Abstract
Oxygen pressure plays an integral role in regulating various aspects of cellular biology. Cell metabolism, proliferation, morphology, senescence, metastasis, and angiogenesis are some instances that are affected by different tensions of oxygen. Hyperoxia or high oxygen concentration, enforces the production of reactive oxygen species (ROS) that disturbs physiological homeostasis, and consequently, in the absence of antioxidants, cells and tissues are directed to an undesired fate. On the other side, hypoxia or low oxygen concentration, impacts cell metabolism and fate strongly through inducing changes in the expression level of specific genes. Thus, understanding the precise mechanism and the extent of the implication of oxygen tension and ROS in biological events is crucial to maintaining the desired cell and tissue function for application in regenerative medicine strategies. Herein, a comprehensive literature review has been performed to find out the impacts of oxygen tensions on the various behaviors of cells or tissues.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oxygen homeostasis is a pivotal factor in preserving the physiological survival of vertebrate species (Semenza 2009a, b). The atmospheric oxygen is estimated about 21.1% (160 mmHg), which is decreased to about 19% (150 mmHg) in the upper airways. However, it has been demonstrated that the oxygen levels could vary between 0 and 19% in non-pathological tissues in a normal state (Carreau et al. 2011). Hyperoxia leads to the production of reactive oxygen species (ROS), including superoxides, hydroxyl radicals, and hydrogen peroxide groups. These chemical groups can cause cellular damage through lipid peroxidation, enzyme destruction and protein/nucleic acid oxidation, leading to apoptosis or necrosis (Jamieson et al. 1986). ROS production causes higher levels of injury in unhealthy cell types; however, ROS toxic effects are needed to suppress the proliferation of cancer cells in tumor tissues (Fang et al. 2009). The mechanism by which ROS enforces cell abnormalities and even death is not clear. Normal cells can neutralize the destructive impacts of ROS by cellular anti-oxidative reactions. For instance, hemeoxygenase-1 (HO-1) catalyzes the breakdown reaction of heme to bilirubin and superoxide dismutase (SOD) converts superoxide ion to hydrogen peroxide. However, anti-cancer drugs target tumor ablation through ROS synthesis. In a study, the effect of ROS production and accumulation was examined in a lung cancer cell line. The results confirmed that the high levels of developed H2O2 cause DNA damage, ATP deficiency, and acute cytotoxicity. In contrast, the low levels of exogenously produced H2O2 resulted in a moderate level of ATP concentration, late toxicity, G2/M cell cycle arrest and cell senescence. The H2O2 produced by mitochondria causes a decline in ATP production, although this reduction is reversible without any negative impact on cell growth except cell senescence due to the cell cycle arrest in G1/S phase. Simultaneously, the glycolysis pathway was inhibited in cells, and subsequently, ATP restoration was inhibited (Panieri et al. 2013). On the other hand, hypoxia has been defined as a state without sufficient oxygen for cellular metabolic reactions. However, oxygen levels are determined in accordance with the metabolic requirements of the cell (Semenza 2000). Chronic wounds remain a global health challenge and hypoxia is one of main causes of delayed wound closure in diabetic wounds. To this end, a variety of oxygen therapeutics, including hyperbaric oxygen therapy, topical pressurized oxygen therapy, topical continuous oxygen therapy, and/or oxygen diffusion enhancer, have been successfully developed over the last decade in order to alleviate the hypoxia for an improved wound healing. In a recent study, a hybrid hydrogen that supply oxygen and provide antibacterial activity through promoting cell proliferation improved healing in diabetic wounds (Lin et al. 2022; Rasouli et al. 2023a, b, c). Hypoxia causes cellular stress through epigenetic-related mechanisms that are regulated by hypoxia induced factors (HIFs). HIFs mainly control oxygen homeostasis in cells by regulating the genes involved in oxygen metabolism, erythrocyte production, angiogenesis, and mitochondrial metabolism (Choudhry and Harris 2018; Al Tameemi et al. 2019). As a whole, oxygen levels could alter different aspects of cell biology through specific mechanisms. Accordingly, achieving an in-depth understanding in this regard will pave the way toward improving tissue regeneration and promoting tissue engineering strategies (Rasouli et al. 2021). In this review, we will concisely discuss the effect of oxygen tension on cell fate and tissue function/regeneration in terms of cell proliferation, angiogenesis, cellular senescence, and cell death.
Oxygen tension and cellular growth and morphology
In general, molecular oxygen is a metabolic and signaling compound for cells, both in vitro and in vivo. One of the most important effects of oxygen tension on cell biology is related to cell proliferation. In normal cell populations, following hypoxia, cell proliferation is weakened since the higher cell population is accompanied by more O2 demand. However, specific cell types sustain their growth in hypoxic conditions. Cancerous cells adapt to hypoxic stress and continue cell growth by decreasing their O2 consumption. However, another important issue is the high levels of non-physiological oxygen that lower cell viability. Therefore, excess oxygen can be toxic to cells because of the increasing influence of ROS (Cipolleschi et al. 1993). Hypoxia induces stem cell proliferation and inhibits their differentiation via stimulation of the Notch signaling pathway (Hubbi and Semenza 2015). For instance, in a study, human embryonic stem cells (hESC) were cultured in both physiological (2–6.5%) and atmospheric (21%) oxygen conditions to evaluate the potential variations in the impacts of different oxygen concentrations on colony formation and genetic stability. It turned out that physiological oxygen conditions better supported clone recovery than atmospheric oxygen conditions, in which the mean increase was six fold. Furthermore, karyotype abnormalities declined in the cultured hESCs under physiological oxygen conditions compared to the cultured hESCs under atmospheric oxygen conditions, which suggests the likelihood of genomic stability during the prolonged in vitro hESCs expansion in physiological oxygen culture conditions (Forsyth et al. 2006).
In certain types of adult stem cells, low oxygen concentrations in vitro cause the multiplication and maintenance of a multi-potency state (Csete 2005; Grayson et al. 2007; Samal et al. 2021). Some researchers believe that hypoxia is a strong stimulus for the differentiation of specific cell lines. For example, hypoxia is a strong stimulus for chondrogenesis by stem cells (Rehman et al. 2004; Sadat et al. 2007; Thangarajah et al. 2009). In most tissue cultures, the oxygen level is approximately 20% in vitro, where cells have much lower oxygen levels. The mean arterial blood oxygen concentration is approximately 12% and about 3% in tissues (Csete 2005). Therefore, optimizing and understanding stem cell growth and function has an important relationship with their condition in the microenvironment of in vivo. Embryonic stem cells live in a low oxygen environment at the time of implantation in the uterus due to the lack of access to maternal circulation. Uterine oxygen levels are 2% in early pregnancy, which increases to only 8% after fetal-maternal blood contact. This relative hypoxia is the natural physiological environment of embryonic stem cells (Genbacev et al. 1997; Cowden Dahl et al. 2005). Similarly, adult stem cells live in conditions of oxygen poverty in the body, such as the environment in which bone marrow-mesenchymal stem cells (BM-MSC) reside (Ma et al. 2009). It was shown, some cells are persistently in hypoxic states between 1 and 2% (Kofoed et al. 1985; Harrison et al. 2002). MSCs are one of the most common candidate cells in clinical trials due to their therapeutic capabilities and ability to be transplanted allogeneically (Rasouli et al. 2022; Shirbaghaee et al. 2023).
Hypoxia can alter the properties of cytoskeleton proteins, resulting in morphological changes in targeted cells (Glass et al. 2015). For example, the phenotype of differentiated chondrocytes could be restored after applying oxygen tension, as shown in vivo. When the encapsulated chondrocytes in alginate were exposed to 20% oxygen, the morphology changed to fibroblastic form, and the expression of specific genes related to human articular chondrocytes like collagen II, aggrecan and sox9 genes were lost in contrast to the cells cultured in 5% oxygen tension. Also, the expression of matrix glycosaminoglycan (GAG) was enhanced statistically significant in physiological oxygen level (5%) compared to the culture condition of 20% oxygen tension (Murphy and Polak 2004).
It seems cell behavior changes in accordance with the with the cell type employed. Regarding this, the adipose-derived MSCs lost their differentiation potential both into chondrocytes and osteocytes after culturing in condition with a lower oxygen level of 2% compared to higher levels of 21% (Malladi et al. 2006). The stem cells derived from rat CNS (Studer et al. 2000), rat neural crest (Chen et al. 2017), murine skeletal muscle (Sharkey 2020), rat bone marrow (Kim et al. 2016), human CD34 + bone marrow (Guitart et al. 2011) and human cord blood (Peng et al. 2016) showed higher proliferation rate when cultured at lower oxygen pressure (2% vs 20%). The addition of antioxidants enhances the self-renewal of hematopoietic cells via the activation of the ataxia telangiectasia mutated (ATM) gene. On the contrary, in the presence of ROS, the self-renewal of hematopoietic progenitor cells was reduced (Ghaffari 2008). The effect of oxygen tension on cell fate is much more complicated. It seems any oxygen response mechanism in case of inducing the differentiation into a lineage suppresses differentiation into other lineages. The oxygen pressure lower than below 20% stimulates myogenesis more than adipogenesis in skeletal muscle stem cells and MSC lines. In the high-oxygen condition the peroxisome proliferator-activated receptor (PPAR)-gamma, a main regulator of adipogenesis, is upregulated, thereby suppressing the activation of the MyoD promoter (Villarroya et al. 2007). In contrast, in the hypoxic condition, the satellite cells undergo an increase in the expression level of multiple myogenic basic-loop-helix genes (Wang et al. 2015). In addition, HIF-1α, an activated factor following diminished oxygenation, inhibits adipogenesis via DEC1/Stra13-mediated repression of PPAR-gamma under hypoxic conditions (Yun et al. 2002). In a study, an innovative channeled scaffold and culture medium containing perfluorocarbon (PFC) were employed to mimic the oxygen carrier function of hemoglobin and capillary network architecture, respectively. Scaffolds are 3D structures that support cell adhesion and proliferation and are commonly used for tissue regeneration purposes (Fattahi et al. 2023; Rasouli et al. 2023a, b, c). Compared to the control group, the oxygen pressure was better maintained, whereas the decline in the control group was twofold. Accordingly, the DNA content, cardiac markers (troponin I, connexin-43) and contractile properties were improved in comparison to the control group. Eventually, the biomimetic approach served as an important strategy to replenish oxygen and increase cardiac differentiation (Radisic et al. 2006). Tissue/organ damage leads to changes in the local microenvironment, including reduced oxygen concentration and degradation of the extracellular matrix (ECM) (Rasouli et al. 2021). The response of surrounding stem cells to these changes is of particular importance. Culture under low-oxygen conditions increased cell proliferation and migration by activating the ERK signaling pathway and related integrins. ECM degradation materials increase ROS levels in cells through mitogenic and chemotactic responses. Under low oxygen conditions, high cell growth rates in progenitor cells were observed without any changes in morphology, differentiation, DNA synthesis process or metabolic activity (Tottey et al. 2011). There is a report that approving the optimum physiological oxygen (5%) inhibits karyotypic abnormalities in cardiac and embryonic stem cells. Aneuploidy occurs following the addition of high doses of antioxidants. Antioxidants also reduce the cellular amount of DNA repair enzymes by removing all intracellular ROS. Therefore, ROS has a physiological value that activates DNA repair pathways and maintains the stem cell genome's stability (Li and Marbán 2010). Since MSCs following implantation are sensitive to microenvironmental stresses such as low oxygen and limited nutrition, In a study, hypoxia preconditioning and/or transforming growth factor-β3 (TGF-β3) were investigated to enhance performance upon their in vivo implantation for cartilage and disc regeneration. Increased MSC survival and extracellular matrix production were observed in a low-oxygen and nutrient-limited environment. The results showed that hypoxic preconditioning led to the upregulation of genes related to growth, cell signaling, metabolism, and cellular stress response pathways and significantly increased the survival of MSCs. These results strongly support the use of hypoxic preconditioning to improve MSC survival after implantation in avascular tissues such as cartilage (Peck et al. 2021). The hypoxia-cultured human MSCs either young and old ones perform better than cultured cells under normoxia. Hypoxia improves MSC's capacity for self-renewal and multipotent differentiation. The underlying mechanisms are Inhibition of miR-627, miR-193a, miR-196a, miR-148b expression and up-regulation of mir-7977 and mir-195 expression (Mohd Ali et al. 2016; Rasouli et al. 2023a, b, c).
