Abstract
The immense potency of nutritional components of human breast milk and importance of breastfeeding is known worldwide. Recent researches had identified stem cells as integral component of human breast milk. Nevertheless, there is little proof of evidence on the stem cell constituents of breast milk. It is imperative to explore the cellular constituents of human breast milk, including of stem cells, to open new avenue in child’s development and regeneration. Thus, we aimed at identifying the cellular constituents of human breast milk by phenotypic characterisation of diverse cell surface markers of hematopoietic stem cells (CD 34, CD 133, CD 117), mesenchymal stem cells (CD 90, CD 105, CD 73), myoepithelial cells (CD 29, CD 44), Immune cells (CD 209, CD 86, CD 83, CD 14, CD 13, HLADR, CD 45), as well as cell adhesion molecules (CD 31, CD 54, CD 166, CD 106, CD 49d), and other markers (ABCG2, CD140b) using flowcytometry. We found a lower expression of CD 34 (13.07 ± 2.0 %), CD 90 (7.79 ± 0.8 %) and CD 73 (2.19 ± 0.41 %), indicating scanty hematopoietic and mesenchymal stem cell population in human breast milk. On contrary, myoepithelial progenitors, cell adhesion molecules, immune cells and growth factors were identified as the major constituents of breast milk. Overall, this study illuminates the benefits of breast feeding as breast milk encompasses heterogeneous cellular components that benefits child’s growth, immunity and development. However, further research on these constituents of human breast milk will widen their applicability in treatment of neonatal disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mesenchymal stem cells have undergone a remarkable evolution sparked by several reports demonstrating its greater plasticity and had generated a great deal of excitement as a potential source for cell based therapies (Fuchs and Segre 2000; Kørbling and Estrov 2003; Pittenger et al. 1999). Bone marrow and adipose tissue had materialized themselves as a promising source of mesenchymal stem cells, being used in a wide range of therapeutics (De Ugarte et al. 2003; Zuk et al. 2002; Kern et al. 2006; Tsai et al. 2004; Gimble et al. 2010). Nevertheless, the existence of MSC derived from different sources such as tendon (Salingcarnboriboon et al. 2003), periodontal ligament (Seo et al. 2004), synovial membrane (De Bari et al. 2001, lung (Sabatini et al. 2005), liver (Campagnoli et al. 2001), endometrial tissue (Musina et al. 2008) and body/tissue fluids such as synovial fluid (Jones et al. 2004), amniotic fluid (Tsai et al. 2004; In ‘t Anker et al. 2003; Kim et al. 2007) and menstrual blood (Gargett et al. 2009; Schwab and Gargett 2007), and their immune potency has been discovered in the past decade. Furthermore, stem cell constituents of breast milk and their therapeutic application has been a fascinating area of research in recent years (Patki et al. 2010; Fan et al. 2010; Cregan et al. 2007).
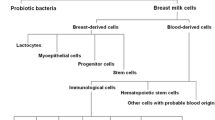
The mammary gland is a metabolically active, dynamic organ that undergoes significant changes during pregnancy, lactation and involution. The self renewing multipotent mammary stem cell has been extensively studied as a prognostic tool for breast cancer (Stingl et al. 2001; Dontu et al. 2003). Breast milk, being the secretary product of the mammary gland in the postpartum period is a complex mixture of interacting compounds like proteins, antibodies, vitamins, growth factors, hormones, cytokines and several immune factors for the newborn (Hamosh 2001; Boutinaud and Jammes 2002; Hanson et al. 2001; Pabst 1997; Hanson 1999). Accumulating evidences suggests that breast milk encompasses epithelial cells, colostral corpuscles, polymorphonuclear leukocytes, mononuclear phagocytes and lymphocytes, with those of epithelial lineage forming the main bulk of cells within 2 weeks of establishing lactation (Fan et al. 2010; Hanson et al. 2001; Labbok et al. 2004).
