton fiber with Fe(III) ions and photocatalytic activity of its Fe complex. Besides, the role of H2O2 and the reuse of the novel catalyst were evaluated during the dye degradation. Finally, the mechanical performance of the citric acid-modified cotton fabric and its Fe complex was examined.
Experimental section
Materials and reagents
Commercially scoured and bleached cotton woven fabric (118.2 gm−2) was used in this study. This fabric was further treated with a solution containing 2.0 g l−1 Na2CO3 and 2.0 g l−1 soap at the boil for 30 min, then thoroughly washed with cold water and dried at ambient temperature before use. Citric acid, NaH2PO412H2O, FeCl3·6H2O and H2O2 (30 %, w/w) were of analytical grade and used as received. A commercial azo dye, C.I. Acid Red 88 (abbr. AR 88), was used after purification by the re-precipitation method; its molecular structure is presented in Scheme 1. This dye was selected as the model dye in this work mainly because of its relatively high consumption rate in China for dyeing silk, wool and nylon textiles. It has been produced by more than 20 chemical companies in China, but has not met China's effluent color standard. Double-distilled and deionized water was used throughout the study.
Modification of cotton fabric
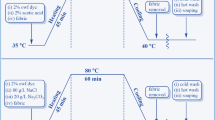
Cotton fabric was first impregnated in an aqueous solution composed of various concentrations of citric acid (1.0–20.0 % w/w) and NaH2PO2 (0.5–9.0 % w/w) at room temperature for 10 min. The impregnated fabric was then padded using a laboratory mangle (Mathis AG, Switzerland). The wet pick-up was 75–80 %. Afterward, the padded fabric was immediately dried at 80 °C for 5 min and cured at different temperatures (100–180 °C) for 1.5 min. The resulting citric acid-modified cotton fabric (denoted as CA-Cotton) was washed thoroughly, soaped, rinsed and finally dried. According to the previous studies (Chattopadhyay et al. 1999; Schindler and Hauser 2004; Yang et al. 1997), citric acid first forms a cyclic anhydride at high temperatures, which then reacts with only one hydroxyl group of cotton fiber to form CA-Cotton containing two carboxyl groups (denoted as CA-Cotton-I) or with two carboxyl groups to form CA-Cotton containing only one carboxyl group (denoted as CA-Cotton-II) for causing the crosslink. The modification of cotton fabric with citric acid is described in Scheme 2.
Determination of carboxyl group contents
First 0.50 g of the dried CA-Cotton pieces (0.50 cm × 0.50 cm) were placed in 50 ml of 100 mmol l−1 NaOH aqueous solution under a nitrogen atmosphere and stirred for 2 h at room temperature. The amount of the unneutralized NaOH in solution was then determined by titration with a standardized 100 mmol l−1 HCl aqueous solution by an automatic titrator (Shanghai Jingmi Instrument Co., China). Phenolphthalein was used as the indicator. The carboxyl group content of CA-Cotton (Q COOH, mmol g−1) was calculated as: Q COOH = (V 1 C 1 − V 2 C 2)/m, where C 1 and C 2 are the molar concentrations of NaOH and HCl aqueous solution (mol l−1), respectively. V 1 is the volume of NaOH solution (50 ml). V 2 is the volume of HCl solution consumed in titration (ml), and m is the mass of dry CA-Cotton used (g).
Determination of the degree of substitution
The degree of substitution (DS) is the average number of hydroxyl groups substituted per anhydroglucose unit in the cellulose molecule (Chi et al. 2008). Hence, the DS value of CA-Cotton was determined using the same method as for its Q COOH value involving the same acid-base titration in this work. It is pointed out that the DS value is of greatly positive relevance to Q COOH since a higher DS value means that a large number of citric acid molecules have reacted with cotton fibers, thus increasing the quantity of carboxyl groups of CA-Cotton. Thus, its DS value was calculated by the following equation:
where 162 is the molecular weight of the anhydroglucose unit and 45 is the molecular weight of the carboxyl group.
Coordination of CA-Cotton with Fe(III) ions
Five grams of CA-Cotton was immersed in 150 ml 0.10 mol l−1 FeCl3 aqueous solution. The mixtures were kept at 50 °C for 3–4 h under continuous agitation. The obtained CA-Cotton Fe complex (denoted as Fe–CA-Cotton) was then taken out, washed with deionized water repeatedly and dried under vacuum at 60 °C for 3 h. The residual concentration of Fe(III) ions in the coordinating solution was determined using a Varian Vista-MPX inductively coupled plasma optical emission spectroscopy (ICP-OES) for calculating the Fe content (Q Fe) of the complex.
