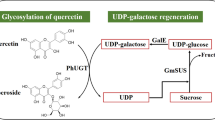
Abstract
Hyperoside is used as the standard to assess the quality of Hyperin perforatum L and exhibits many biological properties. To improve the yield of hyperoside in Escherichia coli, four UDP-galactose synthesis pathways were screened and a recombinant strain was reconstructed by using an efficient UDP-galactose generation system coupled with Petunia hybrida glycosyltransferase to biosynthesis hyperoside from quercetin. By adopting the resting cell transformation, maximal hyperoside production of 4574 mg/L and average productivity of 63.5 mg/L/h were achieved after 72 h of biotranformation. Subsequently through recycling of resting cells, average productivity within 72 h reached 126 mg/L/h, which was 100% higher than that in the batch fermentation. Hyperoside production reached equivalent to 18,000 mg/L by recycling seven times of resting cell in 168 h, which was 393% of that in the batch fermentation and the first to reach 10 g per liter scale in E. coli. Therefore, this study provides an efficient method for construction of UDP-galactose synthesis pathway and hyperoside production in E. coli.
Graphic Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Flavonoids are a large class of over 10,000 natural compounds found in various plants. They are of significant interest due to their varied applications in the fields of flavors, agriculture, nutrition, and pharmaceutics [1,2,3,4]. In recent years, some special flavonoids have attracted scientific attention because of their effective pharmaceutical activities such as antiviral, anti-inflammatory, antidepressant, apoptotic, and antifungal activities [5,6,7,8,9,10,11,12]. For example, hyperoside (quercetin 3-O-galactoside) is used as the standard to assess the quality of Hyperin perforatum L, which is traditional Chinese medicine and exhibits higher bioactivity compared to that of quercetin in some respects [8, 11]. However, it is difficult to produce hyperoside in an industrial scale because of its low concentration and complex composition in the plants [13, 14]. Quercetin has been cheaply produced in an industrial scale from Sophora japonica L. via solvent extraction, hydrolysis, and column chromatography in China [15]. Hyperoside has one more galactose residue at the C-3 position than quercetin. Theoretically, hyperoside can be obtained by glycosylating quercetin at the C-3 position [16].
Structural modifications of flavonoids are an important method for the structural and functional diversification of natural flavonoids [17, 18]. The glycosylation of quercetin can increase its biological activity, bioavailability, and value [5, 16, 19, 20]. In plants, UDP-glycosyltransferases catalyze the glycosylation of flavonoids by transferring a sugar molecule from the UDP-sugar to an acceptor. Many kinds of UDP-glycosyltransferase genes have been cloned and expressed. Some recombinant strains harboring UDP-glycosyltransferase genes have been reconstructed to produce flavonoid glycosides [2, 19,20,21,22,23,24]. De Bruyn et al. developed a glucosylation platform in vivo through expressing sucrose phosphorylase and uridylyltransferase to increasing UDP-glucose supply in E. coli. Subsequently, the recombinant strain with an efficient UDP-galactose supply was reconstructed by overexpressing UDP-galactose/4-epimerase gene [18, 23]. By introducing the UDP-glycosyltransferase gene, titers of hyperoside in the recombinant strain reached 940 mg/L [23]. Although hyperoside production reached almost the gram scale, it is difficult to further improve hyperoside production towards low-cost production because flavonoids can affect the activity of DNA topoisomerase causing the growth inhibition of recombinant strains [25]. Resting cells can overcome these problems and have been used to biotransformation because of separation of growth and transformation. On the other hand, resting cells can be recovered by centrifugation and be used for several rounds of biotransformation [26,27,28,29].
Another UDP-glucose synthesis pathway with cellobiose phosphorolysis has recently been developed in E. coli by introducing the cellobiose phosphorylase and the UTP-glucose-1-phosphate uridylyltransferase genes, which could not only enhance the UDP-glucose supply, but also be helpful for the metabolism and growth of bacteria [30,31,32]. Thus, a novel and efficient UDP-galactose platform in vivo has been developed by using the UDP-glucose synthesis pathway coupled with UDP-galactose/4-epimerase gene. On the basis, a recombinant strain was reconstructed to produce hyperoside. The biotransformation conditions for producing hyperoside by the resting cells were determined.
