Abstract
Developing mild decolorization technology is critical for accomplishing clean pulping to overcome issues of the severe degradation of cellulose during the preparation of cotton pulp from waste cotton textiles and the high energy consumed in this preparation. The sodium hydroxide (NaOH)-sodium dithionite (Na2S2O4) system is widely used for decolorizing cotton fabrics. However, previous reports have only studied decolorizing cotton fabrics using this hybrid system and did not clarify the decolorization mechanism of NaOH and Na2S2O4 on fabric dyed with different types of reactive dyes. Therefore, in this work, according to the chromophore groups and active groups of the reactive dyes, the decolorization of cotton fibers dyed with azo monochlorotriazine reactive dyes, anthraquinone vinyl sulfone reactive dyes and diazobismonochlorotriazine reactive dyes was studied. The decolorization mechanism of NaOH and Na2S2O4 on cotton fabrics dyed with different types of reactive dyes was clarified by employing NaOH and Na2S2O4 separately and in combination. Fourier transform infrared spectroscopy, X-ray diffractometer and extension testing were used to explore the effects of decolorization on the chemical structure, crystalline structure, and physical and mechanical properties of cotton fabrics. This work provides a theoretical basis for the decolorization of cotton fabrics dyed using different reactive dyes.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As people’s living standards have improved, the use cycle of textiles has shortened, and a large number of waste textiles are produced every year. At present, waste textiles are mostly disposed of in landfills or via incineration, which leads to severe waste of resources and environmental pollution (Athanasopoulos and Zabaniotou 2022; Lopatina et al. 2021; Jiang et al. 2022; Pensupa et al. 2017; Sharma et al. 2020). Waste cotton fabric accounts for a large amount of waste textiles, and the supply of cotton fibers has always been limited by “the contradiction between food and cotton” (Dahlbo et al. 2017; Leal et al. 2019). Therefore, recycling waste cotton fabric is of both great economic and environmental interest.
Decolorization is an indispensable process for reusing colored waste cotton fabrics. Oxidative decolorization and reductive decolorization are widely used for decolorizing waste cotton. Oxidative decolorization is accomplished using oxidants, such as sodium hypochlorite, chlorine dioxide, ozone, and hydrogen peroxide. However, in the process of decolorization, sodium hypochlorite produces a large amount of chlorine gas, which is very harmful for human health. It also causes damage to the fabric, resulting in decreased strength and poor reusability of the fabric. Chlorine dioxide and hydrogen peroxide are used at a relatively high temperature and in an excessive amount, so their application is limited. The fabric after ozone treatment turns yellow with the increase in storage time. When the ozone residue remains on the fabric, the yellowing phenomenon becomes more obvious (Arooj et al. 2015). The photocatalysis system avoids the consumption of a large amount of energy and chemicals, achieves satisfactory color stripping effect at atmospheric pressure and low temperature, and has the advantages of high efficiency, energy saving and emission reduction. However, the active substances produced by the photocatalysis system can also affect the macromolecules of the fiber while destroying the dye. This may cause changes in the chemical structure and/or aggregation structure of the fiber, leading to changes in the macroscopic properties of the fiber (Chen et al. 2007). In other words, this oxidative decolorization approach has a significant influence on the strength and molecular weight of fabrics, so it is not suitable for the mild decolorization of waste cotton fabric (Li et al. 2022). Reductive decolorization primarily uses reducing agents, such as sodium dithionite (Na2S2O4) and thiourea dioxide, and has a minimal effect on the mechanical properties of fabrics (He et al. 2021; Uddin et al. 2015). Considering the requirements of cellulose molecular weight and mechanical properties, reductive decolorization is an ideal method for mild decolorizing of waste cotton.