Characterization of Fe–CA-Cotton
The surface morphology of Fe–CA-Cotton was observed using a S-4800 scanning electron microscope (Hitachi High-Tech Co., Japan) operating at 15 kV. The composition of Fe–CA-Cotton was verified using a Nicolet Magna-560 Fourier transform spectrometer (Nicolet Instrument Co., USA) with 4 cm−1 resolution. The binding energy analyses of the complex were performed on a PHI 5600 X-ray photoelectron spectrometer (PekinElmer Inc., USA), and the binding energy of C1s was shifted to 284.8 eV as the reference. The powder X-ray diffraction measurement of the complex was conducted on a Rigaku Xd/Max-2500 X-ray diffractometer (Rigaku Co., Japan) operating with Cu Ka radiation at 40 kV and 20 mA and 2θ ranges from 5° to 80°. The scan rate used was 0.025°min−1. The light adsorption properties of the complex were evaluated by measuring their diffuse reflectance UV-Vis spectra (DRS), which were recorded on a Varian Cary 500 UV-Vis-NIR spectrometer (Varian Inc., USA) in the 200–800-nm range with BaSO4 as the reflectance standard.
Catalytic evaluation
The catalytic performance of Fe–CA-Cotton was evaluated by dye degradation under visible irradiation. The dye degradation was carried out in a photoreaction system presented in our previous studies (Dong et al. 2010; Han et al. 2011) at an initial pH of 6.0 and 25 °C. A 400-W high-pressure mercury lamp was used as the irradiation source for the photocatalytic reaction. A cutoff filter was used to ensure irradiation only by visible light (λ > 420 nm). The intensity of visible irradiation over the test solution surface was measured to be 9.65 mW cm−2 using a radiometer (BNU Light and Electronic Instrumental Co., China). Then 0.50 g of Fe–CA-Cotton was first placed into 50 ml of test solution containing 0.05 mmol l−1 AR 88 and the appropriate amount of H2O2. The photocatalytic degradation of the dye was initialized after the adsorption/desorption equilibrium of the dye on the complex had been reached in the dark for 2 h. At given irradiation time intervals, 1–2 ml of the test solution was taken and analyzed immediately on a UV-2401 Shimadzu spectrophotometer. The decoloration percentage of the dye was expressed as: D% = (1 − C d/C d,0) × 100, where C d,0 and C d are the initial and residual concentration of the dye (mmol l−1), respectively. Moreover, the content of total organic carbon (TOC) was assayed in the dye degradation process by using a Phoenix 8000 TOC analyzer (Tekmar-Dehrmann Inc., USA), and the TOC removal percentage of the dye was also calculated using the following formula: TOCR% = (1 − TOC t /TOC0) × 100, where TOC0 and TOC t are the TOC values (mg l−1) at reaction times 0 and t, respectively. ESR (electron spin resonance) spectra of the radical spin trapped by DMPO (5,5-dimethyl pyridine N-oxide) were examined using a Bruker ESP 300E spectrometer equipped with an irradiation source of the Quanta-Ray ND: YAG laser system (λ = 532 nm). To minimize measurement errors, the same quartz capillary tube was used throughout the ESR measurements.
Mechanical performance testing
Before testing, the modified cotton fabric and its Fe complex samples were conditioned at 25 °C and 65 % RH for at least 24 h. Their breaking strength and elongation at break were then tested using a YG 061 laboratory fabric tensile tester (Laizhou Electronic Instrument Co., China) with a 25-cm-long sample and 10 cm min−1 rate of deformation according to the Chinese Standard GB/T3923.1-2013, based on ISO 13934-1:1999. The data shown correspond to averages of at least five individual tests for each sample.
Results and discussion
Characterization of Fe–CA-Cotton
SEM observation
Figure 1 shows the SEM images of the dry original cotton fiber, CA-Cotton and Fe–CA-Cotton. Many small wrinkles were found on the surface of the original cotton fiber in Fig. 1a, which was similar to the SEM observation by Lewin (2010). Figure 1b shows that after the modification with citric acid, these small wrinkles become much more obvious. When being coordinated with Fe(III) ions, some of these wrinkles were covered by a mud-like layer, which thus seemed to cause the rough and uneven surface of Fe–CA-Cotton in Fig. 1c. The morphological change suggested that carboxyl groups of citric acid have been imparted to the cotton fiber surface and led to the Fe species located on Fe–CA-Cotton.
FTIR analysis
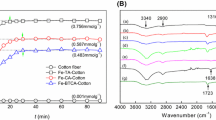
The original cotton fiber, CA-Cotton and its Fe complexes were examined by FTIR spectrometry, and their spectra are presented in Fig. 2.
The major absorption peaks of the original cotton fiber were found to be at 3,340, 2,900, 1,431, 1,316, 1,158, 1,061, 1,033 and 905 cm−1 owing to the stretching of OH, CH, CO and C–O–C at β-1,4 glycosidic linkages, as well as symmetric CH2 bending, respectively (Oh et al. 2005; Sun et al. 2008). Compared with the original cotton fiber, a new peak was found at 1,720 cm−1 in the spectrum of CA-Cotton, which may be attributed to the absorption vibrations of the carboxyl groups and ester carbonyl bands of citric acid with cotton fiber since CA-Cotton was thoroughly washed to remove the unbound citric acid and catalyst. This confirms that the carboxyl groups have been introduced into the molecular structure of the cotton fiber by ester linkage with citric acid. Moreover, it is expected that the peak centered at 1,720 cm−1 became much less intensive in the spectrum of Fe–CA-Cotton. This result suggested that Fe(III) ions coordinated with the carboxyl groups of CA-Cotton.
