Abstract
Adipose tissue often becomes poorly oxygenated in obese subjects. This feature may provide cellular mechanisms involving chronic inflammation processes such as the release of pro-inflammatory cytokines and macrophage infiltration. In this context, the purpose of the present study was to determine whether a hyperoxia exposure on mature adipocytes may influence the expression of some adipokines and involve favorable changes in specific metabolic variables. Thus, 3T3-L1 adipocytes (14 days differentiated) were treated with 95 % oxygen for 24 h. Cell viability, intra and extracellular reactive oxygen species (ROS) content, glucose uptake, as well as lactate and glycerol concentrations were measured in the culture media. Also, mRNA levels of hypoxia-inducible factor (HIF)-1α, leptin, interleukin (IL)-6, monocyte chemotactic protein (MCP)-1, peroxisome proliferator-activated receptor (PPAR)-γ, adiponectin, and angiopoietin-related protein (ANGPTL)4 were analyzed. Hyperoxia treatment increased intra and extracellular ROS content, reduced glucose uptake and lactate release and increased glycerol release. Additionally, a higher oxygen tension led to an upregulation of the expression of IL-6, MCP-1, and PPAR-γ, while ANGPTL4 was downregulated in the hyperoxia group with respect to control. The present data shows that hyperoxia treatment seems to produce an inflammatory response due to the release of ROS and the upregulation of pro-inflammatory adipokines, such as IL-6 and MCP-1. On the other hand, hyperoxia may have an indirect effect on insulin sensitivity due to the upregulation of PPAR-γ signaling as well as a possible modulation of both glucose and lipid metabolic markers. To our knowledge, this is the first study analyzing the effect of hyperoxia in 3T3-L1 adipocytes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity is a major metabolic disorder associated to an excessive fat accumulation. The most common causes for the increase in the prevalence of this disease are overnutrition and a reduction in physical activity, leading to a chronic positive balance between energy intake and energy expenditure. However, other factors such as endocrine disruptions, perinatal malnutrition, environmental effects or epigenetic dysregulations, can also contribute to obesity [5, 27]. This disease often courses with a low-grade chronic inflammation, characterized by changes in the release of inflammation-related adipokines and macrophage infiltration within the white adipose tissue, which may play an important role in the onset and development of obesity-related diseases [17, 25].
Therefore, inflammation could be a target for understanding the etiology and complications of some causes of obesity. Indeed, several hypotheses have been proposed as the cause for the initiation of inflammatory processes during obesity, including oxidative stress [12], endoplasmic reticulum (ER) stress [28], and adipose tissue hypoxia [37]. These possibilities do not necessarily have to be mutually exclusive, since ER stress and hypoxia lead to the release of reactive oxygen species (ROS) and, consequently, to oxidative stress in adipocytes [19]. These reactive molecules can act internally, reacting on proteins, lipids or DNA leading finally to cell death, and externally, provoking specific and nonspecific pro-inflammatory effects [2]. In this sense, therapies trying to manage and counterbalance some of these adipose tissue pro-inflammatory conditions are under investigation [14, 38]. Nevertheless, to our knowledge, there are no studies trying to ameliorate the hypoxic state within adipose tissue. Indeed, recent investigations have suggested that adipose tissue hypoxia provides a cellular explanation for chronic inflammation and macrophage infiltration in white adipose tissue in obesity [18, 46], which have been associated to some complications accompanying obesity-related diseases.
In this context, oxygen is used in current medicine as a treatment for several conditions such as apnea, migraine, and wounds. Moreover, some animal studies have demonstrated that the treatment with hyperoxia might produce beneficial effects in different metabolic disorders, such as protecting the rat brain tissue against ischemia reperfusion injury [4], reducing severity of colitis [9] or ameliorating hemorrhagic shock-induced renal failure by decreasing intrarenal hypoxia and improving renal functions [8]. Additionally, several studies have shown that hyperoxia can decrease the expression of some pro-inflammatory genes in different organs and cell types, since a decrease in several inflammatory markers in alveolar macrophages exposed to hyperoxia has been reported [7]. This effect seems to be consistent with hyperbaric oxygen therapy (HBOT) studies carried out in ex vivo cell cultures [3]. These findings are also in agreement with studies demonstrating that HBOT attenuates pro-inflammatory cytokine production of systemic inflammation in animal models [24, 45].
