Abstract
Stabilized Pd(0) nanoparticles by supported 12-tungstophosphoric acid (Pd(0)-TPA/ZrO2) was explored as a sustainable recyclable catalyst for selective C=C hydrogenation of cyclohexene and crotonaldehyde. The catalyst shows an outstanding performance [catalyst to substrate ratio (1:1.31 × 104)] towards high conversion as well as 100% selectivity of the desired product with high turnover number (> 10,000) and turnover frequency (> 2600 h−1) for both the systems. The use of neat water as a solvent and mild reaction conditions makes the present system environmentally benign and green. Moreover, the catalyst could be recovered and reused up to five cycles without any significant loss in their conversion as well as selectivity. The viability of the catalyst was evaluated towards different aromatic as well as aliphatic arenes and found to be excellent in all the cases. The obtained selectivity, especially butyraldehyde, was correlated with the nature of the catalyst as well as solvent and based on the study, a plausible mechanism for both the reactions was also proposed.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Selective C=C catalytic hydrogenation is a diverse and versatile acceptable route to synthesize precursors for various intermediates [1] and still is fascinating and challenging field. Cyclohexane, a product of cyclohexene C=C hydrogenation, has immense importance for its uses as solvent and to synthesize mainly cyclohexanol and cyclohexanone, which are the chemical commodities to produce adipic acid, caprolactam, nylon 6 and nylon 66. Similarly, n-butyraldehyde, one of the selective products of crotonaldehyde C=C hydrogenation, is the chemical intermediate for n-butyl alcohol, 2-ethylhexanol, trimethylolpropane (TMP), polyvinyl butyral (PVB), pharmaceuticals, pesticides, antioxidants, flavors, synthetic perfumes, vulcanization accelerators, crop protecting agent and many more.
Due to the importance of these products, number of groups have been working on the same and as a result over the last few years, hydrogenation using Pd(0) nanoparticles as catalysts, has gained lot of interest because of their great efficiency towards the selective hydrogenation of C=C bond. However, C=O selectively can be reduced by either designing the suitable bimetallic catalyst in which one of the metals having oxidic species (acidic sites) would attract the lone pair of the carbonyl group, or using the alcohols as preferred solvents which can easily activate the C=O bond. However, use of alcohols can arise the undesirable secondary effect such as formation of acetals and hemiacetals [2].
Cyclohexene hydrogenation reaction on the basis of supported Pd(0) nanoparticles had been carried out by various groups such as, in 2009, Liu et al. immobilized Pd nanoparticles on sepiolite (SH-IL-1.0 wt% Pd) and carried out the reaction in stainless steel autoclave with a magnetic stirrer to obtain 99% conversion in 1 h at 60 °C under 2 Mpa H2 pressure [3]. In 2014, Zhang group immobilized Pd nanoparticles in a microporous/mesoporous composite (Pd/MSS@ZIF-8) to obtain 5.6% conversion at 35 °C in 6 h using ethyl acetate as a solvent [4]. In 2016, Sapi et al. synthesized mesoporous carbon-supported Pd nanoparticles (Pd/CNT) and performed hydrogenation in a continuous flow reactor system at 40 °C, where the ratio of cyclohexene: hydrogen: nitrogen was 1: 10: 90 with a total flow rate of 101 mL min−1 at 1 bar [5]. In 2017, Leng et al. immobilized palladium nanoparticles on nitrogen-doped carbon (Pd@CN) to get 96% conversion in 12 h at 90 °C using formic acid as solvent [6].
As per our knowledge, there are only three reports on hydrogenation of crotonaldehyde to butyraldehyde using supported Pd (0) nanoparticles. In 2003, Zhao et al. reported carbon supported palladium nanoparticles (Pd/C) to achieve 100% conversion with 100% selectivity using supercritical CO2 as a solvent at 100 °C. Here, the reaction was carried out under very harsh condition i.e. 80 bar pressure [7]. In 2005, Iwasa et al. reported the synthesis of palladium nanoparticles supported on CeO2 (Pd/CeO2) and achieved 100% conversion with 40% selectivity of butyraldehyde at 200 °C using fix bed reactor [8]. After 8 years, in 2013, Harraz et al. reported the synthesis of stabilized palladium nanoparticles by polyethylene glycol (Pd/PEG) to obtain 100% conversion and 100% selectivity of butyraldehyde in 90 min at room temperature [9].
Thus, literature survey shows that hydrogenation of the unsaturated compounds, especially in case of crotonaldehyde in harsh conditions, was carried out either in organic solvents or in aqueous solvents. In addition, no study has been carried out in which neat water was used as a solvent. It would be interesting and environmentally benign if hydrogenation reactions would be carried out in neat water.