Sodium hydroxide (NaOH)/Na2S2O4 has been widely used for decolorizing cotton fabrics that are dyed with reactive dyes. However, in this reductive decolorization method, a mixture of NaOH/Na2S2O4 is added to the decolorizing system without considering the chromophore group and active group of dyes, which results in reagent waste and higher manufacturing costs. In particular, it is unclear which reagent (NaOH or Na2S2O4 or a combination of NaOH and Na2S2O4) functions as the decolorizing agent for reactive dyes with distinct chromophores. Thus, in this work, the mechanism of reductive decolorization of cotton textiles dyed with azo-, anthraquinone-, and bis azo-reactive dyes was investigated. This study provides a theoretical reference for decolorizing reactive-colored cotton fabric, which would be important for recycling and reusing waste cotton fabric.
Experimental
Materials
For this work, a woven cotton fabric with the weight of 95 g/m2 was supplied by Lu Tai Group Co., Ltd. (Shandong, China). The commercially available Reactive Red X-3B (C.I. Red 2; CAS: 17804-49-8), Reactive Blue KN-R (Reactive Blue 19; CAS:2580-78-1), and Reactive Red KE-3B (C.I. Reactive Red 120; CAS: 61951-82-4) dyes were purchased from Longsheng Group Co., Ltd. (Zhejiang, China). The chemical structures of these dyes are depicted in Fig. 1. Sodium dithionite (AR) and sodium hydroxide (AR) were provided by Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China).
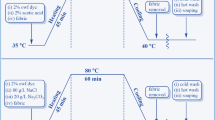
Cotton substrates were colored with each of the Reactive Red X-3B, Reactive Blue KN-R and Reactive Red KE-3B dyes. Specifically, the prepared cotton fabric sample was immersed in a dye bath (4.0%, o.m.f.) at a bath ratio of 1:50. The other dyeing procedures were performed in accordance with the process curves shown in Fig. 2 and the dyeing recipes shown in Table 1.
Decolorization of cotton substrate dyed with reactive dyes
Cotton fabrics dyed with different reactive dyes were decolorized using NaOH/Na2S2O4 system with a bath ratio of 1:20. The decolorized cotton fabrics were thoroughly washed with deionized water until the pH was neutral, and then the fabrics were dried at a temperature of 60 ℃.
Characterization
Lightness values
The color intensity value (K/S value) and the lightness value of the colored and decolorized cotton fabrics were determined using a Datacolor 850 instrument (Datacolor. Co., Ltd., USA) equipped with a simulated D65 light source lamp and 10° visual angle at the maximum characteristic absorption wavenumber (λmax) for each individual reactive dye. Also, a 4-folded form for each colored and decolorized cotton fabric sample and eight-site detections on the individual sample were used throughout the shade determination. Then, the arithmetic mean of the measured lightness values for an individual sample was calculated according to Eq. (1).
Super depth-of-field microscope
Paraffin was melted and smeared on the surface of the dyed and discolored cotton fabrics to prepare samples. A super depth-of-field microscope (DVM6M, Leica Co., Ltd., Germany) was used to investigate decolorizing of cotton fabric samples under different conditions when using NaOH/Na2S2O4 system. Specifically, the surface and section morphological changes of cotton fabric samples were observed.
Fourier transform infrared spectroscopy analysis
The chemical structures of all samples were examined using a Fourier transform infrared spectroscopy (FT-IR) instrument (Nicolet iS50, Thermo Scientific Co., Ltd., US). Samples of the cotton fabrics before and after decolorization were dried and crushed into a homogenous powder. The powdered sample (2 mg) was combined with IR grade KBr (200 mg), and pellets were prepared on a KBr press. The FT-IR spectra of the samples were recorded at ambient conditions from 500.0 to 4000.0 cm−1 with a resolution of 4.0 cm−1.
Wide-angle X-ray diffraction analysis
An X-ray diffractometer (XRD, Smartlab SE, Rigaku Corporation Co., Ltd., Japan) was used to analyze the crystal structures of cotton fabrics before and after decolorization. The cotton fabric samples were dried and crushed into a homogenous powder, and then tested using a 40.0 kV tube voltage and a 40.0 mA tube current. The XRD patterns were recorded over the range of \(2\theta =5\sim 45^\circ \).