2 Materials and Methods
2.1 Strains, Plasmids, Media, and Chemicals
All plasmids and strains used in this research are listed in Table 1. Escherichia coli JM109 and BL21 (DE3) strains were grown at 37 °C in Luria–Bertani medium (LB) and supplemented with antibiotics when required. Quercetin was purchased from Shananxi Huike Botanical Development Co., Ltd. (Shanxi, China). Hyperoside was purchased from MUST Bio-Technology (Chengdu, China). LB medium contained 10 g/L tryptone, 5 g/L yeast extract, and 10 g/L NaCl; TB medium contained 12 g/L tryptone, 24 g/L yeast extract, 2.32 g/L KH2PO4, 12.54 g/L K2HPO4, and 10 g/L glycerol; M9 medium contained 3 g/L Na2HPO4, 1.5 g/L KH2PO4, NH4Cl 0.5 g/L, 1 mM MgSO4, and 2 g/L glycerol.
2.2 Plasmid Construction
DNA manipulations were performed according to standard procedures [33]. The flavonol 3-O-galactosyltransferase gene (PhUGT) was obtained by digesting with NcoI and EcoRI from pACYCDuet-PhUGT [16, 34] and subcloned into the expression vector pCDFDuet-1 at the NcoI and EcoRI sites to create pCDFDuet-phUGT. UDP-galactose/4-epimerase gene (galE) was obtained by digesting with NcoI and EcoRI from pACYCDuet-GalE and subcloned into the expression vector pET-28a at the NcoI and EcoRI sites to create pET-28a-GalE.
2.3 Hyperoside Production from Quercetin by the Recombinant Strains
The recombinant strains were inoculated into 5 mL fresh LB medium containing appropriate antibiotics and were grown at 37 °C until the OD600 reached 0.8. A total of 1000 mg/L quercetin, 5 g/L cellobiose or sucrose, and 0.1 mM IPTG were added to the recombinant strains. The fermentation broths were incubated at 20 °C, 30 °C or 37 °C and 180 rpm for 24 h. Ten volumes of methanol were added directly to the fermentation broths. The supernatant was harvested by centrifugation at 12,000×g for 5 min and analyzed using high-performance liquid chromatography (HPLC).
2.4 Hyperoside Production by the Resting Cells
The recombinant strains were inoculated into 50 mL fresh LB medium containing appropriate antibiotics and were grown at 37 °C for 24 h. The seed culture was then inoculated in 300 mL fresh TB medium containing appropriate antibiotics and were grown at 37 °C until the OD600 reached 1.0. Cellobiose (2 g/L) and IPTG (0.1 mM) were added to the broth incubated at 20 °C for 12 h. The recombinant strains were harvested by centrifugation at 5000×g for 10 min and were re-suspended by LB, TB, or M9 medium. The broth (5 mL) was used in tubes with the silica gel plug. For more oxygen supply, the broth (20 mL) was used in shakes (100 mL) the eight-layer gauze. Different concentrations of recombinant strains were incubated at different temperatures (20 °C, 30 °C, and 37 °C) for 48 h with different IPTG concentrations (0.05 mM, 0.1 mM, 0.2 mM, and 0.4 mM), different cellobiose concentrations (5 g/L, 10 g/L, 15 g/L, and 20 g/L), different DMSO concentrations (1%, 3%, 5%, 6%, 8%, and 10%), and different quercetin concentrations (2 g/L, 3 g/L, 4 g/L, 5 g/L, and 6 g/L). Fifty volumes of methanol were added to the fermentation broths and the samples were measured using a method similar to that described above.
2.5 Hyperoside Production by Recycling the Resting Cells
The recombinant strains were inoculated using a method similar to that described above. M9 medium (200 mL) was used to re-suspend the recombinant strains to OD600 = 20. A total of 2 g/L quercetin, 10 g/L cellobiose, 5% DMSO, 0.1 mM IPTG, and antibiotics were added to the broth incubated at 30 °C for 24 h. The resting cells were recycled by centrifugation at 5000×g for 10 min and were re-suspended by M9 medium (200 mL) with 2 g/L quercetin, 10 g/L cellobiose, 5% DMSO, 0.1 mM IPTG, and antibiotics. The broth incubated at 30 °C for 24 h. The whole process recycles seven times. The samples were measured using a method similar to that described above.
2.6 Product Purification
The reaction solution at first and second recycles was harvested by centrifugation at 10,000 g for 5 min. The supernatant was applied to a AB-8 column macroporous resin (2.5 × 30 cm, Jianghua, China) equilibrated with the distilled water, and was eluted with 50% ethanol. The elution with 50% ethanol was collected and evaporated to dryness, and the product was analyzed by LC/MS and NMR.
