Abstract
Breastmilk is a dynamic, multi-faceted, and complex fluid containing a plethora of biochemical and cellular components that execute developmental effects or differentiation program, providing nourishment and immunity to newborns. Recently, it was reported that breastmilk contains a heterogeneous population of naïve cells, including pluripotent stem cells, multipotent stem cells, immune cells, and non-immune cells. The stem cells derived from breastmilk possess immune privilege and non-tumorigenic properties. Thus, breastmilk may represent an ideal source of stem cells collected by non-perceive procedure than other available sources. Thus, this “maternally originating natural regenerative medicine” may have innumerable applications in clinical biology, cosmetics, and pharmacokinetics. This review describes the efficient integrated cellular system of mammary glands, the impressive stem cell hierarchy of breastmilk, and their possible implications in translational research and therapeutics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human milk is an optimized in vivo product that contains a series of nutritional agents and an array of bioactive factors that confers nutrition and protection to infants (Fig. 1). This relevant source includes various components: (1) genetic materials (such as microRNAs); (2) stem cells; (3) organic substances; and (4) components like immunoglobulins, cytokines, chemokines, cytokine Inhibitors, growth factors, hormones, oligosaccharides, glycans, mucins, and HLA-free antigens (Ballard and Morrow 2013). It has been reported that, compared to maternal serum and plasma the concentration of many growth factors is higher in breastmilk (Kaingade et al. 2017). Among them, vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), epidermal growth factor (EGF), insulin growth factor (IGF), and transforming growth factor-β (TGF-β) have been extensively studied. The sophisticated bioactive factors present in human milk synergistically facilitate the normal development of the offspring via targeted programming.
Recent investigators have revealed that breastmilk contains several cell types, including colostral corpuscles, polymorphonuclear leukocytes, mononuclear phagocytes, and lymphocytes including different types of transitory stem cells such as mesenchymal stem cells (MSCs), mesenchymal stem-like (MSC-like) cells, pluripotent embryonic stem (ES)-like cells, hematopoietic stem cells (HSCs), mammary stem cells (MaSCs), neuroprogenitor cells, and myoepithelial progenitor cells (Aoyama et al. 2010; Kaingade et al. 2017). Stem cells present in breastmilk are able to cross the gut epithelia of the infant in an unknown way and integrate into the newborn’s tissues (Dutta and Burlingham 2010). Thus, this “materno-neonatal relay system” provides protection to the newborn from infection, inflammation, and oxidative stress as well as from overweight and obesity in adolescence and adulthood possibly by the recombination of cells or DNA of maternal origin in offspring via maternal–fetal microchimerism (Melnik et al. 2013; Ozkan et al. 2012).
It is not possible to envisage clearly the cellular environment of lactating breasts by using biopsy of the resting breast tissue. It is also difficult to collect tissues from lactating breast and characterize their complex cellular architecture owing to ethical issues associated with it. Accumulating evidences suggest that the complete cellular hierarchy of lactating breasts is reflected in breastmilk (Hassiotou et al. 2012; Hassiotou and Hartmann 2014).. Thus, breastmilk may act as an excellent model for studying the biology and pathology of breasts. The presence of stem cells makes breastmilk a clinically relevant source for regenerative medicine, cell and tissue engineering, and future stemapeutics. Taking all these into account, the prior work of established milk banks is to develop new methodologies that can store the novelty of breastmilk. This review summarizes the dynamic features of mammary glands, key signaling pathways, and the local cellular cross-talk that orchestrates tissue remodeling and mammary morphogenesis. The cellular components in breastmilk are identified, and the investigation of breastmilk stem cells reveals some of their unique attributes, suggesting potential applications in cell-based therapies, pharmacokinetics, and clinical biology.
Mammary gland development in human
Mammary gland development in females is dynamic and orchestrated in response to systemic hormonal secretion influenced by local paracrine interactions between the developing epithelial ducts and their adjacent embryonic mesenchyme or postnatal stroma (Sternlicht 2006). Mammary glands undergo structural and functional changes at defined stages of human life, namely, embryonic, prepubertal, and pubertal stages, and pregnancy, lactation, and involution (Fig. 2). First a pair of mammary glands appears at 5 weeks as ectodermal mammary streaks that extend bilaterally from the axilla to the groin during embryogenesis. Two weeks later, the mammary ridge or milk line develops in the thoracic portion of the primitive streaks (Fig. 3). Then epithelial mammary gland buds are induced in the ventral epidermis by mammary mesenchyme and ectodermal cells migrate along the mammary line and coalesce to form epithelial placodes (Veltmaat et al. 2004). The mammary placodes go underneath the mesenchyme where epithelial cells advance into the underlying stromal matrix to produce primitive rudimentary ductal structures. This elegant process is chiefly mediated by epithelial–mesenchymal interactions (Macias and Hinck 2012).
The stromal constituents like mesenchymal cells, fibroblasts, blood cells, and leukocytes and extra-cellular matrix (ECM) consisting of laminin, fibronectin, collagen, proteoglycans, etc., further accelerate this process of development (Kass et al. 2007). The temporal and spatial coordination of many signaling pathways requires for directing the change in cell shape and cell movement. These phenomena also play a critical role in establishing communication between cell to cell that is necessary for the development of the mammary gland and tissue morphogenesis (Hens and Wysolmerski 2005). The versatile cellular pattern of the mammary gland is influenced by many signaling pathways from several cell types along with mechanical cues and cell rearrangements (Gjorevski and Nelson 2011).
The tissue architecture of the mammary gland changes with age in response to hormonal and local cues. It undergoes partial remodeling at the onset of puberty and complete remodeling during pregnancy and lactation cycles. The apparent migration, proliferation, and differentiation of cells ultimately results in cellular transformation, rearrangements, commitment, and ultimately converts to a fully functional gland during lactation from a rudimentary organ at birth (Fig. 4a–i). During embryogenesis, cellular morphogenesis is directed by signals from the mesenchyme and is independent of hormonal input (Sternlicht 2006). Thus, at the “resting state,” the mammary gland consists of a network of bilayered epithelial ducts embedded within supporting stromal and adipose tissue (Fig. 5a). At the onset of puberty and subsequent adulthood, the development of the mammary glands is controlled by circulating hormones released from the pituitary and ovary (Macias and Hinck 2012). At the pubertal stage, marked changes in hormonal and local cues rapidly establish a ductal network (Fig. 5b). Onset of pregnancy results in a gradual remodeling of the gland. Dynamic reciprocity (DR) exists between the cells, and the extracellular matrix (ECM), associated change in the hormonal cues, as well as various signaling pathways and growth factors (GFs) further fuel this remodeling process (Thorne et al. 2015). Further, the alveolar development advances toward the establishment of a gland that is profusely filled with alveolar structures by the end of pregnancy (Hovey and Trott 2004). After parturition, milk expulsion is regulated by oxytocin in response to mechanical cues provided by the suckling of newborns from fully functional mammary glands (Fig. 5c).
The complete indulgence and knowledge regarding mammary gland development may significantly contribute to the study of cellular and molecular mechanisms regulating the branching process of other organs and pathways regulating tissue morphogenesis and functional differentiation. Readers are suggested to refer to the excellent review by Javed and Lteif (2013) for a detailed understanding of the precise mechanisms governing the process of development.
Key signaling pathways regulating mammary gland development
The signaling pathways are pivotal players that regulate cellular activities and coordinate different cellular actions through a complex series of events in response to the available cellular microenvironment. β-catenin signaling is imperative for placode formation and its maintenance (Boras-Granic et al. 2006; Zhang et al. 2008). Canonical Wnt signaling is also important for executing the cell movements into the placode. The mammary placode expands and rivets through a series of sequential and reciprocal interactions between the epithelial cells and the surrounding mesenchyme is instructed to form the mammary bud. Furthermore, parathyroid hormone-related protein (PTHrP), Wnt, insulin-like growth factor-1 (IGF1) signaling pathways, and the combination of two homeodomain transcription factors, Msx1 and Msx2, are anticipated to control this event. Sprouting of the mammary buds initiates the formation of the rudimentary ductal tree (Cowin and Wysolmerski 2010). In the case of humans, instead of a single epithelial sprout extending from the mammary bud, several sprouts form, creating multiple mammary trees that unite at the nipple. PTHrP is a critical developmental factor for embryonic mammary development and pubertal ductal morphogenesis (Dunbar and Wysolmerski 1999).
