Abstract
The present article demonstrates a simple method for synthesizing the highly stabilized Pd(0) nanoparticles by using supported 12-tungstophosphoric acid as a stabilizer as well as a carrier. The obtained material was characterized by different methods and the presence of nanoparticles on the surface of the carrier was confirmed, especially by TEM and XPS. As an application, the use of material was explored for the well-known fascinating organic transformation, Suzuki–Miyaura cross coupling reaction in aqueous medium as well as in neat H2O. It was found that the material shows an outstanding activity as the heterogeneous catalyst (0.0096 mol% of Pd) for both aqueous medium (99% conversion, TOF 96958 h−1) and in neat H2O (89% conversion, TOF 46390 h−1) towards biphenyl. The catalyst was recovered by filtration only, regenerated and reused without any significant loss in conversion. Study shows that the present catalyst is truly heterogeneous and sustainable for the said reaction, in either of the medium. The viability of the catalyst was learned toward different substrates and found to be excellent in almost all cases.
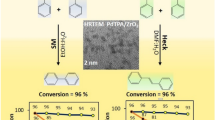
Graphical Abstract
Pd nanoparticles stabilized by supported 12-tungstophosphoric acid is proved to be sustainable and excellent for Suzuki–Miyaura reaction with very high catalyst to substrate ratio as well as TOF.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Pd(0) nanoparticles are one of the most studied catalysts for C–C coupling reactions [1] and over the last few years, synthesis of stabilized Pd(0) nanoparticles is gaining a tremendous attention. As a result number of articles describing various methods with different stabilizing agents [2,3,4,5,6,7,8,9,10,11,12,13] are available in the literature. In addition, Veisi et al. reported use of number of different natural plant extracts as green stabilizing agents for synthesizing Pd(0) nanoparticles [14,15,16,17,18,19,20,21,22,23,24]. Amongst, polyoxometalates (POMs), unique class of inorganic compounds having discrete metal–oxygen cluster composed of transition metals of group V and VI in high oxidation states and acidic form of them are known as heteropolyacids, are very fascinating and excellent as stabilizing agent due to their number of convergent properties such as robust oxoanionic nature, its negative charges, large relative sizes, stability, reducing and encapsulating agent [13, 25,26,27,28]. As a result, number of POMs, including lacunary as well as substituted, are used to stabilized Pd(0) following different methods [25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42]. At the same time, electron transfer ability of the POMs facilitates the oxidative addition and reductive elimination steps [41] making them very attractive candidates for carrying out different coupling reactions [25, 35, 41, 42]. In 2002, Neumann et al. derived palladium nanoparticles from a palladium substituted keggin-type POM [K5[PdPW11O39]·12H2O] and achieved 99% yield of biphenyl using chlorobenzene in ethanol–water system at 85 °C in 12 h. The same catalyst was supported on γ-Al2O3, [K5[PdPW11O39]·12H2O/γ-Al2O3], and applied as a heterogeneous catalyst for the said reaction at 130 °C for 16 h, under the solvent free condition to achieve 99% yield of biphenyl [25]. It is surprising that, even though the reaction is most important, no study was carried out for the same using Pd(0) based POMs for a long time. Almost after 13 years, in 2015, Rafiee et al. developed palladium-poly(N-vinylpyrrolidone)-nano zero valent iron and H5PMo10V2O40(Mo10V2) supported on Fe2O3@SiO2 core–shell nanoparticles to perform in ethanol–water system and achieved 96% yield of biphenyl using bromobenzene at reflux temperature in 30 min. The catalyst was recycled up to four cycles [41]. In 2016, Wang et al. reported nanorolls and hollow spindles based on palladium substituted Wells–Dawson POM (K15[Pd2(α2-P2W17O61)2H]) to obtain 96% yield in ethanol–water system at 30 °C in 20 min with stability up to four catalytic runs [42].
In summary, a literature survey shows that among the available methods to stabilize Pd(0) using different POMs, only one report describes the use of Cs salt of 12-tungstophosphoric acid-supported SiO2 as a platform to produce highly dispersed Pd nanoparticles under UV-light irradiation [40]. It also shows that no report is available where combination of Pd(0) and supported heteropolyacid is used for carrying out Suzuki-Miyaura (SM) reaction. It was also found that the report on the use of one catalyst for both the systems i.e. aqueous medium as well as in neat water is also not available in the art. Keeping in mind all these observations, it was thought of developing a simple method for getting stabilized Pd(0), as well as to evaluate its catalytic activity for SM reaction in different medium in order to check its sustainability.
In the present paper, first time we are reporting the synthesis of Pd(0) nanoparticles stabilized by zirconia supported 12-tungstophosphoric acid (TPA/ZrO2). Here, Pd(II) was exchanged with available protons of supported TPA/ZrO2. The synthesized material was characterized by elemental analysis EDX, TGA, FT-IR, XPS, TEM, BET and XRD and used as a sustainable heterogeneous catalyst for the said reaction in aqueous medium as well as in neat water. Influence of various parameters such as reaction time, reaction temperature, catalyst amount, effect of base, solvent and solvent to water ratio were studied out to obtain maximum % conversion. An attempt was also made to scale up the experiment under the optimized conditions. Scope and limitation of synthesized catalyst was investigated towards different substrates. The catalyst was regenerated, reused and the regenerated catalyst was characterized by TEM and XPS to confirm the stability of Pd(0) nanoparticles.
2 Experimental
2.1 Materials
All chemicals used were of A.R. grade. 12-Tungstophosphoric acid, zirconium oxychloride, ammonia, palladium chloride, iodobenzene, bromobenzene, chlorobenzene, 1-bromo-4-nitrobenzene, 4-bromoacetophenone, p-bromophenol, 4-methoxybenzene boronicacid, phenylboronic acid, potassium carbonate, petroleum ether, ethyl acetate and dichloromethane were obtained from Merck and used as received.