Breaking strength
The breaking strength of cotton fabric samples that were colored and decolorized was determined using a universal material testing machine (INSTRON5967, Instron Co., Ltd., USA) and the strip method of China textile criteria of GB/T3923.1-2013. Strips of cotton fabric samples were cut into dimensions of 5.0 × 25.0 cm in both the warp and weft directions for making measurements. The breaking strength of each cotton fabric sample was reported as the average of three replicate measurements.
Results and discussion
Effect of decolorizing cotton fabrics with different reactive dyes using only NaOH
The mechanism for the proposed decolorization method on a reactive-dyed cotton fabric substrate with different chromophores for different dyed-cotton treated with different concentrations of only NaOH solution in NaOH/Na2S2O4 system was investigated. The K/S value of decolorized cotton fabrics with the bath ratio of 1:20 at different temperatures for 80.0 min duration was measured for this investigation. Figure 3 depicts the K/S value curves of several decolorized cotton materials at the boiling temperature.
Figure 3 shows that when only NaOH was used to treat colored cotton fabrics, the K/S curves of the decolorized cotton fabrics decreased steadily with increase in the dose. Also, the maximum absorption wavelength did not change. At the same time, the hue of the decolorizing solution became darker. The results show that the covalent bond between the dye and the cotton fiber was hydrolyzed, allowing the hydrolyzed dye to be transferred from cotton fabrics to the decolorizing solution. Additionally, the chromophore of the dye was not destroyed when only NaOH was used to treat dyed cotton fabrics.
The lightness value was calculated to assess the effect of NaOH on decolorizing cotton fabrics. Figure 4 depicts the influence of NaOH concentration on the lightness of cotton fabric at different temperatures. Figure 4 clearly shows that when the dose of NaOH and the temperature were increased, the lightness value was enhanced. However, cotton fabrics colored with Reactive Red X-3B were treated with a solution of 70 g/L NaOH at the boiling temperature. After treatment, the decolorization result was unsatisfactory, with a lightness value of just 58.13, as shown in Fig. 4a. Since the active group of Reactive Red X-3B is only slightly active, it can easily react with cotton fabric to form covalent bonds. Hence, the decolorization result was not optimal when only NaOH was used to decolorize Reactive Red X-3B colored cotton fabrics. As seen in Fig. 4b, the impact of decolorizing with various doses of NaOH at the boiling temperature was outstanding for cotton fibers colored with Reactive Blue KN-R dyes. The lightness value of cotton fabric colored with Reactive Blue KN-R was 62.83 when the fabric was treated with a solution of 10 g/L NaOH. The lightness value improved to 71.67 when the NaOH concentration was increased to 30 g/L. The decolorization result was excellent when only NaOH was used to decolorize cotton fibers colored with Reactive Blue KN-R dye because covalent connections between Reactive Blue KN-R and cotton fabric were not stable in alkaline conditions. Decolorization was accomplished with a 70 g/L NaOH solution at the boiling temperature, as shown in Fig. 4c, and the lightness value was around 63.70 for cotton fabric colored with Reactive Red KE-3B dye. It can be inferred that the decolorization effect was average when only NaOH was used to decolorize cotton fabrics colored with Reactive Red KE-3B dye because it was relatively easy to react with cotton fabric to form covalent bonds due to Reactive Red KE-3B’s functional group. Also, the strength of the covalent bonding between the dye and cotton fiber for cotton fabrics dyed with Reactive Red KE-3B dye was between that of the other two reactive dyes.
Effect of decolorizing cotton fabrics with different reactive dyes using only Na2 S 2 O 4
The mechanism for the proposed method was investigated using a reactive-dyed cotton fabric substrate with different chromophores for different dyed-cotton treated with different concentrations of only Na2S2O4 in NaOH/Na2S2O4 system. The K/S value of decolorized cotton fabrics with the bath ratio of 1:20 at different temperatures for 80.0 min duration was used for the investigations. Figure 5 depicts the K/S value curves of several decolorized cotton materials at the boiling temperature.