Reactive oxygen species (ROS) act to regulate various signaling pathways in cell physiology. Previous studies have shown that hydrogen peroxide (H2O2), a non-radical ROS, is required for cytokines, growth factors, insulin, AP-1 and NF-KB signaling (Finkel 1998). H2O2 can also inactivate phosphatase by oxidizing cysteine, which confirms that ROS can interfere with the signaling pathway (Rhee et al. 2000). The physiological flux of H2O2 results in reversible oxidation to a sustained state of specific protein targets. As a result, protein activity changes localization and interactions. On the other hand, it helps coordinate processes such as cell reproduction, differentiation, migration and angiogenesis (Jones and Sies 2015; Siegrist and Sies 2017). The low-level H2O2 maintenance status and the associated physiological redesign signals are called “oxidative stress” (Sies 2017). The total cellular concentration of radical anions is much lower than that of H2O2. Molecular redox initiation in signaling is one of the physiological targets of oxidants that respond to external stress by regulating cells at different levels. In addition to physiological H2O2 levels, which are important for signal transmission, high physiological H2O2 concentrations (approximately over 100 nM) also lead to non-specific protein oxidation, altered reaction patterns, and reversible and irreversible damage to protein biomolecules. Finally, cell death is accompanied by pathological complications (oxidative distress) (Sies and Jones 2020). Most superoxide is generated by the partial reduction of oxygen in the electron transfer chain. In addition, they are formed at different rates in a cell, and their activity is different. In terms of activity, the hydroxyl radical is the most reactive species known and is mainly responsible for the cytotoxic effects of ROS (Bolisetty and Jaimes 2013). Most of the superoxide produced is found either in the matrix or in the inner membrane of the mitochondria facing the matrix. Although hydrogen peroxide is more stable than superoxide, it can be released from the mitochondria into the cytosol through voltage-gated anion channels, thereby reducing the deleterious effects of these reactive species on the mitochondria (MacMillan-Crow et al. 1998; Lacza et al. 2003). Besides mitochondrial metabolism, ROS also produce cellular enzymes known as nicotinamide adenine dinucleotide phosphate oxidases (NADPH) (Lassègue et al. 2012). Other cellular sources of ROS include neutrophils, monocytes, cardiomyocytes, endothelial cells, xanthine oxidases, cytochrome P450, lipoxygenases, and nitric oxide synthase (Izyumov et al. 2010; Cubero and Nieto 2012). Another physiological factor of ROS is aging caused by ROS toxicity through a destructive cycle. Damage from mitochondrial ROS is greater. It was also shown that mitochondrial ROS production is higher in mitochondria isolated from older animals (Sohal and Sohal 1991; Balaban et al. 2005). On the other hand, protein, lipid, and DNA levels in oxidative diseases are strongly associated with aging (Bardaweel et al. 2018). Therefore, a negative relationship between mitochondrial ROS production and longevity can be understood in different organisms (Lambert et al. 2007). In the chronic inflammatory state (Dröge 2002), excess ROS inhibits mitophagy and activates a specific type of autophagy used to eliminate dysfunctional mitochondria (Salminen et al. 2012). Thus, increased levels of ROS are produced, which activate more inflammation (Zhou et al. 2011).However, high concentrations of generated ROS are involved in other biological processes.
It is obvious that the low tension of oxygen forms ROS and finally causes chromosomal instabilities, and the physiological signals try to eliminate the harm. In this manner, VEGF and erythropoietin are increased after hypoxic conditions over injuries and lead to the migration of bone marrow stem cells into the blood circulation and their mobilization into the site (Greenwald et al. 2019). Furthermore, these two factors play a role in the tumor core following hypoxia, where they promote cancer stem cell survival and radiation resistance (Lugano et al. 2020). Moreover, in the ischemic tissues, the stromal cell-derived factor (SDF-1) is expressed, and thereby this factor stimulates the migration of stem cells into the injury sites (Miller et al. 2005). Although, the corresponding factor acts as a metastatic agent, such as other oxygen-regulated factors, matrix metalloproteases and hepatocyte growth factor (Bragg et al. 2019).
When the hypoxic condition is applied to MSCs in an in vivo model, the apoptosis happens through the down-regulation of pro-survival genes like Akt, which causes cell death. In contrast, in in vitro studies, the proliferation rate of the corresponding cells is enhanced and their differentiation into various lineages is started. The lower oxygen tension induces the paracrine activity of MSCs and increases the expression of angiogenic factors such as VEGF and interleukin-6 (IL-6). Also, hypoxia causes the migration and homing of MSCs via the stimulating expression of stromal cell–derived factor-1 (SDF-1) and its receptor, CXCR4 (Das et al. 2010).
The effects of hypoxia on cell morphology lead to different results that could be beneficial or harmful to cell functionality. Yuan et al. illustrated that after culturing the human HCC cell line HepG2 in 1% O2 for 72 h, the cells morphology changed to a spindle shape and their cellular junctions were destroyed (Yuan et al. 2012). Also, neuronal IMR-32 cells rounding and detachment from the growth surface were observed by Huang et al. under hypoxia (Huang et al. 2013). The same result was achieved by Song et al. In this evaluation, three breast cell lines (MDA-MB-231, MCF-7 and MCF-10A) were stored in a hypoxic state for 72 h and their morphology was compared with that of control cells in a normal condition. The cell–cell junctions, form, and shape of cells under normoxia (21% oxygen) were normal, but the cells were flattened and lost their tight cell–cell junctions and looked like fibroblasts. Indeed, the cell phenotype had changed under hypoxia stress (Song et al. 2018). Furuta et al. incubated Lewis lung carcinoma (LLC) cells in hypoxia. They observed that the cell morphology was altered to a spindle-shape under hypoxia (Furuta et al. 2015). However, a reverse report was published by Ahmed et al. Their findings revealed that the dental pulp stem cells (DPSCs) cultured under normoxia condition had a larger and more flattened morphology than under hypoxia. Also, the cells exhibited larger nuclei and were hardly detached from surface culture by trypsinization under hypoxia than the cells cultured under normoxia condition (Ahmed et al. 2016). Peixoto et al. showed that under hypoxia, bladder cancer cell lines (T24) had a morphology with a more elongated semblance and larger intercellular spaces (Peixoto et al. 2016). Moreover, Lim et al. cultured human embryonic stem cells under hypoxia and examined the effects of O2 tension on embryoid body (EB) formation and their morphology. Significant cell death was observed, and also, the smaller EBs were formed under hypoxia than those grown under normoxia (300 ± 50 µm vs. 500 ± 100 µm, respectively) (Lim et al. 2011). On the other hand, the content of extracellular vesicles (EV) obtained from MSCs can be adjusted under different culture conditions. These cells respond to exposure to hypoxia by activating hypoxia-inducible factor (HIF) at low O2 concentrations. HIF has direct and indirect pleiotropic effects and modulates the expression of hundreds of genes involved in processes such as inflammation, migration, proliferation, differentiation, angiogenesis, metabolism, and cell apoptosis. Several studies have shown that MSC-derived EVs subjected to hypoxia have a greater regenerative capacity than those obtained under normoxia (Pulido-Escribano et al. 2022). Morphological studies of 3 cell types have been presented in Fig. 1. Since hypoxia increases the proliferation of BM-MSCs, in a study, the proliferation of BM-MSCs along with zinc was investigated. All tissues, fluids, and organs of the human body contain zinc. Zinc is essential for cell proliferation and differentiation, especially for the regulation of DNA synthesis and mitosis. It significantly increases the proliferation of BM-MSC and reduces the doubling time of the cell population in hypoxic and normoxic environments compared to control cells. MSC migration to the injury site was increased, and HIF1-α expression significantly decreased in hypoxic conditions compared to untreated and control hypoxic cells. Zn-preconditioned mesenchymal cells maintained their spindle-shaped and fibroblast-like morphology at high passage (Rizvi et al. 2022).