XPS analysis
According to the survey scan from Fig. 3, the modified cotton fiber surface was composed mainly of carbon and oxygen elements (the hydrogen element is not detectable in XPS analysis). More importantly, a small amount of the Fe element was found in case of Fe–CA-Cotton. Besides, the binding energy of O1s and C1s in the complex exhibited an increase of 0.27 and 0.14 eV, respectively. Moreover, the high-resolution carbon C1s spectrum was fitted into three symmetric Gaussian components C1 (C–C), C2 (C–O) and C3 (C=O and O–C–O) (Buchert et al. 2001; Fras et al. 2005). The binding energies of three subpeaks for Fe–CA-Cotton were higher than those for CA-Cotton, correspondingly. These results proposed that oxygen atoms in the carboxyl groups on CA-Cotton could coordinate with Fe(III) ions as the electron donors when Fe–CA-Cotton was being prepared. According to the previous works (El-Sawy and Ali 2007; Park and Na 2006), CA-Cotton-I containing both carboxyl groups may react with the Fe(III) ion by inter- and intramolecular coordination, while CA-Cotton-II retaining one carboxyl group has to coordinate through the intermolecular mode (b) because of the space steric hindrance. A possible coordinating mechanism is suggested in Scheme 3.
XRD analysis
Figure 4 shows the XRD patterns of cotton fiber before and after modification and coordination. Three typical characteristic peaks of cotton fiber were found at 14.50°, 16.72° and 22.81°, which agreed well with those published in the literature (Parikh et al. 2007). It was worth noticing that the peak centered at 22.81° had a strong intensity after the modification, indicating that treatment with citric acid increased the crystallinity of cotton fiber. This is because the citric acid molecule can penetrate the amorphous regions in the fiber more easily than it does the crystals when cotton fiber is modified with citric acid. Then they produce crosslinks between the cellulose units in the amorphous regions, thus increasing the crystallinity (Xu 2003, Parikh et al. 2007). However, the intensity of this peak was reduced by subsequent coordination of Fe(III) ions. A possible reason is that although Fe(III) ions may enhance the linkage between the cellulose units in the amorphous regions because of the coordination of Fe(III) ions with carboxyl groups of carboxylic fiber (Li et al. 2014a). The strong acidity of FeCl3 aqueous solution can cause a significant erosion of the crystal surface, which reduces the crystal volume (Xu 2003), thus resulting in a decrease in crystallinity. The pH of 0.10 mol l−1 FeCl3 aqueous solution was measured using a DHS-25C digital pH meter (Shanghai Jingmi Instrument Co., China) to be 1.92 during the coordination in this work.
Light adsorption property
Little light absorption was seen for the original cotton fiber from Fig. 5. CA-Cotton had a very weak light absorption at less than 300 nm. On the contrary, Fe–CA-Cotton showed a much stronger light absorption in the UV and visible light regions, and the characteristic broad band was centered at 289 nm. This may be attributed to the d–d transitions of the Fe(III) ions and the ligand-to-metal charge transfer (LMCT) transitions from CA-Cotton toward Fe(III) ions in the complex, thus often causing adsorption in the visible region (Cotton et al. 1995). Furthermore, the light absorption in the UV region was much stronger than that in the visible region for the complex. This is similar to the light absorption feature of the Fe(III)-alginate fiber complex (Li et al. 2014b). Accordingly, it is likely that the light absorption feature of this complex will ensure the potential utilization of the UV and visible light of solar irradiation when it serves as a heterogeneous photo-Fenton catalyst for the dye degradation.
Catalytic activity of Fe–CA-Cotton
Effect of the citric acid concentration
The cotton fabrics were modified with various concentrations of citric acid (C CA) in the presence of 5.0 % (w/w) NaH2PO412H2O by the pad-dry-cure (180 °C × 1.5 min) process to produce CA-Cotton. The obtained modified fabrics were then coordinated with 0.10 mol l−1 FeCl3 to prepare Fe–CA-Cotton. The Q COOH and DS values of CA-Cotton as well as Q Fe values of their complexes were measured (Table 1). Fe–CA-Cotton was tested as a heterogeneous Fenton photocatalyst for degradation of AR 88 under visible irradiation. The decoloration percentage of the dye within 50 min (D 50%) was measured. Meanwhile, the pseudo-first-order rate constants, k, of dye degradation were also calculated with all regression coefficients greater than 0.95 and are listed in Table 1.