Figures 5a and c reveal that when only Na2S2O4 was used to treat cotton fabrics colored with Reactive Red X-3B and Reactive Red KE-3B dyes, the K/S values of the decolorized cotton fabrics decreased rapidly with an increase in the Na2S2O4 dose. This was especially notable at the maximum absorption wavelength. No new absorption peak was found, and the decolorization solution was very light in color. The results demonstrate that Na2S2O4 immediately reacted with dyes in cotton fabrics, reducing the chromophore -N = N– in the dye to –NH–NH– and resulting in decolorizing the cotton fabric.
To assess the influence of Na2S2O4 on decolorization, the lightness value was determined. Figure 6a depicts the corresponding findings of the influence of NaOH concentration on the lightness of cotton fabric at different temperatures. The results demonstrate that when colored cotton fabric was treated with a 0.2 g/L Na2S2O4 solution at the boiling temperature, the lightness value was as high as 78.73. The lightness value of the cotton fabrics improved to 84.46 when the concentration of the Na2S2O4 solution was 0.6 g/L. However, no further significant improvement was achieved by increasing the Na2S2O4 dose above 0.6 g/L. The reducing ability of Na2S2O4 for the Reactive Red X-3B chromophore was extremely targeted. Thus, it may be concluded that Na2S2O4 can be used to decolorize cotton fabrics without utilizing NaOH. Moreover, the high lightness of cotton fabrics could be achieved with only a small amount of Na2S2O4, possibly because the chromophore of Reactive Red X-3B was mono-azo and the reactive group was very active. Thus, it was indicated that a dosage range of 0.4 to 0.6 g/L for the reducing agent Na2S2O4 was effective for decolorizing reactive-dyed cotton substrate.
Figure 6c depicts similar findings. At the same temperature, the lightness values increased when the Na2S2O4 concentration was increased. Despite the fact that the Reactive Red KE-3B colored cotton fabric was treated with 4.0 g/L Na2S2O4 at the boiling temperature, the lightness value was only 64.62. As a result, Na2S2O4 alone had a poor decolorization effect on colored cotton fibers. From the results of the NaOH decolorization study shown in Fig. 5c and the Na2S2O4 decolorization analysis in Fig. 6c, it is obvious that it was unsuitable to use only NaOH for decolorization of cotton fabrics colored with Reactive Red KE-3B dye. Due to the bis azo structure of Reactive Red KE-3B, NaOH and Na2S2O4 should be used together for treating colored cotton fabrics to achieve a better decolorization effect.
Figure 5b shows that when Na2S2O4 was used to treat cotton fibers colored with Reactive Blue KN-R dye, the maximum absorption wavelength of the dye on the decolorized cotton fabric changed from 600 to 480 nm with an increase in the concentration of Na2S2O4. This phenomenon was significantly different from that using Na2S2O4 to decolorize other colored cotton materials, which indicates that it had different reductive capacities and reductive functions on different chromophores. Reactive Red X-3B has a mono-azo structure, Reactive Red KE-3B has a bis azo structure, and Reactive Blue KN-R has an anthraquinone structure. The K/S value of the decolorized cotton fibers at 600 nm reduced dramatically with an increase in the concentration of Na2S2O4, but the K/S value at 480 nm increased. Figure 6b depicts similar findings. Specifically, it demonstrates that the presence of only Na2S2O4 did not assist in decolorization since the lightness values increased and then decreased with an increase in the Na2S2O4 concentration. The effect of the decolorization treatment using only Na2S2O4 on cotton fabric was not desirable due to the increased reduction potential of the Reactive Blue KN-R chromophore. Therefore, cotton fabrics colored with Reactive Blue KN-R dye should be decolorized using NaOH at a concentration of 30 g/L NaOH.
Synergistic effect of sodium hydroxide and sodium dithionite for cotton fabrics colored with Reactive Red KE-3B dyes
To investigate the synergistic effect of NaOH and Na2S2O4, the decolorization performance of the proposed method on a cotton substrate colored with Reactive Red KE-3B dye was investigated using a dose of 60 g/L NaOH and bath ratio of 1:20 at a temperature of 80.0 ℃ for a duration of 80.0 min. The results of lightness values are depicted in Fig. 7.