2.7 HPLC and Liquid Chromatography-Mass Spectrometry (LC/MS) Analysis
Cellobiose was determined with an HPLC 1200 system (Agilent, USA) and a Prevail Carbohydrate ES 5 μm column (250 mm×4.6 mm; Grace, USA) according to a previous method [30]. HPLC analysis of quercetin and hyperoside was performed using an HPLC 1200 system (Agilent, USA) and a C18 (25×4.6 mm; i.d., 5 μm) column with methanol (A) and distilled water (B) at an A/B ratio of 55:45 for 15 min. The flow rate was 0.8 mL/min, and detection was performed by monitoring the absorbance at 368 nm. LC/MS for quercetin and hyperoside were analyzed in an LTQ Orbitrap XL LC/MS in negative mode with an ion trap analyzer. The ion spray was operated at 25 Arb N2/min, 3.5 kV, and 300°C.
2.8 Structural Identification
The structure of the product was determined using the proton and carbon nuclear magnetic resonance (1H-NMR, 13C-NMR) spectrum method (Bruker AVANCE IIII 400) and DMSO-d6 was used as the solvent. 1H NMR (500 MHz, DMSO-d6) δ 12.63 (s, 1H), 10.84 (s, 1H), 7.67 (dd, J = 8.9, 2.4 Hz, 1H), 7.53 (d, J = 2.5 Hz, 2H), 6.82 (d, J = 8.8 Hz, 1H), 6.40 (d, J = 2.3 Hz, 2H), 6.20 (d, J = 2.3 Hz, 2H), 5.37 (d, J = 8.1 Hz, 1H), 3.65 (d, J = 3.7 Hz, 2H), 3.57 (t, J = 8.8 Hz, 1H), 3.46 (dd, J = 10.4, 5.7 Hz, 1H), 3.39–3.26 (m, 5H). 13C NMR (126 MHz, DMSO-d6) δ 177.48, 164.10, 161.22, 156.29, 156.23, 148.44, 144.81, 133.49, 121.98, 121.09, 115.94, 115.17, 103.92, 101.80, 98.64, 93.48, 75.83, 73.18, 71.19, 67.91, 60.12.
3 Results and Discussion
3.1 Engineering UDP-Galactose Synthesis Pathway for Hyperoside Production
UDP-sugar is an important factor in glycosylation [18, 23, 30, 35]. To increase hyperoside production, it is important to improve the supply of UDP-galactose in the recombinant strains. UDP-galactose is synthesized from UDP-glucose by the UDP-galactose/4-epimerase in E. coli, therefore to increase hyperoside production must increase the supply of UDP-glucose. In this study, four methods for improving the supply of UDP-galactose were adopted by introducing different UDP-glucose synthesis pathways in the recombinant strains (Fig. 1). Hyperoside production in the strain BL-phUGT-I, which was constructed by enhancing the original UDP-glucose synthesis pathway, was 388 mg/L, but it has not been significantly improved in hyperoside production compared with the strain BL-phUGT-0 (Fig. 1). Hyperoside production in the strain BL-phUGT-II was 505 mg/L at 20 °C of conversion temperature, which was 1.4 times higher than that produced in the strain BL-phUGT-0. Hyperoside production in BL-phUGT-II reduced remarkably as the conversion temperature increased. The highest hyperoside production was determined in the strain BL-phUGT-III with 682 mg/L at 30 °C of conversion temperature, which was 135% of that produced in the strain BL-phUGT-II (Fig. 1). The expression levels of phUGT, cep, UgpA, and galE genes inthe strain BL-phUGT-III were determined and the target proteins were identified (Fig. S1). The results showed that the method by the cellobiose phosphorolysis pathway was more effective in the supply of UDP-galactose than those in other strains. Although a recombinant strain was reconstructed by introducing an efficient UDP-galactose biosynthesis pathway to produce hyperoside from quercetin, hyperoside production was still low.