Another study identified that mice lacking growth hormone (GH), Igf1, or estrogen receptor (alpha) (Esr1) genes fail to show pubertal development of the mammary gland (Macias and Hinck 2012). Earlier studies suggest that the administration of GH and prolactin (PRL) can enhance mammogenesis and lactogenesis (Trott et al. 2008). It is presumed that GH and IGF1 mediate the post-natal development of the mammary gland. Both GH and IGF act in a coordinated manner and regulate cell proliferation. These act together with estrogen secreted from the ovary to induce epithelial cell proliferation. Estrogen also shows other functions. Growth and maintenance of alveolar cells is maintained by estrogen during pregnancy (Nandi 1959).
Estrogen signaling occurs through its receptor (ESR1). This works in a paracrine manner to stimulate the release of various GFs. AREG, a member of the epidermal growth factor (EGF) family, proceeds to bind its receptor on stromal cells and induce the expression of fibroblast growth factors (FGFs). FGFs, in turn, stimulating luminal cell proliferation. Other factors, such as TGFß1, Reelin (RELN), Slit2, and Netrin1 (NTN1), contribute significantly to the mammary architecture by either positively or negatively regulating cell proliferation or maintaining cell–cell interactions (Macias and Hinck 2012). Extensive side-branching and alveologenesis is required to create a lactation-competent gland. Progesterone (Pg) and prolactin (PRL) are mainly responsible for activating the “alveolar switch,” a genetic program that synchronizes proliferation, migration, differentiation, and deletion of mammary epithelial cells within the many tissue types of the mammary gland (Oakes et al. 2006). Pg stimulates secondary and tertiary branching, which has been confirmed from a study conducted on mice (Atwood et al. 2000). PRL integrates many signals, including those from the extracellular matrixes (ECMs) by interacting with integrin through the transmembrane signal regulatory protein alpha (SIRPA) (Galbaugh et al. 2010). Again PRL acts as a transducer mediated by the JAK2/STAT5 pathway, whose downstream targets include milk genes casein beta (Csnb) and whey acidic protein (Wap) (Macias and Hinck 2012).
Upon weaning, the gland is remodeled back to its pre-pregnancy state. At involution, the redundancy of the mammary gland occurs as it removes the milk-producing epithelial cells. This occurs in two phases. Stage one is reversible and is regulated largely by STAT3, which is induced by the leukemia inhibitory factor (LIF) and opposes pro-survival STAT5 signaling by upregulating the expression of numerous proteins, including lysosomal proteases, cathepsins, insulin-like growth factor binding protein 5 (IGFBP5), and two regulatory isoforms of phosphatidylinositol 3 kinase, viz., p50alpha (P50A) and p55alpha (P55A). Cell death and limited proteolysis of the extracellular matrix (ECM) occurs during this stage as plasminogen (PLG) is converted to plasmin through the actions of plasma kallikrein (KLK1), yet the alveoli largely retain their shape (Schedin et al. 2004). The changes occur during stage two, which is irreversible and characterized by alveolar collapse and redifferentiation of adipocyte (Watson 2006). The mammary gland returns to its pre-lactation state by active participation of matrix metalloproteinases (MMPs), tissue inhibitors of metalloproteinases (TIMPs) along with plasmin for releasing GFs and remodeling the ECM (Macias and Hinck 2012).
Stem cells in mammary gland
Stem cells are primarily defined as precursor cells of every tissue, which have the potential for unlimited or prolonged self-renewal, as well as the ability to give rise to at least one or more types of mature differentiated cells (Chagastelles and Nardi 2011). Pluripotent stem cells present within the embryo have the ability to give rise to differentiated progeny representatives of all three embryonic germ layers, even to haploid germ cells (Kuijk et al. 2010). In mammals, pluripotency is restricted to the oocyte, blastocyst, early embryonic cells, primordial germ cells, and stem cells of tumors (embryonal carcinoma cells) (Reubinoff et al. 2000). Pluripotent embryonic stem cells (ESCs) are derived from the inner cell mass (ICM) of pre-implanted blastocyst-stage embryo. All these resources provide a unique tool to study the functional expression of developmentally regulated genes and to identify polypeptide factors involved in the differentiation and proliferation of committed embryonic progenitor cells. These can also be used in many therapeutic approaches for treating a wide range of diseases. However, the ethical issues around this pluripotent source of cells restrict the exploration of its full potential.
In the last decade, it was found that somatic cells can revert to the pluripotent state by the reprogramming or reactivation of pluripotency-related genes (Oct4, Sox2, Klf4, c-Myc) and inactivation of tissue-specific genes (Takahashi et al. 2007). Consequently, this pluripotential reprogramming may provide an easily accessible cell source for the treatment of diseases without any ethical debate. However, the mechanisms underpinning cellular reprogramming are still largely unidentified (Kim et al. 2011). Now it is well established that all postnatal organs and tissues contain adult stem cells (ASCs) (Kørbling and Estrov 2003). ASCs chiefly constitute hematopoietic stem cells (HSCs) and non-hematopoietic stem cells (Berz et al. 2007). Current medicine predominantly uses MSCs’a type of non-hematopoietic stem cells may be due to their effective immunomodulatory function and immunotolerant characteristics (Weil et al. 2011). Bone marrow (BM) is supposed to be the most potent source of both HSCs and MSCs. Rather than BM, MSCs can be derived from the adipose tissue, periosteum, synovial membrane, synovial fluid (SF), muscles, dermis, deciduous teeth, pericytes, trabecular bone (TB), infrapatellar fat pad, articular cartilages, umbilical cord blood (UCB), and peripheral blood (Klingemann et al. 2008). However, the collection of tissue samples for the isolation of stem cells from such sources involved an intense invasive procedure. Recently, it has been confirmed that physiological fluids like breastmilk (Hassiotou et al. 2012), menstrual blood (Mou et al. 2013), and urine (Qin et al. 2014; Long et al. 2015) also contain stem cells. Most notably, the samples can be collected via a non-invasive procedure. Breastmilk is supposed to be a novel source for the isolation of different types of stem cells, progenitors, and MSCs that can be used safely in many therapeutic applications than other contemporary sources (Hassiotou 2012).
Development of mammary gland and its homeostasis is a stem-cell-driven process that depends on age-related hormonally driven cues. There is presence of slow cycling stem cells, long- and short-term repopulating cells, and unique fetal MaSC population within the mammary gland (Fu et al. 2014). The cycle of pregnancy, lactation, and involution can repeat itself multiple times during the reproductive lifespan of a woman, suggesting the presence of stem/progenitor cells and original antecedent that supply cells at each new cycle of expansion and for subsequent pregnancy (Smith and Chepko 2001). However, these phenomena are directed in accordance with the niche available to them.
These stem cells are proposed to serve three functions: (1) to give rise to the tissues of the adult mammary gland during development; (2) to allow the enormous tissue expansion and remodeling that occurs in the mammary gland during multiple cycles of pregnancy, lactation, and involution; and (3) rarely, to serve as a reserve for repair in the event of tissue damage. The bipotent mammary stem cells might give rise to both luminal and basal progenitors, from which the morphogenesis of the mammary ductal tree takes place. During pregnancy, alveoli are formed by alveolar precursors to form an external network of myoepithelial cells (Blanpain et al. 2007). Functional expression of milk occurs owing to the contractile properties of mammary myoepithelial cell, which helps to eject milk during lactation (Deugnier et al. 2002). During pregnancy, differentiation of alveolar epithelial cells occurs into groups of small terminal ductal alveolar structures, which are collectively termed as terminal ductal lobular units (TDLUs) (Fig. 6).