2.2 Catalyst Synthesis
The synthesis of stabilized palladium(0) by supported 12-tungstophosphoric acid is shown in scheme 1. To design the catalyst, the advantage of available counter protons of zirconia supported 12-tungstophosphoric acid was exploited.
Zirconia (ZrO2) [43] and zirconia supported 12-tungstophosphoric acid (TPA/ZrO2) [44] was synthesized by following the same method reported by us. Palladium(II) was deposited on supported 12-tungstophosphoric acid via exchanging the available protons of TPA [45]. 1 g of TPA/ZrO2 was soaked with 25 mL 0.05 M aqueous solution of PdCl2 for 24 h with stirring. The solution was filtered, washed with distilled water in order to remove the excess of palladium and dried in air at room temperature. The resulting brown catalyst was designated as Pd(II)-TPA/ZrO2. Then, synthesized catalyst was charged in a Parr reactor under 1 bar H2 pressure, at 40 °C for 30 min to reduce Pd(II) to Pd(0). After that the catalyst was removed from reactor and kept in air to attain the room temperature. The obtained black catalyst was designated as Pd(0)-TPA/ZrO2. The same procedure was followed for the synthesis of Pd(0)/ZrO2.
2.3 Characterization
The analysis of Pd in the filtrate was carried out by gravimetric analysis [46]. Elemental analysis of the solid catalyst was performed by JSM 5610 LV combined with INCA instrument. Leaching of Pd in the reaction mixture was checked by using atomic adsorption spectrometer AAS GBC-902 instrument. Thermo gravimetric analysis (TGA) of the catalyst was performed on Metler Toledo Star SW 7.01 in the temperature range of 50–600 °C with flow rate of 2 mL min−1 under nitrogen atmosphere with heating rate of 10 °C min−1. FT-IR spectrum of the material was performed by using the KBr wafer on a Shimadzu instrument (IRAffinity-1S). X-ray photoelectron spectroscopy (XPS) measurements were performed with Auger electron spectroscopy (AES) Module PHI 5000 Versa Prob II. TEM analysis was carried out on JEOL/EO TEM instrument (model-JEM 1400) with accelerating voltage of 120 kV. Samples were prepared by dropping the dispersed sample on 300 mesh carbon coated Cu grid. Adsorption desorption isotherms of samples were recorded by Micromeritics ASAP 2010 surface area analyzer at − 196 °C. The XRD pattern was performed by using a Philips PW-1830 diffractometer. The conditions were: Cu-Kα radiation (1.54 Å), scanning angle from 10° to 80°. It must be noted that Pd(0)/ZrO2 has not been characterized in detail as the main focus was on the comparison of the catalytic activity.
2.4 Suzuki–Miyaura Reaction
Initially, the glass reactor was flushed using N2 gas. In an experiment, halobenzene (1.96 mmol), phenylboronic acid (2.94 mmol), K2CO3 (3.92 mmol), solvent and catalyst Pd(0)-TPA/ZrO2 (0.0096 mol% Pd) were charged into the glass reactor. The reactor was heated at desired temperature in an oil bath with stirring, under a N2 gas atmosphere. After reaction completion, the reaction mixture was cooled and the organic phases were extracted by using dichloromethane as an extracting solvent. The organic phases were then dried with anhydrous magnesium sulfate and analyzed by a gas chromatograph (Shimadzu-2014) using a capillary column (RTX-5). The obtained products were identified by comparison with the authentic samples. The product was also purified by column chromatography on silica gel with a mixture of ethyl acetate and petroleum ether as an eluent. The isolated product was identified by 1H & 13C NMR (Supplementary Fig. S1) spectroscopic analysis as well as by melting point and the % yield of product was presented in Table 5.
3 Results and Discussion
3.1 Catalyst Characterization
The gravimetric analysis shows 0.4 wt% of Pd in Pd(II)-TPA/ZrO2 [46]. EDX value of W (16.87 wt%) and Pd (0.47 wt%) is in good agreement with calculated one (16.91 wt% of W, 0.4 wt% of Pd). Very low % of Pd indicates that only the protons of TPA were exchanged [45]. EDX elemental mapping of the catalyst is shown in Supplementary Fig. S2.
The TGA curve (Fig. 1) of TPA/ZrO2 shows the 12.6% weight loss in the temperature range of 70–100 °C indicates the loss of absorbed water molecules and then there was no weight loss observed up to 500 °C indicates the stability of the supported catalyst. The TGA of the Pd(0)-TPA/ZrO2 shows the 13% weight loss up to 150 °C due to the loss of adsorbed water molecules. No weight loss was observed up to 500 °C indicating the stability of the catalyst up to 500 °C.
FT-IR spectrum (Supplementary Fig. S3) of ZrO2 indicates two bending vibrations at 1600 and 1370 cm−1 for O–H–O and H–O–H vibrations and weak bending vibration of Zr–O–H at 600 cm−1. The FT-IR spectrum of TPA/ZrO2 shows characteristic stretching vibration bands of W–O–W, W=O and P–O at 812, 964 and 1070 cm−1 respectively indicating the presence of TPA. The FT-IR spectrum of Pd(0)-TPA/ZrO2 shows the characteristic bands at 824, 945 and 1056 cm−1 corresponding to stretching vibration for W–O–W, W=O and P=O but with significant shift may be due to the change in the environment. Here, additional band corresponding to Pd–O band is not observed, this may be due to the merging of band with Zr–O.