Morphological differences of cell cultured under normoxia and hypoxia by light microscopy. A H9C2 cells under hypoxia condition; B H9C2 cells under normoxia condition (Fan et al. 2018); C OCUM-2MD3 (Gastric cells) under normoxia condition; D OCUM-2MD3 (Gastric cells) under hypoxia condition (Matsuoka et al. 2013); E Renal Carcinoma Cell Lines in normoxia condition; F Renal Carcinoma Cell Lines in hypoxia condition (Zhang et al. 2017)
Oxygen and inflammatory pathways
Inflammatory mechanisms are complex biological processes by which tissues respond to combat injurious stimuli and defend the host. This event can be associated with tissue remodeling and metabolic changes, in order to maintain cell homeostasis (Medzhitov 2008). The inflammatory reactions involve multiple specific cell activities that could cause different processes, such as the activation of immune cells (leukocytes, granulocytes, monocytes, lymphocytes, and dendritic cells) and the stimulation of the different bioactive mediators’ production (such as cytokines, chemokines, or prostanoids). Also, this complex biological process leads to epigenetic regulation of the important related genes expression, such as nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB/AP1) activity or IL-6 expression (Martínez et al. 2012; Nayadu et al. 2012). The NF-κB family contains members of transcription factors that regulate inflammation, coordinate immune responses and tissue homeostasis (Vallabhapurapu and Karin 2009). The hypoxia of intestinal ischemia reperfusion causes the activation of NF-κB in intestinal epithelial cells, which in turn leads to the higher production of tumor necrosis factor α (TNF-α); a pro-inflammatory cytokine, but simultaneously reduces intestinal epithelial apoptosis (Chen et al. 2003). Accordingly, the increased TNF-α production strongly inhibits skeletal (Chen et al. 2008) and cardiac (Suematsu et al. 2003) muscle differentiation by decreasing myocyte enhancer factor 2C (MEF2C) (Csete 2005). Another point worth noting is that hypoxia enhances the NF-κB pathway through the increased expression and signaling of Toll-like receptors (TLRs) (Kuhlicke et al. 2007). The TLRs family, as a class of pathogen-recognition receptors, provides antimicrobial factors and stimulates phagocytosis in the first line of defense against pulmonary infection. The function is carried out via the recognition of unique microbial structures and the initiation of inflammatory and adaptive immune responses (Bals and Hiemstra 2004). Macrophages are homed to inflammation site after the activation step by damaged tissue, and they increase the levels of cytokines, chemokines, and other molecules in the blood circulation. These macrophages are called polarized type, having M1 or M2 phenotype. The M1 macrophage after inhibition of arginine metabolism, and the M2 macrophage, under inhibitory induction of cytokines, produce ROS and inflammatory cytokines (Brown et al. 2012).
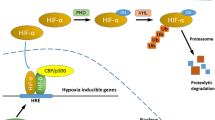
The HIFs are another important factors in hypoxic situations. HIFs are a family of transcription factors whose role is response regulation to hypoxic stimuli. Over 100 target genes whose expression are involved in a broad range of physiological functions, including metabolism, autophagy, and other physiological responses to hypoxia, can be regulated by the HIF signaling pathway (Semenza 2009a, b). Members of this κB (NF-κB) family interact with the members of PHD–HIF pathway associated with inflammation (Taylor 2008). The HIF gene expression in hypoxia and NF-κB in inflammation conditions have been shown to have several common target genes, common regulators, and specifically, common stimuli (Bandarra and Rocha 2013; Nath et al. 2022; Dvornikova et al. 2023). NF-κB activation has been shown to stabilize HIF-1α activation in hypoxia and HIF-1β expression in inflammation (Van Uden et al. 2008). On the other hand, HIF-1α factor has been shown to inhibit NF-κB expression in vivo and in vitro under inflammatory conditions (Bandarra et al. 2015, Dvornikova et al. 2023). Cellular adaptation to hypoxia is based on the transcription factor HIF-1α, which is inactive under normoxic conditions. In mammals, HIF-1α plays an important role in the cellular response to systemic oxygen levels by influencing metabolic and inflammatory pathways (Di Girolamo et al. 2022). It has been confirmed that the concentration of environmental oxygen levels and inflammation as a physiological process are closely related. Inflammation is often accompanied by hypoxia and on the other hand, hypoxia itself can cause inflammation (Corcoran and O’Neill 2016). In spite of the important role of ROS in pathogen killing, it could increase vascular permeability through endothelial cell damage (Tiidus 1998). In one investigation, the adhesion of eosinophils to nerve cells and the release of their products were investigated due to the plausible contribution of eosinophil products in developing some inflammatory diseases. Eosinophils caused neurite contraction in IMR32 neuroblastoma cells, which resulted in tyrosine phosphorylation of some neuronal cell proteins, activation of p38 MAP kinase, and ROS production. If eosinophil adhesion is suppressed, the reaction will stop. However, attenuation of p38 MAP kinase could not stop ROS production despite inhibition of neurite contraction. Thus, there may be two distinct pathways that work separately: by producing tyrosine kinase-dependent MAP kinase and by p38 MAP kinase. Therefore, the results show that the consequences of eosinophil adhesion and degranulation of neurons, change the morphology of neurons with the release of a number of proteins (Kingham et al. 2003). ROSs are produced by cells engaging in host-defense responses. These cells, called polymorphonuclear neutrophils (PMNs), promote endothelial dysfunction by oxidizing crucial cellular signaling proteins. ROS acts as a signaling molecule and also as an inflammation mediator. Thus, the production of ROS is a central occurrence in the progression of many inflammatory diseases (Salvemini et al. 2003). Moreover, recent studies indicated that TLRs expression participates in the response to oxidant stress and that ROS is correlated with the inflammatory signaling pathway of TLR4 in neutrophils (Jiang et al. 2005) (Fig. 2).
Oxygen and inflammation pathways. Hypoxic conditions lead to increased expression and signaling of Toll-like receptors (TLRs), and thereby NF-κB pathway activation. An increased NF-κB signaling pathway is associated with higher levels inflammatory cytokines, including TNF-α, and simultaneously decreased cell apoptosis. Furthermore, expression and activation of both HIF-1α HIF-1β increased as a result of NF-κB activation. However, following HIF-1α activation the NF-κB pathway is suppressed. It is worth noting, TLRs not only activate NF-κB, but also drive ROS production. Subsequently, generated ROS plays a role in promoting inflammation and rising vascular permeability through endothelial cell damage
In some patients, it had been shown that their inflamed tissues had a lower oxygen value than normal. There are several diseases that result from the de-regulation of hypoxia and inflammation pathways such as rheumatoid arthritis (RA), inflammatory bowel disease, and colorectal cancer (CRC) (Taylor 2008; Näthke and Rocha 2011). Recently, a few scientific studies have been focused on attempting to better understand how these pathways are regulated and respond to in diseases. In the gastrointestinal (GI) tract, the mice with the experimental models associated with inflammatory bowel disease (IBD) confirmed that more inflammation was accompanied by lower oxygen levels in their colonic tissues (Karhausen 2004). The result correlates with the pathology that was investigated in IBD patients (Taylor and Colgan 2007). Inspecting the role of hypoxia in epithelial cells and immune cell responses could open a new field for the development of new therapeutics for treating IBD (Lewis et al. 1999). The fact that hypoxia can induce inflammation has attained general acceptance from studies related to hypoxia signaling pathways. In people with mountain sickness, for example, the levels of circulating pro-inflammatory cytokines would be increased subsequently (Grocott et al. 2009). By the examination of all inflammation markers, it was shown that the serum levels of interleukin-6, the interleukin-6 receptor, and C-reactive were increased in healthy volunteers who spent three nights at elevations higher than 3400 m (Hartmann et al. 2000). Furthermore, the increase of inflammatory cells in multiple organs and greater serum levels of cytokines were approved in mice after short-term exposure to low oxygen concentrations (Rosenberger et al. 2009). In various studies, it has been reported that higher oxygen concentrations can regulate the mRNA expression of several genes related to protein secretion. For example, after 5 h of HBOT (90 min at 97.5% O2 at 2.4 ATA), 19 genes involved in adhesion, angiogenesis, inflammation, and oxidative stress were downregulated (Kendall et al. 2012). While, T cells showed different relationships with oxygen when they regulated their function in response to environmental oxygen levels (Ruigrok et al. 2019). In addition, Mitchel et al. showed that necrosis can lead to acute inflammation, and among pro-inflammatory cytokines, the mRNA expression of IL-6 and TNF-α were elevated upon exposure to 80% O2 in 48 h, although they decreased after 96 h (Ruigrok et al. 2019). In an In vivo study of Wang C. et al. the higher expression of IL-1β mRNA after 24 h confirmed by the examination of the blood samples from individuals under hypobaric hypoxia (Wang et al. 2018). At the end, the molecular mechanisms of the interactions between hypoxia and inflammation are intertwined at cellular and clinical levels. Therefore, oxygen-sensing mechanisms and hypoxia signaling pathways could be potential therapeutic targets for the treatment of inflammatory diseases. Moreover, this evaluation could be tested in patients with acute lung injury, myocardial ischemia, inflammatory bowel disease, or cancer. There are studies focused on the impact of oxygen levels on the expression of inflammatory genes, as summarized in Table 1.
Oxygen and cancer/cell senescence
There are two types of cell death, necrosis and apoptosis, that are influenced by different factors. Oxygen depletion is one of the main factors involved in necrosis, but apoptosis is a form of programmed cell death (Elmore 2007). The cells that get necrotic, can be identified with these morphological features. The appearance of flocculent densities in mitochondria was detected with the progressive swelling and degeneration of cellular components. The Madin-Derby canine kidney (MDCK) cell line was cultured in hypoxia and normoxia conditions by Allen et al. (Allen et al. 1992). They showed that the cell death pathway could change from apoptosis to necrosis by adding hypoxia stress to renal tubule structures. Although, this phenomenon has already been proven by Sheridan et al. for human melanoma cells in semi-solid agar medium (Sheridan et al. 1984). It was recently shown that hypoxia can modulate the anti-proliferative response in tumor cells (oncogene-induced senescence). Intracellular levels of ROS increases following hypoxia. ROS acts as a double-edged sword in cancer cells by altering two signaling pathways, such as ras-raf-mek1/2-ERK1/2 and the P38 mitogen-activated protein kinases (MAPK) pathways, that have opposing roles in tumorogenesis. It was concluded that ROS may not be an absolute tumor suppressor or activator factor (Eren 2023).