The existence of putative mammary stem cell population has been reported in breast milk with the expression of markers such as CK5, CK14, CK 19 and nestin (Fan et al. 2010; Cregan et al. 2007). Besides, human breast milk was also identified to possess heterogeneous cellular components such as mesenchymal stem cells (MSC), hematopoietic stem cells (HSC), side population (SP) and endothelial cells (Patki et al. 2010; Fan et al. 2010). Hence, it was believed that breast milk derived stem cells might possess considerable potency for the treatment of a wide horizon of neonatal diseases. Nonetheless, there is little proof of evidence on the stem cell constituents of breast milk. Furthermore, spectrum of cellular constituents of human breast milk is yet to be defined.
In lieu of the above, we aimed at exploring the major cellular constituents of human breast milk and its beneficial effects on child’s growth, immunity, development and regeneration. It was achieved by cell surface marker expression analysis of various stem cell and non-stem cell population such as HSC, MSC, SP, endothelial cell, monocytes, macrophages, dendritic cells and platelet derived growth factor (PDGF) using flowcytometry (n = 26).
Materials and methods
Samples collection
All human milk samples (n = 26) were collected manually in an aseptic fashion from the healthy term delivered lactating mothers aged 21–34, from day 3 to day 7. All samples were processed within 2 h of collection. This study was approved by the institutional ethics regulatory board of Lifeline Multispeciality Hospital, Perungudi, Chennai. The purpose of the research was explained to all patients under study and a formal written consent was obtained before collection.
Processing of human breast milk
The breast milk was diluted with DMEM-Low Glucose (LG) and centrifuged at 400×g for 10 min. The cellular component obtained in form of a pellet was collected and washed twice with PBS. The final cellular constituent pelleted down was subjected to analysis of cell surface antigenic expression profile using flowcytometry.
Flowcytometric characterisation
Flowcytometry was performed on a Becton Dickinson FACS Aria (http://www.bd.com/) using a 488-nm argon ion LASER and 632-nm red LASER for excitation. Fluorescence emission was collected using the corresponding detectors. About 1 × 105 cells were stained with saturating concentrations of fluorochrome–conjugated antibodies. The details on the antibodies, its fluorochromes, and catalogue numbers are listed in Table 1. The cells were incubated in the dark for 20 min at RT, washed thrice with wash flow buffer and resuspended in 500 μl of Phosphate-buffered solution (PBS). Data analysis and acquisition was then performed using DIVA Software (Becton Dickinson). A minimum of 10,000 events were characterized and recorded.
Results
Antigenic expression of assorted cellular constituents in human breast milk
Human breast milk (n = 26) was found to possess heterogenous cellular constituents of both stem and non-stem cell populations characterized by cell surface marker profiles: CD 34, CD 133, CD 117 (hematopoietic stem cells); CD 90, CD 105, CD 73 (mesenchymal stem cells); CD 29, CD 44 (myoepithelial cells); CD 209, CD 86, CD 83, CD 14, CD 13, HLADR, CD 45 (immune cells), as well as cell adhesion molecules (CD 31, CD 54, CD 166, CD 106, CD 49d), and Platelet Derived Growth Factor (CD140b) using flowcytometry (Fig. 1a). The assorted cell surface marker expression of all samples with its exhibited variations is presented as mean ± SEM (Fig. 1b; Table 2). Analysis of each of its constituents was categorized and detailed according to the Mean ± SEM value of their percentage expressions.
Scanty expressions of mesenchymal stem cells
The study provides evidence for the existence of mesenchymal stem cells in human breast milk with the presence of certain markers such as STRO 1, osteonectin, osteocalcin, ALP, CD 29 and CD 44 (Patki et al. 2010; Fan et al. 2010). According to the International Society for Cellular Therapy (ISCT), mesenchymal stem cells are defined by the expression of CD 90, CD 105 and CD 73 and lack of expression of CD 45, CD 34, CD 14, HLA-DR, etc. (Dominici et al. 2006). Surprisingly, we found an absence of the expression of surface markers CD 90 (7.7 ± 0.8 %) and CD 73 (2.1 ± 0.41 %), but expression of CD 105 (47.7 ± 2.95 %) on cells in breast milk (Fig. 2a). The scanty expressions of these MSC markers in all samples provide evidence for the lack of MSCs in human breast milk.