Figure 7 shows that the suggested approach achieved good decolorization, as indicated by the lightness values of the cotton substrate colored with reactive dyes at most of the Na2S2O4 doses. The lightness value rapidly improved from 55.85 to 69.68 with Na2S2O4 dosages in the range from 0 to 1 g/L. The lightness values improved from 73.23 to 76.68 with an increase in the amount of reducing agent from 2 to 5 g/L. Despite the fact that the lightness values exceeded 70, the increase was very slow. It is anticipated that adding Na2S2O4 to the NaOH solution might greatly improve the decolorization impact. Hence, it is suggested that a concentration range from 2 to 4 g/L for Na2S2O4 can obtain a greater decolorization effect. The decolorization performance for a cotton substrate colored with Reactive Red KE-3B dye was then tested with a dose of 2 g/L Na2S2O4 while the rest of the parameters were the same. The results are depicted in Fig. 8.
Figure 8 shows that the lightness value improved rapidly from 63.48 to 70.30 when the Na2S2O4 dose varied in the range from 10 to 40 g/L. However, when the amount of NaOH was in the range from 40 to 60 g/L, the lightness values improved from 70.30 to 72.85, but the improvement was very slow. It can be inferred that adding NaOH to Na2S2O4 solution significantly improves the decolorization effect. Hence, a concentration range of NaOH from 40 to 60 g/L is recommended to obtain a greater decolorization effect.
Moreover, the decolorization mechanism of the NaOH/Na2S2O4 system for a cotton substrate colored with reactive dye is described by Eqs. (2–4). (El-Sakhawy 2005; Malkavaara et al. 2000).
Investigation of decolorization on cotton fabric with super depth of field microscope analysis
Samples of cotton fabrics colored using different dyes were decolorized using the optimal process, and the samples were analyzed using a super depth-of-field microscope. The surface and cross-sectional morphologies were observed. The results are shown in Fig. 9.
Figure 9 shows that before decolorization, reactive dyes with three different types of chromophores were uniformly distributed on the surface of the yarn and on the cotton fiber that was wrapped inside the yarn. It also shows that the color of each of the different decolorized cotton fabrics was white. There was nearly no dye on the cotton fabrics. It is clearly demonstrated that dye can be stripped from cotton fabric and that an excellent decolorization effect can be obtained using NaOH/Na2S2O4 for cotton fabrics dyed with the three dyes with different chromophores.
Investigation of the decolorization on cotton substrates with mechanical properties analysis
The breaking strength data of cotton fabric before decolorization and after using the optimal decoloring process are shown in Fig. 10.
Figure 10 shows that the retention efficiencies of the warp breaking strength and weft breaking strength were respectively 94.52 and 92.30% for Reactive Red X-3B, 89.80 and 88.90% for Reactive Blue KN-R, and 93.33 and 91.46% for Reactive Red KE-3B dyed cotton fabrics. These results indicate that the physical and mechanical properties of the decolorized fabric were not significantly damaged. The results are consistent with a slight decrease in the crystallinity of the cotton fiber. The decolorization treatment of cotton fabrics with NaOH/Na2S2O4 system has a mild decolorization effect on waste cotton fabrics without affecting subsequent pulping.
Investigation of decolorization on cotton substrates with FT-IR spectra analysis
The decolorization reactions occurring on the substrate and on different dyed cotton substrates after they were decolorized using different decolorization conditions were further investigated by using FT-IR analysis. The recorded FT-IR spectra are depicted in Fig. 11.