Risk factors such as cancer, stress, tobacco, environmental pollutants, radiation, viral infection, diet, and bacterial infection produce ROS molecules after their interactions with cells (Zhang et al. 2016). ROS molecules act as a double-edged sword in cancer cells (Schumacker 2006) depending on ROS dosage and type. The site of ROS production can either lead to apoptosis or increase tumorigenesis (Raza et al. 2017; Kumari et al. 2018). The destruction of cancer cells occurs at a high ROS level (Nishikawa 2008), while at a modest level of ROS, cancer cells can survive (Kong et al. 2000). ROS-dependent apoptosis in cancer cells, both in vitro and in vivo, has been shown in many studies in the past years (Trachootham et al. 2006). Both extrinsic and intrinsic pathways of apoptosis are activated through ROS (Ozben 2007). In the intrinsic pathway, apoptosis occurs through the opening of mitochondrial permeability transfer pores and the release of cytochrome C into the cytosol. These events cause the formation of an apoptosome, caspase activation, and the decomposition of intracellular proteins, and finally, cell death occurs (Martindale and Holbrook 2002; Redza-Dutordoir and Averill-Bates 2016). Extrinsic pathways are composed of external stimuli, ligand molecules, and death receptors. In this pathway, extracellular ligands such as Fas-L, TNF, and TNF-related apoptosis-inducing ligand (TRAIL) are attached to death receptors, and a death-inducing signaling complex (DISC) is developed. Then, apoptotic signaling happens and facilitates cell death (Uchikura et al. 2004; Jin et al. 2005). On the other hand, for the role of ROS tumorigenesis, ROS can target transcription factors, and among the various transcription factors that are activated by ROS, HIF-1 α, NF-κB and STAT3 could be introduced. Transcription factors activated by ROS are capable of enhancing the activation of the antioxidant defense system and promoting the expression of cell survival proteins and the genes involved in inflammation, cell transformation, tumor cell death or survival, proliferation, invasion, angiogenesis, and tumor migration (Dewhirst et al. 2008; Gupta et al. 2012). It was shown that the antioxidant compatibility of tumor cells is very limited. Increased activity of antioxidants such as SOD2/MnSOD or inactivation of inhibitory enzymes, including PRX1, are both seen in the development and progression of cancer (Janssen et al. 1999). The summarized pathways of ROS impacts are indicated in Fig. 3.
ROS impacts on cancer and senescence. A ROS, activates the transcription factors HIF-1 α, NF-kB and STAT3. DNA and mitochondrial damage activate the antioxidant defense system and lead to cell death and senescence. B ROS, in response to death-inducing ligands (TNFα and Fas), increases the activation of effective caspases, decreases Bcl-2 activity, or stimulates intracytoplasmic cytochrome release. Cytochrome c interacts with Apaf-1 to form the apoptosis. ROS can also increase p53 expression, which increases ROS levels through an intracellular mechanism. Eventually leads to cell death
Singlet oxygen is one of the most famous non-radical factors, but ROS compounds are active, and their levels decrease after the addition of antioxidants. A balance between the levels of antioxidants and ROS is required to suppress cell damage ranging from signal transduction and gene expression to cell transformation and necrosis/death. Thus, a lower or higher amount of oxygen could, respectively, lead to hypoxia or oxidative stress (Matés and Sánchez-Jiménez 2000). Tumor cells adapt to hypoxia through glucose metabolism, which contributes to an invasive phenotype. Glycolysis plays a role in redox homeostasis in cancer through the production of reducing agents such as NADPH and glutathione (GSH). Also, studies have shown that tumor cells adapt by increasing glycolysis to prevent H2O2-induced cell death (Harris 2002). On the other hand, depending on the type and concentration of ROS they impose complex effects on cells. A slight increase in reactive species causes different cell signaling cascades that enable cell growth and survival (Bäumer et al. 2008). If ROS levels are decreased using antioxidants in tumor tissue, it would induce senescence, although cancer stem cells still remain. The incidence of antioxidant defense signaling in the following could occur and reactivate cancer cells (Achuthan et al. 2011). Moreover, ROS could not only be a reason for cell death but also activate a signaling pathway for cell survival. Hypoxia or oxidative stress induces ROS generation, and autophagy in cancer may lead to a healthy state (Azad et al. 2009). However, it is worth mentioning that hypoxia can participate in tumor progression through alternations in the gene and protein expression of the ECM, and these proteins play a main role in angiogenesis. Hielscher et al. showed that the fibrous structure of ECM in the co-cultures of neonatal fibroblasts (NuFF) with MDAMB-231 breast cancer cells after incubation in hypoxia, converted to more compact and aligned fibers with a larger diameter compared to the fibers formed in the atmospheric oxygen condition. This architecture regulated the expression of angiogenic factors and matrix metalloproteinases (Hielscher et al. 2013).
Singlet oxygen is a toxic molecule and can be implicated in many human diseases. It is derived directly from oxygen gas and relates extremely well to the oxygen level of an in vitro or in vivo environment. Also, when antioxidants such as NAC are used to inhibit HIV-virus replication, oxidative stress starts and disrupts the balance of cellular protein kinase/phosphatase (Waris and Ahsan 2006). Intracellular ROS is produced after higher expression of p53 and induces senescence or apoptosis in normal and cancer cells. While, exogenous ROS in combination with p53's physiological level could change senescence into apoptosis (Macip et al. 2003). There are differences between the reactions of human and mouse cells to oxygen pressures. Human cells showed a senescence-associated secretory phenotype (SASP) for the secretion of cytokines. Both senescent mouse and human fibroblasts were cultured in physiological (3%) and normal cell culture conditions (20%) of oxygen tension. Mouse cells did not expose SASP like human cells. When the mouse cells were incubated in 3% oxygen pressure and induced by radiation, SASP appeared and the synthesis of metalloproteinases started. Furthermore, the cell proliferation of mouse cells was inhibited, although with SA-bGal activity, DNA synthesis, and serum-inducible c-Fos expression (Coppé et al. 2010). In a study, human umbilical vein endothelial cells (HUVECs) were treated with indoxyl sulfate (IS) that had a concentration similar to the serum level in hemodialysis patients. IS caused ROS synthesis and cell senescence through p53 activation (Adelibieke et al. 2012). A group studied the effect of physiological oxygen pressure (3%) vs. high oxygen tension (20%) in an in vitro analysis of mouse embryonic fibroblasts (MEFs) derived from lacZ mice. Senescence and immortalization with a threefold increase in mutations occurred when the cells were cultured in a 20% oxygen condition, contrary to the cells in a 3% oxygen condition. The results clearly exposed the critical role of oxidative conditions on genomic integrity (Busuttil et al. 2003). It has recently been claimed that physiological levels of O2 should be maintained in cell culture to better mimic the in vivo redox reactions associated with specific cell types (Sies et al. 2022). Aging is a stochastic process that happens as a result of genetic and epigenetic changes such as oxidants (Colavitti and Finkel 2005). ROS induces telomerase shortening and premature senescence increases ROS levels as a positive feedback system (Passos and Von Zglinicki 2006). Also, it was reported that the activation of the checkpoint gene CDKN1A (p21) for a long time caused mitochondrial dysfunction and finally ROS synthesis, which is a major proof of DNA damage and then senescence (Passos et al. 2010). The senescence of endothelial cells accelerates in diabetes. The antidiabetic hormone glucagon-like peptide 1 (GLP-1) was studied by a group to reduce the aging rate of these cells which were under oxidative stress. The peptide decreased intracellular H2O2 and activated the transcription factor of cAMP response element-binding (CREB) levels through the cAMP/protein kinase A (PKA) pathway. In addition, the oxidative defense genes HO-1 and NQO1 were activated after applying GLP-1 (Oeseburg et al. 2010). Exogenous hydrogen peroxide at ≥ 1 mM, induced cell death, 100–500 μM caused senescence, and lower values did not lead to cell proliferation. This non-specific behavior of ROS has been related to the random injury of cell components and some activation of specific cell pathways (Lu and Finkel 2008).
Oxygen and angiogenesis
Hypoxia or ischemia conditions could activate the expression of VEGF and angiopoietin-1 genes, triggering the activation of NADPH oxidase. This enzyme produces ROS compounds such as superoxide and the resultant H2O2 leads to the auto-phosphorylation of VEGFR2 and finally angiogenesis. This ROS dependent angiogenesis happens via endogenous antioxidant enzymes such as SOD and thioredoxin. Hence, NADPH oxidase has been introduced as a main producer of ROS. The deep insight into oxygen's role in cell fate may provide a better understanding of stem cell requirements for desirable proliferation, migration, homing, commitment, or differentiation (Ushio-Fukai and Nakamura 2008). The related mechanism is summarized in Fig. 4.
ROS impacts on angiogenesis. In hypoxic conditions, the expression of VEGF and angiopoietin-1 genes is activated, which contributes to the activation of NADPH oxidase. Subsequently, the activated enzyme generates ROS compounds. Thereby, ROS molecules induce the auto-phosphorylation of VEGFR2 and eventually angiogenesis
The singlet oxygen, as a chemical formula of O2 is classified as the non-radical form of ROS that could lack unpaired electrons but is chemically reactive and can be changed to radical ROS through mitochondria, peroxisomes, endoplasmic reticulum, and the NADPH oxidase (NOX) complex in cell membranes (Luis et al. 1992; Inoue et al. 2003). There is a major relationship between tissue oxygenation status and active angiogenesis, in which the accumulation of genes as hypoxia-inducible factor (HIF)-α subunits, plays a major role in the activation of angiogenic transcription factors. HIF-α is regulated by a subset of dioxygenases called prolyl hydroxylase (PHD), which use oxygen as a substrate. Therefore, in the absence of oxygen, HIF-α subunits are gathered due to lower hydroxylation function. After their formation of heterodimers with HIF-1ß, the expression of angiogenic factors is activated and thus, cells could adapt to hypoxia. The regulation of angiogenesis involves two different mechanisms. The mechanism of paracrine (non-endothelial expression of angiogenesis factors) induced by VEGF-A, in which the factor interacts with surface receptors on endothelial cells to initiate angiogenic activity. The autocrine mechanism induces endothelial cells to express VEGF-A, which supports angiogenesis and protects vascular integrity. These two mechanisms interact with each other (Fong 2009). The therapeutic use of MSC transplantation has proposed a new strategy for hypoxic preconditioning through angiogenesis. For example, in ischemic heart disease, a major problem with using this treatment is the low survival of transplanted cells around the infarct, which leads to cell death due to endogenous and environmental factors such as the inflammatory response. Therefore, improving the survival of transplanted cells is crucial to increasing the efficiency and effectiveness of stem cell therapy. Angiogenesis is one of the effective mechanisms for improving the function of stem cells after transplantation. Transplanted MSCs stimulate angiogenesis after myocardial infarction (MI) by secreting multiple angiogenic cytokines and undergoing differentiation into endothelial cells. Hypoxia preconditioning (HP) stimulates endogenous mechanisms to express multiple proteins to protect against hypoxia. This condition can reduce neuronal apoptosis by inducing HIF-1α and protecting myocytes from oxygen-induced damage and reperfusion. As a result, studies show that HP-treated MSCs have better therapeutic effects in ischemic heart cases. The functional advantages of HP-MSC transplantations are as follows: (1) With HP, autocrine and paracrine signaling of MSCs is increased, and it reduces the apoptosis of transplanted cells and endogenous cardiomyocytes; (2) the higher survival of HP-MSCs provides better and longer support in a compensation process; and (3) the higher survival of the transplanted cells increases angiogenesis. These factors generally repair tissue and provide a simple but effective strategy for treating clinical MSC transplantations. The important point in this regard is to study its effect on mortality for long time (Hu et al. 2008). In addition, mechanical stress has been shown in in vivo studies to lead to angiogenesis and vascular regeneration. In this regard, related evidence has shown that blood vessels contain stem/progenitor cells that are activated by physiological and pathophysiological biomechanical pressures and released into the bloodstream (Sharifpanah et al. 2016). Also, hypoxia stimulates the angiogenic effects of fat-derived older MSCs. Since the exact concentration of oxygen in adipose tissue is unknown, hypothetical oxygen levels were used for bone marrow. Hypoxic ventilation has shown beneficial effects on bone marrow-derived MSCs by enhancing their angiogenic properties. Activation of HIF-1 leads to the expression of more genes for angiogenic stimuli such as VEGF, angiopoietin, platelet-derived growth factor B subunit (PDGFB), beta-1 growth factor conversion (TGFb1), and SDF-1. Thus, hypoxic ventilation of human fat-derived MSCs can increase angiogenesis through secretory factors (Efimenko et al. 2011).