Number of methods are available in literature for stabilization of Pd(0) nanoparticles with different stabilizing agents [10,11,12,13,14,15,16,17,18,19,20,21,22,23,24], more recently Scarso et al. also reported anionic surfactant stabilized Pd nanoparticles for selective hydrogenations and dechlorinations in neat water [25], as well as polyoxometalates (POMs) [26,27,28,29]. Amongst these, POMs are very fascinating and excellent as stabilizing agents due to their number of convergent properties such as robust oxoanionic nature, their negative charges, large relative sizes, stability, reducing and encapsulating agent [26,27,28,29]. However, only two reports are available in which palladium and supported heteropolyacid were used for carrying out hydrogenation reaction. First, in 2002, Newmann and co-workers synthesized palladium substituted polyoxometalate (K5PPdW11O39/C) as pre-catalyst for hydrogenation of aromatic compounds [30] and second, in 2013, Parida et al. reported palladium based lacunary phosphotungstate supported mesoporous silica (50LPdW/MCM-41) for hydrogenation of p-nitrophenol to p-aminophenol at room temperature [31]. In both of these reports the use of Pd(0) nanoparticles had not been mentioned. No report is available in the art, in which supported Pd(0) nanoparticles stabilized polyoxometalate were used for the hydrogenation of unsaturated hydrocarbon.
Rrecently we have published, Suzuki-Miyaura cross coupling reaction in aqueous medium as well as in neat water using stabilized Pd(0) nanoparticles by zirconia supported 12-tungstophosphoric acid (Pd(0)-TPA/ZrO2) [32]. The obtained excellent catalytic activity encouraged us to extend our work for another important and challenging transformation i.e. hydrogenation of cyclohexene and crotonaldehye.
In the present paper, efficiency of the catalyst was evaluated for hydrogenation of cyclohexene and crotonaldehyde in neat water by varying different parameters such as catalyst amount, time, temperature, pressure and effect of solvents were studied for maximum conversion as well as selectivity of the desired product. Under optimized condition, heterogeneity test, control experiment as well as catalytic activity of recycled catalyst were performed. Regenerated catalyst was characterized by EDS, FT-IR and XPS techniques to confirm its stability. Plausible reaction mechanism for both the reactions was also proposed.
2 Experimental
2.1 Materials
All chemicals used were of A.R. grade. 12-tungstophosphoric acid, zirconium oxychloride, ammonia, palladium chloride, cyclohexene, crotonaldehyde and dichloromethane were obtained from Merck and used as received.
2.2 Catalyst Synthesis
Zirconia (ZrO2) [33] and Zirconia supported 12-tungstophosphoric acid (TPA/ZrO2) [34] was synthesized by following the same method reported by us. Pd(0) nanoparticles were deposited on supported 12-tungstophosphoric acid via exchanging the available protons of TPA/ZrO2 [32]. 1 g of TPA/ZrO2 was soaked with 25 mL 0.05 M aqueous solution of PdCl2 for 24 h with stirring. The solution was filtered, washed with distilled water in order to remove the excess of palladium and dried in air at room temperature. The resulting brown catalyst was designated as Pd(II)-TPA/ZrO2. Then, synthesized catalyst was charged in a Parr reactor under 1 bar H2 pressure, at 40 °C for 30 min to reduce Pd(II) to Pd(0). After that the catalyst was removed from reactor and kept in air to attain the room temperature. The obtained black colored catalyst was designated as Pd(0)-TPA/ZrO2.
2.3 Characterization
A detailed study on the characterization of Pd(0)-TPA/ZrO2 can be found in our recent publication [32]. However, in the present article EDS, XPS, TEM, BET and XRD are given for reader’s convenience. In addition, we have characterized fresh and regenerated catalyst by XPS for W also in order to confirm its reduction. Elemental analysis of the solid catalyst was performed by JSM 5610 LV combined with INCA instrument. Leaching of Pd in the reaction mixture was checked by using atomic adsorption spectrometer AAS GBC-902 instrument. X-ray photoelectron spectroscopy (XPS) measurements were performed with Auger Electron Spectroscopy (AES) Module PHI 5000 Versa Prob II. TEM analysis was carried out on JEOL/EO TEM instrument (model-JEM 1400) with accelerating voltage of 120 kV. Samples were prepared by dropping the dispersed sample on 300 mesh carbon coated Cu grid. Adsorption–desorption isotherms of samples were recorded by Micromeritics ASAP 2010 surface area analyzer at − 196 °C. XRD pattern was performed by using a Philips PW-1830 diffractometer. The conditions were: Cu-Kα radiation (1.54 Å), scanning angle from 10° to 80°.