FT-IR spectra of cotton cellulose and locally enlarged image for (0) cotton fabric before dying, 1 Reactive Red X-3B dyed cotton fabric and 2 Reactive Red X-3B dyed cotton fabric after decolorization in panel a; 3 Reactive Blue KN-R dyed cotton fabric and 4 Reactive Blue KN-R dyed cotton fabric after decolorization in panel b; 5 Reactive Red KE-3B dyed cotton fabric and 6 Reactive Red KE -3B dyed cotton fabric after decolorization in panel c
Figure 11 reveals some evident variations in the FT-IR spectra of the cotton substrates after they were decolorized using different decolorization conditions compared to the reactive-dyed one. For a sample of the cotton substrate, the strong and typical absorption band at 3351.2 cm−1 was mainly due to the combined O–H (νO-H) stretching vibrations in the macrochains of cotton (Choe et al. 2019; Chung et al. 2004). In addition, the absorption bands at 1061.6 and 1029.8 cm−1 were assigned to absorption peaks of stretching vibration for the C–O–R covalent bond formed between dye molecules and cotton cellulose molecules and the absorption peaks of the C-H in-plane bending vibration in the cellulose molecular structure. Both absorption peaks were weak. The heights of the absorption peaks at the two places were consistent in the IR spectrum for the cotton fabric before it was dyed (Fig. 11). However, the absorption peak at 1061.6 cm−1 was only slightly higher than that at 1029.8 cm−1 in the IR spectrum of cotton fabric colored with different dyes, due to the formation of covalent bonds between the dye and fiber. Moreover, the intensities of these two absorption peaks did not change compared with those of cotton fabrics colored with Reactive Dye X-3B (Curve 2). However, for cotton fiber colored with Reactive Blue KN-R dye and then decolorized by NaOH (Curve 4), and especially for cotton fiber colored with Reactive Red KE-3B dye and decolorized by NaOH/Na2S2O4 (Curve 6), the absorption peak at 1061.6 cm−1 was significantly weaker. It can be inferred that the covalent bond formed between the dye and cotton cellulose was hydrolyzed and broken under the action of NaOH and that Na2S2O4 destroyed the chromophore to achieve decolorization.
Investigation of decolorization on cotton substrates with wide-angle X-ray diffraction analysis
To further explore the decolorization mechanism in NaOH/Na2S2O4 system, both the cotton fiber before decolorization and the decolorized cotton substrate samples were further investigated using wide-angle XRD analysis. The recorded WAXRD patterns are shown in Fig. 12.
WAXRD patterns of cotton samples for 1 Reactive Red X-3B dyed cotton fabric, 2 Reactive Red X-3B dyed cotton fabric after decolorization, 3 Reactive Blue KN-R dyed cotton fabric, 4 Reactive Blue KN-R dyed cotton fabric after decolorization, 5 Reactive Red KE-3B dyed cotton fabric, 6 Reactive Red KE -3B dyed cotton fabric after decolorization
Figure 12 shows that there were some variations in the XRD patterns of the decolorized cotton samples compared to that the dyed control, indicating some changes in the crystalline structure of the cotton fibers during decolorization. As seen in Fig. 12(1), a typical WAXRD pattern of the reactive-dyed cotton fiber with a characteristic and dominant crystalline form of cellulose I was recorded, and there were four broad peaks at Bragg diffraction angles of 2θ = 14.65°, 16.64°, 22.61°, and 34.47° (French 2014; Zhu et al. 2017). The intensity of the diffraction peak for the cotton fibers after decolorization decreased compared to that of the dyed control sample (Fig. 12). These results indicate that only a small amount of the crystal zone of cotton fiber was damaged and that most of the crystal zone and crystal structure were not affected during decolorization. Importantly, these variations also show that the active species were able to diffuse into the inner phase of the cotton fiber to efficiently degrade the reactive dye molecules on the substrate chains, which further confirms that the mechanism for decolorizing Reactive-dyed cotton fabrics was mild.