Different investigations conducted on mouse models demonstrate that a single normal mouse mammary stem cell can regenerate an entire glandular tree capable of producing milk (Kordon and Smith 1998). An experimental study also identifies that a single cell, marked with a LacZ transgene, can reconstitute a complete mammary gland in vivo (Shackleton et al. 2006). The transplanted cell contributed to both the luminal and myoepithelial lineages and generated functional lobuloalveolar units during pregnancy. The self-renewing capacity of these cells was demonstrated by serial transplantation of clonal outgrowths. However, human mammary stem cells seldom form complete ductal trees across the fat pad upon transplantation (Villadsen et al. 2007; Visvader and Stingl 2014). Current experimental analysis has shown that the pragmatic behavior of fully functional mammary gland with all the cellular characteristics is supposed to be expressed in breastmilk.
Cellular nature of breastmilk
Human milk secretion occurs at three distinct phases, namely, colostrum, transitional milk, and mature milk. Colostrum is the first fluid produced by a mother immediately after delivery, which has a distinct volume, appearance, and composition. It is observed that mature milk contains more nutritional products required for children’s growth, but poor cellular components, whereas early milk has rich cellular constituents (Kaingade et al. 2017). The cellular composition of breastmilk is highly heterogeneous. Many internal and external factors, such as genetic makeup, maternal diets, and environment, are responsible for creating variability in the composition of breastmilk.
Some investigators explain this cellular makeup of breastmilk as epithelial, others as immune/mesenchymal or a mix of both types (Engel 1953; Indumathi et al. 2013). Epithelial cells and their cell clusters are predominantly present in human milk. The first evidence of the presence of epithelial cell clusters of various sizes in mammary secretions during pregnancy and the first week postpartum comes from the study of milk smears (Holmquist and Papanicolaou 1956). These epithelial cells can be isolated and cultured in in vitro condition. In specific growth conditions, epithelial cells form three distinct types of colonies, namely, open, closed, and mixed; these colonies are supposed to contain luminal (CK18+), ductal (CK19+), and myoepithelial cells (CK14+/SMA+) expressing specific cell surface markers. The experimental analysis of the breastmilk identified three different epithelial cell types, namely, lactocytes, squamous epithelial cells, and ductal cells. These cells are profusely present in human milk at different stages of lactation (Brooker 1980). This may be because breastmilk epithelial cells originate from different areas of breast epithelium, both ductal and alveolar. These might enter the milk by exfoliation through breast-feeding. However, a study suggests that epithelial detachment is an active phenomenon that occurs owing to the modification in gene expression for a short interval (Hassiotou 2012). This may be because during this interval the gene expression pattern is governed by stages of lactation, requirement of dyads, and mobility of cells.
In human colostrum, macrophages are the predominant leukocyte type (40–50% of total leukocytes) followed by polymorphonuclear neutrophils (40–50% of total leukocytes) and lymphocytes (5–10% of total leukocytes), and T cells constitute the major cell percentage (approximately 83%) in contrast to B cells (4–6%) of lymphocytic population. In breastmilk, B cells displayed a phenotype of class-switched memory B cells, with few IgD(+) memory and naive B cells. All these leukocytes are active and motile and work precisely in an integrated manner. However, recent analysis of milk cells revealed that mature human milk contain only a smaller fraction (< 2%) of leukocytes, when both mother and infant are healthy. Thus, it clearly indicates that the main cell types the infant receives via breastfeeding for the majority of the lactation period are non-immune cells (Hassiotou and Hartmann 2014).
An investigation revealed that there is incidence of CD34+ hematopoietic stem/progenitor cells in the colostrum (Fan et al. 2010). A flow cytometry study also accounted for the fact that breastmilk expresses the markers for HSCs (CD 34, CD 133, CD 117), MSCs (CD 90, CD 105, CD 73), myoepithelial cells (CD 29, CD 44), immune cells (CD 209, CD 86, CD 83, CD 14, CD 13, HLADR, CD 45), as well as cell-adhesion molecules (CD 31, CD 54, CD 166, CD 106, CD 49d) and other markers (ABCG2, CD140b). The data further show a lower expression of CD 34 (13.07 ± 2.0%), CD 90 (7.79 ± 0.8%) and CD 73 (2.19 ± 0.41%), indicating the presence of negligible amount of HSCs and MSCs in human milk. This study further illustrates that human milk is an integral cellular system of putative stem cells, immune cells, and non-immune cells (Indumathi et al. 2013).
Stem cells subsist in many organs, and tissue systems exhibit a high degree of heterogeneity. In part, this heterogeneity may depend on the origin of tissues and stages of development (Villadsen et al. 2007). The same may hold true for breastmilk and its cellular counterparts. Recent studies have demonstrated that stem cells and differentiated cells from the lactating epithelium enter breastmilk either through cell migration and turnover and/or as a consequence of the mechanical shear forces of breastfeeding. Accumulating evidences from in vitro analysis indicates that a number of morphologically distinct stem cells that include HSCs, MaSCs, MSCs, neuro-progenitor cells, epithelial, myoepithelial progenitor cells, ductal, and alveolar progenitors exist in breastmilk (Fan et al. 2010; Indumathi et al. 2013; Hassiotou and Hartmann 2014).
In vitro analysis of breastmilk stem cells
In vitro cultured breastmilk stem cells have been analyzed by using different methods. The general procedure adapted to perform in vitro culture and characterization of stem cells from breastmilk is depicted in Fig. 7. Briefly, after collection, breastmilk was processed, isolated cells were seeded in culture dishes, and cultivated along with a specific growth medium designed by the concerned researchers. Subsequently, investigations were conducted to evaluate the stemness of in vitro cultured cells by subjecting these to different stem cell markers. The expression of these markers was mainly recorded by following different techniques such as immunofluorescence assay, immunocytochemistry, quantitative real-time polymerase chain reaction, and flow cytometry analysis (Briere et al. 2016).
Role of breastmilk stem cells in the infant after ingestion
Do these stem cells possess similar functions and properties like other stem cells in in vivo and as well as in vitro condition? It is hypothesized that these maternal breast-derived stem cells are able to integrate into the newborn’s tissues and differentiate into functional cells. Thus, maternal milk might play a relevant role in the postnatal development of multiple organs, including the brain, of every neonate undergoing breastfeeding (Fig. 8). Notably, this hypothesis has been recently demonstrated in a mouse model where breast-milk-derived maternal stem cells were identified, 3 weeks after birth, in the stomach wall, in the thymus and in the liver of lactating pups (Hassiotou et al. 2014).
Pioneering studies demonstrate that most leukocytes present in the milk bolus were differentiated myeloid cell precursors. The maternal cells transferred to the offspring via breastmilk allocate themselves in crucial anatomical regions of the intestine “Peyer’s patches (PPs)” of the nursed infant, which are involved in immune surveillance of pathogenic microbes entering the intestinal tract. The major constituents of breastmilk leukocytes localized to PPs are T lymphocytes and cytotoxic T cells (CTLs). Maternal CTLs found in breast milk are directed to the PPs to compensate for the immature adaptive immune system of the infant in order to protect it against constant oral infectious risks during the postnatal phase (Cabinian et al. 2016). Breastmilk stem cells integrate into tissues of the neonate and persist for longer periods, potentially providing protection against infection and allergy. Simultaneously this could potentially prevent incidences of necrotizing enterocolitis, obesity, and chronic conditions such as type I diabetes, celiac disease, Crohn’s disease, and even faster psychomotor development (Kramer 2010; Hassiotou and Geddes 2015; Bion et al. 2016; Witkowska-Zimny et al. 2017). The integration of the maternal genome into the neonate genome though ingestion of milk might also result in permanent correction of genetic disorders (Irmak et al. 2012).
Transitional properties of human breastmilk derived stem cells (hBMDSCs)
Similarity with ESCs
Accumulating evidences suggest that hBMDSCs show hallmarks of stemness. These stem cells express characteristics similar to those of ESCs. The colony-forming ability is one of the distinguishing features of human ESCs. Similar to ESCs, hBMDSCs also form compact colonies. Their undifferentiated status of ESCs are marked by the consistent expression of stage-specific embryonic antigen-3 (SSEA-3), stage-specific embryonic antigen-4 (SSEA-4), tumor-rejection antigen-1-60 (TRA-l-60), and tumor-rejection antigen-1-81 (TRA-1-81).