2.4 Catalytic Activity
The catalytic reaction was carried out using Parr reactor instrument having three major components: The batch type reactor of 100 mL capacity is made up of SS-316, H2 reservoir with electronic temperature and pressure controller. For example, in typical reaction, 9.87 mmol of cyclohexene with 50 mL of water as solvent and 20 mg of catalyst were charged to the reactor vessel. The reactor was flushed thrice with H2 gas to remove the air present in the empty part of the vessel. Finally, 10 bar H2 pressure was applied for the reaction. The reaction was set at 80 °C with the stirring rate of 1700 rpm for 4 h. The continuous decrease in pressure inside the vessel was utilized for determination of the reaction progress. After reaction completion, the reaction mixture was cooled to room temperature and then H2 pressure was released from the vent valve. The organic layer was extracted using dichloromethane, while catalyst was collected from the junction of the liquid phases and finally recovered by centrifugation. The organic phase was then dried with anhydrous magnesium sulfate and analyzed by a gas chromatograph (Shimadzu-2014) using a capillary column (RTX-5). The products were recognized by comparison with the standard samples.
3 Results and Discussion
3.1 Characterization
EDX values of W (16.87 wt%) and Pd (0.47 wt%) is in good agreement with calculated one (16.91 wt% of W, 0.4 wt% of Pd). Very low % of Pd indicates that only the protons of TPA were exchanged [35]. EDX elemental mapping of the catalyst is shown in Supplementary Fig. S1.
The XPS of Pd(0)-TPA/ZrO2 is displayed in Fig. 1. A high intense peak at 532 eV (3p3/2) with broad peak at 558 eV (3p1/2) and low intense peak at 335 eV (3d5/2) are in good agreement with available reports [29, 32, 36,37,38] respectively, which confirm the presence of Pd(0) nanoparticles on the surface. However, there is still low amount of Pd(II) present, as evident by corresponding peak at 345.5 eV (3d3/2). This may be because of air oxidation of Pd(0) during the drying process. This is further confirmed by TEM. The W4f peak is composed of a well resolved spin orbit doublet (33.9 eV and 35.9 eV for W4f7/2 and W4f5/2 respectively), typical of W(VI) atoms in agreement with literature data on Keggin-type POMs [29], confirming no reduction of W(VI) during the synthesis of Pd(II)-TPA/ZrO2 to Pd(0)-TPA/ZrO2.
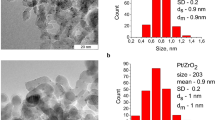
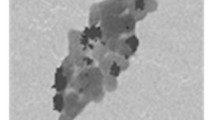
TEM images of fresh catalyst are shown in Fig. 2 at various magnifications. Images (a) and (b) show the dark uniform suspension in the amorphous nature of the catalyst. This indicates the high dispersion of Pd(0) nanoparticles over the surface of the catalyst.
The increase in surface area of the Pd(II)-TPA/ZrO2 (169 m2 g−1) as compared to that of TPA/ZrO2 (146 m2 g−1) indicates high uniform dispersion of the Pd on the surface of TPA/ZrO2 [35]. The observed drastic increase in the value of surface area of Pd(0)-TPA/ZrO2 (203 m2 g−1) as compared to that of Pd(II)-TPA/ZrO2 (169 m2 g−1), conforms the reduction of Pd(II) to Pd(0) and is in good agreement with the known fact that nanoparticles have higher surface area than the parent one. It is very interesting to note down that in spite of different surface areas, the nitrogen adsorption desorption isotherms (Supplementary Fig. S2) are almost similar for all systems confirming no change in the basic structure.
XRD patters of ZrO2, TPA, TPA/ZrO2 and Pd(0)-TPA/ZrO2 are presented in Supplementary Fig. S3. The absence of any crystalline peak in TPA/ZrO2 corresponding to TPA, indicates the high dispersion of material over the surface of the support. The XRD patterns of TPA/ZrO2 and Pd(0)-TPA/ZrO2 are almost identical, indicating the retention of the TPA structure even after exchanging and reduction of palladium. Absence of any crystalline peak corresponds to Pd(0) in Pd(0)-TPA/ZrO2 spectrum, is due to its very low concentration on the surface of the catalyst as well as high dispersion of Pd(0) nanoparticles on the surface of TPA/ZrO2.
3.2 Catalytic Activity
The catalytic activity of Pd(0)-TPA/ZrO2 was evaluated for hydrogenation of cyclohexene and crotonaldehyde. Effect of various reaction parameters like solvent, substrate-catalyst ratio, time and temperature and hydrogen pressure were studied in order to obtain maximum conversion.