Conclusions
In this work, the decolorization properties and mechanism of cotton fabrics colored with dyes having different chromophores and active groups were investigated in the NaOH/Na2S2O4 system in a targeted manner. The results show that NaOH breaks the covalent bond between the dye and cotton fiber via a hydrolysis reaction. In contrast, Na2S2O4 destroys the chromophore via a reduction reaction to achieve decolorization. The proposed targeted decolorization method was very effective for removing reactive dye molecules from cotton fabric substrates. This method achieved mild decolorization and adequately retained the breaking strength, which was more than 90%. The dye on Reactive Red KE-3B dyed cotton fabrics was the most difficult to remove, while the dye on Reactive Red X-3B dyed cotton fabrics was the easiest to remove. Cotton fabric dyed with azo monochlorotriazine reactive dye was efficiently decolorized by adding only Na2S2O4. Cotton fabric dyed with anthraquinone vinyl sulfone reactive dye was efficiently decolorized by adding only NaOH. Cotton fabric dyed with bis azo bis monochlorotriazine reactive dye was efficiently decolorized by adding Na2S2O4 and NaOH simultaneously. Moreover, the surface and cross-sectional morphologies of the fiber were observed using a super depth-of-field microscope, and it was found that the dye on the cotton fabric was basically removed. The chemical structure and crystalline structure of dyed and decolorized cotton fabric were mainly unchanged, according to FT-IR and XRD. All of the investigations also indicated that gentle decolorization of waste cotton fabrics could be achieved under the condition of normal pressure and high temperature in the NaOH/ Na2S2O4 system without affecting subsequent pulping.
References
Arooj F, Ahmad N, Chaudhry MN (2015) A pilot-scale application of ozone to bleach raw cotton fabric using various additives. Ozone-Sci Eng 37:203–215. https://doi.org/10.1080/01919512.2014.956861
Athanasopoulos P, Zabaniotou A (2022) Post-consumer textile thermochemical recycling to fuels and biocarbon: a critical review. Sci Total Environ 834:14. https://doi.org/10.1016/j.scitotenv.2022.155387
Cao HT, Cobb K, Yatvitskiy M, Wolfe M, Shen HQ (2022) Textile and product development from end-of-use cotton apparel: a study to reclaim value from waste. Sustainability 14:20. https://doi.org/10.3390/su14148553
Chen SL, Lu SS, Wei LL, Yao YY, Chen WX (2007) Study on decoloration of reactive dye by chlorine dioxide. J Text Res 28:68–71
Choe EK, Lee M, Park KS, Chung C (2019) Characterization of cotton fabric scouring by Fourier transform-infrared attenuated total reflectance spectroscopy, gas chromatography-mass spectrometry and water absorption measurements. Text Res J 89:2305–2315. https://doi.org/10.1177/0040517518790976
Chung C, Lee M, Choe EK (2004) Characterization of cotton fabric scouring by FT-IR ATR spectroscopy. Carbohyd Polym 58:417–420. https://doi.org/10.1016/j.carbpol.2004.08.005
Dahlbo H, Aalto K, Eskelinen H, Salmenpera H (2017) Increasing textile circulation-consequences and requirements. Sustain Prod Consum 9:44–57. https://doi.org/10.1016/j.spc.2016.06.005
El-Sakhawy M (2005) Effect of bleaching sequence on paper ageing. Polym Degrad Stab 87:419–423. https://doi.org/10.1016/j.polymdegradstab.2004.10.002
French AD (2014) Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 21:885–896. https://doi.org/10.1007/s10570-013-0030-4
He FN, Li X, Zhu MK, Hu JH, Yuan YJ, Li CC, Long JJ (2019) Color stripping of reactive-dyed cotton fabric in a UV/sodium hydrosulfite system with a dipping manner at low temperature. Cellulose 26:4125–4142. https://doi.org/10.1007/s10570-019-02336-w
He JM, Xie CF, Long JJ (2021) Sustainable color stripping of cotton substrate dyed with reactive dyes in a developed UV/K2S2O8 photocatalytic system. J Taiwan Inst Chem Eng 121:241–256. https://doi.org/10.1016/j.jtice.2021.04.023