The X-Ray photoelectron spectrum of Pd(0)-TPA/ZrO2 is displayed in Fig. 2. A high intense peak at 532 eV (3p3/2) is in agreement with reported one [47] and confirms the presence of Pd(0) nanoparticles on the surface. In addition, the peak obtained [28] at 335 eV (3d5/2) as well as broad peak at 558 eV (3p1/2) [47] also confirms the presence of Pd(0) nanoparticles. However, there are still a tiny number of Pd(II) present, as evidence by corresponding peak at 345.5 eV (3d3/2). This may be because of air oxidation of the surface of Pd(0) during the drying process. From these obtained data and the intensities of the peaks, it is confirmed that amount of Pd(0) species is higher with a very trace amount of Pd(II) species in the catalyst. This is further confirmed by TEM.
TEM images of the fresh catalyst are displayed in Fig. 3. SAED Fig. 3a indicates the non-crystalline and uniform dispersion of Pd(0) in the synthesized catalyst. Fig. 3b shows the dark uniform suspension in the amorphous nature of the catalyst. This indicates the high dispersion of Pd(0) nanoparticles over the surface of the catalyst.
The increase in surface area of the Pd(II)-TPA/ZrO2 (169 m2 g−1) as compared to that of TPA/ZrO2 (146 m2 g−1) indicates high uniform dispersion of the Pd on the surface of TPA/ZrO2 [45]. The observed drastic increase in the value of surface area of Pd(0)-TPA/ZrO2 (203 m2 g−1) as compared to that of Pd(II)-TPA/ZrO2 (169 m2 g−1), conforming the reduction of Pd(II) to Pd(0) and in good agreement with the known fact that nanoparticles have higher surface area than the parent one. It is very interesting to note down that in spite of different surface area, the nitrogen adsorption desorption isotherms (Fig. 4) are almost similar for all systems confirming no change in the basic structure.
The XRD patterns of ZrO2, TPA, TPA/ZrO2 and Pd(0)-TPA/ZrO2 catalysts are shown in Supplementary Fig. S4. The XRD patterns of ZrO2 (Supplementary Fig. S4a) shows the amorphous nature of the support. The XRD pattern of TPA/ZrO2 (Supplementary Fig. S4b) does not show any characteristic diffraction line indicating that a high dispersion of TPA in a non-crystalline form on the surface of TPA/ZrO2. In XRD patterns of Pd(0)-TPA/ZrO2 (Supplementary Fig. S4c), absence of any crystalline peak corresponds to Pd(0), is due to the very low concentration of Pd(0) on the surface of the catalyst as well as high dispersion of Pd(0) nanoparticles on the surface of TPA/ZrO2.
3.2 Catalytic Studies
3.2.1 SM Reaction Aqueous Medium
To evaluate the catalytic activity, iodobenzene (1.96 mmol) and phenylboronic acid (2.94 mmol) were taken as the coupling substrates and the effect of various reaction parameters such as time, catalyst amount, base, solvent, solvent ratio and temperature were studied to optimize the conditions for maximum % conversion.
The effect of time on catalytic conversion was screened by varying the time from 10 h to 10 min as shown in Fig. 5. Almost similar % conversion was obtained from 10 h to 30 min, after that it decreases. Hence, 30 min optimized for the reaction.
The concentration effect of the Pd(0) on the reaction is shown in Table 1. The reaction was carried out by varying the catalyst amount in the range of 100–5 mg with concentration of Pd(0) in the range of 37.4 × 10−4 mmol (0.192 mol%) to 1.88 × 10−4 mmol (0.0096 mol%) respectively. Initially, the reaction was carried out using (0.192 mol%). The conversion remained unchanged with decrease in the concentration of Pd(0) up to 1.88 × 10−4 mmol (0.0096 mol%). This indicates that very low concentration of Pd(0) is sufficient for the maximum % conversion.
The effect of base on the reaction is shown in the Fig. 6. The study indicates that organic base triethyl amine (Et3N) is less favorable for coupling reaction compare to that of inorganic base. The highest conversion was found in the case of K2CO3 and NaOH compared to Na2CO3. As K2CO3 is environmentally benign, easy to handle and non-hygroscopic in nature compared to NaOH, further study was carried out with K2CO3.
The effect of different solvents is shown in the Supplementary Table ST1 and it is clear that in case of ethanol the highest conversion was achieved compare to acetonitrile and toluene. The results are in good agreement with reported one [45], stating that polar solvents tend to give the best results for coupling reaction. Hence, ethanol was selected as an appropriate solvent for further study.
Further the effect of solvent to water (C2H5OH:H2O) ratio was also studied and data are presented in Fig. 7. It is seen from the figure that in all the cases, conversion in aqueous medium is better than neat solvents. In case of ethanol alone, the conversion is 53% only, this is because of insolubility of base in reaction mixture. In case of (9:1) mL and (8:2) mL ratios base solubility increases due to increase in water amount. Hence, conversion also increases. The same trend was found for (7:3) mL, (5:5) mL and (3:7) mL ratios. Further, with increase in water amount, conversion decreases due to low solubility of the substrates in water compared to ethanol. Hence, (3:7) mL ratio is optimized for further study. Thus, the presence of water which acts as co-solvent can accelerate the reaction towards high yield of the product. This is expected because, presence of water facilitates the easy ionization of K2CO3 and provides basic medium for the reaction. Similarly, under the optimized (3:7) mL ratio, reactions with (CH3CN:H2O) and (toluene: H2O) were also carried out and 92% and 23% conversion was found respectively.
Finally, the effect of temperature was studied and obtained results are presented in Supplementary Fig. S5. The reaction was carried out by varying the temperature range from 50 to 90 °C. It can be seen from the table that with increase in temperature, the % conversion also increases. 99% conversion was obtained at 80 °C. Further, increase in temperature dose not shows any effect on conversion. Hence, 80 °C was considered as optimum for the maximum % conversion.