3.2.1 Cyclohexene Hydrogenation
To evaluate the efficiency of the catalyst for hydrogenation, cyclohexene was selected as test substrate (Scheme 1). Effect of different reaction parameters such as Pd concentration, solvent, temperature, pressure and time were studied to optimize the conditions for maximum conversion.
3.2.1.1 Effect of Palladium Concentration (Substrate/Catalyst Ratio)
The effect of Pd concentration was evaluated by varying the catalyst amount from 10 to 25 mg (Substrate/ Catalyst ratio from 26259/1 to 10504/1 respectively). Initially, with increase in concentration of Pd, % conversion also increases i.e. 10 mg to 20 mg (Fig. 3). Higher concentration of Pd would means a higher number of active sites to tolerate the substrate and gives higher conversion. With further increasing Pd concentration, the conversion remains constant. The highest, 99% conversion was achieved by 20 mg of catalyst.
3.2.1.2 Effect of Solvent
Effect of various solvents was studied and obtained results are presented in Fig. 4. Comparatively, low % conversion was obtained in case of CH3CN–H2O solvent system due to its oxidizing nature which resists the reduction of cyclohexene. While it was achieved maximum for CH3OH–H2O, IPA–H2O and CH3OH–H2O system and this may be due to their reducing nature. However, it was miracle that higher % conversion was obtained under the identical reaction conditions for neat water as a solvent (i.e. 96%). Hence, water was selected as a solvent for further study.
3.2.1.3 Effect of H2 Pressure
Hydrogen pressure influence was evaluated and obtained results are presented in Supplementary Fig. S4. Initially, with increase in pressure from 5 to 10 bar, the % conversion also increased linearly. Hence, the reaction is first order with respect to H2 pressure. With further increase in pressure, no effect on the conversion was observed. Highest 96% conversion was obtained for 10 bar H2 pressure.
3.2.1.4 Effect of Time
The effect of time was investigated by varying the reaction time from 2 to 5 h (Supplementary Fig. S5). It is seen from the results that initially with increase in time up to 4 h, the % conversion also increases, which is in good agreement with the well-known fact that as time increases, the formation of reactive intermediates from the reactant increases, and finally converted into the products. On further increasing the reaction time, the % conversion decreases. This might be due to occurrence of reversible process.
3.2.1.5 Effect of Temperature
Effect of temperature was screened from 50 to 90 °C and obtained results are presented in Fig. 5. Results shows that % conversion increases with increase in temperature from 50 to 80 °C, as expected. Further increase in the temperature showed no effect on the % conversion.
The optimized conditions for the maximum % conversion (96) are: cyclohexene (9.87 mmol), conc. of Pd(0) (7.48 × 10−4 mmol), H2O (50 mL), H2 pressure (10 bar), time (4 h) and temp. (80 °C), Substrate/Catalyst ratio (13130/1) and TON (12,667).
3.3 Crotonaldehyde Hydrogenation
To evaluate the efficiency of the catalyst for hydrogenation, crotonaldehyde was also selected as test substrates (Scheme 2). Effect of different reaction parameters such as Pd concentration, solvent, temperature, pressure and time were studied to optimize the conditions for maximum conversion.
As described in section of cyclohexene hydrogenation, in present case also, all parameters were varied and results are shown in respective figures (Fig. 6). It should be noted that the explanation will remain the same as given in the section of cyclohexene hydrogenation. Here, in all the cases, butyraldehyde was obtained with 100% selectively. Whereas in case of EtOH: H2O (20: 30) mL solvent system, we achieved 91% conversion with 60% butyraldehyde and 40% crotylalcohol selectively. An explanation for the same is provided in mechanism Sect. 3.7.3.
Reaction conditions. a Effect of Pd concentration: crotonaldehyde (9.87 mmol), H2O (50) mL, Temp. (80 °C), H2 pressure (8 bar), Time (4 h). b Effect of solvent: crotonaldehyde (9.87 mmol), conc. of Pd (7.48 × 10-4 mmol), solvent ratio (20–30) mL, Temp. (80 °C), H2 pressure (8 bar), time (4 h). c Effect of temperature: crotonaldehyde (9.87 mmol), conc. of Pd (7.48 × 10-4 mmol), H2O (50) mL, H2 pressure (8 bar), time (4 h). d Effect of H2 pressure: crotonaldehyde (9.87 mmol), conc. of Pd (7.48 × 10-4 mmol), H2O (50) mL, temp. (80 °C), time (4 h)
Finally, the effect of time on reaction was also studied and obtained results are presented in Supplementary Fig. S6. From the data, it can be seen that reaction progress increases with increase in time up to 4 h. Further increase in time didn’t show any effect on the conversion indicating the saturation point of the reaction. Maximum 89% conversion was obtained in 4 h.