3.2 Optimizing the Bioconversion Conditions by the Resting Cell
Hyperoside production in the strain BL-phUGT-III reached 682 mg/L, while it is inefficient and difficult for the industrial production to extract hyperoside from the fermentation broth. Technology of resting cell transformation has been used to bio-transform because it could effectively increase yield, reduce the cost, and improve the tolerance of substrate, product and chemical reagents [27, 29]. The cell concentration was very important for resting cell transformation. Effects of the cell concentration on hyperoside production were determined. The optimal cell concentrations in LB, TB and M9 media were OD600 = 10, 10, and 5, respectively. The highest hyperoside production was 1308 mg/L in M9 media at OD600 = 10, which was 191% of that produced with the microbial fermentation (Fig. 2a–c). However, it is against our expectation that hyperoside production by the resting cell reduced remarkably as the cell concentration increased in LB, TB or M9 media. Hyperoside production was 310 mg/L in LB medium at OD600 = 30, which was only 24.6% of that at OD600 = 10 (Fig. 2a). Insufficient oxygen supply could lead to the reduction of hyperoside production with high cell concentration, because oxygen was needed for cell growth, the supply of UDP-galactose, and substrate transportation. Moreover, requirement for oxygen in the resting cell increased remarkably as the cell concentration increased. But the bioconversion was in the tubes with the silica gel plug, which could inhibit oxygen supply. When the shakes with the eight-layer gauze were used to produce hyperoside, production increased remarkably as the cell concentration increased, because the eight-layer gauze resulted in a greater supply of oxygen. Highest hyperoside production with more oxygen supply was 3583 mg/L in M9 medium at OD600 = 25, which was 364% of that with lower oxygen supply and was 525% of that with the microbial fermentation (Fig. 3a–c).
The cellobiose concentration, substrate concentration, DMSO concentration, IPTG concentration and induction temperature were important factors in hyperoside production by affecting the strain growth, the supply of UDP-galactose, and the expression of recombinant proteins. Hyperoside production increased remarkably with adding 5 g/L cellobiose, but itwas slightly increased after further increasing of cellobiose concentration (Fig. 4a). Hyperoside production increased linearly when quercetin concentration was no more than 6 g/L. This result showed that the strain BL-phUGT-III displayed high tolerance to quercetin inhibition (Fig. 4b). But hyperoside production decreased in the strain BL-phUGT-II with quercetin concentration exceeding 3 g/L. The substrate tolerance of BL-phUGT-III was significantly higher than that of BL-phUGT-II. The main reason is that the strain BL-phUGT-III harboring cellobiose phosphorylase gene could enhance the substrate resistance of heterologous host. It has been reported that cellobiose phosphorylase-catalyzed phosphorolysis of cellobiose has many advantages such as its circumvention of catabolite repression, better tolerance of common inhibitors under both anaerobic and aerobic conditions, control of favorable energy metabolism and increases in the expression of heterologous genes. Hyperoside production was slightly affected when DMSO concentration was no more than 6%. When DMSO concentration exceeded 8%, hyperoside production was rapidly decreased (Fig. 4c). It has been reported low-level organic solvents could improve efficiency of glycosylation in the whole-cell catalytic system [36]. Hyperoside production was 3977 mg/L with adding 0.05 mM IPTG, which was 121% of that without adding IPTG, while hyperoside production was not significantly affected when IPTG concentration was raised from 0.05 mM to 0.4 mM (Fig. 4d). The optimal conversion temperature was at 30 °C and hyperoside production reached 4015 mg/L, which was 147% that at 20 °C (Fig. 4e).
Optimization of bioconversion conditions for hyperoside production. a The effects of cellobiose concentration on hyperoside production. b The effects of quercetin concentration on hyperoside production. c The effects of DMSO concentration on hyperoside production. d The effects of IPTG concentration on hyperoside production, e The effects of temperature on hyperoside production
3.3 Batch and Recycling of Resting Cell Fermentation on Hyperoside Production
Under the optimal conditions, the time-courses for quercetin and cellobiose consumption and hyperoside production by batch fermentation are given in Fig. 5. After 72 h of bioconversion, maximal hyperoside production of 4574 mg/L and average productivity of 63.5 mg/L/h was achieved with a corresponding molar conversion of 59.5% (Fig. 5). It was the highest yield of hyperoside reported to date in E. coli. However, the specific productivity could not maintain its high level in the whole bioconversion, and the specific productivity decreased sharply from 172 mg/L/h at 3 h to 2.3 mg/L/h at 72 h. In order to effectively analyze the whole bioconversion, the process of conversion was divided into three parts, 0–24 h, 24–48 h and 48–72 h, respectively. Hyperoside production and average productivity are showed in Table 2. Comparison of the whole bioconversion, average productivity of 24–48 h was 45.7% of that at 0–24 h and average productivity of 48–72 h was only 2.29 mg/L/h, which was far lower than that at 0–24 h. Hyperoside production of 24–48 h and 48–72 h was found to be 45.6% and 1.8%, lower than that at 0–24 h (Table 2). In addition, after 30 h of bioconversion, cellobiose was almost completely consumed. But hyperoside production and average productivity could not increase in the strain BL-phUGT-III with adding 10 g/L cellobiose at 36 h. The results showed that cellobiose consumption was not the only reason for reduction of specific productivity in the strain BL-phUGT-III. Hyperoside accumulation and deterioration of bioconversion conditions could also play the major roles in the average productivity.