Because oxygen is an important signaling molecule in stem cells, hypoxia can increase the expression of specific genes, including glycolysis, erythropoiesis, and angiogenesis (Glut-1, Epo, and VEGF, respectively) (Cameron et al. 2008). Satellite cells are muscle progenitor cells that are normally inactive and activated by muscle damage and have a number of growth factors, including hepatocyte growth factor (HGF), fibroblast growth factor (FGF) and VEGF. Their strong VEGF gene expression happens during muscle fiber regeneration. In ischemic muscle, HIF transcribes 100 genes involved in metabolism, erythropoiesis (Rhoads et al. 2009). Exogenous oxygen supply could increase nerve regeneration through Schwan cell survival and angiogenesis. Schwan cells were protected from hypoxia and angiogenesis was improved in vitro by perfluorotributylamine (PFTBA)-VEGF core–shell system. Furthermore, In vivo investigations shown that the VEGF could induce neovascularization, and the emerging blood vessels served as consecutive oxygen supplies for SCs during nerve regeneration when the oxygen transported by PFTBA was depleted (Ma et al. 2022). The physiological niches of MSCs have much less oxygen stress. Therefore, the stimulus of oxygen stress plays an important role in the balance between cell proliferation and commitment to differentiation. The presence of blood vessels and HIF-1α in fetus is not known. HIF-1α shows anomalous evidence about its role in NSCs fate via glycolytic metabolism. In drosophila neuroblasts, during the development of anaerobic metabolism, it is converted to oxidative phosphorylation, and the induction of oxidative phosphorylation is required for cell cycle exit and neuroblast differentiation. Similarly, in adult mammals, the oxygen consumption of NSCs, increases with their differentiation in vitro and accordingly, the inhibition of electron transfer chain enhances the proliferation of these cells (Lange et al. 2016). In a study, the adhesive proteins secreted from oysters (MAPs) were found to be biocompatible natural adhesives due to their attractive physicochemical properties such as non-accumulation in water, low surface tension and strong adhesion underwater. In addition, it enhances angiogenesis(Park et al. 2019). Studies have shown that HP can alter MSC function, aging, and epigenetic patterns by modulating chromatin-modifying enzyme gene expression levels (Isik et al. 2021). Another example is about cancer-like stem cell (CSC) interactions that occur between microvascular endothelial cells and endothelial progenitor cells in hypoxia within tumor niches. These interactions could induce an angiogenic response by the evaluation of microRNA expression in spheroid cells (Klimkiewicz et al. 2017).
Conclusion
Taking everything into account, ROS concentration, as a dependent factor of oxygen tension, plays a pivotal role in the regulation of cell fate and tissue function/regeneration. However, the underlying mechanism varies to some extent depending on the type of cell/tissue. Obviously higher concentrations of ROS alter cell growth, typical cell morphology, tissue angiogenesis, and cancer/senescence pathways. Anyway, these molecules must be neutralized by antioxidants, especially at higher concentrations. Moreover, it seems that the production site of this factor is important to determining the cell's fate. In the case of TNF activation by extrinsic ROS, cells are strictly doomed to die. In contrast, cell's death is not the only fate and their survival/senescence may occur in the case of originating ROS from tumor tissue.
References
Achuthan S, Santhoshkumar TR, Prabhakar J, Nair SA, Pillai MR (2011) Drug-induced senescence generates chemoresistant stemlike cells with low reactive oxygen species. J Biol Chem 286(43):37813–37829
Adelibieke Y, Shimizu H, Muteliefu G, Bolati D, Niwa T (2012) Indoxyl sulfate induces endothelial cell senescence by increasing reactive oxygen species production and p53 activity. J Ren Nutr 22(1):86–89
Ahmed NE-MB, Murakami M, Kaneko S, Nakashima M (2016) The effects of hypoxia on the stemness properties of human dental pulp stem cells (DPSCs). Sci Rep 6:35476
Al Tameemi W, Dale TP, Al-Jumaily RMK, Forsyth NR (2019) Hypoxia-modified cancer cell metabolism. Front Cell Dev Biol 7:4
Ali MH, Schlidt SA, Chandel NS, Hynes KL, Schumacker PT, Gewertz BL (1999) Endothelial permeability and IL-6 production during hypoxia: role of ROS in signal transduction. Am J Physiol Lung Cell Mol Physiol 277(5):L1057–L1065
Allen J, Winterford C, Axelsen RA, GobÉ GC (1992) Effects of hypoxia on morphological and biochemical characteristics of renal epithelial cell and tubule cultures. Ren Fail 14(4):453–460
Azad MB, Chen Y, Gibson SB (2009) Regulation of autophagy by reactive oxygen species (ROS): implications for cancer progression and treatment. Antioxid Redox Signal 11(4):777–790
Balaban RS, Nemoto S, Finkel T (2005) Mitochondria, oxidants, and aging. Cell 120(4):483–495
Bals R, Hiemstra P (2004) Innate immunity in the lung: how epithelial cells fight against respiratory pathogens. Eur Respir J 23(2):327–333
Bandarra D, Rocha S (2013) Tale of two transcription factors: NF-кB and HIF crosstalk. OA Mol Cell Biol 1(1):1–7
Bandarra D, Biddlestone J, Mudie S, Müller H-AJ, Rocha S (2015) HIF-1α restricts NF-κB-dependent gene expression to control innate immunity signals. Dis Model Mech 8(2):169–181
Barazzone-Argiroffo C, Muzzin P, Donati YR, Kan C-D, Aubert ML, Piguet P-F (2001) Hyperoxia increases leptin production: a mechanism mediated through endogenous elevation of corticosterone. Am J Physiol Lung Cell Mol Physiol 281(5):L1150–L1156
Bardaweel SK, Gul M, Alzweiri M, Ishaqat A, ALSalamat HA, Bashatwah RM (2018) Reactive oxygen species: the dual role in physiological and pathological conditions of the human body. Eurasian J Med 50(3):193
Bäumer AT, Ten Freyhaus H, Sauer H, Wartenberg M, Kappert K, Schnabel P et al (2008) Phosphatidylinositol 3-kinase-dependent membrane recruitment of Rac-1 and p47phox is critical for α-platelet-derived growth factor receptor-induced production of reactive oxygen species. J Biol Chem 283(12):7864–7876
Benderro GF, Sun X, Kuang Y, LaManna JC (2012) Decreased VEGF expression and microvascular density, but increased HIF-1 and 2α accumulation and EPO expression in chronic moderate hyperoxia in the mouse brain. Brain Res 1471:46–55
Bolisetty S, Jaimes EA (2013) Mitochondria and reactive oxygen species: physiology and pathophysiology. Int J Mol Sci 14(3):6306–6344
Bragg R, Gilbert W, Elmansi AM, Isales CM, Hamrick MW, Hill WD et al (2019) Stromal cell-derived factor-1 as a potential therapeutic target for osteoarthritis and rheumatoid arthritis. Ther Adv Chron Dis 10:2040622319882531
Brown BN, Ratner BD, Goodman SB, Amar S, Badylak SF (2012) Macrophage polarization: an opportunity for improved outcomes in biomaterials and regenerative medicine. Biomaterials 33(15):3792–3802
Brueckl C, Kaestle S, Kerem A, Habazettl H, Krombach F, Kuppe H et al (2006) Hyperoxia-induced reactive oxygen species formation in pulmonary capillary endothelial cells in situ. Am J Respir Cell Mol Biol 34(4):453–463
Busuttil RA, Rubio M, Dollé ME, Campisi J, Vijg J (2003) Oxygen accelerates the accumulation of mutations during the senescence and immortalization of murine cells in culture. Aging Cell 2(6):287–294
Cameron C, Harding F, Hu W-S, Kaufman DS (2008) Activation of hypoxic response in human embryonic stem cell–derived embryoid bodies. Exp Biol Med 233(8):1044–1057
Carreau A, Hafny-Rahbi BE, Matejuk A, Grillon C, Kieda C (2011) Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. J Cell Mol Med 15(6):1239–1253
Chaddha A, Broytman O, Teodorescu M (2020) Effects of allergic airway inflammation and chronic intermittent hypoxia on systemic blood pressure. Am J Physiol Regul Integr Comp Physiol 319(5):R566–R574
Chen L-W, Egan L, Li Z-W, Greten FR, Kagnoff MF, Karin M (2003) The two faces of IKK and NF-κB inhibition: prevention of systemic inflammation but increased local injury following intestinal ischemia-reperfusion. Nat Med 9(5):575–581
Chen X, Andresen BT, Hill M, Zhang J, Booth F, Zhang C (2008) Role of reactive oxygen species in tumor necrosis factor-alpha induced endothelial dysfunction. Curr Hypertens Rev 4(4):245–255
Chen CC, Hsia CW, Ho CW, Liang CM, Chen CM, Huang KL et al (2017) Hypoxia and hyperoxia differentially control proliferation of rat neural crest stem cells via distinct regulatory pathways of the HIF1α–CXCR4 and TP53–TPM1 proteins. Dev Dyn 246(3):162–185
Choudhry H, Harris AL (2018) Advances in Hypoxia-Inducible Factor Biology. Cell Metab 27(2):281–298
Cipolleschi MG, Dello Sbarba P, Olivotto M (1993) The role of hypoxia in the maintenance of hematopoietic stem cells
Colavitti R, Finkel T (2005) Reactive oxygen species as mediators of cellular senescence. IUBMB Life 57(4–5):277–281
Coppé J-P, Patil CK, Rodier F, Krtolica A, Beauséjour CM, Parrinello S et al (2010) A human-like senescence-associated secretory phenotype is conserved in mouse cells dependent on physiological oxygen. PLoS ONE 5(2):e9188