Taking all this information into account, and in relation to the hypothesis exposed by our group in 2010 [31] where we proposed hyperoxia as a novel therapeutic intervention for the improvement of the obesity state, we performed the current study to determine whether an exposure of mature adipocytes to hyperoxia may regulate the expression of some adipokines and involve favorable changes in specific metabolic variables.
Material and methods
Cell culture and treatment
3T3-L1 mouse preadipocytes were cultured with Dulbecco’s minimal essential media (DMEM) containing 4.5 g/L glucose and supplemented with 10 % calf bovine serum as described elsewhere [11]. Two days before full confluence, cells were cultured in 12-well plates. Differentiation into adipocytes was induced by treating cells for 2 days with 0.5 mM isobutylmethylxanthine, 1 μM dexamethasone, and 10 μg/ml insulin in DMEM supplemented with 10 % fetal bovine serum (FBS), and then for 2 days with 10 μg/ml insulin in the same media. Thereafter, cells were maintained and re-fed every 2 or 3 days with FBS without hormones until 14 days after differentiation induction, when between 80 and 90 % of the cells exhibited the adipocyte phenotype. Media had 100 units/ml penicillin, and 100 μg/ml streptomycin, and cells were always maintained at 37°C in a humidified atmosphere containing 5 % CO2.
Cells of both groups were serum-deprived and consecutively exposed to the different O2 atmospheres for up to 24 h. The cells exposed to hyperoxia were placed in an MIC-101 incubator chamber (Billups-Rosenberg, Del Mar, CA, USA) with an inside concentration of 95 % O2/5 % CO2 at 37°C. Control cells were cultured in a standard incubator (21 % O2/5 % CO2) at 37°C. After the treatment, culture media were collected and stored at −80°C for further measurements.
Cell viability assay and ROS determination
Cell viability was measured with the lactate dehydrogenase (LDH) Cytotoxicity Assay Kit at 24 h according to manufacturer’s instructions (Cayman Chemical Company, Ann Arbor, MI, USA). For determining intracellular and extracellular ROS concentration, 2′,7′-dichlorfluorescein (DCFH) was used [23]. Briefly, cells were incubated with 10 μM DCFH for 40 min at 37°C in 5 % CO2, frozen for at least 1 h at −80°C, and then lysed with 1,000 μl lysis buffer (150 mM NaCl, 0.1 % Triton, and 10 mM Tris). Finally, 200 μl of each lysate were loaded on a 96-well black plate. For extracellular ROS determinations, after treatment, 300 μl of culture media of each sample were also incubated with 10 μM DCFH at 37°C in 5 % CO2 for 40 min, frozen for at least 1 h at −80°C, and then 200 μl of this incubation mix were loaded onto a 96-well black plate. Finally, fluorescence intensity was measured using a POLARstar spectrofluorometer plate reader (BMG Labtechnologies, Offenburg, Germany) at an excitation wavelength of 485 nm and at an emission of 530 nm.
Measurement of metabolic markers
The glucose (HK-CP kit; Horiba, Montpellier, France), lactate (ABX Diagnostic, Montpellier, France) and glycerol (GLY 105; Randox Laboratories, Antrim, UK) concentrations were measured from culture media samples with a PENTRA C200 auto-analyzer (Horiba, Montpellier, France) after the 24-h treatment. The adipocyte glucose uptake was estimated by the difference between the content of glucose in the culture media at the beginning and at the end of the experiment, as previously described [13].
RT-PCR analysis
Total RNA was isolated from all samples using Trizol (Invitrogen, Paisley, UK) according to the manufacturer’s instructions. Thus, purified total RNA from adipocytes was then treated with DNAse (DNAfree kit; Ambion Inc., Austin, USA) and used to generate cDNA with M-MLV reverse transcriptase (Invitrogen, Paisley, UK). Real-time PCR was performed in an ABI PRISM 7,000 HT Sequence Detection System (Applied Biosystems, California, USA). Taqman probes for mouse hypoxia-inducible factor (HIF)-1α (Mm00468875_m1), monocyte chemotactic protein (MCP)-1 (Mm99999056_m1), peroxisome proliferator-activated receptor gamma (PPAR)-γ coactivator 1α (Mm00447183_m1), adiponectin (Mm0456425_m1), and angiopoietin-related protein (ANGPTL)4 (Mm00480431_m1) were also supplied by Applied Biosystems. Leptin and interleukin (IL)-6 primers and probes were synthesized commercially (through Applied Biosystems’ webpage), and the sequences were as follows:
-
Mouse leptin (72-bp product)
-
Sense 5′-CAT CTG CTG GCC TTC TCC AA-3′;
-
antisense 5′-ATC CAG GCT CTC TGG CTT CTG-3′;
-
Taqman probe 5′-FAM-AGC TGC TCC CTG CCT CAG ACC AGT G-TAMRA-3′.