Recycling of resting cell fermentation can overcome above problems by separating resting cells and transformation product [29]. Hyperoside solubility was higher than that of quercetin because of glycosylation. Appropriate concentration of hyperoside could dissolve the fermentation broth by adding appropriate DMSO, so hyperoside can easily separate with resting cell and quercetin by centrifugation, and the resting cell could be used to produce hyperoside again by adding fresh M9 medium and quercetin. To avoid the inhibition and insolubility of hyperoside on the strain BL-phUGT-III, we recycled the resting cell and added fresh 2000 mg/L quercetin every 24 h of the bioconversion cycle. As shown in Fig. 6, the resting cell was recycled seven times within 168 h. Hyperoside production and average productivity in previous five recycles were more than 2900 mg/L and 125 mg/L/h, respectively (Table 2). Hyperoside production at sixth and seventh recycles was only 1642 mg/L and 904 mg/L, which was less than that at first recycle and average productivity was also found to be 54.3% and 29.9% of that at first recycle. Average productivity within 72 h was 3031 mg/L/h, which was 100% higher than that in the batch fermentation (Table 2). Hyperoside production and average productivity remained the high level in previous five recycles when the fresh M9 medium was added into the resting cell. The results showed that the inhibitory effect of the product in the bioconversion could be released by recycling of resting cell fermentation. However, hyperoside production and average productivity decreased sharply at sixth and seventh recycles. The main reason was that hyperoside accumulated in precipitation and could not separate with the resting cell from the fourth recycles. As shown in Fig. 6, hyperoside in precipitation remained no more than 750 mg/L at previous three recycles and hyperoside production and average productivity remained the high level. But hyperoside began to largely accumulate in precipitation from fourth recycle and hyperoside in precipitation reached 7544 mg/L and 8164 mg/L at sixth and seventh recycles. At the same time, hyperoside production and average productivity decreased sharply at sixth and seventh recycles, which could cause the bioconversion inhibition of recombinant strains (Table 2). It is very interesting that hyperoside began to largely accumulate in precipitation from fourth recycle, although DMSO concentration was the same at every time cycle. The results suggested that the changes of bacterial surface molecules caused hyperoside accumulation in precipitation. Thus, recycling the resting cell was ended at the seventh recycle, and the total hyperoside production reached equivalent to 18,000 mg/L with a corresponding molar conversion of 83.7%, which was 393% of that in the batch fermentation and the first to reach 10 g per liter scale in E. coli. Therefore, this study provides an efficient method for construction of UDP-galactose synthesis pathway and hyperoside production in E. coli.
3.4 Structural Elucidation of Hyperoside
The molecular weight and structure of the bioconversion product by the strain BL-phUGT-III were determined through LC/MS and NMR. A comparison of the m/z of molecular ions [M-H]− of the bioconversion product (463.0839) showed that differences corresponded to a d-galactose residue in quercetin (301.0324) (Fig. S2), and the bioconversion product had a retention time similar to the authentic hyperoside (Fig. S3) Furthermore, the 1H-NMR and 13C-NMR spectra were analyzed and compared to the reference compounds [16] (Fig. S4). These results confirmed that the bioconversion product was hyperoside.
4 Conclusion
In this study, a recombinant strain was reconstructed by introducing an efficient UDP-galactose biosynthesis pathway and E. coli UDP-galactose/4-epimerase (GalE) to produce hyperoside from quercetin. Recycling of resting cell fermentation was used to improve hyperoside production. Finally, maximal hyperoside production reached equivalent to 18,000 mg/L by recycling 7 times of resting cell in 168 h, which was 393% of that in the batch fermentation and the first to reach 10 g per liter scale in E. coli.
