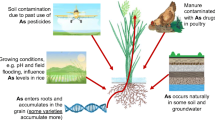
Abstract
Cardiovascular risk has traditionally been defined by modifiable and non-modifiable risk factors, such as tobacco use, hyperlipidemia, and family history. However, chemicals and pollutants may also play a role in cardiovascular disease (CVD) risk. Arsenic is a naturally occurring element that is widely distributed in the Earth’s crust. Inorganic arsenic (iAs) has been implicated in the pathogenesis of atherosclerosis, with chronic high-dose exposure to iAs (> 100 µg/L) being linked to CVD; however, whether low-to-moderate dose exposures of iAs (< 100 µg/L) are associated with the development of CVD is unclear. Due to limitations of the existing literature, it is difficult to define a threshold for iAs toxicity. Studies demonstrate that the effect of iAs on CVD is far more complex with influences from several factors, including diet, genetics, metabolism, and traditional risk factors such as hypertension and smoking. In this article, we review the existing data of low-to-moderate dose iAs exposure and its effect on CVD, along with highlighting the potential mechanisms of action.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epidemiological studies have demonstrated that an individual’s susceptibility to developing cardiovascular disease (CVD) is related to both “non-modifiable” cardiovascular risk factors, such as age, family history, genetics, and sex, and “modifiable” risk factors including smoking, hypertension, diabetes, hyperlipidemia, and chronic kidney disease [1, 2]. Despite attempts to address these modifiable risk factors, CVD remains the leading cause of death in the United States (US) and costs the healthcare system $216 billion per year [3, 4].
Generally, CVD results from a relationship between genetics and environmental factors. Environmental factors are traditionally thought of in terms of lifestyle choices including physical activity, diet, and tobacco use [1]. However, the influence of chemicals and pollutants in our environment on cardiovascular health is less clear. One environmental exposure that has been implicated in the development of CVD is arsenic [5]. Arsenic can be found in combination with organic or inorganic substances. Organic arsenic species, such as those found in seafood, are less toxic, while inorganic arsenic (iAs) species are very reactive intracellularly [6, 7]. Most exposure to iAs in the current era is dietary through ingestion of contaminated food and water at high dose levels (> 100 µg/L) and is more prevalent in countries such as Bangladesh, India, China, and Taiwan [8, 9]. However, even in the US, iAs has been detected in the drinking water supply. In a national study of groundwater quality, the US Geological Survey found iAs to be present in half of the sampled wells used for drinking water supply at a concentration greater than 1 µg/L, with higher prevalence in the western US [10].
The potential toxicity of iAs is far-reaching with significant impacts on cardiovascular health. For many decades, higher doses have been linked to Blackfoot disease, a form of arteriosclerosis obliterans [11], though studies have also suggested correlations between lower exposure of iAs and CVD [12]. In this review, we provide a contemporary appraisal of the existing data related to low-to-moderate iAs exposure (< 100 µg/L) and the effect on CVD. We also review the potential pathogenesis of iAs and CVD in order to help guide clinicians in risk stratification and counseling of patients exposed to arsenic.
Arsenic Exposures and Metabolism
In 2001, the US Environmental Protection Agency (EPA) reduced the maximum contaminant level for iAs in potable, or drinking water, (regulations in the US have only pertained to potable water) from 50 to 10 µg/L [13]. However, even when potable water sources demonstrate iAs below the current EPA limit, urinary excretion, which reflects dietary intake, has indicated persistent high level exposure presumably from dietary food sources; this has been demonstrated in studies from both Bangladesh and the USA [14, 15]. Major dietary sources of iAs include vegetables, fruits, and particularly rice, suggesting that ground water contamination is an important source of iAs [16,17,18,19]. The efficient silicon uptake pathway in rice allows for concomitant uptake of iAs due to chemical similarity, leading to high iAs content in rice. Rice also has high iAs content due to the agricultural process of growing rice in flooded soil where iAs is more mobile [20]. Other food sources, such as fish and shellfish, contribute significantly to total arsenic exposure, but their contribution to iAs exposure is not as significant [18, 21].
iAs is methylated in the liver into dimethylarsinate (DMA) and monomethylarsonate (MMA) metabolites, whose major pathway of elimination is through the kidney. The nuances of the methylation steps of metabolism are beyond the scope of this review; however, it is important to note that there are cross-species differences in iAs metabolism and therefore the doses exposed to in animal model studies do not directly translate to human exposures in water and dietary sources [22].
iAs exposure via water or dietary intake can be quantified in various ways, including measurement of iAs levels in urine and nail samples. A study from Pakistan demonstrated a strong relationship between arsenic intake from water and the concentrations of iAs in urine and toenail samples. However, the study specifically found toenail samples to be the most valuable biomarker of past exposure to iAs of dietary origin [23]. Therefore, it is important to note these differences when considering the studies described in this review.