The optimized conditions for the maximum % conversion (89) are: crotonaldehyde (9.87 mmol), conc. of Pd(0) (7.48 × 10 −4 mmol), H2O (50 mL), temp. (80 °C), H2 pressure (8 bar) and time (4 h). Substrate/Catalyst ratio (13130/1), TON (11744).
3.4 Control Experiment
In both the cases, control experiments were carried out with ZrO2, PdCl2, TPA/ZrO2 and Pd(0)-TPA/ZrO2 under optimized conditions and results are shown in Supplementary Table S1. It is seen from the data that ZrO2 and TPA/ZrO2 were inactive towards the reactions. Almost same conversions were found in the case of PdCl2 and Pd(0)-TPA/ZrO2 in both the cases. This indicates that Pd is real active species responsible for the reaction.
3.5 Leaching and Recycling Test
The leaching of Pd from Pd(0)-TPA/ZrO2 was confirmed by carrying out an elemental analysis of the recovered catalyst by EDS as well as of the reaction mixtures by Atomic Adsorption Spectroscopy (AAS). The analysis of the recovered catalyst and reaction mixture did not show appreciable loss in the Pd content. To evaluate the efficiency of Pd(0)-TPA/ZrO2 towards recyclability, after reaction completion, the catalyst was recovered by simple centrifugation, washed with dichloromethane followed by water and then dried in oven at 100 °C for an hour and finally used for the next cycle. Obtained constant results (Fig. 7) shows the stable activity up to number of cycles without leaching of Pd(0) nanoparticles. Recycling test was carried out for both reactions and almost same trend was found upto 5 cycles. However, we have reported here only the results for cyclohexene substrate.
3.6 Heterogeneity Test
In both the cases, reaction was carried out for 2 h, catalyst was removed and then filtrate was allowed to react up for 4 h. After that, obtained reaction mixtures were extracted by using dichloromethane and analyzed by Gas chromatography. Obtained results (Supplementary Table S2) showed that there was no significant change in % conversion of the reactants after removing the catalyst, indicating the absence of active species in the reaction mixture which proves that there was no leaching of Pd during the reactions. This study indicates that TPA/ZrO2 stabilized Pd nanoparticles and does not allow to leach it into reaction mixture, making it a true heterogeneous catalyst to recycle and reuse. Here, heterogeneous nature of the catalyst and withholding of the active species on the support during the reaction shows that present catalyst is of category C [32]. In both the cases, the same trend was found and hence we have reported here only the results of cyclohexene.
3.7 Characterization of Regenerated Catalyst
In order to check the stability, the regenerated catalyst was characterized for EDS, FT-IR and XPS.
EDX values of W (16.90 wt%) and Pd (0.46 wt%) of regenerated Pd(0)-TPA/ZrO2 is in good agreement with values of fresh catalyst (16.87 wt% of W, 0.47 wt% of Pd) confirming no leaching of TPA from ZrO2. This indicates that TPA plays an important role as a stabilizer to keep the Pd(0) active and also prevent its leaching from the support.
The XPS of fresh and regenerated Pd(0)-TPA/ZrO2 is displayed in Fig. 8. The obtained spectra are identical with fresh one, which confirms that Pd(0) nanoparticles are stabilized by TPA and catalyst is sustainable during the reaction. Here, the intensity of regenerated catalyst spectrum is low, which may be due to the sticking of the substrates on the surface, although this might not be significant in the reutilization of the catalyst.
3.7.1 Substrate Study
In order to see the viability of the present catalyst, hydrogenation of different substrates was carried out over Pd(0)-TPA/ZrO2 and the results are presented in Table 1. From the results it is clear that the catalyst was found to be active for selective hydrogenation of C=C bond without tolerating the C=O bond.
3.7.2 Comparison with Reported Catalysts
Catalytic activity of the present catalyst is also compared with reported catalysts in both the cases.
In case of cyclohexene hydrogenation (Table 2), Liu et al. [3] reported very high conversion at lower temperature with very small amount of catalyst but they had utilized very high H2 pressure (20 bar) for the reaction compared to present work. Zhang et al. [4] reported the same reaction at 35 °C with very low conversion, moreover use of catalyst amount was too high. Leng et al. [6] achieved high conversion using formic acid as a sovent with very high amount of the catalyst compared to the present catalytic system.
In case of crotonaldehyde hydrogenation (Table 3), Iwasa et al. [8] reported 100% conversion at 200 °C temperature with very high amount of catalyst, moreover selectivity of the desired product butyraldehyde was only 31%. Here, reason for the low selectivity was weaker adsorption of formed product butyraldehyde over the surface of the catalyst, which easily gets disorb and hydrogenated to form another products alcohol. Zhao et al. [7] achieved 100% conversion at 100 °C with very low amount of Pd, but they had used super critical CO2 as solvent under 80 bar pressure (very harsh condition). Here, the use of CO2 favours to obtain 100% selectivity of butyraldehyde.