-
-
Mouse IL-6 (129-bp product)
-
Sense 5′-ACA ACC ACG GCC TTC CCT ACT T-3′;
-
antisense 5′-CAC GAT TTC CCA GAG AAC ATG TG-3′;
-
Taqman probe 5′-FAM-TTC ACA GAG GAT ACC ACT CCC AAC AGA ACC T-TAMRA-3′
-
All the expression levels of the target genes studied were normalized by the expression of 18s (Hs99999901_s1) as the selected internal control (housekeeping gene), since it demonstrated that no significant changes in its expression were detected after 95 % O2 exposition against control (data not shown). All procedures were performed according to a previously described protocol [14].
Statistical analysis
The results are expressed as mean±SD. Statistical significance between groups was assessed by U Mann–Whitney test. A probability of p < 0.05 was setup for determining statistically significant differences. The statistical analyses were performed using SPSS 15 (Chicago, USA) and Graphpad Prism 5.0 (San Diego, USA) software.
Results
Cell viability and ROS
The activity of LDH was determined in the conditioned cell media in order to investigate the potential cytotoxicity of the applied treatment. The 24-h hyperoxic treatment did not affect differently cell integrity with respect to control (data not shown). Furthermore, intracellular and extracellular ROS secretions, corrected by cell viability [14], were both significantly increased by the hyperoxia exposure (Fig. 1a, b).
Intracellular and extracellular ROS content in control and hyperoxia treatments at 24 h in 3T3-L1 adipocytes (14 days postdifferentiation). White bar control group, gray bar hyperoxia group. Data (n = 6) are expressed as mean±SD. U Mann–Whitney test was performed to identify statistical effects. **P < 0.01 vs untreated cells
Culture media determinations
Glucose uptake, lactate production and glycerol release of isolated adipocytes from both groups were determined after the 24 h treatment, and were corrected by cell viability. Glucose uptake and lactate production were significantly reduced (p < 0.05 and p < 0.01, respectively), while glycerol release was significantly increased (p < 0.01) in the hyperoxia group when compared to the control (Fig. 2).
Effect of 95 % oxygen exposure on glucose uptake, glycerol, and lactate release in 3T3-L1 adipocytes (14 days postdifferentiation). White bars control group, gray bars hyperoxia group. Data (n = 6) are expressed as mean±SD. U Mann–Whitney test was performed to identify statistical effects. *P < 0.05 and **P < 0.001 vs untreated cells
Gene expression
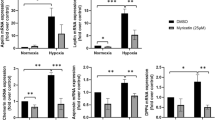
Regarding gene expression, a significant increase in the expression of IL-6, MCP-1, and PPAR-γ (p < 0.05, p < 0.01, and p < 0.01, respectively) and a significant decrease in the expression of ANGPTL4 (p < 0.01) were observed. Also, a strong correlation between MCP-1 mRNA expression and ROS release was found (r=0.800; p < 0.01). No statistically significant differences were found in HIF-1α, leptin and adiponectin mRNA expression between the experimental groups (Fig. 3).
Gene expression analysis of HIF-1α, leptin, IL-6, MCP-1, PPAR-γ, adiponectin, and ANGPTL4 at 24 h of 95 % oxygen treatment in 3T3-L1 adipocytes (14 days postdifferentiation). White bars control group, gray bars hyperoxia group (HPx). A mean value of triplicates was used for relative mRNA level. Data (n = 6) are expressed as mean±SD. U Mann–Whitney test was performed to identify statistical effects. *P < 0.05 and **P < 0.001 vs untreated cells
Discussion
In the current trial, the effects of hyperoxia in adipocytes were investigated as a potential approach trying to counteract the hypoxic state supposed to occur in an excessively expanded adipose tissue. Several studies have reported a positive relationship between low oxygen concentrations and cell death in adipocytes and other cell types [47]. In our experiment, 3T3-L1 adipocytes incubated under hyperoxic conditions (95 % O2) did not show a significant decrease or increase in cell death. However, an enhanced generation of both intracellular and extracellular ROS was observed after a 24-h exposure. This finding seems to be consistent with another study that described an increase of ROS production in 3T3/J2 fibroblasts exposed to 100 % O2 at 2.5 absolute atmosphere (ATA), where it was suggested that intracellular ROS generation could be directly related to cell apoptosis [6].
