Abstract
Objective
To produce high concentrations of hyperoside from quercetin using recombinant Escherichia coli with in situ regeneration of UDP-galactose.
Results
Sucrose synthase from Glycine max (GmSUS) was co-expressed with UDP-glucose epimerase from E. coli (GalE) in E. coli for regenerating UDP-galactose from UDP and sucrose. Glycosyltransferase from Petunia hybrida (PhUGT) was introduced to synthesize hyperoside from quercetin through the regeneration system of UDP-galactose. Co-expressing with molecular chaperones GroEL/ES successfully enhanced the catalytic efficiency of the recombinant strain, which assisted the soluble expression of PhUGT. By using a fed-batch approach, the production of hyperoside reached 863.7 mg L−1 with a corresponding molar conversion of 93.6% and a specific productivity of 72.5 mg L−1 h−1.
Conclusion
The method described herein for hyperoside production can be widely applied for the synthesis of isorhamnetin-3-O-galactoside, kaempferol-3-O-galactoside and other flavonoids.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hyperoside (quercetin 3-O-galactoside) is a kind of flavonoid-O-glycoside, which has have attracted extensive research interests due to its powerful antioxidant, cytoprotective effects (Choi et al. 2011; Piao et al. 2008) and antiviral activity against hepatitis B (Wu et al. 2007), SARS (Chen et al. 2006) viruses. Hyperoside is purified from Zanthoxylum bungeanum or Hypericum perforatum L. via solvent extraction, column chromatography, crystallization and so on (Cao et al. 2011; Fengyuan et al. 2016). However, it is a complicated process and therefore improper for mass production.
Due to environmental concerns, there is a growing interest for the biotechnological production of hyperoside (Lin et al. 2014; Putignani et al. 2013; Wang et al. 2011). The flavonol glycosyltransferase from Petunia hybrida (PhUGT) has been cloned and expressed in Escherichia coli (Miller et al. 1999), and recombinant strains harboring the PhUGT gene have been constructed to produce hyperoside (Kim et al. 2015; Li et al. 2022). However, flavonoids show inhibitory effects on cell growth, influencing further improvement of the flavonoids production (De Bruyn et al. 2015b; Pei et al. 2016). These problems can be solved by enzymatic methods, but the process needs to consume UDP-galactose.
Sucrose synthase (SUS), which coverts sucrose and uridine 5′-diphosphate (UDP) into UDP-glucose and fructose, has been utilized for constructing the UDP-glucose regeneration system (Gutmann et al. 2014). UDP-galactose 4-epimerase (GalE) catalyzes the interconversion between UDP-glucose and UDP-galactose (Chen et al. 1999). Sucrose synthase can be coupled with GalE to provide a simple and efficient method that can use sucrose as an inexpensive and sustainable carbon source for the synthesis of UDP-galactose.
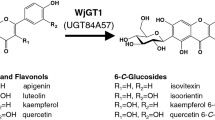
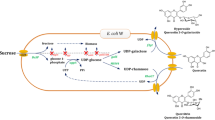
In the present study, we described the co-expression of a SUS gene from Glycine max (GmSUS) and GalE from E. coli in E. coli cells for efficient in situ regeneration of UDP-galactose. Then, PhUGT was coupled with the UDP-galactose regeneration system for hyperoside production (Fig. 1). The efficiency of the recombinant strain was further improved by increasing soluble expression of PhUGT, which is a limiting step in the biocatalysis. Finally, hyperoside production using a substrate fed-batch strategy was investigated.
Material and methods
Plasmids, strains and chemicals
All plasmids and strains applied in this study were listed in Table 1. All the chemicals were purchased from Macklin (Shanghai, China) or Sangon Biotech (Shanghai, China). All chemicals were analytical or HPLC grade.