Current Evidence of Arsenic and CVD
Overview
Numerous epidemiologic surveys, prospective analyses, and observational studies from endemic regions have demonstrated an association between iAs and CVD, especially with chronic high-dose exposures [24,25,26,27,28,29,30,31]. iAs exposure has been linked to ischemic heart disease [32, 33], hypertension [34, 35], and carotid artery atherosclerosis [36]. Most studies have focused on chronic high-dose iAs exposure, while few studies characterize the relationship between CVD and low-to-moderate dose iAs exposure, which are more commonly seen in the US. A 2012 meta-analysis by Moon et al. demonstrated a causal association between chronic iAs exposure (mean iAs in drinking water > 50 µg/L) and CVD (coronary heart disease and peripheral artery disease), but the results were inconsistent at lower iAs exposures [37]. Another meta-analysis showed associations between chronic iAs exposure and CVD incidence (stroke, coronary heart disease, and heart failure), mortality, and carotid atherosclerosis at both low-to-moderate and high levels of iAs exposure from drinking water. However, there was wide variation in the relative risks, highlighting the limitations of these analyses which were comprised of studies that differed in methodology and exposure assessment [38]. The studies discussed in the subsequent part of this review, unless otherwise specified, pertain to low-to-moderate dose iAs exposure (defined as < 100 µg/L).
Low-to-Moderate Dose iAs Exposure and CVD
A few small-scale studies have demonstrated associations between low-to-moderate levels of iAs exposure (10–100 µg/L) and CVD [39, 40], hypertension [41], and stroke [42]. In two prospective studies from Bangladesh, there was a trend towards low-to-moderate iAs exposure and incident CVD though this was not statistically significant [25, 26]. In addition, in a population of patients from the Danish Diet, Cancer, and Health cohort, there was no overall association found between average concentration of iAs in drinking water and risk of myocardial infarction, but there was an association in one specific geographic area for iAs exposures at 2.21–25.34 μg/L, with an incidence rate ratio of 1.48 (95% confidence interval [CI]: 1.19–1.83) when compared with very low iAs exposure (0.05–1.83 μg/L). However, the authors were unable to rule out whether this association was caused by risk factors for myocardial infarction being more prevalent among participants in the geographic area that showed the positive association [43]. Socioeconomic factors may play a role in the differences in outcomes, especially at low levels of iAs exposure, though the exact extent is often unclear in studies. Given the paucity of high-quality studies, establishing an association between CVD and iAs at lower levels relevant to the US population is challenging. However, several recent analyses have provided more evidence on low-to-moderate dose iAs exposure and may allow clinicians in the US to begin to identify a threshold at which iAs exposure contributes significantly to CVD [44,45,46,47].
A case-cohort study of 555 individuals from Southern Colorado who were exposed to low-to-moderate levels of iAs in drinking water throughout their lives found that there was an increased risk of coronary heart disease (HR: 1.38, 95% CI: 1.09–1.78, per every 15 μg/L increase) [44]. In the North American Heart Study, a prospective study conducted in Native American communities in Oklahoma, Arizona, and North and South Dakota, a significant increase in CVD incidence (HR: 1.32, 95% CI 1.09–1.59; p = 0.002) and mortality (HR: 1.65, 95% CI 1.20–2.27; p < 0.001) was found when comparing the quartile with the highest urine iAs levels to the lowest after adjusting for confounders [12, 45]. As discussed above, most studies have focused on the association between iAs exposure from drinking water and CVD risk; however, rice intake also represents a significant exposure to iAs, even in developed countries. In a recent ecological study from England and Wales, a non-linear dose–response relationship was found for the relationship between rice intake and CVD and indicated that CVD risk increased with iAs exposure from rice at exposures above 0.3 μg/person/day [47]. Another study evaluated the comprehensive cardiovascular risk due to low-to-moderate levels of iAs exposure in patients with baseline hypertension from the 2003–2012 National Health and Nutrition Examination Survey (NHANES) by utilizing the 10-year atherosclerotic CVD (ASCVD) risk score from the pooled cohort equations. After adjustment for sociodemographic factors and ASCVD risk factors, male participants in the highest quartile of urine iAs had higher 10-year ASCVD risk (24% increase in 10-year ASCVD risk; 95% CI: 2–53%), but there was no association of urine iAs with ASCVD risk score in women participants (5% increase in 10-year ASCVD risk in highest quartile of urine iAs; 95% CI: − 15 to 29%) [46].