From the above study, the optimized conditions for the maximum % conversion (99) are: iodobenzene (1.96 mmol), phenylboronic acid (2.94 mmol), K2CO3 (3.92 mmol), conc. of Pd(0) (0.0096 mol%), C2H5OH:H2O (3:7) mL, catalyst/substrate ratio (9.59E−5), TOF (20,642 h−1), 30 min, 80 °C.
Further, the obtained product was purified by column chromatography on silica gel with a mixture of ethyl acetate and petroleum ether as eluent. Isolated yield (99%) was found to be the same with the conversion (99%) found by GC.
3.2.2 SM Reaction in Neat Water
Here also, various parameters have been studied in order to achieve the maximum % conversion using iodobenzene (1.96 mmol) and phenylboronic acid (2.94 mmol) as the coupling substrates. The effect of time was screened by varying the time from 30 min to 2 h and the maximum conversion was obtained in 1 h. The effect of concentration of Pd(0) was studied by varying the amount from 5 to 15 mg with concentration of Pd(0) from 1.88 × 10−4 mmol (0.0096 mol%) to 5.61 × 10−4 mmol (0.0288 mol%) respectively and the conversion remains unchanged with increase in the concentration of Pd(0). Results indicate that very low concentration of Pd(0) (0.0096 mol%) is sufficient for the maximum % conversion. Effect of various bases was also carried out and K2CO3 was found to be the most suitable base for the maximum % conversion. Similarly, effect of temperature was studied from the range of 80–100 °C. Conversion increases with increase in temperature and was found to be maximum at 100 °C. Obtained results are summarized in Fig. 8.
Optimization of parameters for SM reaction. Reaction conditions: a effect of time: K2CO3 (3.92 mmol), conc. of Pd(0) (0.0096 mol%), H2O (6 mL), catalyst/substrate ratio (9.59E−5), 100 °C. b Effect of catalyst amount: K2CO3 (3.92 mmol), H2O (6 mL), 1 h, 100 °C. c Effect of base: base (3.92 mmol), conc. of Pd(0) (0.0096 mol%), H2O (6 mL), catalyst/substrate ratio (9.59E−5), 1 h, 100 °C. d Effect of temperature: K2CO3 (3.92 mmol), conc. of Pd(0) (0.0096 mol%), H2O (6 mL), catalyst/substrate ratio (9.59E−5), 1 h
Finally, the effect of water amount on the reaction was also studied by keeping all other parameters constant. Obtained results are presented in Supplementary Fig. S6, it is seen from the figure that with increase in the volume of water, initially conversion also increases. This may be due to the increase in the solubility of substrates as well as base. However, with further increase in volume of water, the conversion eventually decreases. This is because of the dilution of base [48]. Under the optimized condition, 6 mL of water is the appropriate volume for the maximum % conversion.
From the above study, the optimized conditions for the maximum % conversion (89) are: iodobenzene (1.96 mmol), phenylboronic acid (2.94 mmol), K2CO3 (3.92 mmol), conc. of Pd(0) (0.0096 mol%), H2O (6 mL), catalyst/substrate ratio (9.59E−5), TOF (9383 h−1), 1 h, 100 °C.
In addition, the obtained product was purified by column chromatography on silica gel with a mixture of ethyl acetate and petroleum ether as eluent. Isolated yield (89%) was found to be the same with the conversion (89%) found by GC.
An attempt was also made to scale up the reaction in both the cases. This was performed under the optimized temperature and concentration of Pd(0) by increasing the substrates (iodobenzene and phenylboronic acid) as well as solvent (five times) in aqueous medium as well in neat water. An almost identical conversion (93% in aqueous medium and 89% in neat water) was obtained, indicating very low amount of the catalyst (0.00192 mol%) is capable to tolerate high amount of the substrates without affecting the conversion with high TOF (96,958 h−1 in aqueous medium and 46,390 h−1 in neat water).
In both the cases, control experiments were carried out with PdCl2, Pd(0)/ZrO2 and TPA/ZrO2 under optimized conditions and results are shown in Table 2. It is seen from the table that TPA/ZrO2 is inactive towards the reaction. Almost same conversion was found in the case of PdCl2, Pd(0)/ZrO2 and Pd(0)-TPA/ZrO2 in both the medium. This indicates that Pd is real active species responsible for the reaction.
In both the cases, heterogeneity test was carried out for Pd(0)-TPA/ZrO2 and Pd(0)/ZrO2 by centrifuging the catalyst from the reaction mixture. In the case of aqueous medium, reaction was carried out for 10 min and then filtrate was allowed to react up to 30 min. Similarly, in neat water, reaction was carried out up to 20 min, then reaction mixture was centrifuged and filtrate was allowed to react up to 60 min. Reaction mixtures were analyzed by gas chromatogram (Table 3). No change in % conversion for Pd(0)-TPA/ZrO2 indicates no leaching of palladium during the reaction and the catalyst is truly heterogeneous. While in the case of Pd(0)/ZrO2 the conversion increases even after removal of the catalyst, indicating the leaching of Pd from the catalyst. The leaching of palladium from the catalyst was also confirmed by carrying out EDX analysis of the used catalyst as well as the product mixtures by AAS. The analysis of the recycled Pd(0)-TPA/ZrO2 catalyst did not show appreciable loss in the Pd content (0.39 wt%) as compared to the fresh catalyst (0.4 wt%). In the mixture whereas in case of Pd(0)/ZrO2, recycled catalyst showed appreciable loss in the Pd content (3.69 wt%) as compared to the fresh catalyst (6.05 wt%). This study also indicates that TPA plays an important role in binding the palladium very strongly and thus does not allow the leaching of palladium into the reaction mixture, making it, a true heterogeneous catalyst to recycle and reuse.