It is quite interesting to note that positive expressions of SSEA4, SSEA3, and TRA-1-60/TRA-1-81 are shown by hBMDSCs. These colonies typically express the pluripotency genes OCT4, SOX2, NANOG, and downstream targets KLF4, REX1, and GDF3 (Hassiotou et al. 2012; Twigger et al. 2015) as expressed by ESCs (Thomson et al. 1998; Ginis et al. 2004). Subsequently, another study observed that a subpopulation of hBMDSCs express TRA 60-1, Oct4, Nanog, and Sox2 but not SSEA-1 or SSEA-4 (Sani et al. 2015). Concordantly, like other multipotent stem cells, hBMDSCs also exhibit extensive proliferation, differentiate into multiple lineages, and undergo replicative senescence after a limited number of cell doubling. The differential gene expression pattern shown by hBMDSCs ranging from early-stage pluripotent stem cells to more committed progenitors to terminally differentiated cells might represent peculiar stem cell heterogeneity that is observed in in vivo lactating mammary glands. This may be due to the fact that the generation of cells that produce milk also takes place at the time of conception as governed by hormonal cues (share the same timing with the embryo from which ESCs are derived) and functionally expressed mammary epithelial stem cells, a type of ASCs after parturition (Pang and Hartmann 2007). Thus, breastmilk may represent a valuable source of a series of transitory stem cells shuttling between two different functionally active cellular compartments. The other reason for this peculiar property shown by hBMDSCs could be that the expression of pluripotent genes at a certain stage of mammary gland development may be controlled by the epigenetic mechanism (Hassiotou 2012).
Similarity with MSCs
The approach of examining the cell fate of in vitro cultured human milk indicates that these cells initially form irregularly shaped epithelial-like cells, but later attain typical slender fibroblast-like MSC phenotypes. The presence of MSCs was first hypothesized in the late nineteenth century by Cohnheim (1867). A century later, for the first time isolation of MSCs and elucidation of its in vitro characteristics was done from BM (Friedenstein et al. 1968). These cells are plastic adherent, show multilineage differentiation potential, and express a cluster of designation or classification determinant (CD) molecules such as CD90, CD44, CD271, and CD146. CD73, and CD105 and lack the expression of CD 14, CD19, CD31, CD34, CD45, and HLA-DR (Sani et al. 2015). However, to date, MSCs lack a unique identifying phenotypic marker (Baer et al. 2013).
The immunofluorescence study of hBMDSCs for specific cell surface markers clearly indicates that these cells express mesenchymal markers like CD44, CD29, and Sca-1 and cytoskeletal protein markers such as smooth muscle actin (SMA), vimentin, and nestin. Negative expression of CD33, CD34, CD45, and CD73 was observed, further confirming their identity as MSCs (Buescher and Pickering 1986). Further, these cells also manifest the presence of E-Cadherin, an epithelial-to-mesenchymal transition marker in their early passages (Patki et al. 2010; Pichiri et al. 2016). It may be because the phenomenon of epithelial to mesenchymal transition (EMT) that occurs during mammary gland branching morphogenesis also recapitulates in the in vitro culture of breastmilk. During EMT, epithelial cells lose their apico-basal polarity (Micalizzi et al. 2010). Tight junctions that typically maintain apico-basal polarity dissolve, allowing the mixing of apical and basolateral membrane proteins.
Adherens and gap junctions are disassembled and cell surface proteins such as E-cadherin and epithelial-specific integrins are replaced by N-cadherin and integrins specific to extracellular components. The actin cytoskeleton is remodeled into stress fibers, which accumulate at areas of cell protrusions. The epithelial intermediate filaments, cytokeratins, are replaced by vimentin. Meanwhile, the underlying basement membrane is degraded and the cell invades and moves into the surrounding stroma, devoid of cell–cell contacts (Fig. 9). A recent study also reports that in vitro breastmilk-derived MSCs are likely to be mediated through epithelial–mesenchymal transition (Kaingade et al. 2016).
Analysis of pluripotent gene expression profile of breastmilk stem cells versus normal resting breast
It has also been observed that a rare subpopulation of the normal resting breast (from non-pregnant, non-lactating women) also express pluripotent genes (OCT4, SOX2, NANOG) and display many features of pluripotency, such as formation of teratoma and capability to differentiate into cell types of the primary three-germ layer in both in vitro and in vivo conditions (Dontu et al. 2003; Roy et al. 2013). It is noteworthy that embryonic TFs like OCT4, SOX2, NANOG, and KLF4 are also expressed in the resting mammary tissue of males (Richter et al. 2013). However, during pregnancy and lactation, a significant upregulation of these genes occurs at specific cell populations of the female breast, an event that is potentially driven by hormone and a fuel to transform the gland into a milk-secretory organ (Seymour et al. 2015).
Breastmilk gene expression varies with characteristics of infant and mother
The demographic gene expression profile of breastmilk varies with infant and maternal characteristics. Infant gestational age at delivery and changes in maternal bra cup size between pre-pregnancy and postpartum lactation were also associated with expression of genes controlling stemness as well as milk synthesis. A study demonstrates that higher expression of α-lactalbumin (α-LA) and NESTIN was observed for infants with greater gestational age at delivery (Twigger et al. 2015). This may be due the fact that the mammary gland is more mature in mothers of term infants, containing more milk-secretory cells, more cells with progenitor properties that are able to maintain some level of plasticity. Additionally, the expression levels of the stem cell marker SOX2 were much higher in milk from mothers of preterm infants and associated with lower-level expression of α-LA and NESTIN than in mothers of full-term infant (Rasmussen 2007).
Moreover, women with a larger body mass index (BMI) have less epithelial tissue that is capable of synthesizing milk. A number of studies report that maternal obesity before conception leads to several complications in the postnatal breastfeeding period, including delayed onset of lactogenesis, impairment of lactogenesis II, late initiation of breastfeeding to infant, and short breastfeeding duration (Rasmussen et al. 2001). A recent study revealed a speculative fact that changes occurring in bra cup size in women between pre- and post-pregnancy periods were associated with lower expression of SOX2 and higher expression of REX1, α-LA, and epithelial cell adhesion molecule (EPCAM) (Twigger et al. 2015). This may be because there is an increase in breast volume during the lactation period that reflects the presence of more epithelial cells resulting in higher milk production.
Differentiation of hBMDSCs
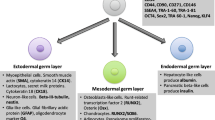
Upon induction of appropriate inducers, hBMDSCs could be able to differentiate and generate into cell types representative of three embryonic germ layers under conditions. During initial period, it was illustrated that MSCs could differentiate only into tissues of mesodermal origin (Friedenstein et al. 1968). However, recent experimental analysis on MSCs has transformed this doctrine. It has been observed that MSCs could be successfully differentiated into a variety of cell lineages like neurons, muscle cells, epithelial cells, hepatocytes, including osteoblasts, chondrocytes, and adipocytes. This may result owing to the presence of particular soluble factors in the culture medium that induce reprogramming of gene expression patterns leading to differentiation of specific lineages. The recent tracking of differentiation hierarchy of hBMDSCs concludes that hBMDSCs also exhibit “stem cells plasticity.” These cells contribute to neurons, myoepithelial cells, luminal cells, pancreatic beta cells, and hepatocytes, apart from traditional MSCs differentiation into osteoblasts, chondroblasts, and adipoblast. These differentiated cells also express lineage-specific markers (Patki et al. 2010; Hassiotou et al. 2012).
Several studies conducted on in vitro cultured breastmilk cells demonstrate that when hBMDSCs are cultured on ultralow-binding plates, these actively participate to form spheroids. These spheroids express elevated levels of pluripotent genes associated with ESCs (Hassiotou et al. 2012). Three-dimensional (3D) multicellular spheroids resemble “embryoid bodies (EBs) to a certain extent. These 3D structures provide a congenial environment that increases structural integrity, high surface area, and porosity, which further promotes tissue remodeling and facilitates cell–cell regulatory mechanisms and signaling networks. These have improved capacity of differentiation into mammary, neuroectodermal, mesodermal, and endodermal lineages (Cui et al. 2017). In addition, the differentiated cell types releases cytokines and angiogenic factors, facilitates angiogenesis, replenishes damaged tissues, and enhances cell survival rate after implantation. Thus, hBMDSCs may be considered as a therapeutically effective cell source for clinical applications. Various strategies, such as hanging drops, spinner flasks, non-adherent surfaces, and micro-fabricated scaffolds, have been followed for efficient and reliable generation of spheroids (Han et al. 2015).