As can be seen from Table 1, the Q COOH and DS values gradually increased with the enhanced C CA from 1.0 % (w/w) to 20.0 % (w/w). This is because the higher C CA could increase the modification degree of the cotton fiber with citric acid (Dong et al. 2001), thus incorporating more carboxyl groups on CA-Cotton. Besides, the highest Q Fe was found when C CA was 10.0 % (w/w), and further increasing C CA led to a reduced Q Fe. This can be explained by the fact that the modified cotton fiber with an excessive concentration of citric acid may have become more crystalline and less amorphous (Parikh et al. 2007), which inhibits the penetration and diffusion of the Fe(III) ion aqueous solution into CA-Cotton, thus retarding the reaction between them. More importantly, Table 1 shows that the higher C CA gave rise to an increment in both D% and k, and there is an optimum C CA level [about 10.0 % (w/w) in this test]. This result suggested that Fe–CA-Cotton exhibited a significant photocatalytic activity for degradation of AR 88 in the solution. The reason is that CA-Cotton is a complicated modified cotton fiber with a crosslink structure formed with the carboxyl groups between both cotton fiber chains due to the incorporation of citric acids (Scheme 2). Furthermore, CA-Cotton can react with Fe(III) ions to form Fe–CA-Cotton through the covalent bonds between its carboxyl groups and Fe(III) ion (Scheme 3). The introduction of Fe(III) ions may significantly enhance the distortion of Fe–CA-Cotton due to the twist of cotton fiber chains, thus resulting in the creation of defects and unsaturated coordination (Skårman et al. 2002; Zhu et al. 2008). When Fe–CA-Cotton is employed as a catalyst, it may react with H2O2 to generate ·OOH radicals. Meanwhile, the reduction of Fe(III) ions to Fe(II) ions loaded on Fe–CA-Cotton is carried out. The formed Fe(II) ions react with H2O2 to produce the ·OH radicals (Fig. 6), which can be responsible for the dye degradation. A possible reaction process is expressed by Eqs. 1–4.
Increasing the Q Fe of Fe–CA-Cotton may cause more active sites on their surface, which can enhance the H2O2 decomposition and produce a relatively high concentration of ·OH radicals in solution by the photo-Fenton reaction, thus accelerating the dye degradation (Dhananjeyan et al. 2001; Dong et al. 2010). In order to further understand the photocatalytic performance of Fe–CA-Cotton, the degradation of AR 88 was carried out under visible irradiation in the presence of 3.0 mmol l−1 H2O2 and Fe–CA-Cotton (Q Fe = 0.53 mmol g−1) and examined by the UV-Vis spectrum and TOC measurement, respectively, and the results are presented in Fig. 7.
It is obvious from Fig. 7 that both characteristic absorption bands (214 and 505 nm) of AR 88 decreased, whereas the TOCR% increased with reaction time. These results suggested that Fe–CA-Cotton had a dramatic photocatalytic activity for the degradation and mineralization of AR 88 under visible irradiation, which promoted the breaking of azo linkages and naphthalene rings in its molecular structure, then converting its intermediates into H2O, CO2 and inorganic salts.
On the other hand, the Fe(III)-alginate fiber complex as a cellulose fiber-supported catalyst was found to be effective for the dye degradation (Li et al. 2014b). Comparing Fe–CA-Cotton and Fe(III)-alginate fiber complex, k values (3.51–5.13 × 10−2 min−1) of Fe–CA-Cotton prepared with C CA above 3.0 % (w/w) are higher than those (2.27 × 10−2 min−1) of Fe(III)-alginate fiber complex (Li et al. 2014b) under similar conditions. Moreover, a similar TOCR% value was also observed within 40 min when either of the two catalysts was used.
Effect of the NaH2PO4 concentration
NaH2PO4 is the most effective catalyst usable for the modification of cotton fabric with citric acid. In this experiment, the concentrations of NaH2PO412H2O (C SHP) were set from 0.50 % (w/w) to 9.0 % (w/w) for preparing the padding solutions. Meanwhile, citric acid was fixed at 10.0 % (w/w). Afterward, the cotton fabrics were treated with the padding solutions by cure treatment (180 °C × 1.5 min) and coordinated with 0.10 mol l−1 Fe(III) ion to produce Fe–CA-Cotton for the photocatalytic degradation of the dye under visible irradiation. The results are provided in Table 2.
It is evident from Table 2 that increasing C SHP is accompanied by increasing Q COOH and DS values, since a high concentration of NaH2PO4 may accelerate the formation of cyclic anhydride from polycarboxylic acid, which is subsequently trapped by cellulose hydroxyl groups to form the ester linkage (Yang and Wang 1997). Thus, this can lead to more intensive modification of cotton fiber with citric acid to impart a large number of hydroxyl groups. However, higher Q COOH of CA-Cotton results in an insignificant increment in the Q Fe of the resulting complex. This is because the excessive amount of NaH2PO4 may significantly increase the crosslinkage degree of the modified cotton fiber through a conversion of CA-Cotton-I to CA-Cotton-II (Scheme 2), thus blocking the coordination of Fe(III) ion in solution by its low amorphous region and different coordination mode (Scheme 3). Additionally, D 50% and k values increase as C SHP increasing from 0.50 % (w/w) to 5.0 % (w/w), and the increasing tendency becomes level at C SHP over 5.0 % (w/w). This may mainly be determined by Q Fe of the obtained Fe–CA-Cotton.
Effect of curing temperature
Cotton fabric was first padded with an aqueous formulation composed of 10.0 % (w/w) citric acid and 5.0 % (w/w) NaH2PO412H2O. Then the padded fabric was cured at different curing temperatures. The obtained modified fabric was coordinated with 0.10 mol l−1 Fe(III) ion to produce Fe–CA-Cotton, which was evaluated as a Fenton catalyst in the photocatalytic degradation of the dye under visible irradiation, and Q COOH, DS, Q Fe, D 50% and k values at different curing temperatures were tested and are listed in Table 3.