The recovery and reusability of the catalyst is a key issue for the sustainability of any catalytic process. After completion of reaction, the organic layer was extracted by dichloromethane and the catalyst was recovered by simple centrifugation, washed with dichloromethane followed by water and then dried in oven at 100 °C for an hour and finally used for the next cycle. Results of recyclability for Pd(0)-TPA/ZrO2 are shown in Table 4. As seen from results, the recycled catalyst did not show any appreciable change in the % conversion up to two cycles, indicating that the catalyst is stable and can be regenerated and reused. Here, the activity of the catalyst might be due to the stabilization of the Pd nanoparticles by POM.
In order to check the stability, the regenerated catalyst was characterized by elemental analysis (EDX), XPS, TEM, XRD and BET.
EDX values of Pd (0.46 wt%) and W (16.90 wt%) of regenerated Pd(0)-TPA/ZrO2 is in good agreement with values of fresh catalyst (16.87 wt% of W, 0.47 wt% of Pd) confirming no emission of TPA from ZrO2. This indicates that TPA plays an important role as a stabilizer to keep the Pd(0) active and also prevent it to leach from the support.
The X-ray photoelectron spectrum of fresh and regenerated Pd(0)-TPA/ZrO2 is displayed in Fig. 9. The obtained spectrum is identical with fresh one confirms that Pd(0) species are stabilized by TPA and catalyst is sustainable during the reaction. The presence of Pd(0) was further supported by TEM.
TEM images of the regenerated catalyst are displayed in Fig. 10. The SAED Fig. 10a shows the poorly crystalline nature of the catalyst whereas Fig. 10b, c shows the bright dark suspension, indicates the aggregate formation of the Pd(0) nanoparticles on the surface of the catalyst, as expected. Here, presence of formed aggregates in regenerated catalyst reveals the strong interaction of Pd(0) nanoparticles with 12-Tungstophosphoric acid, which prevent the leaching of Pd(0) from the surface of the catalyst. It is very interesting to note down that that PXRD of the regenerated catalyst (Supplementary Fig. S6) still indicates that the catalyst is amorphous, which may be due to the presence of very less amount of Pd(0) on the surface of the catalyst.
The drastic decrease in BET surface area of regenerated catalyst (104 m2 g−1) is as compare to that of fresh catalyst (204 m2 g−1) indicates that during the reaction Pd(0) nanoparticles undergo aggregate formation. This is an expected and known fact [36]. However, no change in nitrogen adsorption desorption isotherm (Fig. 11) as compare to that of fresh catalyst indicates that surface phenomena remains intact even after the completion of the reaction. This result is in good agreement with TEM analysis.
Thus from the TEM and BET, it can be concluded that during the reaction, Pd(0) nanoparticles undergo aggregate formation without any change in basic structure. Though aggregation, regenerated catalyst shows constant activity up to two cycles confirming the role of TPA as mentioned above.
Under the optimized condition, the scope and limitations of substrates were investigated by using different halobenzenes and phenylboronic acid (Table 5). Coupling of iodobenzene with phenylboronic acid gives higher conversion compared to bromobenzene and chlorobenzene as expected, because iodide is a bulky group and easily get detached, making the reaction more feasible compared to bromide and chloride groups. The found reactivity order was Ph–I > Ph–Br > Ph–Cl. Presence of strongly electron donating group like –OH and –OCH3 are less favorable for the coupling reaction, though in the case of p-bromophenol, high conversion was obtained in both the medium. This may be due to the complete solubility of the substrate in reaction medium at optimized temperature which facilitates the reaction. 100% conversion was obtained in the case of p-methoxyphenylboronic acid. This indicates the conversion of the reaction may be greatly dependent on halobenzene substrate. Substituted bromobenzene with strongly electron withdrawing group such as –NO2 facilitates the reaction, and hence p-bromonitrobenzene gives the 99% conversion in aqueous medium but almost negligible in neat water because of its low solubility. Similarly, p-bromoacetophenone gives the high conversion in aqueous medium due to the presence of moderate electron withdrawing group –COCH3 but shows low conversion in neat water may be due to its low solubility in water.

Catalytic activity of the present catalyst is also compared with reported catalysts in aqueous medium (Table 6) as well as in neat water (Table 7) in terms of iodobenzene as one of the substrate. Li and co-workers [49], Hong and co-workers [50] and Bai and co-workers [51] reported the reaction in aqueous medium with high conversion but the catalyst amount was very high compared to the present work. Mirkhani et al. [52] also reported the reaction in aqueous medium with a low amount of catalyst but prolong time for the completion of the reaction compared to the present catalytic system. Gholinejad et al. [53], Veisi and co-workers [54] and Azadi and co-workers [55] have reported the reaction in aqueous medium with high conversion but they required higher concentration of palladium.
Karimi et al. [56], Dutta and co-workers [57], Siril and co-workers [58], Yang et al. [59] and Dyson et al. [60] have reported the reaction in neat water with high conversion but required more time comparatively for the completion of the reaction. Sabounchei et al. [61] reported the reaction with high conversion and fewer time but used high concentration of Pd. Overall, the present catalytic system is superior to all the reported system in terms of concentration of Pd(0), 0.0096 mol%, is required to run the reaction. Moreover, very high TOF is obtained than all the reported systems.
In the present work, we are also expecting the well-known mechanism, which involves oxidative addition, transmetalation and reductive elimination [62]. During the oxidative addition and transmetalation steps, we could not isolate the Pd(II) species due to the high rate of the reaction. The steric and electronic characteristics of TPA are important for catalysis. In general, the oxidative addition is governed by electronic factors, whereas the transmetalation and reductive elimination processes are controlled by a mixture of both electronic and steric effect [63]. Thus, TPA would achieve a suitable balance between these two factors.