Effect Modification
Epidemiologic evidence indicates there is a more nuanced relationship between iAs exposure and CVD, with effect modification from diet, genetics, metabolism, socioeconomic factors, and traditional cardiovascular risk factors such as hypertension and smoking. Clinical nutritional research is ongoing into whether an individual’s diet modifies iAs effect on cardiovascular health. Adequate dietary folate intake has been linked to more robust methylation capacity and could potentially lower the risk of iAs toxicity [48]. Selenium is another nutritional factor that may modify the effect of iAs on cardiovascular health [49, 50], as adequate dietary selenium may curtail lipid peroxidation, thereby mitigating cardiovascular toxicity [51,52,53]. Higher urinary selenium concentrations have been linked to the somewhat less toxic intermediate, DMA, implying selenium aids in detoxification of iAs [54]. Thus, to better understand the public health implications of iAs exposure and recognize patient populations that are susceptible to its toxic effects, it is important to understand gene-environment interactions. A study from Mexico demonstrated that a specific genetic susceptibility (PON1 Q192 R polymorphism) modified the CVD risk from iAs exposure in drinking water, as assessed by biomarkers of cardiovascular disease — asymmetric dimethylarginine (ADMA) and fatty acid-binding protein 4 (FABP4) [55].
The role of iAs in cardiovascular health does not exist in isolation, as traditional CVD risk factors may modify the effects of iAs exposure and further lead to susceptibility to iAs toxicity. A prospective analysis of 2,939 participants in the New Hampshire Skin Cancer Study demonstrated that iAs exposure measured from toenail clipping samples was related to an increased risk of ischemic heart disease mortality among smokers, with a higher hazard ratio in participants with ≥ 30 pack-years (HR: 1.66, 95% CI: 1.12−2.45) [56]. This study highlights a synergistic relationship between iAs exposure and smoking. However, the Hispanic Community Health Study/Study of Latinos which investigated the relationship between iAs exposure through rice consumption and hypertension found an association in non-smokers rather than in smokers. Among never smokers who had high rice-consumption, less efficient iAs metabolism (higher % iAs as compared to % of arsenic metabolites — DMA and MMA) was associated with increased systolic blood pressure (BP) (1.96 mmHg/percentage point increase in % iAs; 95% CI: 0.13–3.80; P = 0.034), and there was no association in smokers [57]. The authors of the study hypothesize that the difference in effect may be due to an interaction between smoking and iAs metabolism, though future studies delving further into this mechanism are needed.
In an analysis from the Strong Heart Family Study of adolescents and young adults, exposure to low-to-moderate iAs levels was associated with increased left ventricular (LV) wall thickness and LV mass, predominantly in patients with hypertension or pre-hypertension [58]. Another risk factor for CVD is metabolic syndrome, comprised of elevated glucose, hypertension, elevated triglycerides, low high-density lipoprotein cholesterol, and high waist circumference. In a study from the US NHANES 2013–2014 data, there was no association found between iAs methylation and metabolic syndrome; however, gender and body mass index (BMI) significantly modified the effect of iAs methylation on metabolic syndrome [59].
Proposed Mechanisms of Action
Framework to Approach Animal Studies
Researchers have attempted to identify a molecular pathway between low-to-moderate dose iAs exposure and CVD toxicity in basic science models. Developing a plausible mechanism of action for iAs toxicity requires determining if and how iAs disrupts cell physiology to generate a chronic inflammatory state that sustains atherosclerosis pathogenesis. Two proposed mechanisms of action are oxidative stress and neovascularization [60]. As we discuss both human and animal studies to explore these two mechanisms, we should note animal models may have an alternative process for metabolizing iAs not directly applicable to metabolism in humans [5, 8].
Oxidative Stress and Endothelial Dysfunction
In analyses exploring mechanistic links between heavy metals and CVD, disturbances in nitric oxide (NO) generating systems and the vascular endothelium are repeatedly identified as a potential culprit [61]. In vitro studies suggest iAs can increase the local production of reactive oxygen species and alter the function of critical antioxidants [62,63,64]. In reviewing animal models investigating endothelial dysfunction, we have to evaluate whether iAs plausibly leads to endothelial nitric oxide synthase inhibition, exhausting local pools of available NO and subsequently increasing oxidative stress to a point beyond which it drives lipid peroxidation, smooth muscle contraction, and other steps in the development of atherosclerosis [61, 65].
The pathway from oxidative stress to oxidative damage leading to atherosclerosis and eventually clinical manifestations of CVD presumably requires a milieu of chronic inflammation; thus, markers of chronic inflammation have been used as surrogates to connect disparate mechanisms of cardiovascular toxicity to iAs. Markers of chronic inflammation linked to high-dose iAs exposure include endothelial adhesion molecules, particularly soluble intercellular adhesion molecule-1 (sICAM-1) and soluble vascular cell adhesion molecule (sVCAM-1) [66], which may be potential biomarkers of CVD [67,68,69]. For instance, a recent study of Bangladeshi adults revealed a positive relationship between increased sICAM-1 and VCAM-1 with iAs in drinking water after adjustments for BMI, hypertension, and other CVD risk factors, though iAs exposure had an interquartile range of 2.98 to 186 μg/L [70]. The investigators also indicated that iAs exposure in drinking water led to increased oxidative stress, specifically measured by plasma levels of oxidized low-density lipoprotein and C-reactive protein (CRP) [70]. In a cross-sectional study population from the New Hampshire Health Study, comprised of adults with low-to-moderate iAs exposure, iAs was positively associated with biomarkers related to CVD pathogenesis, including markers of endothelial dysfunction such as vascular and cellular adhesion molecules (VCAM-1 and ICAM-1) [71].