The data in Table 3 indicated that Q COOH and DS values became gradually higher as the curing temperature rose from 100 to 180 °C, implying that the elevation of the curing temperature promoted the combination of citric acid with cotton fibers. The main reason is that an increase in curing temperature increased the esterification rate. Consequently, both the quantity of ester groups formed and number of carboxyl groups formed per each acid molecule increased at a higher curing temperature (Yang 1991). Although high Q COOH could increase the Q Fe of Fe–CA-Cotton, Fe–CA-Cotton with the highest Q Fe was obtained for CA-Cotton prepared at 140–160 °C. This is because that first carboxyl group in the citric acid molecule is easily esterified with cotton fiber through the formation of a cyclic anhydride at lower temperature to generate CA-Cotton-I containing two carboxyl groups (Yang 1991), which can combine with Fe(III) ions by inter- and intramolecular modes (Scheme 3). A higher curing temperature was able to enhance the crosslinkage of the modified cotton fiber through the formation of CA-Cotton-II, thus reducing the coordination rate of the Fe(III) ion as a consequence of the increased crystallinity and different coordination mode of the modified cotton fiber mentioned earlier. Moreover, D 50% and k values also varied remarkably with curing temperature and reached their maximum levels at 140 °C. Accordingly, there is a consistent match between the D 50% or k value and Q Fe of Fe–CA-Cotton. In addition, the low hydrophilicity of Fe–CA-Cotton prepared at high temperature may hinder the adsorption of both dye and H2O2 molecules during the degradation because a good heterogeneous catalyst should have the ability to adsorb reactant molecules strongly enough for them to react (Dong et al. 2010).
Initial H2O2 concentration
The photocatalytic degradation of AR 88 was performed in the presence of 10.0 g l−1 Fe–CA-Cotton (Q Fe = 0.46 mmol g−1) and varied the initial H2O2 concentration from 0 to 6.0 mmol l−1 under visible irradiation. The results are shown in Fig. 8.
Figure 8 shows that D% value increased with the prolongation of reaction time. Moreover, the initial D% value gradually increased with increasing initial H2O2 concentrations, indicating that a high initial concentration could enhance the D% value. The highest D% value was achieved at an initial H2O2 concentration of 4.5 mmol l−1. Further increasing the initial H2O2 concentration decreased the D% value. This is mainly ascribed to the role of H2O2 in the Fenton reaction depending on its concentration. H2O2 molecules on the photocatalyst could not generate enough ·OH radicals for dye degradation at a low initial concentration of H2O2. When the initial H2O2 concentration was over the optimum value, surplus H2O2 molecules served as hydroxyl scavengers (Muruganandham and Swaminathan 2004; So et al. 2002), thus reducing the numbers of ·OH radicals, which was responsible for the slow decomposition of the dye in the system. Accordingly, the initial H2O2 concentration of 4.5 mmol l−1 was considered to be the optimum value for completing the degradation of 0.05 mmol l−1 AR 88 in this work.
Recycling capacity of Fe–CA-Cotton
The recycling property of Fe–CA-Cotton was assessed as a stable photocatalyst by the additional degradation process of fresh AR 88 solution with the Fe–CA-Cotton used from the previous runs. The used Fe–CA-Cotton was thoroughly washed with distilled water after each run, and AR 88 and H2O2 were added before the next run. Fe–CA-Cotton (Q Fe = 0.46 mmol g−1) was reused up to five times. The result is presented in Fig. 9.
Figure 9 shows the degradation curves in four successive runs were exactly similar to those in the first run, proposing that the catalytic activity of Fe–CA-Cotton had little deactivation in four successive runs. A possible explanation is that the excessive adsorption of reactant molecules and their products formed during the reaction may decrease the activity of the catalyst by the poisoning effect (Soon and Hameed 2011; Moulijn et al. 2001). In a heterogeneous Fenton reaction catalyzed by Fe(III)-carboxylic fibrous complex, the adsorption as well as coordination of H2O2 is a crucial step to generate ·OH radicals by Fe ions on the catalyst (Li et al. 2014a). Moreover, the most strongly adsorbing dyes or organic species hinder the adsorption of less strongly adsorbing H2O2, which may limit its activation. In this study, the crosslink of citric acid with cotton fiber could cause the decrease in accessibility of dyes to adsorption sites (Blanchard et al. 1996). Moreover, the water contact angle of Fe–CA-Cotton was tested to be 95.3° using a liquid-solid contact angle analyzer (DSA100; Krüss, Germany) equipped with a high-speed camera, which indicates that Fe–CA-Cotton has a relatively low hydrophilicity. These may inhibit the excessive adsorption of the water-soluble AR 88 or its degradation products into Fe–CA-Cotton, thus preventing poisoning of the active catalytic sites. Additionally, the leaching tests were conducted to examine the potential of leaching of Fe(III) ions from Fe–CA-Cotton using the ICP-OES method to be less than 1.0 mg l−1. These results confirmed that the photocatalytic activity of Fe–CA-Cotton could be kept from leaching Fe(III) ions and poisoning of the active catalytic sites owing to the adsorbed organic species.