4 Conclusions
Here, we have first time reported very simple method for the synthesis of stabilized Pd(0) nanoparticles using 12-tungstophosphoric acid supported on to zirconia. Studies also reveal that TPA plays an important role to stabilize and prevent the leaching of Pd(0) nanoparticles during the reaction. It is also interesting to note down that very small amount of palladium has great potential to tolerate high amount of substrates under the identical experimental condition for practically large scale biphenyl production with high turnover frequency (TOF in aqueous medium 96,958 h−1 and 46,390 h−1 in neat water). We believe that the proposed synthesis method of the catalyst can be extended to synthesize other precious metal nanoparticles as well as applicability in other C–C coupling reactions in future.
References
Astruc D, Lu F, Aranzaes JR (2005) Nanoparticles as recyclable catalysts: the frontier between homogeneous and heterogeneous catalysis. Angew Chem Int Ed 44(48):7852–7872
Deshmukh RR, Rajagopal R, Srinivasan KV (2001) Ultrasound promoted C–C bond formation: Heck reaction at ambient conditions in room temperature ionic liquids. Chem Commun 17:1544–1545
Hamill NA, Hardacre C, McMath SEJ (2002) In situ XAFS investigation of palladium species present during the Heck reaction in room temperature ionic liquids. Green Chem 4(2):139–142
Reetz MT, Breinbauer R, Wanninger K (1996) Suzuki and Heck reactions catalyzed by preformed palladium clusters and palladiumnickel bimetallic clusters. Tetrahedron Lett 37(26):4499–4502
Li Y, Hong XM, Collard DM, El-Sayed MA (2000) Suzuki cross-coupling reactions catalyzed by palladium nanoparticles in aqueous solution. Org Lett 2(15):2385–2388
Li Y, Boone E, El-Sayed MA (2002) Size effects of PVP–Pd nanoparticles on the catalytic Suzuki reactions in aqueous solution. Langmuir 18(12):4921–4925
Li Y, El-Sayed MA (2001) The effect of stabilizers on the catalytic activity and stability of Pd colloidal nanoparticles in the Suzuki reactions in aqueous solution†. J Phys Chem B 105(37):8938–8943
Moreno-Mañas M, Pleixats R, Villarroya S (2001) Fluorous phase soluble palladium nanoparticles as recoverable catalysts for Suzuki cross-coupling and Heck reactions. Organometallics 20(22):4524–4528
Strimbu L, Liu J, Kaifer AE (2003) Cyclodextrin-capped palladium nanoparticles as catalysts for the Suzuki reaction. Langmuir 19(2):483–485
Pathak S, Greci MT, Kwong RC, Mercado K, Prakash GKS, Olah GA et al (2000) Synthesis and applications of palladium-coated poly(vinylpyridine) nanospheres. Chem Mater 12(7):1985–1989
Rocaboy C, Gladysz JA (2002) Highly active thermomorphic fluorous palladacycle catalyst precursors for the Heck reaction; evidence for a palladium nanoparticle pathway. Org Lett 4(12):1993–1996
Jana NR, Wang ZL, Pal T (2000) Redox catalytic properties of palladium nanoparticles: surfactant and electron donor–acceptor effects. Langmuir 16(6):2457–2463
Özkar S, Finke RG (2016) Palladium(0) nanoparticle formation, stabilization, and mechanistic studies: Pd(acac)2 as a preferred precursor, [Bu4N]2HPO4 stabilizer, plus the stoichiometry, kinetics, and minimal, four-step mechanism of the palladium nanoparticle formation and subsequent agglomeration reactions. Langmuir 32(15):3699–3716
Veisi H, Faraji AR, Hemmati S, Gil A (2015) Green synthesis of palladium nanoparticles using Pistacia atlantica kurdica gum and their catalytic performance in Mizoroki–Heck and Suzuki–Miyaura coupling reactions in aqueous solutions. Appl Organomet Chem 29(8):517–523
Lebaschi S, Hekmati M, Veisi H (2017) Green synthesis of palladium nanoparticles mediated by black tea leaves (Camellia sinensis) extract: catalytic activity in the reduction of 4-nitrophenol and Suzuki-Miyaura coupling reaction under ligand-free conditions. J Colloid Interface Sci 485:223–231
Veisi H, Mirzaee N (2018) Ligand-free Mizoroki–Heck reaction using reusable modified graphene oxide-supported Pd(0) nanoparticles. Appl Organomet Chem 32(2):e4067
Veisi H, Pirhayati M, Kakanejadifard A (2017) Immobilization of palladium nanoparticles on ionic liquid-triethylammonium chloride functionalized magnetic nanoparticles: as a magnetically separable, stable and recyclable catalyst for Suzuki-Miyaura cross-coupling reactions. Tetrahedron Lett 58(45):4269–4276
Farzad E, Veisi H (2018) Fe3O4/SiO2 nanoparticles coated with polydopamine as a novel magnetite reductant and stabilizer sorbent for palladium ions: synthetic application of Fe3O4/SiO2@PDA/Pd for reduction of 4-nitrophenol and Suzuki reactions. J Ind Eng Chem 60:114–124
Veisi H, Najafi S, Hemmati S (2018) Pd(II)/Pd(0) anchored to magnetic nanoparticles (Fe3O4) modified with biguanidine-chitosan polymer as a novel nanocatalyst for Suzuki-Miyaura coupling reactions. Int J Biol Macromol 113:186–194