In adipocytes, it has been reported that low oxygen concentrations induce an increase on lipolysis [47]. In our study, hyperoxia also increased glycerol release, an episode that perhaps may lead to pro-inflammatory responses inducing ROS production, as it has been shown in other studies [40]. Furthermore, Yin et al. [47] demonstrated that hypoxia increases glucose metabolism in adipocytes through both insulin-dependent and -independent pathways. In our experiment, glucose uptake was significantly inhibited by hyperoxic conditions compared to control. Thus, decreased glucose utilization in hyperoxia would be expected to result in a lower production and release of lactate, as it was observed in the present trial. This outcome seems to go in the same direction as some studies have observed in blood and other tissues [16, 36]. A possible explanation for this finding could be that an excessive amount of oxygen might induce the adenosine-5′-triphosphate production fully through mitochondrial respiration, leading to suppression of the anaerobic pathway and therefore resulting in a minor lactate release. It has been recently proposed that within white adipose tissue, an autocrine/paracrine loop occurs in which lactate derived from glucose mediates the antilipolytic effect of insulin [33]. Thus, the increased production of lactate by adipocytes in obesity, as a consequence of adipose tissue hypoxia, might promote this anti-lipolytic action of insulin, constituting another link between this disease and its associated pathologies [29]. In this sense, a decreased lactate release in hyperoxic adipocytes may ameliorate this detrimental loop and, therefore, some obesity-associated complications.
Several groups have investigated the effects of normobaric (more than 21 % O2 at 1 ATA) and hyperbaric (100 % oxygen at a pressure greater than 1 ATA) O2 therapies (NBOT and HBOT, respectively) in both, animal and cell culture models, regarding the expression of some inflammatory genes. Thus, Desmarquest et al. [7] observed a decrease in TNF-α, IL-1β, and IL-6 expression in alveolar macrophages exposed to 48 h of 95 % O2. This effect seems to be consistent with other HBOT studies carried out in ex vivo cell cultures [3, 22, 42]. All these findings are also in agreement with studies demonstrating that HBOT attenuates pro-inflammatory cytokine production in animal models of systemic inflammation [24, 44, 45]. However, significant increases in the expression of IL-6, IL-1, and TNF-α have been also reported [1, 20]. Interestingly, these effects were not evident until the animals were treated for at least 48 h of hyperoxia, suggesting that inflammation is dependent on the duration of the oxygen exposure. Overall, these studies suggest that oxygen availability may improve oxygen utilization by body organs.
Apparently, there are no data available regarding the effect of hyperoxia on gene expression of isolated adipocytes. However, in adipose cells exposed to hypoxia, it has been shown that pro-inflammatory adipokines are increased, while anti-inflammatory adipokines are decreased [21, 46]. In our study, mRNA levels of pro-inflammatory markers IL-6 and MCP-1 were upregulated by hyperoxia. In this context, it has been demonstrated that lipolysis can produce inflammatory responses in endothelial cells [39]; it is therefore possible that free fatty acids may induce the expression of pro-inflammatory markers in 3T3-L1 adipocytes. Interestingly, a strong correlation between MCP-1 mRNA expression and ROS release was found, which is in accordance with other studies that showed ROS production could increase MCP-1 expression [30]. It is known that IL-6 suppresses adiponectin gene expression [10], but mRNA levels of this anti-inflammatory adipokine did not show a significant change. Adiponectin is also an important selective controlled modulator of insulin sensitivity and it has been demonstrated that its expression is enhanced by PPAR-γ [35]. Furthermore, it has been also reported that the regulation of these two molecules could be influenced by terminal products of lipid peroxidation [41]. In our experiment, an increase of PPAR-γ mRNA expression was observed, and perhaps this may prevent the decrease of adiponectin expression induced by IL-6. Indeed, PPAR-γ is a transcription factor preferentially expressed in adipose tissue, and it is known that its activation, as we have observed in adipocytes exposed to hyperoxia, could improve insulin resistance [35].