Construction of plasmids and strains
GmSUS from G. max (Accession No. NM_001250596.2) and PhUGT from P. hybrida (Accession No. AF165148.1) were cloned into pET28a between Hind III and Noc I (Sangon Biotech, Shanghai) for getting pET28a-GmSUS as well as pET28a-PhUGT (Table 1). The primers GalE-R and GalE-F (Table S1) were used to amplify GalE from genome of E. coli MG1655. The resulting PCR products were inserted into the expression vector pET28a between Hind III and Noc I on the basis of the standard One Step Cloning Kit (Vazyme Biotech, Nanjing) for constructing recombinant plasmid pET28a-GalE (Table 1). The primers PhUGT-mcs1-R and PhUGT-mcs1-F, GalE-mcs2-R and GalE-mcs2-F (Table S1) were applied to amplify PhUGT and GalE with pET28a-PhUGT and pET28a-GalE as templets, and the fragments were inserted into MCS1 and MCS2 of pETDuet-1between Hind III and Nco I, Xho I and Nde I to obtain pACYCDuet-PhUGT-GalE. GmSUS and GroEL/ES were amplified using pET28a-GmSUS, pGro7 as templets, GmSUS-mcs1-R and GmSUS-mcs1-F, GroEL/ES-mcs2-R and GroEL/ES-mcs2-F (Table S1) as the primers, and these fragments were inserted into MCS1 and MCS2 of pETDuet-1 between Hind III and Nco I, Xho I and Nde I, resulting pETDuet-GmSUS-GroEL/ES.
The recombinant strains BL-GalE and BL-GmSUS were respectively obtained when plasmids pET28a-GalE and pET28a-GmSUS were transformed into E. coli BL21(DE3). Plasmids pACYCDuet-PhUGT-GalE and pET28a-PhUGT were converted into E. coli BL21(DE3) to obtain recombinant strain BL-I and BL-II (Table 1). Plasmids pET28a-GmSUS and pETDuet-GmSUS-GroEL/ES were transformed into BL-II, respectively, to obtained BL-III and BL-IV (Table 1). Chaperone plasmids pGro7, pG-KJE8, pTf16, pG-Tf2, along with pKJE7 were transformed into BL-I, respectively to obtain BL-I-pG-KJE8, BL-I-pGro7, BL-I-pKJE7, BL-I-pTf16, and BL-I-pG-Tf2 (Table 1).
Protein production using recombinant strains
Several recombinant strains such as BL-GalE, BL-GmSUS, BL-III, BL-I, BL-IV, and BL-II were cultured on 50 ml of LB medium containing certain antibiotics under the temperature of 37 °C. 0.1 mM isopropyl-d-1-thiogalactopyranoside (IPTG) was added to induce the expression of protein at 20 °C for 24 h when the absorbance at 600 nm achieved 0.8. Recombinant strains BL-I-pG-KJE8, BL-I-pGro7, BL-I-pKJE7, BL-I-pTf16, and BL-I-pG-Tf2 were initially inoculated under the temperature of 37 °C in LB medium of 50 mL containing 34 mg mL−L chloramphenicol and 50 mg mL−L kanamycin. When OD600 achieved 0.5, 5 ng mL−1 tetracycline or 0.5 mg mL−1 l-arabinose was increased. When OD600 achieved 1.0, 0.1 mM IPTG was then added to induce PhUGT expression at 20 °C for 24 h. The cells were subsequently gathered through centrifugation at 8000 rpm under a temperature of 4 °C for fifteen minutes. The supernatants were achieved by ultra-sonication (50% amplitude, 3 s pulse and 7 s pause for 15 min) and centrifugation (10,000 rpm, 4 °C for 20 min). Cell dry weight (CDW) was calculated from the OD600 value using a ratio of 0.33 g (CDW) L−1 per OD600.
Enzyme activity
Enzyme activity of PhUGT, GmSUS and GalE were measured as described previously (Diricks et al. 2015; Miller et al. 1999; Pei et al. 2017).
Hyperoside production by recombinant E. coli
Batch reactions using BL-I and BL-II were performed in a 50 mL shake flask with 10 mL working-volume, which contained quercetin of 0.5 mM, 0.81 g (CDW) L−1 cells, phosphate buffer of 50 mM (pH 7.5), as well as 10 g L−1 glucose. Reactions were conducted at 200 rpm under the temperature of 30 °C for 12 h.
Batch reactions using BL-III and BL-IV were performed in a 50 mL shake flask with 10 mL working-volume, which contained quercetin of 0.5 mM, 0.81 g (CDW) L−1, phosphate buffer of 50 mM (pH 7.5), 0.25 mM UDP as well as 500 mM sucrose. Stock solutions of quercetin (50 mM) was made up in 100% DMSO. Reactions were conducted at 200 rpm under the temperature of 30 °C for 3 h. Substrate fed-batch was conducted under the same conditions. After depletion of quercetin added initially, 0.5 mM quercetin was fed. Samples were harvested at intervals and ten volumes of methanol were added directly to stop the rection. The supernatant was harvested by centrifugation at 12,000×g for 10 min and analyzed using high-performance liquid chromatography (HPLC).