Mechanical performance
Better mechanical performance is necessary for effective use of the fibers as the supporting material for Fenton catalysts, since their mechanical performance usually plays an important role in their durability and regenerative property (Ishtchenko et al. 2003b). In this work, CA-Cotton (Q Fe = 0.46 mmol g−1) was first coordinated with different concentrations of Fe(III) ions, then breaking strength and elongation at break of the resulting Fe–CA-Cotton samples were measured and are presented in Fig. 10.
Figure 10 shows that after the modification with citric acid, the breaking strength and elongation at break of CA-Cotton are reduced by about 27 and 56 %, respectively. This is mainly attributed to the lesser uniformity of citric acid crosslinks distributed on the cotton fabric, thus decreasing the breaking strength and elongation at break, as a result of the stress concentration. Moreover, the crosslinking property of citric acid can also effectively limit the activity of the fiber molecular chains and restrict the slippage of molecular chains in case of experiencing stress, which is responsible for the lower elongation. The variation of breaking strength and elongation at break of the resulting Fe–CA-Cotton with increasing Fe(III) ion concentration is also observed in Fig. 10. Its maximum values are obtained when the Fe(III) ion concentration is 0.04 mol l−1 in the solution. A reason for this may be that according to our previous study (Li et al. 2014a, b), when the coordination of carboxylic fibers with the Fe(III) ion occurred, the Fe(III) ion was able to react with two or three carboxyl groups on the adjacent molecular chains in the amorphous regions of the fibers to link the chains to each other, thus enhancing the mechanical properties. On the other hand, as described above, the strong acidity of FeCl3 aqueous solution can cause significant erosion of the modified cotton fabric. Moreover, increasing the FeCl3 concentration may enhance the acidic erosion, thus balancing out the increase in mechanical properties. Accordingly, 0.04 mol l−1 is considered the critical concentration of Fe(III) ions for obtaining Fe–CA-Cotton with better mechanical properties. Subsequently, Fe–CA-Cotton prepared with 0.04 mol l−1 Fe(III) ion solution was reused for dye degradation five times as mentioned above to investigate its damage during the degradation procedure. Its breaking strength after reuse was then tested to be 284.5 N, indicating that Fe–CA-Cotton was insignificantly destroyed with ·OH radicals from the photo-Fenton reaction. This result confirms that Fe–CA-Cotton is robust with a strength sufficient for use as a cellulose fiber-supported catalyst.
Advantages of citric acid-modified cotton fabric
In spite of possessing excellent properties for use as a supporting material for immobilizing Fe(III) ions to produce the heterogeneous Fenton catalysts (Li et al. 2014b), alginate fibers can rarely be used alone because of their relatively poor mechanical strength and high price. In contrast, cotton is a well-known polysaccharide-based cellulose fiber. The cellulose molecules are linearly arranged and pass through the crystalline and amorphous regions of the cotton fibers. Hence, cotton fiber has been the commonly used cellulose fiber in the modern textile industry throughout the world owing to its better mechanical strength, chemical resistance, spinnability for textile processes, etc. (Grace et al. 2009). In this study, cotton fabric was modified easily and economically with citric acid using the regular pad-dry-cure process widely applied for textile finishing. Citric acid is preferable for the modification of cotton fabric because a low concentration (5–10 % w/w) is required. Furthermore, it can be derived from fermentation and can therefore be considered as a green chemical (Reddy and Yang 2010). Citric acid modification caused cotton fabric strength loss. However, the coordination of Fe(III) ions was found to partly recoup its loss by linking carboxylic cotton fiber chains. More importantly, comparing the Fe(III)-modified cotton fiber complex with the Fe(III)-alginate fiber complex with similar Fe contents, the photocatalytic activity of the former is higher than that of the latter at the same conditions. Thus, this makes citric acid-modified cotton fiber a better candidate for replacing the alginate fiber used to prepare a heterogeneous photo-Fenton catalyst.
Conclusion
FTIR and XPS analysis confirmed that cotton fabric was modified with citric acid by the pad-dry-cure process to incorporate the carboxyl groups in its structure, which could coordinate with Fe(III) ions to prepare the Fe(III)-modified cotton fiber complex (Fe–CA-Cotton). DRS measurement revealed that Fe–CA-Cotton was activated throughout the UV and visible light regions, ensuring the potential utilization of UV and visible light of solar irradiation when it was used as a heterogeneous photo-Fenton catalyst for dye degradation. The modification process exhibited a great effect on the performance of CA-Cotton and its Fe complex. Increasing the concentrations of citric acid and NaH2PO4 or high curing temperature could enhance the DS value and carboxyl group content of CA-Cotton as well as the Fe content and photocatalytic activity of its complex. However, an excessive amount of citric acid and NaH2PO4 or curing temperature higher than 140 °C led to a reduction in the Fe content and catalytic performance of the obtained Fe–CA-Cotton due to its low amorphous region and hydrophilicity as well as the different coordination mechanism. Increasing the initial H2O2 concentration significantly accelerated the dye degradation. Therefore, the catalytic activity of Fe–CA-Cotton could be optimized toward the dye degradation by adjusting the modification process and initial H2O2 concentration. Fe–CA-Cotton was also stable in the reusing processes. It should be noticed that although the modification of citric acid reduced the mechanical property of cotton fabric, the coordination of Fe(III) ions was found to partly recoup its loss by linking carboxylic cotton fiber chains. After reuse the breaking strength of Fe–CA-Cotton is reduced by about 20 %. Because of its advantages such as the simple and easy modification with citric acid, and the high catalytic activity and regenerative property of its Fe complex, citric acid-modified cotton fiber is regarded as a proper substitute for alginate fiber as a novel cellulose fiber supporting material with low-cost and good performance to prepare a heterogeneous photo-Fenton catalyst usable for practical treatment of wastewater on an industrial scale.