Hematopoietic stem/progenitor cells
Our results demonstrated higher expressions of hematopoietic progenitor population markers (43.1 ± 2.1 % of CD 133 and 23.2 ± 1.51 % of CD 117) than of CD 34 + marker of hematopoietic stem cells (13.07 ± 2.0 %) (Fig. 2b). The CD 34 + HSCs were consistently low and CD 117+, CD133+ progenitor cells were consistently higher in all twenty-six samples analysed using flowcytometry with negligible variations exhibited in few samples (Fig. 2b).
Myoepithelial progenitors and other unique markers
Our results elucidated the wide prevalence of myoepithelial progenitors such as CD 29 and CD 44 marker positive cells with a mean ± SEM of 60.5 ± 3.04 % and 44.4 ± 3.75 %, respectively. All analysed samples (n = 26) showed higher expression of CD 29 and CD 44, with certain exhibited variation among the samples (Fig. 3a). Similarly, it was identified that breast milk also possesses cells with higher expression of CD 140b (Platelet Derived Growth Factor), an important growth factor responsible for angiogenesis with a mean expression of 43.3 ± 3.34 %. Similar to the expressions of myoepithelial markers, CD 140b also showed a variation in expression pattern among all twenty-six samples (Fig. 3b). In addition, we identified that human breast milk does not possess side population, which is evident from lack of expression of ABCG2 in all samples (Fig. 3b).
Versatile cell adhesion molecules
The samples showed high expressions of certain cell adhesion molecules such as CD 31, CD 54, CD 166 and CD 49d but not CD 106 (Fig. 4a). The markers such as CD 166, CD 49d and CD 31, that favour leukocyte-endothelial transmigration, showed a high disparity of increase and decrease in expression profiles among the twenty-six samples. Of all cell adhesion molecules, CD 54 was highly expressed in all samples with slight variations evidenced (Fig. 4a).
Adequacy of immune cellular components
Contrary to the scanty expression of HSC and MSC markers, human breast milk was found to possess higher and certain remarkable expressions of immune markers of monocytes, macrophages, dendritic cells and leucocytes such as CD 86, CD 14, CD 13, HLADR and CD 45 in all samples (Fig. 4b), indicating adequacy of significant immune cell components in breast milk. On the other hand, cells in human breast milk were found to lack the expression of immune markers, CD 209 and CD 83 in all samples (Fig. 4b).
Discussion
Regardless of the accumulating evidences on the existence of stem cells in human breast milk (Patki et al. 2010; Fan et al. 2010; Cregan et al. 2007), there seems to be paucity of information pertaining to its cellular constituents. Besides, it was believed that breast milk derived stem cells possess a colossal regenerative potency that can be used for treating a wide horizon of neonatal disorders. Hence, our work aimed at identifying whether or not breast milk possesses hematopoietic and mesenchymal stem cells. Furthermore, the research work also aimed at exploring the spectrum of cellular constituents of human breast milk that probably contribute to the child’s growth, immunity, development and regeneration.
From our research work, it was identified that cells in breast milk express higher percentage of hematopoietic progenitor cell markers than those of hematopoietic stem cells. In addition, it was also identified from our work that human breast milk does not possess MSC in our series of samples (n = 26), which is evident from the scanty expression of MSC markers CD 90 and CD 73, that are supposed to likely be the markers of MSC according to the ISCT standards (Dominici et al. 2006). The results obtained from our study show a disparity to that of earlier reports (Patki et al. 2010; Fan et al. 2010). This disparity lies in lieu of the presence of HSC and MSC in human breast milk in facets of the hematopoietic stem cell marker, CD 34 and mesenchymal stem cell markers, CD 90 and CD 73. Patki et al. (2010) had reported the absence of CD 73 as confirmed by our study and concluded the presence of MSCs in human breast milk, however, which is contradictory to the minimal criteria of MSC put forward by the ISCT (Dominici et al. 2006). Similarly, Fan et al. (2010), reported a very reduced expression of CD 34 (1.1 ± 0.15 %) and CD 133 (2.6 ± 0.79 %) in discrepancy to that of our results showing the expression of CD 34 and CD 133 as 43.1 ± 2.1 % and 13.07 ± 2.0 %, respectively.