The gene expression analysis of breastmilk cells identified core genes such as cytokeratin (CK) CK5, CK14, ESRRB, and α-LA responsible for organizing the behavior of the mammary gland during lactation and development of infants. In in vivo conditions, breastmilk stem cells may integrate and differentiate into neural cells as well as other cell types in the infant’s body, potentially secreting neurotrophic factors. Further, these integrated stem cells are involved in tissue homeostasis and development (Twigger et al. 2015). Evidence suggests that a common regulator TBX3 plays a central role in the development of both the mammary gland and neuroepithelium. This is also involved in ESCs differentiation and self-renewal (Esmailpour and Huang 2012). It is demonstrated that hBMDSCs are extensively differentiated into neural-like cell lineages including neurons, oligodendrocytes, and astrocytes. These neural-like cells show exclusive features of neuronal lineages and express neural stem cells markers nestin and CD 133, neuron marker β-tubulin, oligodendrocyte marker O4, and astrocyte marker GFAP (Hosseini et al. 2014).
Cregan et al. (2007) identified the expression of CK5 (marker mammary stem cell), CK14 (marker of myoepithelial cells), CK18 [marker of alveolar cells (lactocytes)], and CK19 (marker of ductal non-secretory epithelial cells) in breast-milk-derived cell culture for the first time. hBMDSCs also express markers of differentiated cells like epithelial progenitor marker p63, mammary stem-like cell markers α-6 integrin (CD49f), epithelial cell marker (EPCAM), myoepithelial cells marker (SMA), and milk proteins such as α-lactalbumin (α-LA)15 and β-casein are also identified (Thomas et al. 2011; Twigger et al. 2015). The overwhelming characteristics of hBMDSCs to differentiate into hepatocytes, cardiomyocytes, neuronal cells, and pancreatic lineages intricately by appropriate stimulus may provide a suitable alternative to replace classical animal models that are currently being used for pharmacological studies (Hassiotou 2012).
Role of stem cells in preterm breastmilk
Premature babies suffer from several complications such as lower birth weight, cerebral palsy, sensory deficits, learning disabilities, respiratory illness, gastrointestinal disorders, and other diseases. Breastfeeding is the most preferred way to fulfill the requirement of preterm infants from the nutritional, gastrointestinal, immunological, developmental, and psychological point of view. The composition of preterm milk depends on the degree of prematurity. As the preterm milk enhances immunity, facilitates neurological development, smoothens gastrointestinal function, and improves delayed developmental responses that occur in premature infants.
Continuous studies reported that if preterm infants feed on breastmilk so often, it improves the feeding tolerance, lowers infection risks, and a decreased rate of necrotizing enterocolitis. All these behaviors exhibited by preterm breastmilk may be due to the presence of ideal cellular/non-cellular constituents like epithelial/mammary stem cell population, hematopoietic/mesenchymal stem/progenitor cells, cell adhesion molecules, and pluripotent markers that most likely favor immunity, growth, and cell fate development of premature infants (Kaingade et al. 2017).
Current status and future prospective of breastmilk stem cells
The remarkable presence of stem cells from both hematopoietic stem cells (HSCs) and mammary origin reveal that hBMDSCs may be a suitable alternative to MSCs derived from BM (BMMSCs) (Hassiotou and Geddes 2015; Hassiotou et al. 2015). Recent technological advances further characterize the cell types present in breastmilk from protein and messenger RNA levels (Irmak et al. 2012). The details regarding significant work conducted thus far on stem cells present in breastmilk are given in Table 1.
Breastmilk banking
Milk acts as an inexhaustible source in a breastfeeding mother. Establishment of a breastmilk bank is another approach to save the life and intellectual abilities of premature babies, as they have not received mother’s milk in sufficient amount right after birth. Such a milk bank should follow the rules and regulations laid by the government. The major concern to establish such a bank is that the (1) donation of milk should be done voluntarily, (2) selection of donors includes healthy and well-nourished women, with no incidence of tuberculosis/human immunodeficiency virus (HIV)/hepatitis or any other infection. Donors should not be taking any kind of medication such as hormonal treatment or drugs. Thus, donated milk distributed through milk banks is becoming an alternative to formula feeding for working ladies and considered an ideal nourishment for hospitalized preterm infants in North America, Germany, and Australia (Ewaschuk et al. 2011; Smith 2015). The process of banking of milk starts with expressions and collection of milk and collection in the specified container. Each container must be labeled with the name, date, and time of collection. In the bank, milk is preserved at − 20 °C. On the day before processing, milk is thawed by keeping the containers in a refrigerator (4 °C) overnight. After pooling essential nutrients such as protein, fat, and other substances, the pasteurization of milk is conducted in a water bath at 62.5 °C for 30 min (Underwood 2012). Pasteurized milk stored in the freezer should be used within 6 months from the date of expression. Therefore, new preservation methods should be develop that can store all the cellular and biochemical ingredients of breastmilk. The first mother’s milk bank in Asia was established in Mumbai on November 27, 1989. Efforts should be undertaken to establish such banks worldwide. From the observations of rich cellular content in breastmilk and its significant effects on neonates in the long term, it is concluded that every mother’s priority should be to breastfeed her child. Therefore, the need of the hour is to educate families, especially mothers, regarding the advantages of breastfeeding, as this support babies at the physical and intellectual levels. The advantages of such milk banks may be seen especially in very low weight babies (LBW) in the first few days, lower segment Caesarean section (LSCS) deliveries, multiple pregnancies, babies of mothers with problems, mothers who are not in a position to feed, babies with some diseases such as necrotizing enterocolitis, etc. (Kaingade et al. 2017). However, the technology regarding extraction and long-term preservation of stem cells from breastmilk is not yet well established. This may be due to isolation of stem cells from breastmilk being at an initial stage in the field of stem cell science. Investigations are going on globally to preserve this invaluable product that has potential use in future regenerative medicine (Hassiotou 2012).
Implication of breastmilk in current practice and research
The beneficial effects of breastfeeding for infants and mothers are well documented. Statements like “breast feeding saves lives” and “breastmilk is the best food for babies” have been proved true (Dieterich et al. 2013). However, the recent finding of stem cells in breastmilk introduces a novel aspect in the field of human milk research. After this evidence, it is most appealing for all healthcare professionals, clinical practitioners, and medicos to make the public aware of the potential of breastmilk and its impact on infant health.
hBMDSCs may be a pertinent source that can help to replenish, repair, and regenerate damaged brain tissue. In regenerative approaches for neurological diseases, MSCs are delivered via intracerebral or intrathecal injection to patients (Joyce et al. 2010). Upon transplantation into the brain, MSCs promote endogenous neuronal growth, decrease apoptosis, reduce levels of free radicals, encourage synaptic connection from damaged neurons, and regulate inflammation, primarily through paracrine actions (Fig. 10). It may be because the mammary gland and nervous system share the same developmental origin (ectoderm). Thus, breastmilk cells may be a pertinent contender for neural cell lineage differentiation and enteric neurons differentiation (Witkowska-Zimny and Kaminska-El-Hassan 2017).
Recent study also show that when the spheroids-derived cells were injected into severe combined immune deficient mice (SCID), there was no sign of teratoma formation. This feature is quite similar to that of the Multilineage Differentiating Stress Enduring Cell (MUSE cells) characteristic (Simerman et al. 2016). Thus, hBMDSCs are anticipated to be of great medical importance, from advanced clinical deliberation to diseases and development, and may create novel therapeutic possibilities for treating many congenital abnormalities and developmental disorders in contrast to ESCs and iPSCs. As accumulating evidence suggests that MSCs contribute the lower level of side effects without provoking immunological response after engraftment (Dehghanifard et al. 2013). Furthermore, a recent study reported that genetically modified MSCs are able to overexpress antitumor genes, indicating that MSCs can be used in anticancer therapy effectively (Sage et al. 2016). Thus, human milk may be a novel source for the derivation of MSCs that can be used to treat neurodegenerative diseases, chronic disorders, acute brain injuries, and cancer.