In the Multi-Ethnic Study of Atherosclerosis (MESA), frequent rice intake was not associated with several markers of inflammation including high sensitivity CRP, interleukin-6, and fibrinogen or subclinical atherosclerosis; however, two markers of inflammation that have been previously associated with iAs exposure (E-selectin and ICAM-1) were positively associated with rice intake [72]. Using rice as a marker for iAs exposure has limitations, and this study was unable to consider an individual’s capacity to metabolize iAs. In addition, evidence detailing what level of iAs exposure leads to clinically relevant lipid peroxidation is lacking [73]. Furthermore, recent human studies in iAs-exposed populations have failed to demonstrate a relationship between iAs ingestion (ranging from less than 10 μg/L up to more than 300 μg/L) and urinary markers of oxidative stress [74].
Several of the early animal models exploring iAs exposure and risk of developing CVD in apolipoprotein E deficient mice revealed that high dose exposures, including either a dose of 2,000 or 10,000 μg/L sodium arsenite in drinking water over the course of 24 weeks in one study [75] or a fixed exposure of 13,300 μg/L in another [76], led to increased atherosclerotic lesions in the vasculature [75, 76]. Other studies have linked high dose exposures of iAs in utero or in the early postnatal period to the increased development of atheroma [77,78,79], but equivalent studies relevant to human exposures are absent. Although biomarkers reflective of perturbations in the NO-generating systems and indicative of chronic inflammation have been identified in animal models with high-dose iAs exposures, animal models of iAs at exposures that would be considered low-to-moderate for studies applicable to human exposures on CVD risk have not been completed. Further animal models defining the role of iAs on pro-thrombotic factors, such as fibrinogen and plasminogen activator inhibitor-1 (PAI-1), are needed as a recent cross-sectional analysis of the Strong Heart Study unexpectedly demonstrated that low-to-moderate iAs exposure was positively associated with baseline fibrinogen levels and inversely associated with PAI-1 [80].
Neovascularization
Neovascularization, the process of generating microvasculature that may supply developing plaques with inflammatory factors and nutrients from the systemic circulation to support its development, is one of the processes proposed in the pathogenesis of atherosclerosis [81]. As the intima thickens, passive diffusion of nourishing factors from the lumen diminishes and these microvessels may play a critical role in sustaining plaque development. One animal model tested whether chronic exposure to iAs in drinking water enhances neovascularization and saw a dose–response relationship between 0–5 and 50 μg/L iAs. However, the response diminished over time, conceivably a result of tolerance to iAs, which suggests that neovascularization may be a less plausible mechanism of action for arsenic-induced CVD [82, 83].
Cardiac Hypertrophy
Along with the effect of iAs on atherosclerosis described above, arsenic has also been shown to induce pathological cardiac hypertrophy; multiple studies have shown exposure to iAs leading to myocyte apoptosis, fibrosis, and subsequent left ventricular hypertrophy [84, 85]. In one study, 8-week iAs exposure in male mice was associated with an increase in systolic pressure and altered cardiac geometry; iAs induced hypertrophic gene expression in ventricular myocytes via a calcineurin-nuclear factor of activated T cells pathway [86].
“Two-Hit” Hypothesis of Cardiovascular Disease
Several studies have evaluated if in utero exposure to iAs in mice alters hepatic development and genetic expression, creating a pro-inflammatory state that accelerates atherosclerosis [87,88,89]. This “two-hit” model of arsenic-induced CVD suggests that epigenetic modifications prime the mouse model with a chronic, low-grade inflammation that can be exacerbated by another insult or “hit” leading to atherosclerosis [87]. For example, one study found in utero iAs exposure was linked with increased production of the lipid modulator sterol regulatory element binding protein (SREBP) 1 [87], a potential factor in the development of diabetes and rheumatoid arthritis, as well as a possible modulator of other chronic inflammatory diseases. Another study found a transient postnatal elevation of heat shock protein 70 (HSP70) in prenatally iAs exposed mice [88], again proposing that iAs exposure alone may not create significant inflammation, but aggravates the effects of another toxic insult [89]. While intriguing, these studies were conducted with iAs doses much higher than is typical for human exposures. In addition, whether HSP70, SREBP1, and other intermediate biomarkers can be reliable indicators of low-grade inflammation remains speculative. However, in the Strong Heart Study prospective cohort, the cardiovascular risk from low-to-moderate level iAs exposure was significantly higher in participants with diabetes compared to those without diabetes [12]. This study suggests the pro-inflammatory state created by diabetes potentially augmented the impact of low-to-moderate level iAs exposure on atherosclerosis, with further evidence in experimental models with healthy donor whole blood [90]. Thus, iAs may have a modifying effect on other cardiovascular risk factors but at what level of exposure and by what mechanism remains unclear.