Corcoran SE, O’Neill LA (2016) HIF1α and metabolic reprogramming in inflammation. J Clin Investig 126(10):3699–3707
Cowden Dahl KD, Fryer BH, Mack FA, Compernolle V, Maltepe E, Adelman DM et al (2005) Hypoxia-inducible factors 1α and 2α regulate trophoblast differentiation. Mol Cell Biol 25(23):10479–10491
Csete M (2005) Oxygen in the cultivation of stem cells. Ann N Y Acad Sci 1049(1):1–8
Cubero FJ, Nieto N (2012) Arachidonic acid stimulates TNFα production in Kupffer cells via a reactive oxygen species-pERK1/2-Egr1-dependent mechanism. Am J Physiol Gastrointest Liver Physiol 303(2):G228–G239
Das R, Jahr H, van Osch GJ, Farrell E (2010) The role of hypoxia in bone marrow–derived mesenchymal stem cells: considerations for regenerative medicine approaches. Tissue Eng Part B Rev 16(2):159–168
Desmarquest P, Chadelat K, Corroyer S, Cazals V, Clement A (1998) Effect of hyperoxia on human macrophage cytokine response. Respir Med 92(7):951–960
Dewhirst MW, Cao Y, Moeller B (2008) Cycling hypoxia and free radicals regulate angiogenesis and radiotherapy response. Nat Rev Cancer 8(6):425–437
Di Girolamo FG, Fiotti N, Sisto UG, Nunnari A, Colla S, Mearelli F et al (2022) Skeletal muscle in hypoxia and inflammation: insights on the COVID-19 pandemic. Front Nutr 9:865402
Dröge W (2002) Free radicals in the physiological control of cell function. Physiol Rev 82(1):47–95
Dvornikova KA, Platonova ON, Bystrova EY (2023) Hypoxia and intestinal inflammation: common molecular mechanisms and signaling pathways. Int J Mol Sci 24(3):2425
Efimenko A, Starostina E, Kalinina N, Stolzing A (2011) Angiogenic properties of aged adipose derived mesenchymal stem cells after hypoxic conditioning. J Transl Med 9(1):1–13
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35(4):495–516
Eren, M. K. (2023) Hypoxia and senescence: role of oxygen in modulation of tumor suppression. In: Hypoxia in cancer: significance and impact on cancer therapy, pp 89–117
Fan F, Sun L, Zhang D, Zhu L, Wang S, Wang D (2018) Effects of red blood cell supernatants on hypoxia/reoxygenation injury in H9C2 cells. Int J Clin Exp Med 11(4):3612–3619
Fang J, Seki T, Maeda H (2009) Therapeutic strategies by modulating oxygen stress in cancer and inflammation. Adv Drug Deliv Rev 61(4):290–302
Fattahi R, Soleimani M, Khani M-M, Rasouli M, Hosseinzadeh S (2023) A three-dimensional structure with osteoconductive function made of O-carboxymethyl chitosan using aspirin as a cross-linker. Int J Polym Mater Polym Biomat 1–17
Finkel T (1998) Oxygen radicals and signaling. Curr Opin Cell Biol 10(2):248–253
Fong G-H (2009) Regulation of angiogenesis by oxygen sensing mechanisms. J Mol Med 87(6):549–560
Forsyth NR, Musio A, Vezzoni P, Simpson AHR, Noble BS, McWhir J (2006) Physiologic oxygen enhances human embryonic stem cell clonal recovery and reduces chromosomal abnormalities. Cloning Stem Cells 8(1):16–23
Furuta C, Miyamoto T, Takagi T, Noguchi Y, Kaneko J, Itoh S et al (2015) Transforming growth factor-β signaling enhancement by long-term exposure to hypoxia in a tumor microenvironment composed of L Ewis lung carcinoma cells. Cancer Sci 106(11):1524–1533
Genbacev O, Zhou Y, Ludlow JW, Fisher SJ (1997) Regulation of human placental development by oxygen tension. Science 277(5332):1669–1672
Ghaffari S (2008) Oxidative stress in the regulation of normal and neoplastic hematopoiesis. Antioxid Redox Signal 10(11):1923–1940
Glass JJ, Phillips PA, Gunning PW, Stehn JR (2015) Hypoxia alters the recruitment of tropomyosins into the actin stress fibres of neuroblastoma cells. BMC Cancer 15:712
Grayson WL, Zhao F, Bunnell B, Ma T (2007) Hypoxia enhances proliferation and tissue formation of human mesenchymal stem cells. Biochem Biophys Res Commun 358(3):948–953
Greenwald AC, Licht T, Kumar S, Oladipupo SS, Iyer S, Grunewald M et al (2019) VEGF expands erythropoiesis via hypoxia-independent induction of erythropoietin in noncanonical perivascular stromal cells. J Exp Med 216(1):215–230
Grocott MP, Martin DS, Levett DZ, McMorrow R, Windsor J, Montgomery HE (2009) Arterial blood gases and oxygen content in climbers on Mount Everest. N Engl J Med 360(2):140–149
Guitart A, Debeissat C, Hermitte F, Villacreces A, Ivanovic Z, Boeuf H et al (2011) Very low oxygen concentration (0.1%) reveals two FDCP-Mix cell subpopulations that differ by their cell cycling, differentiation and p27 KIP1 expression. Cell Death Differ 18(1):174–182
Gupta SC, Hevia D, Patchva S, Park B, Koh W, Aggarwal BBJA et al (2012) Upsides and downsides of reactive oxygen species for cancer: the roles of reactive oxygen species in tumorigenesis, prevention, and therapy. Antioxid Redox Signal 16(11):1295–1322
Harris AL (2002) Hypoxia—a key regulatory factor in tumour growth. Nat Rev Cancer 2(1):38–47
Harrison JS, Rameshwar P, Chang V, Bandari P (2002) Oxygen saturation in the bone marrow of healthy volunteers. Blood J Am Soc Hematol 99(1):394–394
Hartmann G, Tschöp M, Fischer R, Bidlingmaier C, Riepl R, Tschöp K et al (2000) High altitude increases circulating interleukin-6, interleukin-1 receptor antagonist and C-reactive protein. Cytokine 12(3):246–252
Hielscher A, Qiu C, Porterfield J, Smith Q, Gerecht S (2013) Hypoxia affects the structure of breast cancer cell-derived matrix to support angiogenic responses of endothelial cells. J Carcinog Mutagen 005
Hsu Y-H, Lin R-M, Chiu Y-S, Liu W-L, Huang K-Y (2020) Effects of IL-1β, IL-20, and BMP-2 on intervertebral disc inflammation under hypoxia. J Clin Med 9(1):140
Hu X, Yu SP, Fraser JL, Lu Z, Ogle ME, Wang J-A et al (2008) Transplantation of hypoxia-preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. J Thorac Cardiovasc Surg 135(4):799–808
Huang Y, Zitta K, Bein B, Steinfath M, Albrecht M (2013) An insert-based enzymatic cell culture system to rapidly and reversibly induce hypoxia: investigations of hypoxia-induced cell damage, protein expression and phosphorylation in neuronal IMR-32 cells. Dis Model Mech 6(6):1507–1514
Huang D, Fang F, Xu F (2016) Hyperoxia induces inflammation and regulates cytokine production in alveolar epithelium through TLR2/4-NF-κB-dependent mechanism. Eur Rev Med Pharmacol Sci 20(7):1399–1410
Hubbi ME, Semenza GL (2015) Regulation of cell proliferation by hypoxia-inducible factors. Am J Physiol Cell Physiol 309(12):C775-782
Inoue M, Sato EF, Nishikawa M, Park A-M, Kira Y, Imada I et al (2003) Mitochondrial generation of reactive oxygen species and its role in aerobic life. Curr Med Chem 10(23):2495–2505
Isik B, Thaler R, Goksu BB, Conley SM, Al-Khafaji H, Mohan A et al (2021) Hypoxic preconditioning induces epigenetic changes and modifies swine mesenchymal stem cell angiogenesis and senescence in experimental atherosclerotic renal artery stenosis. Stem Cell Res Ther 12(1):1–13
Izyumov D, Domnina L, Nepryakhina O, Avetisyan A, Golyshev S, Ivanova OY et al (2010) Mitochondria as source of reactive oxygen species under oxidative stress. Study with novel mitochondria-targeted antioxidants—the “Skulachev-ion” derivatives. Biochemistry 75(2):123–129
Jamieson D, Chance B, Cadenas E, Boveris A (1986) The relation of free radical production to hyperoxia. Annu Rev Physiol 48(1):703–719
Janssen A, Bosman C, Kruidenier L, Griffioen G, Lamers C, Van Krieken J et al (1999) Superoxide dismutases in the human colorectal cancer sequence. J Cancer Res Clin Oncol 125(6):327–335
Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y et al (2005) Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med 11(11):1173–1179
Jin Z, El-Deiry WS (2005) Overview of cell death signaling pathways. Cancer Biol Ther 4(2):147–171
Jones DP, Sies H (2015) The redox code. Antioxid Redox Signal 23(9):734–746
Kammerer T, Faihs V, Hulde N, Stangl M, Brettner F, Rehm M et al (2020) Hypoxic-inflammatory responses under acute hypoxia: in Vitro experiments and prospective observational expedition trial. Int J Mol Sci 21(3):1034
Karhausen J, Furuta GT, Tomaszewski JE, Johnson RS, Colgan SP, Haase VH (2004) Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J Clin Invest 114:1098–1106
Kendall AC, Whatmore JL, Harries LW, Winyard PG, Smerdon GR, Eggleton P (2012) Changes in inflammatory gene expression induced by hyperbaric oxygen treatment in human endothelial cells under chronic wound conditions. Exp Cell Res 318(3):207–216
Kim DS, Ko YJ, Lee MW, Park HJ, Park YJ, Kim D-I et al (2016) Effect of low oxygen tension on the biological characteristics of human bone marrow mesenchymal stem cells. Cell Stress Chaperones 21(6):1089–1099
Kingham PJ, McLean WG, Walsh M-T, Fryer AD, Gleich GJ, Costello RW (2003) Effects of eosinophils on nerve cell morphology and development: the role of reactive oxygen species and p38 MAP kinase. Am J Physiol Lung Cell Mol Physiol 285(4):L915–L924
Klimkiewicz K, Weglarczyk K, Collet G, Paprocka M, Guichard A, Sarna M et al (2017) A 3D model of tumour angiogenic microenvironment to monitor hypoxia effects on cell interactions and cancer stem cell selection. Cancer Lett 396:10–20
Kofoed H, Sjøntoft E, Siemssen SO, Olesen HP (1985) Bone marrow circulation after osteotomy: blood flow, po2, pCO2, and pressure studied in dogs. Acta Orthop Scand 56(5):400–403