Yet, they reported the existence of hematopoietic stem cell population in the uncultured whole cell population of human breast milk. In contrary, our study concluded that CD 133+ hematopoietic progenitors were at higher levels than the hematopoietic stem cell population.
This study focused, for the first time, on identifying the existence of cell adhesion molecules using specific cell surface markers in human breast milk. This is because of the fact that the molecular mechanisms that underlay the roots of migration of stem cells in vivo to the site of injury, necessitates a complex multistep cascade of events capable of resisting shear forces coupled with transendothelial migration. This clearly emphasizes the imminent activity of cell adhesion molecules in transendothelial migration and homing of stem cells. It was identified that human breast milk possesses specific CAM such as CD 166, CD 31, CD 49d, CD 54, but lack CD 106. In addition, human breast milk was also identified to possess lymphocytes, monocytes and macrophages, which is coherent with earlier reports (Hamosh 2001; Boutinaud and Jammes 2002; Pabst 1997; Hanson 1999). Furthermore, the existence of CD 105 and CD 31 revealed the presence of endothelial progenitor cells and endothelial cells in breast milk, enhancing the property of transendothelial migration. In addition, the work also demonstrated the expression of certain unique markers of dendritic cells, and of myoepithelial progenitors and of platelet derived growth factor that are responsible for immunity, growth, migration and angiogenesis. Overall, the percentages of expressions of all cell surface markers analysed in our study was found to be much higher than that in earlier reports (Patki et al. 2010; Fan et al. 2010; Cregan et al. 2007). Moreover, there was also a variation seen in the percentage of expression within the twenty-six samples for all markers. From these variations, it became apparent that, although human breast milk possesses heterogenous cellular constituents potentially benefitting neonates, the percentage expression of these cellular constituents might vary according to individuals.
Conclusion
In summary, based on the series of samples analyzed in our study we identified that breast milk possesses scanty mesenchymal and hematopoietic stem cells. Furthermore, the higher levels of the progenitors positive for immunological components, progenitors, cell adhesion molecules and growth factors identified from our study can probably be explained by the higher needs of neonates for immunological protection, growth, leucocyte-transendothelial migration, protein synthesis, neuro-cognitive development and angiogenesis. This observation strengthens the policy of breast feeding, which is promoted by organizations like WHO and UNICEF. However, further research on cellular constituents of human breast milk may well throw light on the therapeutic potential in the treatment of neonatal disorders.
References
Boutinaud M, Jammes H (2002) Potential uses of milk epithelial cells: a review. Reprod Nutr Dev 42:133–147
Campagnoli C, Roberts IA, Kumar S, Bennett PR, Bellantuono I, Fisk NM (2001) Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood 98:2396–2402
Cregan MD, Fan Y, Appelbee A, Brown ML, Klopcic B, Koppen J, Mitoulas LR, Piper KM, Choolani MA, Chong YS, Hartmann PE (2007) Identification of nestin-positive putative mammary stem cells in human breastmilk. Cell Tissue Res 329:129–136
De Bari C, Dell’Accio F, Tylzanowski P, Luyten FP (2001) Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum 44:1928–1942
De Ugarte DA, Morizono K, Elbarbary A, Alfonso Z, Zuk PA, Zhu M, Dragoo JL, Ashjian P, Thomas B, Benhaim P, Chen I, Fraser J, Hedrick MH (2003) Comparison of Multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs 174:101–109
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini FC, Krause DS, Deans RJ, Keating A, Prockop DJ, Horwitz EM (2006) POSITION PAPER minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8:315–317
Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, Wicha MS (2003) In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev 17:1253–1270
Fan Y, Chong YS, Choolani MA, Cregan MD, Chan JKY (2010) Unravelling the mystery of stem/progenitor cells in human breast milk. PLoS One 5:e14421
Fuchs E, Segre JA (2000) Stem cells: a new lease on life. Cell 100:143–155
Gargett CE, Schwab KE, Zillwood RM, Nguyen HPT, Wu D (2009) Isolation and culture of epithelial progenitors and mesenchymal stem cells from human endometrium. Biol Reprod 80:1136–1145
Gimble J, Guilak F, Bunnell B (2010) Clinical and preclinical translation of cell-based therapies using adipose tissue-derived cells. Stem Cell Res Ther 1:19
Hamosh M (2001) Bioactive factors in human milk. Pediatr Clin North Am 48:69–86
Hanson LA (1999) Human milk and host defence: immediate and long-term effects. Acta Paediatr Suppl 88:42–46
Hanson L, Silfverdal SA, Strömbäck L, Erling V, Zaman S, Olcén P, Telemo E (2001) The immunological role of breast feeding. Pediatr Allergy Immunol 12:15–19
In’t Anker PS, Scherjon SA, Kleijburg-van der Keur C, Noort WA, Claas FH, Willemze R, Fibbe WE, Kanhai HH (2003) Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood 102:1548–1549
Jones EA, English A, Henshaw K, Kinsey SE, Markham AF, Emery P, McGonagle D (2004) Enumeration and phenotypic characterisation of synovial fluid multipotential mesenchymal progenitor cells in inflammatory and degenerative arthritis. Arthritis Rheum 50:817–827
Kern S, Eichler H, Stoeve J, Kluter H, Bieback K (2006) Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 24:1294–1301
Kim J, LeeY Kim H, Hwang KJ, Kwon HC, Kim SK, Cho DJ, Kang SG, You J (2007) Human amniotic fluid-derived stem cells have characteristics of multipotent stem cells. Cell Prolif 40:75–90
Kørbling M, Estrov Z (2003) Adult stem cells for tissue repair—a new therapeutic concept? N Engl J Med 349:570–582
Labbok MH, Clark D, Goldman AS (2004) Breastfeeding: maintaining an irreplaceable immunological resource. Nat Rev Immunol 4:565–572
Musina RA, Belyavski AV, Tarusova OV, Solovyova EV, Sukhikh GT (2008) Endometrial mesenchymal stem cells isolated from the menstrual blood. Bull Exp Biol Med 145:539–543
Pabst HF (1997) Immunomodulation by breast-feeding. Pediatr Infect Dis J 16:991–995
Patki S, Kadam S, Chandra V, Bhonde R (2010) Human breast milk is a rich source of multipotent mesenchymal stem cells. Hum Cell 23:35–40
Sabatini F, Petecchia L, Tavian M, Jodon de Villeroché V, Rossi GA, Brouty-Boyé D (2005) Human bronchial fibroblasts exhibit a mesenchymal stem cell phenotype and multilineage differentiating potentialities. Lab Invest 85:962–971
Salingcarnboriboon R, Yoshitake H, Tsuji K, Obinata M, Amagasa T, Nifuji A, Noda M (2003) Establishment of tendon-derived cell lines exhibiting pluripotent mesenchymal stem cell-like property. Exp Cell Res 287:289–300
Schwab KE, Gargett CE (2007) Co-expression of two perivascular cell markers isolates mesenchymal stemlike cells from human endometrium. Hum Reprod 22:2903–2911
Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, Young M, Robey PG, Wang CY, Shi S (2004) Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 364:149–155
Stingl J, Eaves C, Zandieh I, Emerman JT (2001) Characterisation of bipotent mammary epithelial progenitor cells in normal adult human breast tissue. Breast Cancer Res Treat 67:93–109
Tsai MS, Lee J-L, Chang YJ, Hwang SM (2004) Isolation of human multipotent mesenchymal stem cells from second-trimester amniotic fluid using a novel two-stage culture protocol. Hum Reprod 19:1450–1456
Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH (2002) Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13:4279–4295
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Indumathi, S., Dhanasekaran, M., Rajkumar, J.S. et al. Exploring the stem cell and non-stem cell constituents of human breast milk. Cytotechnology 65, 385–393 (2013). https://doi.org/10.1007/s10616-012-9492-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-012-9492-8