HPLC analysis
The specimen needed to analyze by the high-performance liquid chromatography (HPLC) (HP1100, Agilent 1100 series) with a C18 column (5 μm, 4.6 × 250 mm, Agilent). The experiment was analyzed under the column temperature of 30 °C at 368 nm with a flow rate of 0.6 mL min−1 in 55% methanol. All the assays were conducted in triplicate.
Product purification and structural identification
The fed-batch reaction solution was harvested by centrifugation at 20,000×g for 10 min. The supernatant was applied to an AB-8 column macroporous resin (2.5 × 30 cm, Jianghua, China) equilibrated with distilled water, and was eluted with 20 and 50% ethanol, respectively. The elution with 50% ethanol was collected and evaporated to dryness, and the product was analyzed using 1H-NMR and 13C-NMR (Bruker AVANCE IIII 400) using DMSO-d6 as the solvent.
Results
Co-expression of PhUGT and GalE in E. coli
PhUGT from P. hybrida was reported to be efficient for glycosylating quercetin at the 3C-O position (Kim et al. 2015; Pei et al. 2017). In this study, PhUGT was cloned into pET-28a(+) and expressed in E. coli BL21 (DE3). However, hyperoside produced using the resulted recombinant strain BL-I could not be detected (Fig. 2a). Genome analysis suggested GalE is absent in BL21 (DE3). Therefore, GalE from E. coli MG1655 was expressed in BL21 (DE3) (Fig. 2b) and the cell extracts were added to the reaction system. The results showed that 62.3 mg L−1 hyperoside was produced, indicating the successful conversion of UDP-glucose to UDP-galactose in BL21 (DE3). To simplify the expression process, GalE was co-expressed with PhUGT using pACYCDuet-1 (Fig. 2b). After 12 h of reaction, the production of hyperoside using the resulted strain BL-II reached 76.5 mg L−1 with a molar conversion rate of 26.8%. Thus, the recombinant strain BL-II is potential to be used in the production of hyperoside.
Construction and performance of recombinant E. coli. a Production of Hyperoside with 151.1 mg L−1 quercetin using different biocatalysts. BL-I, recombinant BL21(DE3) with pET28a-PhUGT; BL-I+GalE, BL-I with exogenous addition of 10 mU mL−1 GalE; BL-II, recombinant BL21(DE3) with pETDuet-PhUGT-GalE. b SDS-PAGE analysis of protein expression of various recombinant strains. M, Marker; Lane 1, BL21(DE3); Lane 2, BL-I; Lane 3, BL-GalE; Lane 4, BL-II
Construction of UDP-galactose regeneration system
UDP-sugar is a key factor in glycosylation (De Bruyn et al. 2015a, 2015b; Miller et al. 1999). Increasing the supply of UDP-galactose in the recombinant strains is essential to improve hyperoside production. UDP-galactose is produced from UDP-glucose via GalE in BL21-II and thus the supply of UDP-glucose must be increased. Two methods have been reported to enhance the synthesis of UDP-glucose in E. coli. One is the overexpression of two key enzymes (phosphoglucomutase, Pgm, and UDP-glucose pyrophosphorylase, GalU) in E. coli (Yao et al. 2006) and the other is the reconstruction of a novel UDP-glucose synthesis pathway via simultaneous expression of Basp (a sucrose phosphorylase from Bifidobacterium adolescentis) and UgpA (a uridylyltransferase from Bifidobacterium bifidum) (De Bruyn et al. 2015b). However, both methods require fine regulations of gene expression and long-time transformation.
Sucrose synthase has been utilized to construct the regeneration of UDP-glucose (Gutmann et al. 2014). When GmSUS was coupled in the reaction, we could even use sucrose and UDP instead of UDP-glucose as starting materials to produce glucosides. In this research, to improve the supply of UDP-galactose, GmSUS was overexpressed in BL-II by pET28a. According to SDS-PAGE analysis, PhUGT, GalE, and GmSUS were successfully expressed in the resulted strain BL-III containing pET28a-GmSUS and pACYCDuet-PhUGT-GalE (Fig. 3a). Hyperoside production using the BL-III reached 172.3 mg L−1, which was 2.3 times higher than that produced in BL-II (Fig. 3b). These results indicated that by introducing an UDP-galactose regeneration system, BL-III is able to offer more UDP-galactose for hyperoside synthesis.