Veisi H, Pirhayati M, Kakanejadifard A, Mohammadi P, Abdi MR, Gholami J et al (2018) In situ green synthesis of Pd nanoparticles on tannic acid-modified magnetite nanoparticles as a green reductant and stabilizer agent: its application as a recyclable nanocatalyst (Fe3O4@TA/Pd) for reduction of 4-nitrophenol and Suzuki reactions. ChemistrySelect 3(6):1820–1826
Veisi H, Mohammadi Biabri P, Falahi H (2017) l-Arginine as a base and ligand for the palladium-catalyzed C-C and C-N cross-coupling reactions in aqueous media. Tetrahedron Lett 58(35):3482–3486
Veisi H, Nasrabadi NH, Mohammadi P (2016) Biosynthesis of palladium nanoparticles as a heterogeneous and reusable nanocatalyst for reduction of nitroarenes and Suzuki coupling reactions. Appl Organomet Chem 30(11):890–896
Veisi H, Mirshokraie SA, Ahmadian H (2018) Synthesis of biaryls using palladium nanoparticles immobilized on metformine-functionalized polystyrene resin as a reusable and efficient nanocatalyst. Int J Biol Macromol 108:419–425
Heidari F, Hekmati M, Veisi H (2017) Magnetically separable and recyclable Fe3O4@SiO2/isoniazide/Pd nanocatalyst for highly efficient synthesis of biaryls by Suzuki coupling reactions. J Colloid Interface Sci 501:175–184
Kogan V, Aizenshtat Z, Popovitz-Biro R, Neumann R (2002) Carbon–carbon and carbon–nitrogen coupling reactions catalyzed by palladium nanoparticles derived from a palladium substituted keggin-type polyoxometalate. Org Lett 4(20):3529–3532
Zhang J, Keita B, Nadjo L, Mbomekalle IM, Liu T (2008) Self-assembly of polyoxometalate macroanion-capped Pd0 nanoparticles in aqueous solution. Langmuir 24(10):5277–5283
D’Souza L, Noeske M, Richards RM, Kortz U (2013) Palladium (0) metal clusters: novel Krebs type polyoxoanions stabilized, extremely active hydrogenation catalyst. Appl Catal A 453:262–271
Villanneau R, Roucoux A, Beaunier P, Brouri D, Proust A (2014) Simple procedure for vacant POM-stabilized palladium (0) nanoparticles in water: structural and dispersive effects of lacunary polyoxometalates. RSC Adv 4(50):26491–26498
Izumi Y, Tanaka Y, Urabe K (1982) Selective catalytic hydrogenation of, propargyl alcohol with heteropoly acid-modified palladium. Chem Lett 11(5):679–682
Izumi Y, Satoh Y, Kondoh H, Urabe K (1992) Reductive carbonylation of nitrobenzene catalyzed by heteropolyanion-modified palladium. J Mol Catal 72(1):37–46
Liu H, Sun W, Yue B, Jin S, Deng J, Xie G (1997) Preparation and characterization of keggin type polyoxotungstates containing palladium or iridium. Synth React Inorg Met-Org Chem 27(4):551–566
Maksimov GM, Zaikovskii VI, Matveev KI, Likholobov VA (2000) Preparation of polyoxometalate-stabilized colloidal solutions of palladium metal and catalysts supported on them. Kinet Catal 41(6):844–852
Troupis A, Hiskia A, Papaconstantinou E (2002) Synthesis of metal nanoparticles by using polyoxometalates as photocatalysts and stabilizers. Angew Chem Int Ed 41(11):1911–1914
Kogan V, Aizenshtat Z, Neumann R (2002) Preferential catalytic hydrogenation of aromatic compounds versus ketones with a palladium substituted polyoxometalate as pre-catalyst. New J Chem 26(3):272–274
Mandal S, Das A, Srivastava R, Sastry M (2005) Keggin ion mediated synthesis of hydrophobized Pd nanoparticles for multifunctional catalysis. Langmuir 21(6):2408–2413
De bruyn M, Neumann R (2007) Stabilization of palladium nanoparticles by polyoxometalates appended with alkylthiol tethers and their use as binary catalysts for liquid phase aerobic oxydehydrogenation. Adv Synth Catal 349(10):1624–1628
Keita B, Zhang G, Dolbecq A, Mialane P, Sécheresse F, Miserque F et al (2007) MoV–MoVI mixed valence polyoxometalates for facile synthesis of stabilized metal nanoparticles: electrocatalytic oxidation of alcohols. J Phys Chem C 111(23):8145–8148
Sun M, Zhang J, Zhang Q, Wang Y, Wan H (2009) Polyoxometalate-supported Pd nanoparticles as efficient catalysts for the direct synthesis of hydrogen peroxide in the absence of acid or halide promoters. Chem Commun 34:5174–5176
Liu R, Li S, Yu X, Zhang G, Zhang S, Yao J et al (2012) A general green strategy for fabricating metal nanoparticles/polyoxometalate/graphene tri-component nanohybrids: enhanced electrocatalytic properties. J Mater Chem 22(8):3319–3322
Mori K, Furubayashi K, Okada S, Yamashita H (2012) Synthesis of Pd nanoparticles on heteropolyacid-supported silica by a photo-assisted deposition method: an active catalyst for the direct synthesis of hydrogen peroxide. RSC Adv 2(3):1047–1054
Rafiee E, Kahrizi M, Joshaghani M, Ghaderi-Sheikhi Abadi P (2016) New strategy by a two-component heterogeneous catalytic system composed of Pd–PVP–Fe and heteropoly acid as co-catalyst for Suzuki coupling reaction. Res Chem Intermed 42(6):5573–5585
He P, Xu B, Xu X, Song L, Wang X (2016) Surfactant encapsulated palladium-polyoxometalates: controlled assembly and their application as single-atom catalysts. Chem Sci 7(2):1011–1015