References
Dixon RA, Paiva NL (1995) Stress-induced phenylpropanoid metabolism. Plant Cell 7:1085–1097
Yonekura-Sakakibara K, Tohge T, Niida R, Saito K (2007) Identification of a flavonol 7-O-rhamnosyltransferase gene determining flavonoid pattern in Arabidopsis by transcriptome coexpression analysis and reverse genetics. J Biol Chem 282:14932–14941
Nijveldt RJ, van Nood E, van Hoorn DE, Boelens PG, van Norren K, van Leeuwen PA (2001) Flavonoids: a review of probable mechanisms of action and potential applications. Am J Clin Nutr 74:418–425
Wu X, Chu J, Wu B, Zhang S, He B (2013) An efficient novel glycosylation of flavonoid by beta-fructosidase resistant to hydrophilic organic solvents. Bioresour Technol 129:659–662
An DG, Yang SM, Kim BG, Ahn JH (2016) Biosynthesis of two quercetin O-diglycosides in Escherichia coli. J Ind Microbiol Biotechnol 43:841–849
Kim SJ, Um JY, Lee JY (2011) Anti-inflammatory activity of hyperoside through the suppression of nuclear factor-kappaB activation in mouse peritoneal macrophages. Am J Chin Med 39:171–181
Comalada M, Camuesco D, Sierra S, Ballester I, Xaus J, Galvez J, Zarzuelo A (2005) In vivo quercitrin anti-inflammatory effect involves release of quercetin, which inhibits inflammation through down-regulation of the NF-kappaB pathway. Eur J Immunol 35:584–592
Zheng M, Liu C, Pan F, Shi D, Zhang Y (2012) Antidepressant-like effect of hyperoside isolated from apocynum venetum leaves: possible cellular mechanisms. Phytomedicine 19:145–149
Butterweck V, Jurgenliemk G, Nahrstedt A, Winterhoff H (2000) Flavonoids from hypericum perforatum show antidepressant activity in the forced swimming test. Planta Med 66:3–6
Cincin ZB, Unlu M, Kiran B, Bireller ES, Baran Y, Cakmakoglu B (2015) Apoptotic effects of quercitrin on DLD-1 colon cancer cell line. Pathol Oncol Res 21:333–338
Li S, Zhang Z, Cain A, Wang B, Long M, Taylor J (2005) Antifungal activity of camptothecin, trifolin, and hyperoside isolated from Camptotheca acuminata. J Agric Food Chem 53:32–37
Fang X, Dong Y, Xie Y, Wang L, Wang J, Liu Y, Zhao L, Cao F (2019) Effects of β-glucosidase and α-rhamnosidase on the contents of flavonoids, ginkgolides, and aroma components in ginkgo tea drink. Molecules. https://doi.org/10.3390/molecules24102009
Cao X, Wang Q, Li Y, Bai G, Ren H, Xu C, Ito Y (2011) Isolation and purification of series bioactive components from Hypericum perforatum L. by counter-current chromatography. J Chromatogr B Analyt Technol Biomed Life Sci 879:480–488
He F, Li D, Wang D, Deng M (2016) Extraction and purification of quercitrin, hyperoside, rutin, and afzelin from Zanthoxylum Bungeanum Maxim leaves using an aqueous two-phase system. J Food Sci 81:C1593–1602
Li X, Zhang Y, Yuan ZJC (2002) Separation and determination of rutin and quercetin in the flowers of Sophora japonica L. by capillary electrophoresis with electrochemical detection. Chromatographia 55:243–246
Pei J, Chen A, Zhao L, Cao F, Ding G, Xiao W (2017) One-pot synthesis of hyperoside by a three-enzyme cascade using a UDP-galactose regeneration system. J Agric Food Chem 65:6042–6048
Zang Y, Zha J, Wu X, Zheng Z, Ouyang J, Koffas MAG (2019) In vitro naringenin biosynthesis from p-coumaric acid using recombinant enzymes. J Agr Food Chem 67:13430–13436
De Bruyn F, De Paepe B, Maertens J, Beauprez J, De Cocker P, Mincke S, Stevens C, De Mey M (2015) Development of an in vivo glucosylation platform by coupling production to growth: production of phenolic glucosides by a glycosyltransferase of Vitis vinifera. Biotechnol Bioeng 112:1594–1603
Kim BG, Kim HJ, Ahn JH (2012) Production of bioactive flavonol rhamnosides by expression of plant genes in Escherichia coli. J Agric Food Chem 60:11143–11148