Conclusion
The profound cellular hierarchy of breastmilk represents a valuable model to study the paraphernalia of mammary gland and pathologies of breast. However, the substantial use of hBMDSCs in regenerative medicine relies on revealing the detailed mechanisms of interaction, integration, and incorporation of stem cells of maternal origin in the infants. In addition to the available information in literature, clinical studies are necessary to determine the efficacy of hBMDSCs in treating those disorders for which no treatment are available in current medicine.
References
Aoyama K, Matsuoka K, Teshima T (2010) Breastmilk and transplantation tolerance. Chimerism 1(1):9–20. https://doi.org/10.4161/chim.1.1.11996
Atwood CS, Hovey RC, Glover JP, Chepko G, Ginsburg E, Robison WG, Vonderhaar BK (2000) Progesterone induces side-branching of the ductal epithelium in the mammary glands of peripubertal mice. J Endocrinol 167(1):39. https://doi.org/10.1677/joe.0.1670039
Baer PC, Kuçi S, Krause M, Kuçi Z, Zielen S, Geiger H, Bader P, Schubert R (2013) Comprehensive phenotypic characterization of human adipose-derived stromal/stem cells and their subsets by a high throughput technology. Stem Cells Dev 22(2):330–339. https://doi.org/10.1089/scd.2012.0346
Ballard O, Morrow AL (2013) Human milk composition: nutrients and bioactive factors. Pediatr Clin North Am 60(1):49–74. https://doi.org/10.1016/j.pcl.2012.10.002
Berz D, McCormack EM, Winer ES, Colvin GA, Quesenberry PJ (2007) Cryopreservation of hematopoietic stem cells. Am J Hematol 82(6):463–472. https://doi.org/10.1002/ajh.20707
Bion V, Lockett GA, Soto-Ramírez N, Zhang H, Venter C, Karmaus W, Holloway JW, Arshad SH (2016) Evaluating the efficacy of breastfeeding guidelines on long-term outcomes for allergic disease. Allergy 71(5):661–670. https://doi.org/10.1111/all.12833
Blanpain C, Horsley V, Fuchs E (2007) Epithelial stem cells: turning over new leaves. Cell 128(3):445–458. https://doi.org/10.1016/j.cell.2007.01.014
Boras-Granic K, Chang H, Grosschedl R, Hamel PA (2006) Lef1 is required for the transition of Wnt signaling from mesenchymal to epithelial cells in the mouse embryonic mammary gland. Dev Biol 295(1):219–231
Briere CE, McGrath JM, Jensen T, Matson A, Finck C (2016) Breast milk stem cells: current science and implications for preterm infants. Adv Neonatal Care 16(6):410–419. https://doi.org/10.1097/ANC.0000000000000338
Briere CE, Jensen T, McGrath JM, Young EE, Finck C (2017) Stem-like cell characteristics from breast milk of mothers with preterm infants as compared to mothers with term infants. Breastfeed Med 12:174–179. https://doi.org/10.1089/bfm.2017.0002
Brooker BE (1980) The epithelial cells and cell fragments in human milk. Cell Tissue Res 210(2):321–332
Buescher ES, Pickering LK (1986) Polymorphonuclear leukocytes in human colostrum and milk. In: Howell RR, Morriss FH, Pickering LK (eds) Human milk in infant nutrition and health. Charles C. Thomas Publisher, Springfield, pp 160–173
Cabinian A, Sinsimer D, Tang M, Zumba O, Mehta H, Toma A, Sant’Angelo D, Laouar Y, Laouar A (2016) Transfer of maternal immune cells by breastfeeding: maternal cytotoxic T lymphocytes present in breast milk localize in the Peyer’s patches of the nursed infant. PLoS ONE 11:e0156762
Chagastelles PC, Nardi NB (2011) Biology of stem cells: an overview. Kidney Int Suppl 1(3):63–67. https://doi.org/10.1038/kisup.2011.15
Cohnheim J (1867) Ueber entzundung und eiterung. Path Anat Physiol Klin Med 40: 1–79. 95059459; Beneke, Rudolf, 1861?- nr 00037756
Cowin P, Wysolmerski J (2010) Molecular mechanisms guiding embryonic mammary gland development. Cold Spring Harb Perspect Biol 2:a003251. https://doi.org/10.1101/cshperspect.a003251
Cregan MD, Fan Y, Appelbee A, Brown ML, Klopcic B, Koppen J, Mitoulas LR, Piper KM, Choolani MA, Chong YS et al (2007) Identification of nestin-positive putative mammary stem cells in human breastmilk. Cell Tissue Res 329(1):129–136. https://doi.org/10.1007/s00441-007-0390-x
Cui X, Hartanto Y, Zhang H (2017) Advances in multicellular spheroids formation. J R Soc Interface 14(127):20160877. https://doi.org/10.1098/rsif.2016.0877
Dehghanifard A, Shahjahani M, Soleimani M, Saki N (2013) The emerging role of mesenchymal stem cells in tissue engineering. Int J Hematol Oncol Stem Cell Res 7(1):46–47
Deugnier M-A, Teulière J, Faraldo MM, Thiery JP, Glukhova MA (2002) The importance of being a myoepithelial cell. Breast Cancer Res 4(6):224–230
Dieterich CM, Felice JP, O’Sullivan E, Rasmussen KM (2013) Breastfeeding and health outcomes for the mother-infant dyad. Pediatr Clin North Am 60(1):31–48. https://doi.org/10.1016/j.pcl.2012.09.010
Dontu G, Al-Hajj M, Abdallah WM, Clarke MF, Wicha MS (2003) Stem cells in normal breast development and breast cancer. Cell Prolif 36(suppl1):59–72
Dunbar ME, Wysolmerski JJ (1999) Parathyroid hormone-related protein: a developmental regulatory molecule necessary for mammary gland development. J Mammary Gland Biol Neoplasia 4(1):21–34
Dutta P, Burlingham WJ (2010) Stem cell microchimerism and tolerance to non-inherited maternal antigens. Chimerism 1(1):2–10. https://doi.org/10.4161/chim.1.1.12667
Engel S (1953) An investigation of the origin of the colostrum cells. J Anat 87(4):362–366
Esmailpour T, Huang T (2012) TBX3 promotes human embryonic stem cell proliferation and neuroepithelial differentiation in a differentiation stage-dependent manner. Stem Cells 30(10):2152–2163. https://doi.org/10.1002/stem.1187
Ewaschuk JB, Unger S, Harvey S, O’Connor DL, Field CJ (2011) Effect of pasteurization on immune components of milk: implications for feeding preterm infants. Appl Physiol Nutr Metab 36(2):175–182. https://doi.org/10.1139/h11-008
Fan Y (2011) The search for stem cells in human breast milk. Ph.D. thesis, National University of Singapore
Fan Y, Chong YS, Choolani MA, Cregan MD, Chan JK (2010) Unravelling the mystery of stem/progenitor cells in human breast milk. PLoS ONE 5(12):e14421. https://doi.org/10.1371/journal.pone.0014421
Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP (1968) Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation 6(2):230–247
Fu N, Lindeman GJ, Visvader JE (2014) The mammary stem cell hierarchy. Curr Top Dev Biol 107:133–160. https://doi.org/10.1016/B978-0-12-416022-4.00005-6
Galbaugh T, Feeney YB, Clevenger CV (2010) Prolactin receptor-integrin cross-talk mediated by SIRP alpha in breast cancer cells. Mol Cancer Res 8(10):1413–1424. https://doi.org/10.1158/1541-7786.MCR-10-0130
Ginis I, Luo Y, Miura T, Thies S, Brandenberger R, Gerecht-Nir S, Amit M, Hoke A, Carpenter MK, Itskovitz-Eldor J et al (2004) Differences between human and mouse embryonic stem cells. Dev Biol 269(2):360–380. https://doi.org/10.1016/j.ydbio.2003.12.034
Gjorevski N, Nelson CM (2011) Integrated morphodynamic signalling of the mammary gland. Nat Rev Mol Cell Biol 12(8):581–593. https://doi.org/10.1038/nrm3168