Conclusion
Although measures of cardiovascular prevention have been traditionally targeted towards conventional risk factors, there is increasing evidence that environmental exposure to iAs may increase risk of CVD (Fig. 1). The pathogenesis of atherosclerosis and its downstream clinical manifestations are complex processes, and associations between high levels of chronic iAs exposures and markers of various stages of atheroma development and CVD have been demonstrated, with some studies also presenting evidence of CVD risk with low-to-moderate levels of iAs exposure which are more generalizable to the US population. Therefore, clinicians should consider iAs exposure when evaluating cardiovascular risk in patients. While the EPA regulates arsenic in community water systems, a threshold level of iAs exposure has not been clearly identified in the literature; therefore, it is important to maintain caution when advising patients on safe levels of iAs exposure. Patients using well water, which is not regulated by the EPA, could be advised to test their water for arsenic which could inform conversations with their physicians regarding risk of CVD. However, individual risk mitigation strategies may not always be feasible, and therefore policy-level change is needed to adequately address environmental arsenic exposure. Finally, the modifying effect of conventional risk factors, such as smoking, diabetes, and hypertension, on iAs toxicity emphasizes the importance of managing traditional CVD risk factors.
Availability of Data and Material
Not applicable.
Code Availability
Not applicable.
References
Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S76-99.
Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2010;122:2748–64.
Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123:933–44.
Virani SS, Alonso A, Aparicio HJ, et al. Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation. 2021;143:e254–743.
States JC, Srivastava S, Chen Y, Barchowsky A. Arsenic and cardiovascular disease. Toxicol Sci. 2009;107:312–23.
Hughes MF. Arsenic toxicity and potential mechanisms of action. Toxicol Lett. 2002;133:1–16.
Wu X, Cobbina SJ, Mao G, et al. A review of toxicity and mechanisms of individual and mixtures of heavy metals in the environment. Environ Sci Pollut Res Int. 2016;23:8244–59.
Hughes MF, Beck BD, Chen Y, Lewis AS, Thomas DJ. Arsenic exposure and toxicology: a historical perspective. Toxicol Sci. 2011;123:305–32.
Rahman M, Sohel N, Yunus FM, et al. Arsenic exposure and young adult’s mortality risk: a 13-year follow-up study in Matlab. Bangladesh Environ Int. 2019;123:358–67.
DeSimone LA, McMahon, P.B., Rosen, M.R. The quality of our Nation’s waters—water quality in Principal Aquifers of the United States, 1991–2010. US Geological Survey Circular 1360:151.
Tseng CH. Blackfoot disease and arsenic: a never-ending story. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2005;23:55–74.
Moon KA, Guallar E, Umans JG et al. Association between exposure to low to moderate arsenic levels and incident cardiovascular disease. A prospective cohort study. Ann Intern Med. 2013;159:649–59.
Kurzius-Spencer M, Burgess JL, Harris RB, et al. Contribution of diet to aggregate arsenic exposures-an analysis across populations. J Expo Sci Environ Epidemiol. 2014;24:156–62.
Kurzius-Spencer M, O’Rourke MK, Hsu CH, et al. Measured versus modeled dietary arsenic and relation to urinary arsenic excretion and total exposure. J Expo Sci Environ Epidemiol. 2013;23:442–9.
Kile ML, Houseman EA, Breton CV, et al. Dietary arsenic exposure in Bangladesh. Environ Health Perspect. 2007;115:889–93.
Gilbert-Diamond D, Cottingham KL, Gruber JF, et al. Rice consumption contributes to arsenic exposure in US women. Proc Natl Acad Sci U S A. 2011;108:20656–60.
Karagas MR, Punshon T, Sayarath V, et al. Association of rice and rice-product consumption with arsenic exposure early in life. JAMA Pediatr. 2016;170:609–16.
Xue J, Zartarian V, Wang SW, Liu SV, Georgopoulos P. Probabilistic modeling of dietary arsenic exposure and dose and evaluation with 2003–2004 NHANES data. Environ Health Perspect. 2010;118:345–50.
Chung JY, Yu SD, Hong YS. Environmental source of arsenic exposure. J Prev Med Public Health. 2014;47:253–7.
Chen Y, Han YH, Cao Y, et al. Arsenic transport in rice and biological solutions to reduce arsenic risk from rice. Front Plant Sci. 2017;8:268.
Seo MN, Lee SG, Eom SY, et al. Estimation of total and inorganic arsenic intake from the diet in Korean adults. Arch Environ Contam Toxicol. 2016;70:647–56.
Cohen SM, Arnold LL, Eldan M, Lewis AS, Beck BD. Methylated arsenicals: the implications of metabolism and carcinogenicity studies in rodents to human risk assessment. Crit Rev Toxicol. 2006;36:99–133.
Rasheed H, Kay P, Slack R, Gong YY. Assessment of arsenic species in human hair, toenail and urine and their association with water and staple food. J Expo Sci Environ Epidemiol. 2019;29:624–32.