Kong Q, Beel J, Lillehei K (2000) A threshold concept for cancer therapy. Med Hypotheses 55(1):29–35
Kuhlicke J, Frick JS, Morote-Garcia JC, Rosenberger P, Eltzschig HK (2007) Hypoxia inducible factor (HIF)-1 coordinates induction of Toll-like receptors TLR2 and TLR6 during hypoxia. PLoS ONE 2(12):e1364
Kumari S, Badana AK, Malla R (2018) Reactive oxygen species: a key constituent in cancer survival. Biomark Insights 13:1177271918755391
Kwak DJ, Kwak SD, Gauda EB (2006) The effect of hyperoxia on reactive oxygen species (ROS) in rat petrosal ganglion neurons during development using organotypic slices. Pediatr Res 60(4):371–376
Lacza Z, Snipes JA, Zhang J, Horváth EM, Figueroa JP, Szabó C et al (2003) Mitochondrial nitric oxide synthase is not eNOS, nNOS or iNOS. Free Radical Biol Med 35(10):1217–1228
Lambert AJ, Boysen HM, Buckingham JA, Yang T, Podlutsky A, Austad SN et al (2007) Low rates of hydrogen peroxide production by isolated heart mitochondria associate with long maximum lifespan in vertebrate homeotherms. Aging Cell 6(5):607–618
Lange C, Turrero Garcia M, Decimo I, Bifari F, Eelen G, Quaegebeur A et al (2016) Relief of hypoxia by angiogenesis promotes neural stem cell differentiation by targeting glycolysis. EMBO J 35(9):924–941
Lassègue B, San Martín A, Griendling KK (2012) Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ Res 110(10):1364–1390
Lewis J, Lee J, Underwood J, Harris A, Lewis C (1999) Macrophage responses to hypoxia: relevance to disease mechanisms. J Leukoc Biol 66(6):889–900
Li TS, Marbán E (2010) Physiological levels of reactive oxygen species are required to maintain genomic stability in stem cells. Stem Cells 28(7):1178–1185
Lim H-J, Han J, Woo D-H, Kim S-E, Kim S-K, Kang H-G et al (2011) Biochemical and morphological effects of hypoxic environment on human embryonic stem cells in long-term culture and differentiating embryoid bodies. Mol Cells 31(2):123–132
Lin Y-J, Chien B-YC, Lee Y-H (2022) Injectable and thermoresponsive hybrid hydrogel with Antibacterial, Anti-inflammatory, oxygen Transport, and enhanced cell growth activities for improved diabetic wound healing. Eur Polym J 175:111364
Lu T, Finkel T (2008) Free radicals and senescence. Exp Cell Res 314(9):1918–1922
Lugano R, Ramachandran M, Dimberg A (2020) Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell Mol Life Sci 77(9):1745–1770
Luis A, Sandalio LM, Palma J, Bueno P, Corpas J (1992) Metabolism of oxygen radicals in peroxisomes and cellular implications. Free Radic Biol Med 13(5):557–580
Ma T, Grayson WL, Fröhlich M, Vunjak-Novakovic G (2009) Hypoxia and stem cell-based engineering of mesenchymal tissues. Biotechnol Prog 25(1):32–42
Ma T, Hao Y, Li S, Xia B, Gao X, Zheng Y et al (2022) Sequential oxygen supply system promotes peripheral nerve regeneration by enhancing Schwann cells survival and angiogenesis. Biomaterials 289:121755
Macip S, Igarashi M, Berggren P, Yu J, Lee SW, Aaronson SA (2003) Influence of induced reactive oxygen species in p53-mediated cell fate decisions. Mol Cell Biol 23(23):8576–8585
MacMillan-Crow LA, Crow JP, Thompson JA (1998) Peroxynitrite-mediated inactivation of manganese superoxide dismutase involves nitration and oxidation of critical tyrosine residues. Biochemistry 37(6):1613–1622
Malladi P, Xu Y, Chiou M, Giaccia AJ, Longaker MT (2006) Effect of reduced oxygen tension on chondrogenesis and osteogenesis in adipose-derived mesenchymal cells. Am J Physiol Cell Physiol 290(4):C1139–C1146
Martindale JL, Holbrook NJ (2002) Cellular response to oxidative stress: signaling for suicide and survival. J Cell Physiol 192(1):1–15
Martínez JA, Cordero P, Campión J, Milagro FI (2012) Interplay of early-life nutritional programming on obesity, inflammation and epigenetic outcomes. Proc Nutr Soc 71(2):276–283
Matés JM, Sánchez-Jiménez FM (2000) Role of reactive oxygen species in apoptosis: implications for cancer therapy. Int J Biochem Cell Biol 32(2):157–170
Matsuoka J, Yashiro M, Doi Y, Fuyuhiro Y, Kato Y, Shinto O et al (2013) Hypoxia stimulates the EMT of gastric cancer cells through autocrine TGFβ signaling. PLoS ONE 8(5):e62310
Medzhitov R (2008) Origin and physiological roles of inflammation. Nature 454(7203):428–435
Miller JT, Bartley JH, Wimborne HJ, Walker AL, Hess DC, Hill WD et al (2005) The neuroblast and angioblast chemotaxic factor SDF-1 (CXCL12) expression is briefly up regulated by reactive astrocytes in brain following neonatal hypoxic-ischemic injury. BMC Neurosci 6(1):1–11
Mohd Ali N, Boo L, Yeap SK, Ky H, Satharasinghe DA, Liew WC et al (2016) Probable impact of age and hypoxia on proliferation and microRNA expression profile of bone marrow-derived human mesenchymal stem cells. PeerJ 4:e1536
Murphy CL, Polak JM (2004) Control of human articular chondrocyte differentiation by reduced oxygen tension. J Cell Physiol 199(3):451–459
Nath A, Chakrabarti P, Sen S, Barui A (2022) Reactive oxygen species in modulating intestinal stem cell dynamics and function. Stem Cell Rev Rep 18(7):2328–2350
Näthke I, Rocha S (2011) Antagonistic crosstalk between APC and HIF-1α. Cell Cycle 10(10):1545–1547
Nayadu S, Kaur G, Gudi G, Addepalli V (2012) The potentials of selected therapeutic targets for inflammation: a snapshot. Recent Pat Inflamm Allergy Drug Discov 6(2):137–146
Nishikawa M (2008) Reactive oxygen species in tumor metastasis. Cancer Lett 266(1):53–59
Oeseburg H, de Boer RA, Buikema H, van der Harst P, van Gilst WH, Silljé HH (2010) Glucagon-like peptide 1 prevents reactive oxygen species-induced endothelial cell senescence through the activation of protein kinase A. Arterioscler Thromb Vasc Biol 30(7):1407–1414
Ozben T (2007) Oxidative stress and apoptosis: impact on cancer therapy. J Pharm Sci 96(9):2181–2196
Panieri E, Gogvadze V, Norberg E, Venkatesh R, Orrenius S, Zhivotovsky B (2013) Reactive oxygen species generated in different compartments induce cell death, survival, or senescence. Free Radic Biol Med 57:176–187
Park TY, Jeon EY, Kim HJ, Choi B-H, Cha HJ (2019) Prolonged cell persistence with enhanced multipotency and rapid angiogenesis of hypoxia pre-conditioned stem cells encapsulated in marine-inspired adhesive and immiscible liquid micro-droplets. Acta Biomater 86:257–268
Passos JF, Von Zglinicki T (2006) Oxygen free radicals in cell senescence: are they signal transducers? Free Radic Res 40(12):1277–1283
Passos JF, Nelson G, Wang C, Richter T, Simillion C, Proctor CJ et al (2010) Feedback between p21 and reactive oxygen production is necessary for cell senescence. Mol Syst Biol 6(1):347
Peck SH, Bendigo JR, Tobias JW, Dodge GR, Malhotra NR, Mauck RL et al (2021) Hypoxic preconditioning enhances bone marrow-derived mesenchymal stem cell survival in a low oxygen and nutrient-limited 3D microenvironment. Cartilage 12(4):512–525
Peixoto A, Fernandes E, Gaiteiro C, Lima L, Azevedo R, Soares J et al (2016) Hypoxia enhances the malignant nature of bladder cancer cells and concomitantly antagonizes protein O-glycosylation extension. Oncotarget 7(39):63138
Peng L, Shu X, Lang C, Yu X (2016) Effects of hypoxia on proliferation of human cord blood-derived mesenchymal stem cells. Cytotechnology 68(4):1615–1622
Pulido-Escribano V, Torrecillas-Baena B, Camacho-Cardenosa M, Dorado G, Gálvez-Moreno M, Casado-Díaz A (2022) Role of hypoxia preconditioning in therapeutic potential of mesenchymal stem-cell-derived extracellular vesicles. World J Stem Cells 14(7):453–472
Quintero P, Gonzalez-Muniesa P, Garcia-Diaz DF, Martinez JA (2012) Effects of hyperoxia exposure on metabolic markers and gene expression in 3T3-L1 adipocytes. J Physiol Biochem 68(4):663–669
Radisic M, Park H, Chen F, Salazar-Lazzaro JE, Wang Y, Dennis R et al (2006) Biomimetic approach to cardiac tissue engineering: oxygen carriers and channeled scaffolds. Tissue Eng 12(8):2077–2091
Rasouli M, Rahimi A, Soleimani M, Keshel SH (2021) The interplay between extracellular matrix and progenitor/stem cells during wound healing: Opportunities and future directions. Acta Histochem 123(7):151785
Rasouli M, Vakilian F, Ranjbari J (2022) Therapeutic and protective potential of mesenchymal stem cells, pharmaceutical agents and current vaccines against COVID-19. Curr Stem Cell Res Ther 17(2):166–185
Rasouli M, Hosseinzadeh S, Mortazavi SM, Fattahi R, Ranjbari J, Soleimani M (2023a) Do carboxymethyl cellulose and pal-KTTKS make bacterial cellulose a superior wound dressing or skin scaffold? Polym Plast Tech Mat 62(8):974–988
Rasouli M, Naeimzadeh Y, Hashemi N, Hosseinzadeh S (2023b) Age-related alterations in mesenchymal stem cell function: understanding mechanisms and seeking opportunities to bypass the cellular aging. Curr Stem Cell Res Ther. Epub ahead of print. https://doi.org/10.2174/1574888X18666230113144016
Rasouli M, Soleimani M, Hosseinzadeh S, Ranjbari J (2023c) Bacterial cellulose as potential dressing and scaffold material: toward improving the antibacterial and cell adhesion properties. J Polym Environ. Epub ahead of print. https://doi.org/10.1007/s10924-023-02779-0