Here in both the cases, high activity as compared to reported systems, may be due to its single atom catalytic (SAC) properties. Recently, in SAC, it is reported that the activity of the catalyst as well as high density of the active sites can be increased by either increasing the dispersion of the metal atoms to the support or reducing the size of metal by converting it into nanoparticles [39, 40].
3.7.3 Plausible Mechanism
Hydrogenation of crotonaldehyde gives three products selectively (i) Butyraldehyde (ii) Crotyl alcohol and (iii) n-butanol. It is well known that thermodynamic parameters favor the hydrogenation of C=C over the C=O bond [41] due to high activation energy of C=O tolerance to yield selectively butyraldehyde. However, C=O selectively can be reduced by either designing the suitable bimetallic catalyst or using organic solvent as discussed in introduction part.
In the present work, we achieved single selective product that is butyraldehhyde instead of product mixture. There might be two reasons for the selective reduction of C=C bond. (i) The presence of only water as a solvent instead of any organic solvent as discussed above. Here, the absence of organic solvent would not activate C=O bond to selectively produce butyraldehyde only. To check the same, we have carried out the same reaction under the optimized condition using EtOH: H2O (20:30) mL as a solvent. Obtained results showed 91% conversion with 60% butyraldehyde and 40% crotylalcohol selectivity, indicating the activation of C=O bond for hydrogenation due to the presence of EtOH as solvent. (ii) The high adsorption capacity of the catalyst towards the substrates and initially formed products [42,43,44,45,46,47,48]. In present case, initially formed product butyraldehyde got adsorbed on the surface of the catalyst, and did not allow it to desorb for further hydrogenation to generate other product butanol. Hence, we are expecting the well-known mechanism for both the reactions. The proposed mechanism for both the reactions is shown in Scheme 3.
4 Conclusions
First time, we report the selective C=C hydrogenation of cyclohexene and crotonaldehyde over a heterogeneous catalyst, (Pd(0)-TPA/ZrO2) consisting of stabilized Pd(0) nanoparticles, in neat water. The superiority of present work lies in obtaining higher conversion and selectivity of desired product (96% for cyclohexene with 100% selectivity of cyclohexane and 89% for crotonaldehyde with 100% selectivity of butyraldehyde) as well as high turnover number (12667, 11744 for cyclohexene and crotonaldehyde respectively). The catalyst can be recovered by centrifugation and can be reused up to 5 cycles and more without any significant change in conversion as well as selectivity. Present catalytic system was found to be viable under sustainable reaction condition to tolerate variety of the substrate.
References
Panpranot J, Phandinthong K, Praserthdam P, Hasegawa M, S-i Fujita, Arai M (2006) A comparative study of liquid-phase hydrogenation on Pd/SiO2 in organic solvents and under pressurized carbon dioxide: activity change and metal leaching/sintering. J Mol Catal A 253:20–24
Campo BC, Volpe MA, Gigola CE (2009) Liquid-phase hydrogenation of crotonaldehyde over platinum- and palladium-based catalysts. Ind Eng Chem Res 48:10234–10239
Tao R, Miao S, Liu Z, Xie Y, Han B, An G, Ding K (2009) Pd nanoparticles immobilized on sepiolite by ionic liquids: efficient catalysts for hydrogenation of alkenes and Heck reactions. Green Chem 11:96–101
Zhang T, Li B, Zhang X, Qiu J, Han W, Yeung KL (2014) Pd nanoparticles immobilized in a microporous/mesoporous composite ZIF-8/MSS: a multifunctional catalyst for the hydrogenation of alkenes. Microporous Mesoporous Mater 197:324–330
Puskas R, Sápi A, Kukovecz Á, Kónya Z (2016) Understanding the role of post-CCVD synthetic impurities, functional groups and functionalization-based oxidation debris on the behaviour of carbon nanotubes as a catalyst support in cyclohexene hydrogenation over Pd nanoparticles. RSC Adv 6:88538–88545
Zhang C, Leng Y, Jiang P, Li J, Du S (2017) Immobilizing palladium nanoparticles on nitrogen-doped carbon for promotion of formic acid dehydrogenation and alkene hydrogenation. ChemistrySelect 2:5469–5474