Chen Y, Hakim ME, Parvez F, et al. Arsenic exposure from drinking-water and carotid artery intima-medial thickness in healthy young adults in Bangladesh. J Health Popul Nutr. 2006;24:253–7.
Sohel N, Persson LA, Rahman M, et al. Arsenic in drinking water and adult mortality: a population-based cohort study in rural Bangladesh. Epidemiology. 2009;20:824–30.
Chen Y, Graziano JH, Parvez F, et al. Arsenic exposure from drinking water and mortality from cardiovascular disease in Bangladesh: prospective cohort study. BMJ. 2011;342: d2431.
Chen Y, Wu F, Liu M, et al. A prospective study of arsenic exposure, arsenic methylation capacity, and risk of cardiovascular disease in Bangladesh. Environ Health Perspect. 2013;121:832–8.
Chen Y, Wu F, Parvez F, et al. Arsenic exposure from drinking water and QT-interval prolongation: results from the Health Effects of Arsenic Longitudinal Study. Environ Health Perspect. 2013;121:427–32.
Wang YH, Wu MM, Hong CT, et al. Effects of arsenic exposure and genetic polymorphisms of p53, glutathione S-transferase M1, T1, and P1 on the risk of carotid atherosclerosis in Taiwan. Atherosclerosis. 2007;192:305–12.
Wade TJ, Xia Y, Wu K, et al. Increased mortality associated with well-water arsenic exposure in Inner Mongolia, China. Int J Environ Res Public Health. 2009;6:1107–23.
Wu MM, Chiou HY, Chen CL, et al. GT-repeat polymorphism in the heme oxygenase-1 gene promoter is associated with cardiovascular mortality risk in an arsenic-exposed population in northeastern Taiwan. Toxicol Appl Pharmacol. 2010;248:226–33.
Hsueh YM, Wu WL, Huang YL, et al. Low serum carotene level and increased risk of ischemic heart disease related to long-term arsenic exposure. Atherosclerosis. 1998;141:249–57.
Chen CJ, Chiou HY, Chiang MH, Lin LJ, Tai TY. Dose-response relationship between ischemic heart disease mortality and long-term arsenic exposure. Arterioscler Thromb Vasc Biol. 1996;16:504–10.
Chen CJ, Hsueh YM, Lai MS, et al. Increased prevalence of hypertension and long-term arsenic exposure. Hypertension. 1995;25:53–60.
Rahman M, Tondel M, Ahmad SA, et al. Hypertension and arsenic exposure in Bangladesh. Hypertension. 1999;33:74–8.
Wang CH, Jeng JS, Yip PK, et al. Biological gradient between long-term arsenic exposure and carotid atherosclerosis. Circulation. 2002;105:1804–9.
Moon K, Guallar E, Navas-Acien A. Arsenic exposure and cardiovascular disease: an updated systematic review. Curr Atheroscler Rep. 2012;14:542–55.
Kuo CC, Moon KA, Wang SL, Silbergeld E, Navas-Acien A. The association of arsenic metabolism with cancer, cardiovascular disease, and diabetes: a systematic review of the epidemiological evidence. Environ Health Perspect. 2017;125: 087001.
Medrano MA, Boix R, Pastor-Barriuso R, et al. Arsenic in public water supplies and cardiovascular mortality in Spain. Environ Res. 2010;110:448–54.
Meliker JR, Wahl RL, Cameron LL, Nriagu JO. Arsenic in drinking water and cerebrovascular disease, diabetes mellitus, and kidney disease in Michigan: a standardized mortality ratio analysis. Environ Health. 2007;6:4.
Gong G, O’Bryant SE. Low-level arsenic exposure, AS3MT gene polymorphism and cardiovascular diseases in rural Texas counties. Environ Res. 2012;113:52–7.
Lisabeth LD, Ahn HJ, Chen JJ, et al. Arsenic in drinking water and stroke hospitalizations in Michigan. Stroke. 2010;41:2499–504.
Monrad M, Ersbøll AK, Sørensen M, et al. Low-level arsenic in drinking water and risk of incident myocardial infarction: a cohort study. Environ Res. 2017;154:318–24.
James KA, Byers T, Hokanson JE, et al. Association between lifetime exposure to inorganic arsenic in drinking water and coronary heart disease in Colorado residents. Environ Health Perspect. 2015;123:128–34.
Chen Y, Karagas MR. Arsenic and cardiovascular disease: new evidence from the United States. Ann Intern Med. 2013;159:713–4.
Nong Q, Zhang Y, Guallar E, Zhong Q. Arsenic exposure and predicted 10-year atherosclerotic cardiovascular risk using the pooled cohort equations in U.S. hypertensive adults. Int J Environ Res Public Health. 2016;13.
Xu L, Polya DA, Li Q, Mondal D. Association of low-level inorganic arsenic exposure from rice with age-standardized mortality risk of cardiovascular disease (CVD) in England and Wales. Sci Total Environ. 2020;743: 140534.