Kim HJ, Kim BG, Ahn JH (2013) Regioselective synthesis of flavonoid bisglycosides using Escherichia coli harboring two glycosyltransferases. Appl Microbiol Biot 97:5275–5282
Paquette S, Moller BL, Bak S (2003) On the origin of family 1 plant glycosyltransferases. Phytochemistry 62:399–413
Devaiah SP, Owens DK, Sibhatu MB, Sarkar TR, Strong CL, Mallampalli VK, Asiago J, Cooke J, Kiser S, Lin Z, Wamucho A, Hayford D, Williams BE, Loftis P, Berhow M, Pike LM, McIntosh CA (2016) Recombinant expression, and biochemical analysis of putative secondary product glucosyltransferases from Citrus paradisi. J Agr Food Chem 64:1957–1969
De Bruyn F, Van Brempt M, Maertens J, Van Bellegem W, Duchi D, De Mey M (2015) Metabolic engineering of Escherichia coli into a versatile glycosylation platform: production of bio-active quercetin glycosides. Microb Cell Fact 14:138
Lim EK, Ashford DA, Hou B, Jackson RG, Bowles DJ (2004) Arabidopsis glycosyltransferases as biocatalysts in fermentation for regioselective synthesis of diverse quercetin glucosides. Biotechnol Bioeng 87:623–631
Bernard FX, Sable S, Cameron B, Provost J, Desnottes JF, Crouzet J, Blanche F (1997) Glycosylated flavones as selective inhibitors of topoisomerase IV. Antimicrob Agents Chemother 41:992–998
Jackson E, Ripoll M (2019) Efficient glycerol transformation by resting Gluconobacter cells. Microbiol Open 8(12):e926
Ramakrishnan GG, Nehru G, Suppuram SP, Balasubramaniyam S, Gulab BR, Subramanian R (2015) Bio-transformation of glycerol to 3-hydroxypropionic acid using resting cells of Lactobacillus reuteri. Curr Microbiol 71:517–523
Schrewe M, Julsing MK, Bühler B, Schmid A (2013) Whole-cell biocatalysis for selective and productive C-O functional group introduction and modification. Chem Soc Rev 42(15):6346–6377
Lin B, Tao Y (2017) Whole-cell biocatalysts by design. Microbial Cell Fact 16:106
Pei J, Sun Q, Zhao L, Shi H, Tang F, Cao F (2019) Efficient biotransformation of luteolin to isoorientin through adjusting induction strategy, controlling acetic acid and increasing UDP-glucose supply in Escherichia coli. J Agric Food Chem 67:331–340
Sekar R, Shin HD, Chen R (2012) Engineering Escherichia coli cells for cellobiose assimilation through a phosphorolytic mechanism. Appl Environ Microb 78:1611–1614
Shin HD, Wu J, Chen R (2014) Comparative engineering of Escherichia coli for cellobiose utilization: hydrolysis versus phosphorolysis. Metab Eng 24:9–17
J Sambrook, EF Fritsch, T Maniatis (1982) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor.
Miller KD, Guyon V, Evans JN, Shuttleworth WA, Taylor LP (1999) Purification, cloning, and heterologous expression of a catalytically efficient flavonol 3-O-galactosyltransferase expressed in the male gametophyte of Petunia hybrid. J Biol Chem 274:34011–34019
Pei J, Dong P, Wu T, Zhao L, Fang X, Cao F, Tang F, Yue Y (2016) Metabolic engineering of Escherichia coli for astragalin biosynthesis. J Agric Food Chem 64:7966–7972
Yang Y, Liu M, Cao Y, Li C, Wang W (2019) Low-level organic solvents improve multienzyme whole-cell catalytic synthesis of myricetin-7-O-glucuronide. Catalysts 9:970
Acknowledgements
This work was supported by the National Key R&D Program of China (2017YFD0600805), the National Natural Science Foundation of China (21978135), the Open Foundation of Jiangsu Provincial Engineering Laboratory for Biomass Conversion and Process Integration (JPELBCPI2017002) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gu, N., Qiu, C., Zhao, L. et al. Efficient Production Hyperoside from Quercetin in Escherichia coli Through Increasing UDP-Galactose Supply and Recycling of Resting Cell. Catal Lett 151, 1202–1211 (2021). https://doi.org/10.1007/s10562-020-03373-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-020-03373-y