Han C, Takayama S, Park J (2015) Formation and manipulation of cell spheroids using a density adjusted PEG/DEX aqueous two phase system. Sci Rep 5:11891. https://doi.org/10.1038/srep11891
Hassiotou F (2012) The cellular hierarchy of human breastmilk: novel insights to breastmilk stem cells. Ph.D. thesis, The University of Western Australia
Hassiotou F, Geddes DT (2015) Immune cell–mediated protection of the mammary gland and the infant during breastfeeding. Adv Nutr 6(3):267–275. https://doi.org/10.3945/an.114.007377
Hassiotou F, Hartmann PE (2014) At the dawn of a new discovery: the potential of breastmilk stem cells. Adv Nutr 5(6):770–778. https://doi.org/10.3945/an.114.006924
Hassiotou F, Beltran A, Chetwynd E, Stuebe AM, Twigger A-J, Metzger P, Hartmann PE (2012) Breastmilk is a novel source of stem cells with multilineage differentiation potential. Stem Cells (Dayton, Ohio) 30(10):2164–2174. https://doi.org/10.1002/stem.1188
Hassiotou F, Heath B, Ocal O, Filgueira L, Geddes D, Hartmann P, Wilkie T (2014) Breastmilk stem cell transfer from mother to neonatal organs (216.4). FASEB J 28(suppl 1):216
Hassiotou F, Geddes DT, Blancafort P, Filgueira L, Hartmann PE (2015) Breastmilk stem cells: recent advances and future prospects. In: Bhattacharya N, Stubblefield P (eds) Regenerative medicine. Springer, London. https://doi.org/10.1007/978-1-4471-6542-2_18
Hens JR, Wysolmerski JJ (2005) Key stages of mammary gland development: molecular mechanisms involved in the formation of the embryonic mammary gland. Breast Cancer Res 7(5):220–224. https://doi.org/10.1186/bcr1306
Holmquist DG, Papanicolaou GN (1956) The exfoliative cytology of the mammary gland during pregnancy and lactation. Ann NY Acad Sci 63(6):1422–1435
Hosseini SM, Talaei-khozani T, Sani M, Owrangi B (2014) Differentiation of human breast-milk stem cells to neural stem cells and neurons. Neurol Res Int. https://doi.org/10.1155/2014/807896
Hovey RC, Trott JF (2004) Morphogenesis of mammary gland development. Adv Exp Med Biol 554(2):219–228
Indumathi S, Dhanasekaran M, Rajkumar JS, Sudarsanam D (2013) Exploring the stem cell and non-stem cell constituents of human breast milk. Cytotechnology 65(3):385–393. https://doi.org/10.1007/s10616-012-9492-8
Irmak MK, Oztas Y, Oztas E (2012) Integration of maternal genome into the neonate genome through breast milk mRNA transcripts and reverse transcriptase. Theor Biol Med Model 9:20. https://doi.org/10.1186/1742-4682-9-20
Javed A, Lteif A (2013) Development of the Human Breast. Semin Plast Surg 27(1):5–12. https://doi.org/10.1055/s-0033-1343989
Joyce N, Annett G, Wirthlin L, Olson S, Bauer G, Nolta JA (2010) Mesenchymal stem cells for the treatment of neurodegenerative disease. Regen Med 5(6):933–946. https://doi.org/10.2217/rme.10.72
Kaingade PM, Somasundaram I, Nikam AB, Sarang SA, Patel JS (2016) Breastmilk-derived mesenchymal stem cells in vitro are likely to be mediated through epithelial-mesenchymal transition. Breastfeed Med 11:152. https://doi.org/10.1089/bfm.2016.0023
Kaingade P, Somasundaram I, Nikam A, Behera P, Kulkarni S, Patel J (2017) Breast milk cell components and its beneficial effects on neonates: need for breast milk cell banking. J Pediatr Neonat Individual Med 6:e060115. https://doi.org/10.7363/060115
Kass L, Erler JT, Dembo M, Weaver VM (2007) Mammary epithelial cell: influence of extracellular matrix composition and organization during development and tumorigenesis. Int J Biochem Cell Biol 39(11):1987–1994
Kim JS, Choi HW, Choi S, Do JT (2011) Reprogrammed pluripotent stem cells from somatic cells. Int J Stem Cells 4(1):1–8
Klingemann H, Matzilevich D, Marchand J (2008) Mesenchymal stem cells—sources and clinical applications. Transfus Med Hemoth 35(4):272–277. https://doi.org/10.1159/000142333
Kørbling M, Estrov ZN (2003) Adult stem cells for tissue repair—a new therapeutic concept? New Engl J Med 349(6):570–582. https://doi.org/10.1056/NEJMra022361
Kordon EC, Smith GH (1998) An entire functional mammary gland may comprise the progeny from a single cell. Development 125(10):1921–1930
Kramer MS (2010) “Breast is best”: the evidence. Early Hum Dev 86(11):729–732. https://doi.org/10.1016/j.earlhumdev.2010.08.005
Kuijk EW, de Sousa Chuva, Lopes SM, Geijsen N, Macklon N, Roelen BA (2010) The different shades of mammalian pluripotent stem cells. Hum Reprod Update 17(2):254–271. https://doi.org/10.1093/humupd/dmq035
Long T, Wu R, Lu X, Deng J, Qin D, Zhang Y (2015) Urine-derived stem cells for tissue repair in the genitourinary system. J Stem Cell Res Ther 5:317. https://doi.org/10.4172/2157-7633.1000317
Macias H, Hinck L (2012) Mammary gland development. Wiley Interdiscip Rev Dev Biol 1(4):533–557. https://doi.org/10.1002/wdev.35
Melnik BC, John SM, Schmitz G (2013) Milk is not just food but most likely a genetic transfection system activating mTORC1 signaling for postnatal growth. Nutr J 12:103. https://doi.org/10.1186/1475-2891-12-103
Micalizzi DS, Farabaugh SM, Ford HL (2010) Epithelial-mesenchymal transition in cancer: parallels between normal development and tumor progression. J Mammary Gland Biol Neoplasia 15(2):117–134. https://doi.org/10.1007/s10911-010-9178-9
Mou XZ, Lin J, Chen JY, Li YF, Wu XX, Xiang BY, Li CY, Ma JM, Xiang C (2013) Menstrual blood-derived mesenchymal stem cells differentiate into functional hepatocyte-like cells. J Zhejiang Univ Sci B 14(11):961–972. https://doi.org/10.1631/jzus.B1300081
Nandi S (1959) Hormonal control of mammogenesis and lactogenesis in the C3H/He Crgl mouse. Univ Calif Publ Zool 65:1–128
Oakes SR, Hilton HN, Ormandy CJ (2006) The alveolar switch: coordinating the proliferative cues and cell fate decisions that drive the formation of lobuloalveoli from ductal epithelium. Breast Cancer Res 8(2):207. https://doi.org/10.1186/bcr1411
Ozkan H, Tuzun F, Kumral A, Duman N (2012) Milk kinship hypothesis in light of epigenetic knowledge. Clin Epigenetics 4:14. https://doi.org/10.1186/1868-7083-4-14
Pang WW, Hartmann PE (2007) Initiation of human lactation: secretory differentiation and secretory activation. J Mammary Gland Biol Neoplasia 12(4):211–221. https://doi.org/10.1007/s10911-007-9054-4
Patki S, Kadam S, Chandra V, Bhonde R (2010) Human breastmilkis a rich source of multipotent mesenchymal stem cells. Hum Cell 23(2):35–40. https://doi.org/10.1111/j.1749-0774.2010.00083.x
Pichiri G, Lanzano D, Piras M, Dessì A, Reali A, Puddu M, Noto A, Fanos V, Coni C, Faa G, Coni P et al (2016) Human breast milk stem cells: a new challenge for perinatologists. J Pediatr Neonat Individual Med 5:e050120. https://doi.org/10.7363/050120
Qin D, Long T, Deng J, Zhang Y (2014) Urine-derived stem cells for potential use in bladder repair. Stem Cell Res Ther 5(3):69. https://doi.org/10.1186/scrt458
Rasmussen KM (2007) Association of maternal obesity before conception with poor lactation performance. Annu Rev Nutr 27:103–121. https://doi.org/10.1146/annurev.nutr.27.061406.093738
Rasmussen KM, Hilson JA, Kjolhede CL (2001) Obesity may impair lactogenesis II. J Nutr 131(11):3009S–3011S. https://doi.org/10.1093/jn/131.11.3009S