Construction and performance of UDP-galactose regeneration system. a SDS-PAGE analysis of protein expression in BL-III harboring both pACYCDuet-PhUGT-GalE and pETDuet-GmSUS. M, Marker; Lane 2, supernatant of BL-III; Lane 3, precipitates of BL-III; Lane 1, E. coli BL21(DE3); b Hyperoside production with 151.1 mg L−1 quercetin using BL-II and BL-III as whole cell biocatalysts
Screening appropriate molecular chaperones to improve PhUGT expression
Although recombinant strain BL-III was constructed via the introduction of UDP-galactose regeneration system, hyperoside production was still low. Exogenous addition of crude extracts of 50 mU mL−1 PhUGT, GalE and GmSUS to the reaction system showed that the inadequate activity of PhUGT was the bottleneck (Fig. 4a). Analysis of SDS-PAGE indicated that about half of the PhUGT was expressed in the form of inclusion bodies (Fig. 3a). Thus, it is necessary to reduce PhUGT inclusion bodies. Reportedly, co-expression of molecular chaperones can enhance soluble expression of different recombinant proteins in E. coli (Ashraf et al. 2017; Zhang et al. 2017). In this research, five chaperone plasmids pG-Tf2, pTf16, pKJE7, pGro7 and pG-KJE8 were transformed into BL-I. SDS-PAGE analysis of the supernatant of cell extracts showed both PhUGT and molecular chaperones were successfully expressed (Fig. S1). Compared with the recombinant E. coli BL21 strain without a chaperone plasmid, the soluble PhUGT co-expressed with pGro7 was significantly increased (Fig. S1), indicating that molecular chaperones GroEL/ES improved the soluble expression of PhUGT. GroEL/ES was then co-expressed with PhUGT using pETDuet-1. The resulted plasmid pETDuet-GmSUS-GroEL/ES was transformed into BL-II to obtained recombinant strain BL-IV (Fig. 4b). As expected, PhUGT activity was significantly increased from 11.1 to 23.6 mU mL−1 (Fig. 4c), indicating the successful introduction of GroEL/ES. Finally, hyperoside production using BL-IV as biocatalyst reached 222.9 mg L−1 with a corresponding molar conversion of 96.0% (Fig. 4c).
Enhancing hyperoside production by improving soluble expression of PhUGT. a Production of hyperoside by BL-III with exogenous addition of crude extracts of PhUGT, GalE and GmSUS. b Construction of recombinant strain BL-IV harboring pACYCDuet-PhUGT-GalE and pETDuet-GmSUS-GroEL/ES. c PhUGT activity after introduction and hyperoside production using BL-III and BL-IV as whole cell biocatalysts
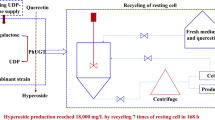
Fed-batch reaction for hyperoside production
To improve the final concentration of hyperoside and prevent the inhibition of a high concentration of quercetin on activity of GmSUS (Pei et al. 2017), we added 0.5 mM fresh quercetin to the reaction mixture every time once the substrate was consumed. As shown in Fig. 5, a kinetic analysis of hyperoside production along with quercetin consumption over time was investigated. The specific productivity was 102.3 mg L−1 h−1 during the first batch period. The specific productivity gradually decreased as the reaction proceeded with 98.7 mg L−1 h−1 during the second batch, 80.4 mg L−1 h−1 during the third batch, and 56.7 mg L−1 h−1 during the fourth batch. Product inhibition may be the major reason of the decrease in the specific productivity. Finally, 869.4 mg L−1 hyperoside was produced with the corresponding molar conversion of 93.6% and a specific productivity of 72.5 mg L−1 h−1.