HIF-1α and leptin are important regulators of hundreds of target genes involved in several biological functions, such as cellular metabolism, cell growth and apoptosis, and restoration of the oxygen supply [32, 34]; nevertheless, their expression was not modified by hyperoxia. Finally, ANGPTL4 is a gene involved in glucose and lipid metabolism, which mainly regulates plasma triacylglycerides metabolism by inhibiting LPL [26]. It has been observed that hypoxia stimulates its expression and secretion in human adipocytes [15]. In contrast, a significant decrease in ANGPTL4 mRNA expression was observed in mouse adipocytes exposed to hyperoxia. This outcome proves that oxygen negatively regulates ANGPTL4 mRNA expression in adipocytes. Yamada et al. also demonstrated a downregulation of ANGPTL4 induced by insulin [43], suggesting the possibility that elevated ANGPTL4 expression might be involved in hypertriglyceridemia in insulin resistant states within 3T3-L1 cells. Thus, a downregulation in ANGPTL4, as it occurs with hyperoxia, might contribute to ameliorate these metabolic disorders. Nevertheless, these results should be examined with care since in vitro data not always translate the same outcome than in vivo conditions.
In summary, the current study shows for the first time the effect of hyperoxia on 3T3-L1 adipocytes. The exposure to 95 % O2 seemed to provoke an inflammatory response due to the release of intra and extracellular ROS and the upregulation of pro-inflammatory adipokines such as IL-6 and MCP-1. Perhaps, these adverse effects were produced by the high amount of oxygen used. On the other hand, hyperoxia may play an indirect role in the improvement of insulin sensitivity, due to the upregulation of PPAR-γ signaling, as well as may produce possible beneficial effects on both glucose and lipid metabolism.
References
Barazzone C, Tacchini-Cottier F, Vesin C, Rochat AF, Piguet PF (1996) Hyperoxia induces platelet activation and lung sequestration: an event dependent on tumor necrosis factor-alpha and CD11a. Am J Respir Cell Mol Biol 15:107–114
Bartz RR, Piantadosi CA (2010) Clinical review: oxygen as a signaling molecule. Crit Care 14:234
Benson RM, Minter LM, Osborne BA, Granowitz EV (2003) Hyperbaric oxygen inhibits stimulus-induced proinflammatory cytokine synthesis by human blood-derived monocyte-macrophages. Clin Exp Immunol 134:57–62
Bigdeli MR, Hajizadeh S, Froozandeh M, Rasulian B, Heidarianpour A, Khoshbaten A (2007) Prolonged and intermittent normobaric hyperoxia induce different degrees of ischemic tolerance in rat brain tissue. Brain Res 1152:228–233
Campion J, Milagro FI, Martinez JA (2009) Individuality and epigenetics in obesity. Obes Rev 10:383–392
Conconi MT, Baiguera S, Guidolin D, Furlan C, Menti AM, Vigolo S, Belloni AS, Parnigotto PP, Nussdorfer GG (2003) Effects of hyperbaric oxygen on proliferative and apoptotic activities and reactive oxygen species generation in mouse fibroblast 3T3/J2 cell line. J Investig Med 51:227–232
Desmarquest P, Chadelat K, Corroyer S, Cazals V, Clement A (1998) Effect of hyperoxia on human macrophage cytokine response. Respir Med 92:951–960
Efrati S, Berman S, Ben Aharon G, Siman-Tov Y, Averbukh Z, Weissgarten J (2008) Application of normobaric hyperoxia therapy for amelioration of haemorrhagic shock-induced acute renal failure. Nephrol Dial Transplant 23:2213–2222
Ercin CN, Yesilova Z, Korkmaz A, Ozcan A, Oktenli C, Uygun A (2009) The effect of iNOS inhibitors and hyperbaric oxygen treatment in a rat model of experimental colitis. Dig Dis Sci 54:75–79
Fasshauer M, Kralisch S, Klier M, Lossner U, Bluher M, Klein J, Paschke R (2003) Adiponectin gene expression and secretion is inhibited by interleukin-6 in 3T3-L1 adipocytes. Biochem Biophys Res Commun 301:1045–1050