Reubinoff BE, Pera MF, Fong CY, Trounson A, Bongso A (2000) Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotechnol 18(4):399–404. https://doi.org/10.1038/74447
Richter A, Nissen N, Mailänder P, Stang F, Siemers F, Kruse C, Danner S (2013) Mammary gland-derived nestin-positive cell populations can be isolated from human male and female donors. Stem Cell Res Ther 4:2681–2694. https://doi.org/10.1186/scrt229
Roy S, Gascard P, Dumont N, Zhao J, Pan D, Petrie S, Margeta M, Tlsty TD (2013) Rare somatic cells from human breast tissue exhibit extensive lineage plasticity. Proc Natl Acad Sci USA 110(12):4598–4603. https://doi.org/10.1073/pnas.1218682110
Sage EK, Thakrar RM, Janes SM (2016) Genetically modified mesenchymal stromal cells in cancer therapy. Cytotherapy 18(11):1435–1445. https://doi.org/10.1016/j.jcyt.2016.09.003
Sani M, Hosseini SM, Salmannejad M, Aleahmad F, Ebrahimi S, Jahanshahi S, Talaei-Khozani T (2015) Origins of the breast milk-derived cells; an endeavor to find the cell sources. Cell Biol Int 39(5):611–618. https://doi.org/10.1002/cbin.10432
Schedin P, Mitrenga T, McDaniel S, Kaeck M (2004) Mammary ECM composition and function are altered by reproductive state. Mol Carcinog 41(4):207–220. https://doi.org/10.1002/mc.20058
Seymour T, Twigger A-J, Kakulas F (2015) Pluripotency genes and their functions in the normal and aberrant breast and brain. Int J Mol Sci 16(11):27288–27301. https://doi.org/10.3390/ijms161126024
Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, Wu L, Lindeman GJ, Visvader JE (2006) Generation of a functional mammary gland from a single stem cell. Nature 439(7072):84–88. https://doi.org/10.1038/nature04372
Simerman AA, Phan JD, Dumesic DA, Chazenbalk GD (2016) Muse cells: nontumorigenic pluripotent stem cells present in adult tissues—a paradigm shift in tissue regeneration and evolution. Stem Cells Int. https://doi.org/10.1155/2016/1463258
Smith JP (2015) Markets, breastfeeding and trade in mothers’ milk. Int Breastfeed 10:9. https://doi.org/10.1186/s13006-015-0034-9
Smith GH, Chepko G (2001) Mammary epithelial stem cells. Microsc Res Tech 52(2):190–203. https://doi.org/10.1002/1097-0029(20010115)52:2
Sternlicht MD (2006) Key stages in mammary gland development: the cues that regulate ductal branching morphogenesis. Breast Cancer Res 8(1):201. https://doi.org/10.1186/bcr1368
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131(5):861–872. https://doi.org/10.1016/j.cell.2007.11.019
Thomas E, Zeps N, Cregan M, Hartmann P, Martin T (2011) 14-3-3σ (sigma) regulates proliferation and differentiation of multipotent p63-positive cells isolated from human breastmilk. Cell Cycle 10(2):278–284. https://doi.org/10.4161/cc.10.2.14470
Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM (1998) Embryonic stem cell lines derived from human blastocysts. Science 282(5391):1145–1147
Thorne JT, Segal TR, Chang S, Jorge S, Segars JH, Leppert PC (2015) Dynamic reciprocity between cells and their microenvironment in reproduction. Biol Reprod 92(1):25. https://doi.org/10.1095/biolreprod.114.121368
Trott JF, Vonderhaar BK, Hovey RC (2008) Historical perspectives of prolactin and growth hormone as mammogens, lactogens and galactagogues—agog for the future! J Mammary Gland Biol Neoplasia 13(1):3–11. https://doi.org/10.1007/s10911-008-9064-x
Twigger A-J, Hepworth AR, Tat Lai C, Chetwynd E, Stuebe AM, Blancafort P, Hartmann PE, Geddes DT, Kakulas F (2015) Gene expression in breastmilk cells is associated with maternal and infant characteristics. Sci Rep 5:12933. https://doi.org/10.1038/srep12933
Underwood MA (2012) Human milk for the premature infant. Pediatr Clin North Am 60(1):189–207. https://doi.org/10.1016/j.pcl.2012.09.008
Veltmaat JM, Van Veelen W, Thiery JP, Bellusci S (2004) Identification of the mammary line in mouse by Wnt10b expression. Dev Dyn 229(2):349–356. https://doi.org/10.1002/dvdy.10441
Villadsen R, Fridriksdottir AJ, Ronnov-Jessen L, Gudjonsson T, Rank F, LaBarge MA, Bissell MJ, Petersen OW (2007) Evidence for a stem cell hierarchy in the adult human breast. J Cell Biol 177(1):87–101. https://doi.org/10.1083/jcb.200611114
Visvader JE, Stingl J (2014) Mammary stem cells and the differentiation hierarchy: current status and perspectives. Genes Dev 28(11):1143–1158. https://doi.org/10.1101/gad.242511.114
Watson CJ (2006) Key stages in mammary gland development—involution: apoptosis and tissue remodelling that convert the mammary gland from milk factory to a quiescent organ. Breast Cancer Res 8(2):203. https://doi.org/10.1186/bcr1401
Weil BR, Manukyan MC, Herrmann JL, Abarbanell AM, Poynter JA, Wang Y, Meldrum DR (2011) The immunomodulatory properties of mesenchymal stem cells: implications for surgical disease. J Surg Res 167(1):78–86. https://doi.org/10.1016/j.jss.2010.07.019
Witkowska-Zimny M, Kaminska-El-Hassan E (2017) Cells of human breast milk. Cell Mol Biol Lett 22:11. https://doi.org/10.1186/s11658-017-0042-4
Witkowska-Zimny M, Kamińska-El-Hassan E, Wójtowicz J (2017) Human breastmilk as a source of progenitor/stem cells. Post N Med 09:460–463
Zhang Y, Andl T, Yang SH, Teta M, Liu F, Seykora JT, Tobias JW, Piccolo S, Schmidt-Ullrich R, Nagy A et al (2008) Activation of β-catenin signaling programs embryonic epidermis to hair follicle fate. Development 135(12):2161–2172. https://doi.org/10.1242/dev.017459
Acknowledgements
The authors are thankful to the Department of Science and Technology, Government of India, for providing financial support vide reference number SR/WOS-A/LS-13/2016 dated 06.09.2016 under the Women Scientist Scheme to carry out this work. They are grateful to Ms. Swati Singh, Ms. Karisma Agrawal, and Ms. Aishwarya Nayak for critical reading and editing of the manuscript. Thanks are due to Dr. Gulam Hussain Syed (Scientist-D, Institute of Life Science, Bhubaneswar) and Dr. Rajkumar Joshi (Associate Professor, Ramadevi Women’s University, Bhubaneswar) for their valuable suggestions to improve the quality of the manuscript. The authors are indebted to the Head of the Department, Centre for Biotechnology, Siksha ‘O’ Anusandhan University, Kalinga Nagar, Ghatikia, Bhubaneswar-751 003, India, for providing the necessary facilities. The authors also acknowledge the contribution of Dr. Ritendra Mishra, Mumbai, India who helped in the copy editing and proofreading of the manuscript.
Funding
This study was funded by Department of Science and Technology, Government of India, for providing financial support under vide reference number SR/WOS-A/LS-13/2016 dated 06.09.2016 under Women Scientist Scheme.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors do not have any conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tripathy, S., Singh, S. & Das, S.K. Potential of breastmilk in stem cell research. Cell Tissue Bank 20, 467–488 (2019). https://doi.org/10.1007/s10561-019-09791-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-019-09791-6