Structural identification of hyperoside
1H NMR (400 MHz, DMSO-d6): 12.58 (s, 1H), 10.82 (s, 1H), 9.69 (s, 1H), 9.11 (s, 1H), 7.62 (dd, J = 1.8, 8.4 Hz, 1H, H-6′), 7.47 (d, J = 1.8 Hz, 1H, H-2′), 6.76 (d, J = 8.4 Hz, 1H, H-5′), 6.35 (d, J = 1.6 Hz, 1H, H-8), 6.15 (d, J = 1.6 Hz, 1H, H-6), 5.33 (d, J = 7.6 Hz, 1H, H-1″), 3.20–5.10 (11H) (Fig. S2). 13C NMR (100 MHz, DMSO-d6): 178.0 (C-4), 164.6 (C-7), 161.7 (C-5), 156.8 (C-2), 156.7 (C-9), 148.9 (C-4′), 145.3 (C-3′), 134.0 (C-3), 122.5 (C-6′), 121.6 (C-1′), 116.4 (C-5′), 115.6 (C-2′), 104.4 (C-10), 102.3 (C-1″), 99.1 (C-6), 94.0 (C-8), 76.3 (C-5″), 73.7 (C-3″), 71.7 (C-2″), 68.4 (C-4″), 60.6 (C-6″) (Fig. S3).
Conclusion
In this study, a recombinant E. coli co-expressing PhUGT and GalE was first constructed for hyperoside production (62.3 mg L−1). When additional enzyme GmSUS was introduced, the regenerative UDP-galactose catalyzed by GmSUS coupled with GalE increased hyperoside production to 172.3 mg L−1. By introducing molecular chaperone GroES/EL, the soluble expression of PhUGT was successfully improved and hyperoside production reached 222.9 mg L−1. By using a substrate fed-batch strategy, hyperoside production reached 869.4 mg L−1 with a conversion rate of 93.6% and a specific productivity of 72.5 mg L−1 h−1. In general, enzymatic glycosylation is one of the most promising methods for producing glycosides. Nevertheless, the method is restricted by the high-cost UDP-sugar. Multienzyme cascade reactions have been established for the regeneration of UDP-galactose as well as the synthesis of glycosides (Pei et al. 2017; Tsai et al. 2013). To date, the highest hyperoside production reached 2134 mg L−1 through the in vitro regeneration of UDP-galactose. However, the process requires the addition of various crude enzyme extracts, which is complex and time-consuming. The whole cell biocatalyst constructed herein for hyperoside production stands out because of its simplicity.
References
Ashraf R, Muhammad MA, Rashid N, Akhtar M (2017) Cloning and characterization of thermostable GroEL/GroES homologues from Geobacillus thermopakistaniensis and their applications in protein folding. J Biotech 254:9–16
Cao X, Wang Q, Li Y, Bai G, Ren H, Xu C, Ito Y (2011) Isolation and purification of series bioactive components from Hypericum perforatum L. by counter-current chromatography. J Chromatogr B 879:480–488
Chen X, Kowal P, Hamad S, Fan HN, Wang PG (1999) Cloning, expression and characterization of a UDP-galactose 4-epimerase from Escherichia coli. Biotech Lett 21:1131–1135
Chen L, Li J, Luo C, Liu H, Xu W, Chen G, Liew OW, Zhu W, Puah CM, Shen X, Jiang H (2006) Binding interaction of quereetin-3-beta-galactoside and its synthetic derivatives with SARS-CoV 3CL(pro): structure-activity relationship studies reveal salient pharmacophore features. Bioorgan Med Chem 14:8295–8306
Choi J-H, Kim D-W, Yun N, Choi J-S, Isam N, Kim Y-S, Lee S-M (2011) Protective effects of hyperoside against carbon tetrachloride-induced liver damage in mice. J Nat Prod 74:1055–1060
De Bruyn F, De Paepe B, Maertens J, Beauprez J, De Cocker P, Mincke S, Stevens C, De Mey M (2015a) Development of an in vivo glucosylation platform by coupling production to growth: production of phenolic glucosides by a glycosyltransferase of Vitis vinifera. Biotech Bioeng 112:1594–1603
De Bruyn F, Van Brempt M, Maertens J, Van Bellegem W, Duchi D, De Mey M (2015b) Metabolic engineering of Escherichia coli into a versatile glycosylation platform: production of bio-active quercetin glycosides. Microb Cell Fact 14:138–149
Diricks M, De Bruyn F, Van Daele P, Walmagh M, Desmet T (2015) Identification of sucrose synthase in nonphotosynthetic bacteria and characterization of the recombinant enzymes. App Microbi Biotech 99:8465–8474