Raza MH, Siraj S, Arshad A, Waheed U, Aldakheel F, Alduraywish S et al (2017) ROS-modulated therapeutic approaches in cancer treatment. J Cancer Res Clin Oncol 143(9):1789–1809
Redza-Dutordoir M, Averill-Bates DA (2016) Activation of apoptosis signalling pathways by reactive oxygen species. Biochim Biophys Acta (BBA) Mol Cell Res 1863(12):2977–2992
Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE et al (2004) Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 109(10):1292–1298
Rhee SG, Bae YS, Lee S-R, Kwon J (2000) Hydrogen peroxide: a key messenger that modulates protein phosphorylation through cysteine oxidation. Sci STKE 2000(53):pe1
Rhoads RP, Johnson RM, Rathbone CR, Liu X, Temm-Grove C, Sheehan SM et al (2009) Satellite cell-mediated angiogenesis in vitro coincides with a functional hypoxia-inducible factor pathway. Am J Physiol Cell Physiol 296(6):C1321–C1328
Rizvi SFA, Wasim B, Usman S, Borges KJJ, Sahibdad I, Salim A et al (2022) Zinc and hypoxic preconditioning: a strategy to enhance the functionality and therapeutic potential of bone marrow-derived mesenchymal stem cells. Mol Cell Biochem 477(12):2735–2749
Rosenberger P, Schwab JM, Mirakaj V, Masekowsky E, Mager A, Morote-Garcia JC et al (2009) Hypoxia-inducible factor–dependent induction of netrin-1 dampens inflammation caused by hypoxia. Nat Immunol 10(2):195–202
Ruigrok MJ, Tomar J, Frijlink HW, Melgert BN, Hinrichs WL, Olinga P (2019) The effects of oxygen concentration on cell death, anti-oxidant transcription, acute inflammation, and cell proliferation in precision-cut lung slices. Sci Rep 9(1):1–13
Sadat S, Gehmert S, Song Y-H, Yen Y, Bai X, Gaiser S et al (2007) The cardioprotective effect of mesenchymal stem cells is mediated by IGF-I and VEGF. Biochem Biophys Res Commun 363(3):674–679
Salminen A, Kaarniranta K, Kauppinen A (2012) Inflammaging: disturbed interplay between autophagy and inflammasomes. Aging (albany NY) 4(3):166
Salvemini D, Ischiropoulos H, Cuzzocrea S (2003) Roles of nitric oxide and superoxide in inflammation. Inflammation Protocols. Springer, pp 291–303
Samal JRK, Rangasami VK, Samanta S, Varghese OP, Oommen OP (2021) Discrepancies on the role of oxygen gradient and culture condition on mesenchymal stem cell fate. Adv Healthc Mater 10(6):e2002058
Schumacker PT (2006) Reactive oxygen species in cancer cells: live by the sword, die by the sword. Cancer Cell 10(3):175–176
Semenza GL (2000) HIF-1 and human disease: one highly involved factor. Genes Dev 14(16):1983–1991
Semenza GL (2009a) Regulation of cancer cell metabolism by hypoxia-inducible factor 1. Seminars in cancer biology. Elsevier
Semenza GL (2009b) Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology 24(2):97–106
Sharifpanah F, Behr S, Wartenberg M, Sauer H (2016) Mechanical strain stimulates vasculogenesis and expression of angiogenesis guidance molecules of embryonic stem cells through elevation of intracellular calcium, reactive oxygen species and nitric oxide generation. Biochim Biophys Acta (BBA) Mol Cell Res. 1863(12):3096–3105
Sharkey JV (2020) Effect of acute low oxygen exposure on the proliferation rate, viability, and gene expression of C2C12 myoblasts in vitro. BioRxiv
Sheridan J, Bishop C, Simmons R (1984) Effects of hypoxia on the kinetic and morphological characteristics of human melanoma cells grown as colonies in semi-solid agar medium. Br J Exp Pathol 65(2):171
Shirbaghaee Z, Keshel SH, Rasouli M, Valizadeh M, Nazari SSH, Hassani M et al (2023) Report of a phase 1 clinical trial for safety assessment of human placental mesenchymal stem cells therapy in patients with Critical limb ischemia (CLI)
Siegrist J, Sies H (2017) Disturbed redox homeostasis in oxidative distress: a molecular link from chronic psychosocial work stress to coronary heart disease? Circ Res 121(2):103–105
Sies H (2017) Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: oxidative eustress. Redox Biol 11:613–619
Sies H, Jones DP (2020) Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat Rev Mol Cell Biol 21(7):363–383
Sies H, Belousov VV, Chandel NS, Davies MJ, Jones DP, Mann GE et al (2022) Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat Rev Mol Cell Biol 23(7):499–515
Sohal R, Sohal BH (1991) Hydrogen peroxide release by mitochondria increases during aging. Mech Ageing Dev 57(2):187–202
Song J, Miermont A, Lim CT, Kamm RD (2018) A 3D microvascular network model to study the impact of hypoxia on the extravasation potential of breast cell lines. Sci Rep 8(1):1–11
Studer L, Csete M, Lee S-H, Kabbani N, Walikonis J, Wold B et al (2000) Enhanced proliferation, survival, and dopaminergic differentiation of CNS precursors in lowered oxygen. J Neurosci 20(19):7377–7383
Suematsu N, Tsutsui H, Wen J, Kang D, Ikeuchi M, Ide T et al (2003) Oxidative stress mediates tumor necrosis factor-α–induced mitochondrial DNA damage and dysfunction in cardiac myocytes. Circulation 107(10):1418–1423
Taylor CT (2008) Interdependent roles for hypoxia inducible factor and nuclear factor-κB in hypoxic inflammation. J Physiol 586(17):4055–4059
Taylor CT, Colgan SP (2007) Hypoxia and gastrointestinal disease. J Mol Med 85(12):1295–1300
Thangarajah H, Vial IN, Chang E, El-Ftesi S, Januszyk M, Chang EI et al (2009) IFATS collection: adipose stromal cells adopt a proangiogenic phenotype under the influence of hypoxia. Stem Cells 27(1):266–274
Tiidus PM (1998) Radical species in inflammation and overtraining. Can J Physiol Pharmacol 76(5):533–538
Tottey S, Corselli M, Jeffries EM, Londono R, Peault B, Badylak SF (2011) Extracellular matrix degradation products and low-oxygen conditions enhance the regenerative potential of perivascular stem cells. Tissue Eng Part A 17(1–2):37–44
Trachootham D, Zhou Y, Zhang H, Demizu Y, Chen Z, Pelicano H et al (2006) Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by β-phenylethyl isothiocyanate. Cancer Cell 10(3):241–252
Uchikura K, Wada T, Hoshino S, Nagakawa Y, Aiko T, Bulkley GB et al (2004) Lipopolysaccharides induced increases in fas ligand expression by kupffer cells via mechanisms dependent on reactive oxygen species. Am J Physiol Gastrointest Liver Physiol 287(3):G620–G626
Ushio-Fukai M, Nakamura Y (2008) Reactive oxygen species and angiogenesis: NADPH oxidase as target for cancer therapy. Cancer Lett 266(1):37–52
Vallabhapurapu S, Karin M (2009) Regulation and function of NF-κB transcription factors in the immune system. Annu Rev Immunol 27:693–733
Van Uden P, Kenneth NS, Rocha S (2008) Regulation of hypoxia-inducible factor-1α by NF-κB. Biochem J 412(3):477–484
Villarroya F, Iglesias R, Giralt M (2007) PPARs in the control of uncoupling proteins gene expression. PPAR research 2007
Wang C, Liu W, Liu Z, Chen L, Liu X, Kuang S (2015) Hypoxia inhibits myogenic differentiation through p53 protein-dependent induction of Bhlhe40 protein. J Biol Chem 290(50):29707–29716
Wang C, Jiang H, Duan J, Chen J, Wang Q, Liu X et al (2018) Exploration of acute phase proteins and inflammatory cytokines in early stage diagnosis of acute mountain sickness. High Alt Med Biol 19(2):170–177
Waris G, Ahsan H (2006) Reactive oxygen species: role in the development of cancer and various chronic conditions. J Carcinog 5:14
Yuan G-J, Li Q-W, Shan S-L, Wang W-M, Jiang S, Xu X-M (2012) Hyperthermia inhibits hypoxia-induced epithelial-mesenchymal transition in HepG2 hepatocellular carcinoma cells. World J Gastroenterol WJG 18(34):4781
Yun Z, Maecker HL, Johnson RS, Giaccia AJ (2002) Inhibition of PPARγ2 gene expression by the HIF-1-regulated gene DEC1/Stra13: a mechanism for regulation of adipogenesis by hypoxia. Dev Cell 2(3):331–341
Zangl Q, Martignoni A, Jackson SH, Ohta A, Klaunberg B, Kaufmann I et al (2014) Postoperative hyperoxia (60%) worsens hepatic injury in mice. Anesthesiol J Am Soc Anesthesiol. 121(6):1217–1225
Zhang J, Ahn KS, Kim C, Shanmugam MK, Siveen KS, Arfuso F et al (2016) Nimbolide-induced oxidative stress abrogates STAT3 signaling cascade and inhibits tumor growth in transgenic adenocarcinoma of mouse prostate model. Antioxid Redox Signal 24(11):575–589
Zhang N, Hong B, Zhou C, Du X, Chen S, Deng X et al (2017) Cobalt chloride-induced hypoxia induces epithelial-mesenchymal transition in renal carcinoma cell lines. Ann Clin Lab Sci 47(1):40–46
Zhou R, Yazdi AS, Menu P, Tschopp J (2011) A role for mitochondria in NLRP3 inflammasome activation. Nature 469(7329):221–225
Acknowledgements
This study is related to the project No. 1400/62981 from Student Research Committee, Shahid Beheshti University of Medical Sciences, Tehran, Iran. We also appreciate the “Student Research Committee” and “Research & Technology Chancellor” in Shahid Beheshti University of Medical Sciences for their financial support of this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rasouli, M., Fattahi, R., Nuoroozi, G. et al. The role of oxygen tension in cell fate and regenerative medicine: implications of hypoxia/hyperoxia and free radicals. Cell Tissue Bank 25, 195–215 (2024). https://doi.org/10.1007/s10561-023-10099-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-023-10099-9