Jiang XY, Zhao ZY, Liao YX, Tang CC, Tremblay PL, Zhang T (2022) A recyclable colorimetric sensor made of waste cotton fabric for the detection of copper ions. Cellulose 29:5103–5115. https://doi.org/10.1007/s10570-022-04572-z
Leal W, Ellams D, Han S, Tyler D, Boiten VJ, Paco A, Moora H, Balogun AL (2019) A review of the socio-economic advantages of textile recycling. J Clean Prod 218:10–20. https://doi.org/10.1016/j.jclepro.2019.01.210
Li RJ, Yang JJ, Zhang GQ, Zhu P (2022) Decolorization of dark-colored waste cotton fabric using redox decoloring agents. RSC Adv 12:17689–17700. https://doi.org/10.1039/d2ra02071h
Long JJ, Liu B, Wang GF, Shi W (2017) Photocatalitic stripping of fixed reactive red X-3B dye from cotton with nano-TiO2/UV system. J Clean Prod 165:788–800. https://doi.org/10.1016/j.jclepro.2017.07.149
Lopatina A, Anugwom I, Blot H, Conde AS, Manttari M, Kallioinen M (2021) Re-use of waste cotton textile as an ultrafiltration membrane. J Environ Chem Eng 9:7. https://doi.org/10.1016/j.jece.2021.105705
Lu L, Fan W, Meng X, Xue L, Ge S, Wang C, Foong SY, Tan CSY, Sonne C, Aghbashlo M et al (2022) Current recycling strategies and high value utilization of waste cotton. The Science of the total environment. https://doi.org/10.1016/j.scitotenv.2022.158798
Malkavaara P, Isoaho JP, Alen R, Soininen J (2000) Dithionite bleaching of thermomechanical pulp: factors having effects on bleaching efficiency. J Chemom 14:693–698. https://doi.org/10.1002/1099-128x(200009/12)14:5/6%3c693::Aid-cem612%3e3.0.Co;2-7
Pensupa N, Leu SY, Hu YZ, Du CY, Liu H, Jing HD, Wang HM, Lin CSK (2017) Recent trends in sustainable textile waste recycling methods: current situation and future prospects. Top Curr Chem 375:40. https://doi.org/10.1007/s41061-017-0165-0
Ribul M, Lanot A, Pisapia CT, Purnell P, McQueen-Mason SJ, Baurley S (2021) Mechanical, chemical, biological: moving towards closed-loop bio-based recycling in a circular economy of sustainable textiles. J Clean Prod 326:13. https://doi.org/10.1016/j.jclepro.2021.129325
Sharma K, Khilari V, Chaudhary BU, Jogi AB, Pandit AB, Kale RD (2020) Cotton based composite fabric reinforced with waste polyester fibers for improved mechanical properties. Waste Manage 107:227–234. https://doi.org/10.1016/j.wasman.2020.04.011
Uddin MG, Islam MM, Islam MR (2015) Effects of reductive stripping of reactive dyes on the quality of cotton fabric. Fash Text 2:1–12. https://doi.org/10.1186/s40691-015-0032-y
Wang C, Li YZ, Yu HY, Abdalkarim SYH, Zhou JP, Yao JM, Zhang LN (2021) Continuous meter-scale wet-spinning of cornlike composite fibers for eco-friendly multifunctional electronics. ACS Appl Mater Interfaces 13:40953–40963. https://doi.org/10.1021/acsami.1c12012
Xie C, Liu B, Sun J, Tang R, Long J (2017) Photocatalytic color stripping of cotton fabric dyed with reactive dye by employing UV/H2O systerm. J Text Res 38:81–88. https://doi.org/10.13475/j.fzxb.20170100308
Zhu CH, Shi J, Xu SJ, Ishimori M, Sui JH, Morikawa H (2017) Design and characterization of self-cleaning cotton fabrics exploiting zinc oxide nanoparticle-triggered photocatalytic degradation. Cellulose 24:2657–2667. https://doi.org/10.1007/s10570-017-1289-7
Acknowledgments
These works were supported by the National Key Research and Development Project “Solid Waste Recycling” Earmarked Project of China (No. 2020YFC1910301)
Author information
Authors and Affiliations
Contributions
WW has done the main job of this study. WW was responsible for investigation, data curation, and original draft. YY was responsible for review and editing. ZX, HW, XG, ZG and PZ were responsible for methodology and investigation. CZ was responsible for conceptualization, methodology, supervision, and funding acquisition. All authors read, revised, and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, W., Xu, Z., Yin, Y. et al. Decolorization properties and mechanism of reactive-dyed cotton fabrics with different structures utilized to prepare cotton pulp. Cellulose 30, 4735–4748 (2023). https://doi.org/10.1007/s10570-023-05153-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-023-05153-4