Fengyuan H, Dengwu L, Dongmei W, Ming D (2016) Extraction and purification of quercitrin, hyperoside, rutin, and afzelin from Zanthoxylum bungeanum Maxim leaves using an aqueous two-phase system. J Food Sci 81:C1593–C1602
Gutmann A, Bungaruang L, Weber H, Leypold M, Breinbauer R, Nidetzky B (2014) Towards the synthesis of glycosylated dihydrochalcone natural products using glycosyltransferase-catalysed cascade reactions. Green Chem 16:4417–4425
Kim SY, Lee HR, Park K-s, Kim B-G, Ahn J-H (2015) Metabolic engineering of Escherichia coli for the biosynthesis of flavonoid-O-glucuronides and flavonoid-O-galactoside. Appl Microb Biotech 99:2233–2242
Li G, Zhu F, Wei P, Xue H, Chen N, Lu B, Deng H, Chen C, Yin X (2022) Metabolic engineering of Escherichia coli for hyperoside biosynthesis. Microorganisms 10:628–639
Lin YH, Jain R, Yan YJ (2014) Microbial production of antioxidant food ingredients via metabolic engineering. Curr Opin Biotech 26:71–78
Miller KD, Guyon V, Evans JNS, Shuttleworth WA, Taylor LP (1999) Purification, cloning, and heterologous expression of a catalytically efficient flavonol 3-O-galactosyltransferase expressed in the male gametophyte of Petunia hybrida. J Biol Chem 274:34011–34019
Pei J, Dong P, Wu T, Zhao L, Fang X, Cao F, Tang F, Yue Y (2016) Metabolic engineering of Escherichia coli for astragalin biosynthesis. J Agr Food Chem 64:7966–7972
Pei J, Chen A, Zhao L, Cao F, Ding G, Xiao W (2017) One-pot synthesis of hyperoside by a three -enzyme cascade using a UDP-galactose regeneration system. J Agr Food Chem 65:6042–6048
Piao MJ, Kang KA, Zhang R, Ko DO, Wang ZH, You HJ, Kim HS, Kim JS, Kang SS, Hyun JW (2008) Hyperoside prevents oxidative damage induced by hydrogen peroxide in lung fibroblast cells via an antioxidant effect. BBA-Gen Subjects 1780:1448–1457
Putignani L, Massa O, Alisi A (2013) Engineered Escherichia coli as new source of flavonoids and terpenoids. Food Res Int 54:1084–1095
Tsai T-I, Lee H-Y, Chang S-H, Wang C-H, Tu Y-C, Lin Y-C, Hwang D-R, Wu C-Y, Wong C-H (2013) Effective sugar nucleotide regeneration for the large-scale enzymatic synthesis of globo H and SSEA4. J Am Chem Soc 135:14831–14839
Wang YC, Chen S, Yu O (2011) Metabolic engineering of flavonoids in plants and microorganisms. App Microb and Biotech 91:949–956
Wu L-l, Yang X-b, Huang Z-m, Liu H-z, Wu G-x (2007) In vivo and in vitro antiviral activity of hyperoside extracted from Abelmoschus manihot (L) medik. Acta Pharmacol Sin 28:404–409
Yao QJ, Song J, Xia CF, Zhang WP, Wang PG (2006) Chemoenzymatic syntheses of iGb3 and Gb3. Org Lett 8:911–914
Zhang Y, Qiao X, Yu X, Chen J, Hou L, Bi Z, Zheng Q, Hou J (2017) Enhanced soluble production of cholera toxin B subunit in Escherichia coil by co-expression of SKP chaperones. Protein Expres Purif 138:1–6
Acknowledgements
This research was funded by High-level talent project of West Anhui University (WGKQ2021025) and Natural Science Foundation of Anhui Province (2008085QB96).
Suplementary Information
Supplementary Table 1 Primers used in the present study.
Supplementary Fig. 1 Expression of PhUGT in E. coli BL21(DE3) assisted by different chaperones.
Supplementary Fig. 2 1H NMR spectra of purified hyperoside.
Supplementary Fig. 3 13C NMR spectra of purified hyperoside.
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, GS., Zhu, FC., Wei, PP. et al. Development of an Escherichia coli whole cell biocatalyst for the production of hyperoside. Biotechnol Lett 44, 1073–1080 (2022). https://doi.org/10.1007/s10529-022-03285-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-022-03285-4