References
Blanchard EJ, Reinhardt RM, Graves EE (1996) Factors affecting the dyeability of cotton crosslinked with polycarboxylic acid. J Soc Dyers Colour 112:108–113
Blanco J, Torrades F, Morón M, Brouta-Agnésa M, García-Montaño J (2014) Photo-Fenton and sequencing batch reactor coupled to photo-Fenton processes for textile wastewater reclamation: feasibility of reuse in dyeing processes. Chem Eng J 240:469–475
Buchert J, Pere J, Johansson L-S, Campbell J (2001) Analysis of the surface chemistry of linen and cotton fabrics. Text Res J 71:626–629
Budimir A, Vukusic SB, Flincec SG (2012) Study of antimicrobial properties of cotton medical textiles treated with citric acid and dried/cured by microwaves. Cellulose 19:289–296
Chattopadhyay D, Sharma D, De P (1999) Studies on formaldehyde-free crease-resistant finishing of cotton fabric using citric acid and selactive chemical adeditives. Indian J Fibre Text Res 24:284–289
Chi H, Xu K, Wu X, Chen Q, Xue D, Song C, Zhang W, Wang P (2008) Effect of acetylation on the properties of corn starch. Food Chem 106:923–928
Cotton AF, Wilkinson G, Gaus PL (1995) Basic Inorganic chemistry. Wiley, New York
Dhananjeyan MR, Kiwi J, Albers P, Enea O (2001) Photo-assisted immobilized Fenton degradation up to pH 8 of azo dye Orange II mediated by Fe3+/Nafion/Glass fibers. Helv Chim Acta 84:3433–3445
Dong Y, Wang J, Liu P (2001) Dyeing and finishing of cotton fabric in a single bath with reactive dyes and citric acid. Color Technol 117:262–265
Dong Y, Han Z, Liu C, Du F (2010) Preparation and photocatalytic performance of Fe(III)-amidoximated PAN fiber complex for oxidative degradation of azo dye under visible light irradiation. Sci Total Environ 408:2245–2253
El-Sawy NM, Ali Z (2007) Iron(III) complexed with radiation-grafted acrylic acid onto poly (tetrafluoroethylene-co-perfluorovinyl ether) films. J Appl Polym Sci 103:4065–4071
Fras L, Johansson L-S, Stenius P, Laine J, Stana-Kleinschek K, Ribitsch V (2005) Analysis of the oxidation of cellulose fibres by titration and XPS. Colloid Surf A 260:101–108
Gong R, Hu Y, Chen J, Chen F, Liu Z (2007) A cellulose-based carboxyl cotton chelator having citric acid as an anchored ligand: preparation and application as solid phase extractant for copper determination by flame atomic absorption spectrometry. Microchim Acta 158:315–320
Grace M, Chand N, Bajpai SK (2009) Copper alginate-cotton cellulose (CACC) fibers with excellent antibacterial properties. J Eng Fiber Fabr 4:24–35
Han Z, Dong Y, Dong S (2011) Copper–iron bimetal modified PAN fiber complexes as novel heterogeneous Fenton catalysts for degradation of organic dye under visible light irradiation. J Hazard Mater 189:241–248
Hartmann M, Kullmann S, Keller H (2010) Wastewater treatment with heterogeneous Fenton-type catalysts based on porous materials. J Mater Chem 20:9002–9017
Ishtchenko V, Huddersman K, Vitkovskaya R (2003a) Part 1. Production of a modified PAN fibrous catalyst and its optimisation towards the decomposition of hydrogen peroxide. Appl Catal A 242:123–137
Ishtchenko VV, Vitkovskaya RF, Huddersman KD (2003b) Investigation of the mechanical and physico-chemical properties of a modified PAN fibrous catalyst. Appl Catal A 242:221–231
Karthik T, Rathinamoorthy R, Murugan R (2012) Enhancement of wrinkle recovery angle of cotton fabric using citric acid cross-linking agent with nano-TiO2 as a co-catalyst. J Ind Text 42:99–117
Lewin M (2010) Handbook of fiber chemistry. CRC Press, Boca Raton
Li B, Dong Y, Li M, Ding Z (2014a) Comparative study of different Fe(III)-carboxylic fiber complexes as novel heterogeneous Fenton catalysts for dye degradation. J Mater Sci 49:7639–7647
Li B, Dong Y, Zou C, Xu Y (2014b) Iron (III)-alginate fiber complex as a highly effective and stable heterogeneous Fenton photocatalyst for mineralization of organic dye. Ind Eng Chem Res 53:4199–4206
Liu X, Tang R, He Q, Liao X, Shi B (2010) Fe(III)-loaded collagen fiber as a heterogeneous catalyst for the photo-assisted decomposition of Malachite Green. J Hazard Mater 174:687–693
Ma W, Huang Y, Li J, Cheng M, Song W, Zhao J (2003) An efficient approach for the photodegradation of organic pollutants by immobilized iron ions at neutral pHs. Chem Commun 13:1582–1583