Gamble MV, Liu X, Slavkovich V, et al. Folic acid supplementation lowers blood arsenic. Am J Clin Nutr. 2007;86:1202–9.
Argos M, Rahman M, Parvez F, et al. Baseline comorbidities in a skin cancer prevention trial in Bangladesh. Eur J Clin Invest. 2013;43:579–88.
Spallholz JE, Mallory Boylan L, Rhaman MM. Environmental hypothesis: is poor dietary selenium intake an underlying factor for arsenicosis and cancer in Bangladesh and West Bengal, India? Sci Total Environ. 2004;323:21–32.
Brown BG, Zhao XQ, Chait A, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med. 2001;345:1583–92.
Nyyssönen K, Porkkala E, Salonen R, Korpela H, Salonen JT. Increase in oxidation resistance of atherogenic serum lipoproteins following antioxidant supplementation: a randomized double-blind placebo-controlled clinical trial. Eur J Clin Nutr. 1994;48:633–42.
Salonen JT, Salonen R, Seppänen K, et al. Effects of antioxidant supplementation on platelet function: a randomized pair-matched, placebo-controlled, double-blind trial in men with low antioxidant status. Am J Clin Nutr. 1991;53:1222–9.
Zwolak I. The role of selenium in arsenic and cadmium toxicity: an updated review of scientific literature. Biol Trace Elem Res. 2020;193:44–63.
Ochoa-Martínez ÁC, Araiza-Gamboa Y, Varela-Silva JA, et al. Effect of gene-environment interaction (arsenic exposure - PON1 Q192R polymorphism) on cardiovascular disease biomarkers in Mexican population. Environ Toxicol Pharmacol. 2021;81: 103519.
Farzan SF, Chen Y, Rees JR, Zens MS, Karagas MR. Risk of death from cardiovascular disease associated with low-level arsenic exposure among long-term smokers in a US population-based study. Toxicol Appl Pharmacol. 2015;287:93–7.
Scannell Bryan M, Sofer T, Mossavar-Rahmani Y, et al. Mendelian randomization of inorganic arsenic metabolism as a risk factor for hypertension- and diabetes-related traits among adults in the Hispanic Community Health Study/Study of Latinos (HCHS/SOL) cohort. Int J Epidemiol. 2019;48:876–86.
Pichler G, Grau-Perez M, Tellez-Plaza M, et al. Association of arsenic exposure with cardiac geometry and left ventricular function in young adults. Circ Cardiovasc Imaging. 2019;12: e009018.
Pace C, Smith-Gagen J, Angermann J. Arsenic Methylation capacity and metabolic syndrome in the 2013–2014 U.S. National Health and Nutrition Examination Survey (NHANES). Int J Environ Res Public Health. 2018;15. doi:.
Sidhu MS, Desai KP, Lynch HN, et al. Mechanisms of action for arsenic in cardiovascular toxicity and implications for risk assessment. Toxicology. 2015;331:78–99.
Bhatnagar A. Environmental cardiology: studying mechanistic links between pollution and heart disease. Circ Res. 2006;99:692–705.
Barchowsky A, Klei LR, Dudek EJ, Swartz HM, James PE. Stimulation of reactive oxygen, but not reactive nitrogen species, in vascular endothelial cells exposed to low levels of arsenite. Free Radic Biol Med. 1999;27:1405–12.
Chen YC, Lin-Shiau SY, Lin JK. Involvement of reactive oxygen species and caspase 3 activation in arsenite-induced apoptosis. J Cell Physiol. 1998;177:324–33.
Wang Z, Dey S, Rosen BP, Rossman TG. Efflux-mediated resistance to arsenicals in arsenic-resistant and -hypersensitive Chinese hamster cells. Toxicol Appl Pharmacol. 1996;137:112–9.
Kumagai Y, Pi J. Molecular basis for arsenic-induced alteration in nitric oxide production and oxidative stress: implication of endothelial dysfunction. Toxicol Appl Pharmacol. 2004;198:450–7.
Chen Y, Santella RM, Kibriya MG, et al. Association between arsenic exposure from drinking water and plasma levels of soluble cell adhesion molecules. Environ Health Perspect. 2007;115:1415–20.
Blankenberg S, Rupprecht HJ, Bickel C, et al. Circulating cell adhesion molecules and death in patients with coronary artery disease. Circulation. 2001;104:1336–42.
Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–43.
Ridker PM, Hennekens CH, Roitman-Johnson B, Stampfer MJ, Allen J. Plasma concentration of soluble intercellular adhesion molecule 1 and risks of future myocardial infarction in apparently healthy men. Lancet. 1998;351:88–92.
Karim MR, Rahman M, Islam K, et al. Increases in oxidized low-density lipoprotein and other inflammatory and adhesion molecules with a concomitant decrease in high-density lipoprotein in the individuals exposed to arsenic in Bangladesh. Toxicol Sci. 2013;135:17–25.