Zhao F, Ikushima Y, Chatterjee M, Shirai M, Arai M (2003) An effective and recyclable catalyst for hydrogenation of α, β-unsaturated aldehydes into saturated aldehydes in supercritical carbon dioxide. Green Chem 5:76–79
Iwasa N, Takizawa M, Arai M (2005) Palladium-based alloy and monometallic catalysts for gas phase hydrogenation of crotonaldehyde: effects of alloying and alloy crystallite size. Appl Catal A 283:255–263
Harraz FA, El-Hout SE, Killa HM, Ibrahim IA (2013) Catalytic hydrogenation of crotonaldehyde and oxidation of benzene over active and recyclable palladium nanoparticles stabilized by polyethylene glycol. J Mol Catal A 370:182–188
Özkar S, Finke RG (2016) Palladium(0) nanoparticle formation, stabilization, and mechanistic studies: Pd(acac)2 as a preferred precursor, [Bu4 N]2HPO4 stabilizer, plus the stoichiometry, kinetics, and minimal, four-step mechanism of the palladium nanoparticle formation and subsequent agglomeration reactions. Langmuir 32:3699–3716
Strimbu L, Liu J, Kaifer AE (2003) Cyclodextrin-capped palladium nanoparticles as catalysts for the Suzuki reaction. Langmuir 19:483–485
Narayanan R, El-Sayed MA (2003) Effect of catalysis on the stability of metallic nanoparticles: suzuki reaction catalyzed by PVP-Palladium nanoparticles. J Am Chem Soc 125:8340–8347
Hamill NA, Hardacre C, McMath SEJ (2002) In situ XAFS investigation of palladium species present during the heck reaction in room temperature ionic liquids. Green Chem 4:139–142
Rocaboy C, Gladysz JA (2002) Highly active thermomorphic fluorous palladacycle catalyst precursors for the heck reaction; evidence for a palladium nanoparticle pathway. Org Lett 4:1993–1996
Li Y, Boone E, El-Sayed MA (2002) Size effects of PVP−Pd nanoparticles on the catalytic Suzuki reactions in aqueous solution. Langmuir 18:4921–4925
Deshmukh RR, Rajagopal R, Srinivasan KV (2001) Ultrasound promoted C-C bond formation: heck reaction at ambient conditions in room temperature ionic liquids. Chem Commun 17:1544–1545
Li Y, El-Sayed MA (2001) The effect of stabilizers on the catalytic activity and stability of Pd colloidal nanoparticles in the suzuki reactions in aqueous solution. Org Lett 105:8938–8943
Moreno-Mañas M, Pleixats R, Villarroya S (2001) Fluorous phase soluble palladium nanoparticles as recoverable catalysts for suzuki cross-coupling and heck reactions. Organometallics 20:4524–4528
Crooks RM, Zhao M, Sun L, Chechik V, Yeung LK (2001) Dendrimer-encapsulated metal nanoparticles: synthesis, characterization, and applications to catalysis. Acc Chem Res 34:181–190
Li Y, Hong XM, Collard DM, El-Sayed MA (2000) Suzuki cross-coupling reactions catalyzed by palladium nanoparticles in aqueous solution. Org Lett 2:2385–2388
Pathak S, Greci MT, Kwong RC, Mercado K, Prakash GKS, Olah GA, Thompson ME (2000) Synthesis and applications of palladium-coated Poly(vinylpyridine) nanospheres. Chem Mater 12:1985–1989
Jana NR, Wang ZL, Pal T (2000) Redox catalytic properties of palladium nanoparticles: surfactant and electron donor−acceptor effects. Langmuir 16:2457–2463
Teranishi T, Miyake M (1998) Size control of palladium nanoparticles and their crystal structures. Chem Mater 10:594–600
Reetz MT, Breinbauer R, Wanninger K (1996) Suzuki and Heck reactions catalyzed by preformed palladium clusters and palladiumnickel bimetallic clusters. Tetrahedron Lett 37:4499–4502
La Sorella G, Sperni L, Canton P, Coletti L, Fabris F, Strukul G, Scarso A (2018) Selective hydrogenations and dechlorinations in water mediated by anionic surfactant-stabilized pd nanoparticles. J Org Chem 83:7438–7446
Kogan V, Aizenshtat Z, Popovitz-Biro R, Neumann R (2002) Carbon−carbon and carbon−nitrogen coupling reactions catalyzed by palladium nanoparticles derived from a palladium substituted Keggin-type polyoxometalate. Organic Lett. 4:3529–3532
Zhang J, Keita B, Nadjo L, Mbomekalle IM, Liu T (2008) Self-assembly of polyoxometalate macroanion-capped Pd0 nanoparticles in aqueous solution. Langmuir 24:5277–5283
D’Souza L, Noeske M, Richards RM, Kortz U (2013) Palladium (0) metal clusters: novel krebs type polyoxoanions stabilized, extremely active hydrogenation catalyst. Appl Catal A 453:262–271