Fernandez-Galilea M, Perez-Matute P, Prieto-Hontoria P, Martinez JA, Moreno-Aliaga MJ (2011) Effects of lipoic acid on apelin in 3T3-L1 adipocytes and in high-fat fed rats. J Physiol Biochem 67:479–486
Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I (2004) Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 114:1752–1761
Garcia-Diaz DF, Arellano AV, Milagro FI, Moreno-Aliaga MJ, Portillo MP, Martinez JA, Campion J (2011) Glucose and insulin modify thrombospondin 1 expression and secretion in primary adipocytes from diet-induced obese rats. J Physiol Biochem 67:453–461
Garcia-Diaz DF, Campion J, Milagro FI, Boque N, Moreno-Aliaga MJ, Martinez JA (2010) Vitamin C inhibits leptin secretion and some glucose/lipid metabolic pathways in primary rat adipocytes. J Mol Endocrinol 45:33–43
Gonzalez-Muniesa P, de Oliveira C, Perez de Heredia F, Thompson MP, Trayhurn P (2011) Fatty acids and hypoxia stimulate the expression and secretion of the adipokine ANGPTL4 (angiopoietin-like protein 4/fasting-induced adipose factor) by human adipocytes. J Nutrigenet Nutrigenomics 4:146–153
Graham TE, Pedersen PK, Saltin B (1987) Muscle and blood ammonia and lactate responses to prolonged exercise with hyperoxia. J Appl Physiol 63:1457–1462
Gregor MF, Hotamisligil GS (2011) Inflammatory mechanisms in obesity. Annu Rev Immunol 29:415–445
Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, Furukawa S, Tochino Y, Komuro R, Matsuda M, Shimomura I (2007) Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes 56:901–911
Hotamisligil GS (2006) Inflammation and metabolic disorders. Nature 444:860–867
Jensen JC, Pogrebniak HW, Pass HI, Buresh C, Merino MJ, Kauffman D, Venzon D, Langstein HN, Norton JA (1992) Role of tumor necrosis factor in oxygen toxicity. J Appl Physiol 72:1902–1907
Kamiya T, Hara H, Inagaki N, Adachi T (2010) The effect of hypoxia mimetic cobalt chloride on the expression of EC-SOD in 3T3-L1 adipocytes. Redox Rep 15:131–137
Lahat N, Bitterman H, Yaniv N, Kinarty A, Bitterman N (1995) Exposure to hyperbaric oxygen induces tumour necrosis factor-alpha (TNF-alpha) secretion from rat macrophages. Clin Exp Immunol 102:655–659
LeBel CP, Ischiropoulos H, Bondy SC (1992) Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol 5:227–231
Luongo C, Imperatore F, Cuzzocrea S, Filippelli A, Scafuro MA, Mangoni G, Portolano F, Rossi F (1998) Effects of hyperbaric oxygen exposure on a zymosan-induced shock model. Crit Care Med 26:1972–1976
Martinez JA (2006) Mitochondrial oxidative stress and inflammation: an slalom to obesity and insulin resistance. J Physiol Biochem 62:303–306
Mattijssen F, Kersten S (2012) Regulation of triglyceride metabolism by Angiopoietin-like proteins. Biochim Biophys Acta 1821(5):782–789
McAllister EJ, Dhurandhar NV, Keith SW, Aronne LJ, Barger J, Baskin M, Benca RM, Biggio J, Boggiano MM, Eisenmann JC, Elobeid M, Fontaine KR, Gluckman P, Hanlon EC, Katzmarzyk P, Pietrobelli A, Redden DT, Ruden DM, Wang C, Waterland RA, Wright SM, Allison DB (2009) Ten putative contributors to the obesity epidemic. Crit Rev Food Sci Nutr 49:868–913
Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS (2004) Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306:457–461
Perez de Heredia F, Wood IS, Trayhurn P (2010) Hypoxia stimulates lactate release and modulates monocarboxylate transporter (MCT1, MCT2, and MCT4) expression in human adipocytes. Pflugers Arch 459:509–518
Quan Y, Jiang CT, Xue B, Zhu SG, Wang X (2011) High glucose stimulates TNFalpha and MCP-1 expression in rat microglia via ROS and NF-kappaB pathways. Acta Pharmacol Sin 32:188–193
Quintero P, Milagro FI, Campion J, Martinez JA (2010) Impact of oxygen availability on body weight management. Med Hypotheses 74:901–907
Rocha S (2007) Gene regulation under low oxygen: holding your breath for transcription. Trends Biochem Sci 32:389–397