Marshall WE, Akin DE, Wartelle LH, Annis PA (2007) Citric acid treatment of flax, cotton and blended nonwoven mats for copper ion absorption. Ind Crops Prod 26:8–13
Maulik SR, Das D, Bhattacharya S (2011) Concurrent dyeing and finishing of cotton with natural colour and citric acid in the presence of NaH2PO4 as catalyst under thermal treatment. J Text Inst 102:491–499
Moulijn JA, Van Diepen AE, Kapteijn F (2001) Catalyst deactivation: is it predictable? What to do? Appl Catal A 212:3–16
Muruganandham M, Swaminathan M (2004) Photochemical oxidation of reactive azo dye with UV–H2O2 process. Dyes Pigm 62:269–275
Oh SY, Yoo DI, Shin Y, Kim HC, Kim HY, Chung YS, Park WH, Youk JH (2005) Crystalline structure analysis of cellulose treated with sodium hydroxide and carbon dioxide by means of X-ray diffraction and FTIR spectroscopy. Carbohydr Res 340:2376–2391
Parikh D, Thibodeaux D, Condon B (2007) X-ray crystallinity of bleached and crosslinked cottons. Text Res J 77:612–616
Park H-J, Na C-K (2006) Preparation of anion exchanger by amination of acrylic acid grafted polypropylene nonwoven fiber and its ion-exchange property. J Colloid Interface Sci 301:46–54
Parra S, Nadtotechenko V, Albers P, Kiwi J (2004) Discoloration of azo-dyes at biocompatible pH-values through an Fe-histidine complex immobilized on Nafion via Fenton-like processes. J Phys Chem B 108:4439–4448
Pignatello JJ, Oliveros E, MacKay A (2006) Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry. Crit Rev Environ Sci Technol 36:1–84
Reddy N, Yang Y (2010) Citric acid cross-linking of starch films. Food Chem 118:702–711
Schindler WD, Hauser PJ (2004) Chemical finishing of textiles. Elsevier, Amsterdam
Shao H, Sun J, Meng W, Qing F (2004) Water and oil repellent and durable press finishes for cotton based on a perfluoroalkyl-containing multi-epoxy compound and citric acid. Text Res J 74:851–855
Skårman B, Grandjean D, Benfield RE, Hinz A, Andersson A, Wallenberg LR (2002) Carbon monoxide oxidation on nanostructured CuO x /CeO2 composite particles characterized by HREM, XPS, XAS, and high-energy diffraction. J Catal 211:119–133
So C, Cheng M, Yu J, Wong P (2002) Degradation of azo dye Procion Red MX-5B by photocatalytic oxidation. Chemosphere 46:905–912
Soon AN, Hameed BH (2011) Heterogeneous catalytic treatment of synthetic dyes in aqueous media using Fenton and photo-assisted Fenton process. Desalination 269:1–16
Sun Y, Lin L, Deng H, Li J, He B, Sun R, Ouyang P (2008) Structural changes of bamboo cellulose in formic acid. BioResources 3:297–315
Wu K, Xie Y, Zhao J, Hidaka H (1999) Photo-Fenton degradation of a dye under visible light irradiation. J Mol Catal A 144:77–84
Xu W (2003) Effect of crosslinking treatment on the crystallinity, crystallite size, and strength of cotton fibers. Text Res J 73:433–436
Yang C (1991) FT-IR spectroscopy study of the ester crosslinking mechanism of cotton cellulose. Text Res J 61:433–440
Yang C, Wang X (1997) Infrared spectroscopy studies of the cyclic anhydride as the intermediate for the ester crosslinking of cotton cellulose by polycarboxylic acids. III. Molecular weight of a crosslinking agent. J Polym Sci A Polym Chem 35:557–564
Yang C, Wang X, Kang I (1997) Ester crosslinking of cotton fabric by polymeric carboxylic acids and citric acid. Text Res J 67:334–342
Zhu J, Gao Q, Chen Z (2008) Preparation of mesoporous copper cerium bimetal oxides with high performance for catalytic oxidation of carbon monoxide. Appl Catal B 81:236–243
Acknowledgments
The authors thanks the Tianjin Municipal Science and Technology Committee for the Research Program of the Application Foundation and Advanced Technology (11JCZDJC24600). This research was also supported in part by a grant from the Natural Science Foundation of China (20773093).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, B., Dong, Y. & Li, L. Preparation and catalytic performance of Fe(III)-citric acid-modified cotton fiber complex as a novel cellulose fiber-supported heterogeneous photo-Fenton catalyst. Cellulose 22, 1295–1309 (2015). https://doi.org/10.1007/s10570-015-0562-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-015-0562-x