Villanneau R, Roucoux A, Beaunier P, Brouri D, Proust A (2014) Simple procedure for vacant POM-stabilized palladium (0) nanoparticles in water: structural and dispersive effects of lacunary polyoxometalates. RSC Adv 4:26491–26498
Kogan V, Aizenshtat Z, Neumann R (2002) Preferential catalytic hydrogenation of aromatic compounds versus ketones with a palladium substituted polyoxometalate as pre-catalyst. New J Chem 26:272–274
Rana S, Parida KM (2012) A simple and efficient protocol using palladium based lacunary phosphotungstate supported mesoporous silica towards hydrogenation of p-nitrophenol to p-aminophenol at room temperature. Catal Sci Technol 2:979–986
Patel A, Patel A (2018) Stabilized palladium nanoparticles: synthesis, multi-spectroscopic characterization and application for Suzuki–Miyaura reaction. Catal Lett 148:3534–3547
Patel S, Purohit N, Patel A (2003) Synthesis, characterization and catalytic activity of new solid acid catalysts, H3PW12O40 supported on to hydrous zirconia. J Mol Catal A 192:195–202
Bhatt N, Shah C, Patel A (2007) 12-tungstophosphoric and 12-tungstosilicicacid supported onto hydrous zirconia for liquid phase tert-butylation of m-cresol. Catal Lett 117:146–152
Pathan S, Patel A (2012) Heck coupling catalyzed by Pd exchanged supported 12-tunstophosphoric acid—an efficient ligand free, low Pd-loading heterogeneous catalyst. RSC Adv 2:116–120
Militello MC, Simko SJ (1994) Elemental palladium by XPS. Surf Sci Spectra 3:387–394
Abbas Khakiani B, Pourshamsian K, Veisi H (2015) A highly stable and efficient magnetically recoverable and reusable Pd nanocatalyst in aqueous media heterogeneously catalysed Suzuki C-C cross-coupling reactions. Appl Organomet Chem 29:259–265
Patel A, Patel A, Narkhede N (2019) Hydrogenation of cyclohexene in aqueous solvent mixture over a sustainable recyclable catalyst comprising palladium and monolacunary silicotungstate anchored to MCM-41. Eur J Inorg Chem 2019:423–429
Liu J-H, Yang L-M, Ganz E (2018) Efficient and selective electroreduction of CO2 by single-atom catalyst two-dimensional TM–Pc monolayers. ACS Sustain Chem Eng 6:15494–15502
Xu L, Yang L-M, Ganz E (2018) Mn–graphene single-atom catalyst evaluated for CO oxidation by computational screening. Theor Chem Acc 137:98
Ruiz-Martinez J, Fukui Y, Komatsu T, Sepulveda-Escribano A (2008) Ru–Ti intermetallic catalysts for the selective hydrogenation of crotonaldehyde. J Catal 260:150–156
Gallezot P, Richard D (1998) Selective hydrogenation of α, β-unsaturated aldehydes. Catal Rev 40:81–126
Vannice MA, Sen B (1989) Metal-support effects on the intramolecular selectivity of crotonaldehyde hydrogenation over platinum. J Catal 115:65–78
Abid M, Ehret G, Touroude R (2001) Pt/CeO2 catalysts: correlation between nanostructural properties and catalytic behaviour in selective hydrogenation of crotonaldehyde. Appl Catal A 217:219–229
Hubaut R, Daage M, Bonnelle JP (1986) Selective hydrogenation on copper chromite catalysts IV. Hydrogenation selectivity for α, β-unsaturated aldehydes and ketones. Appl Catal 22:231–241
Augustine RL (1976) Organic functional group hydrogenation. Catal Rev 13:285–316
Touroude R (1980) Catalytic behavior of group VIII transition metals in the deuterium-acrolein reaction. J Catal 65:110–120
Shirai M, Tanaka T, Arai M (2001) Selective hydrogenation of α, β-unsaturated aldehyde to unsaturated alcohol with supported platinum catalysts at high pressures of hydrogen. J Mol Catal A 168:99–103
Acknowledgements
We are thankful to Department of Atomic Energy (DAE) and Board of Research in Nuclear Science (BRNS), Project No. 37(2)/14/34/2014-BRNS, Mumbai, for the financial support. One of the authors Mr. Anish Patel is thankful to the same for the grant of JRF. We are also thankful to Department of Chemistry, The Maharaja Sayajirao University of Baroda for BET surface area analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Patel, A., Patel, A. Selective C=C Hydrogenation of Unsaturated Hydrocarbons in Neat Water Over Stabilized Palladium Nanoparticles Via Supported 12-Tungstophosphoric Acid. Catal Lett 149, 1476–1485 (2019). https://doi.org/10.1007/s10562-019-02763-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-019-02763-1