Farzan SF, Howe CG, Zens MS et al. Urine arsenic and arsenic metabolites in U.S. adults and biomarkers of inflammation, oxidative stress, and endothelial dysfunction: a cross-sectional study. Environ Health Perspect. 2017;125:127002
Sobel MH, Sanchez TR, Jones MR, et al. Rice intake, arsenic exposure, and subclinical cardiovascular disease among US adults in MESA. J Am Heart Assoc. 2020;9: e015658.
Wu F, Molinaro P, Chen Y. Arsenic exposure and subclinical endpoints of cardiovascular diseases. Curr Environ Health Rep. 2014;1:148–62.
Harper KN, Liu X, Hall MN, et al. A dose-response study of arsenic exposure and markers of oxidative damage in Bangladesh. J Occup Environ Med. 2014;56:652–8.
Simeonova PP, Hulderman T, Harki D, Luster MI. Arsenic exposure accelerates atherogenesis in apolipoprotein E(-/-) mice. Environ Health Perspect. 2003;111:1744–8.
Bunderson M, Brooks DM, Walker DL, et al. Arsenic exposure exacerbates atherosclerotic plaque formation and increases nitrotyrosine and leukotriene biosynthesis. Toxicol Appl Pharmacol. 2004;201:32–9.
Srivastava S, Vladykovskaya EN, Haberzettl P, et al. Arsenic exacerbates atherosclerotic lesion formation and inflammation in ApoE-/- mice. Toxicol Appl Pharmacol. 2009;241:90–100.
Srivastava S, D’Souza SE, Sen U, States JC. In utero arsenic exposure induces early onset of atherosclerosis in ApoE-/- mice. Reprod Toxicol. 2007;23:449–56.
Lemaire M, Lemarié CA, Molina MF, et al. Exposure to moderate arsenic concentrations increases atherosclerosis in ApoE-/- mouse model. Toxicol Sci. 2011;122:211–21.
Moon KA, Navas-Acien A, Grau-Pérez M, et al. Low-moderate urine arsenic and biomarkers of thrombosis and inflammation in the Strong Heart Study. PLoS ONE. 2017;12: e0182435.
Moreno PR, Purushothaman KR, Zias E, Sanz J, Fuster V. Neovascularization in human atherosclerosis. Curr Mol Med. 2006;6:457–77.
Soucy NV, Mayka D, Klei LR, et al. Neovascularization and angiogenic gene expression following chronic arsenic exposure in mice. Cardiovasc Toxicol. 2005;5:29–41.
Larsson A, Sköldenberg E, Ericson H. Serum and plasma levels of FGF-2 and VEGF in healthy blood donors. Angiogenesis. 2002;5:107–10.
Sanchez-Soria P, Broka D, Monks SL, Camenisch TD. Chronic low-level arsenite exposure through drinking water increases blood pressure and promotes concentric left ventricular hypertrophy in female mice. Toxicol Pathol. 2012;40:504–12.
Phan NN, Wang CY, Lin YC. The novel regulations of MEF2A, CAMKK2, CALM3, and TNNI3 in ventricular hypertrophy induced by arsenic exposure in rats. Toxicology. 2014;324:123–35.
Kabir R, Sinha P, Mishra S, et al. Inorganic arsenic induces sex-dependent pathological hypertrophy in the heart. Am J Physiol Heart Circ Physiol. 2021;320:H1321–36.
States JC, Singh AV, Knudsen TB, et al. Prenatal arsenic exposure alters gene expression in the adult liver to a proinflammatory state contributing to accelerated atherosclerosis. PLoS ONE. 2012;7: e38713.
Ngalame NN, Micciche AF, Feil ME, States JC. Delayed temporal increase of hepatic Hsp70 in ApoE knockout mice after prenatal arsenic exposure. Toxicol Sci. 2013;131:225–33.
Tan M, Schmidt RH, Beier JI, et al. Chronic subhepatotoxic exposure to arsenic enhances hepatic injury caused by high fat diet in mice. Toxicol Appl Pharmacol. 2011;257:356–64.
Newman JD, Echagarruga CT, Ogando YM, et al. Hyperglycemia enhances arsenic-induced platelet and megakaryocyte activation. J Transl Med. 2017;15:55.
Author information
Authors and Affiliations
Contributions
Conception of the idea for the review: Dr. Desai, Dr. Venditti, and Dr. Sidhu.
Performed the literature search and drafted the review: Dr. Kaur, Dr. Desai, and Ms. Chang.
Critical revision of drafts: Dr. Kaur, Dr. Desai, Ms. Chang, Dr. Newman, Dr. Mathew, Dr. Bangalore, Dr. Venditti, and Dr. Sidhu.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kaur, G., Desai, K.P., Chang, I.Y. et al. A Clinical Perspective on Arsenic Exposure and Development of Atherosclerotic Cardiovascular Disease. Cardiovasc Drugs Ther 37, 1167–1174 (2023). https://doi.org/10.1007/s10557-021-07313-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-021-07313-9