Patel S, Purohit N, Patel A (2003) Synthesis, characterization and catalytic activity of new solid acid catalysts, H3PW12O40 supported on to hydrous zirconia. J Mol Catal A 192(1):195–202
Bhatt N, Shah C, Patel A (2007) 12-tungstophosphoric and 12-tungstosilicicacid supported onto hydrous zirconia for liquid phase tert-butylation of m-cresol. Catal Lett 117(3):146–152
Pathan S, Patel A (2012) Heck coupling catalyzed by Pd exchanged supported 12-tunstophosphoric acid—an efficient ligand free, low Pd-loading heterogeneous catalyst. RSC Adv 2(1):116–120
Vogel AI, Jeffery GH (1989) Vogel’s textbook of quantitative chemical analysis. Longman, London
Militello MC, Simko SJ (1994) Elemental palladium by XPS. Surf Sci Spectra 3(4):387–394
Röhlich C, Wirth AS, Köhler K (2012) Suzuki coupling reactions in neat water as the solvent: where in the biphasic reaction mixture do the catalytic reaction steps occur? Chem Eur J 18(48):15485–15494
Guan Z, Hu J, Gu Y, Zhang H, Li G, Li T (2012) PdCl2(py)2 encaged in monodispersed zeolitic hollow spheres: a highly efficient and reusable catalyst for Suzuki–Miyaura cross-coupling reaction in aqueous media. Green Chem 14(7):1964–1970
Zhang L, Su Z, Jiang F, Zhou Y, Xu W, Hong M (2013) Catalytic palladium nanoparticles supported on nanoscale MOFs: a highly active catalyst for Suzuki–Miyaura cross-coupling reaction. Tetrahedron 69(44):9237–9244
Shi Z, Bai X-F (2015) Novel Pd nanocubes supported on activated carbon as a catalyst for the Suzuki-Miyaura coupling reaction. Open Mater Sci J 9:173–177
Isfahani AL, Mohammadpoor-Baltork I, Mirkhani V, Khosropour AR, Moghadam M, Tangestaninejad S et al (2013) Palladium nanoparticles immobilized on nano-silica triazine dendritic polymer (Pdnp-nSTDP): an efficient and reusable catalyst for Suzuki–Miyaura cross-coupling and Heck reactions. Adv Synth Catal 355(5):957–972
Gholinejad M, Razeghi M, Najera C (2015) Magnetic nanoparticles supported oxime palladacycle as a highly efficient and separable catalyst for room temperature Suzuki–Miyaura coupling reaction in aqueous media. RSC Adv 5(61):49568–49576
Abbas Khakiani B, Pourshamsian K, Veisi H (2015) A highly stable and efficient magnetically recoverable and reusable Pd nanocatalyst in aqueous media heterogeneously catalysed Suzuki C–C cross-coupling reactions. Appl Organomet Chem 29(5):259–265
Sarvestani M, Azadi R (2016) Palladium nanoparticles deposited on a graphene–benzimidazole support as an efficient and recyclable catalyst for aqueous-phase Suzuki–Miyaura coupling reaction. Appl Organomet Chem 31(8):e3667
Karimi B, Mansouri F, Vali H (2014) A highly water-dispersible/magnetically separable palladium catalyst based on a Fe3O4@SiO2 anchored TEG-imidazolium ionic liquid for the Suzuki–Miyaura coupling reaction in water. Green Chem 16(5):2587–2596
Borah BJ, Borah SJ, Saikia K, Dutta DK (2014) Efficient Suzuki–Miyaura coupling reaction in water: stabilized Pdo-montmorillonite clay composites catalyzed reaction. Appl Catal A 469:350–356
Dutt S, Kumar R, Siril PF (2015) Green synthesis of a palladium–polyaniline nanocomposite for green Suzuki–Miyaura coupling reactions. RSC Adv 5(43):33786–33791
Yang F, Chi C, Dong S, Wang C, Jia X, Ren L et al (2015) Pd/PdO nanoparticles supported on carbon nanotubes: a highly effective catalyst for promoting Suzuki reaction in water. Catal Today 256:186–192
Ghazali-Esfahani S, Păunescu E, Bagherzadeh M, Fei Z, Laurenczy G, Dyson PJ (2016) A simple catalyst for aqueous phase Suzuki reactions based on palladium nanoparticles immobilized on an ionic polymer. Sci China Chem 59(4):482–486
Sabounchei SJ, Hosseinzadeh M, Panahimehr M, Nematollahi D, Khavasi HR, Khazalpour S (2015) A palladium–phosphine catalytic system as an active and recycable precatalyst for Suzuki coupling in water. Transition Met Chem 40(6):657–663
Miyaura N, Suzuki A (1995) Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem Rev 95(7):2457–2483
Das P, Linert W (2016) Schiff base-derived homogeneous and heterogeneous palladium catalysts for the Suzuki–Miyaura reaction. Coord Chem Rev 311:1–23
Acknowledgements
We are thankful to Department of Atomic Energy (DAE) and Board of Research in Nuclear Science (BRNS), Project No. 37(2)/14/34/2014-BRNS, Mumbai, for the financial support. One of the authors Mr. Anish Patel is thankful to the same for the grant of JRF. We are also thankful to Department of Chemistry, The Maharaja Sayajirao University of Baroda for BET surface area analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Patel, A., Patel, A. Stabilized Palladium Nanoparticles: Synthesis, Multi-spectroscopic Characterization and Application for Suzuki–Miyaura Reaction. Catal Lett 148, 3534–3547 (2018). https://doi.org/10.1007/s10562-018-2559-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-018-2559-1