Rooney K, Trayhurn P (2011) Lactate and the GPR81 receptor in metabolic regulation: implications for adipose tissue function and fatty acid utilisation by muscle during exercise. Br J Nutr 106:1310–1316
Seufert J (2004) Leptin effects on pancreatic beta-cell gene expression and function. Diabetes 53(Suppl 1):S152–S158
Spiegelman BM (1998) PPAR-gamma: adipogenic regulator and thiazolidinedione receptor. Diabetes 47:507–514
Stellingwerff T, Leblanc PJ, Hollidge MG, Heigenhauser GJ, Spriet LL (2006) Hyperoxia decreases muscle glycogenolysis, lactate production, and lactate efflux during steady-state exercise. Am J Physiol Endocrinol Metab 290:E1180–E1190
Trayhurn P, Wood IS (2004) Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr 92:347–355
Valdecantos MP, Perez-Matute P, Quintero P, Martinez JA (2010) Vitamin C, resveratrol and lipoic acid actions on isolated rat liver mitochondria: all antioxidants but different. Redox Rep 15:207–216
Wang L, Gill R, Pedersen TL, Higgins LJ, Newman JW, Rutledge JC (2009) Triglyceride-rich lipoprotein lipolysis releases neutral and oxidized FFAs that induce endothelial cell inflammation. J Lipid Res 50:204–213
Wang L, Sapuri-Butti AR, Aung HH, Parikh AN, Rutledge JC (2008) Triglyceride-rich lipoprotein lipolysis increases aggregation of endothelial cell membrane microdomains and produces reactive oxygen species. Am J Physiol Heart Circ Physiol 295:H237–H244
Wang Z, Dou X, Gu D, Shen C, Yao T, Nguyen V, Braunschweig C, Song Z (2012) 4-Hydroxynonenal differentially regulates adiponectin gene expression and secretion via activating PPARgamma and accelerating ubiquitin-proteasome degradation. Mol Cell Endocrinol 349:222–231
Weisz G, Lavy A, Adir Y, Melamed Y, Rubin D, Eidelman S, Pollack S (1997) Modification of in vivo and in vitro TNF-alpha, IL-1, and IL-6 secretion by circulating monocytes during hyperbaric oxygen treatment in patients with perianal Crohn’s disease. J Clin Immunol 17:154–159
Yamada T, Ozaki N, Kato Y, Miura Y, Oiso Y (2006) Insulin downregulates angiopoietin-like protein 4 mRNA in 3T3-L1 adipocytes. Biochem Biophys Res Commun 347:1138–1144
Yamashita M, Yamashita M (2000) Hyperbaric oxygen treatment attenuates cytokine induction after massive hemorrhage. Am J Physiol Endocrinol Metab 278:811–816
Yang ZJ, Bosco G, Montante A, Ou XI, Camporesi EM (2001) Hyperbaric O2 reduces intestinal ischemia-reperfusion-induced TNF-alpha production and lung neutrophil sequestration. Eur J Appl Physiol 85:96–103
Ye J, Gao Z, Yin J, He Q (2007) Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am J Physiol Endocrinol Metab 293:E1118–E1128
Yin J, Gao Z, He Q, Zhou D, Guo Z, Ye J (2009) Role of hypoxia in obesity-induced disorders of glucose and lipid metabolism in adipose tissue. Am J Physiol Endocrinol Metab 296:E333–E342
Acknowledgments
The authors wish to thank the Ministry for Education and Science (MEC, Spain; grant AGL2006-04716/ALI), the Education Department of the Navarra Government (Spain), the Carlos III Health Institute (CIBER project grant CB06/03/1017 and RETIC project grant RD06/0045/0011; Spain), the “Línea Especial” (LE/97) from the University of Navarra (Spain), IBERCAJA and the “Asociación de Amigos de la Universidad de Navarra” (Spain, Diego Garcia-Diaz and Pablo Quintero pre-doctoral grants, respectively) for financial support. Finally, the authors are grateful for the expert technical assistance of Verónica Ciaurriz and to Richard Marks (University of Surrey, Guilford) for technical support and for reviewing the English of the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Quintero, P., González-Muniesa, P., García-Díaz, D.F. et al. Effects of hyperoxia exposure on metabolic markers and gene expression in 3T3-L1 adipocytes. J Physiol Biochem 68, 663–669 (2012). https://doi.org/10.1007/s13105-012-0169-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-012-0169-8