Abstract
Over the last few decades, there has been a growing body of epidemiologic evidence linking chronic toxic metal exposure to cardiovascular disease-related morbidity and mortality. The recent and unexpectedly positive findings from a randomized, double-blind, multicenter trial of metal chelation for the secondary prevention of atherosclerotic cardiovascular disease (Trial to Assess Chelation Therapy (TACT)) have focused the discussion on the role of chronic exposure to toxic metals in the development and propagation of cardiovascular disease and the role of toxic metal chelation therapy in the secondary prevention of cardiovascular disease. This review summarizes the most recent evidence linking chronic toxic metal exposure to cardiovascular disease and examines the findings of TACT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metal chelation involves the use of specific drugs to remove toxic metals from the human body. These drugs, known as chelators, perform their action by creating molecular bonds with metal ions, mobilizing them from tissues and enhancing their excretion in urine or bile [1]. Chelation has taken root in modern medicine for the treatment of metal poisoning such as seen in lead toxicity, in which high blood levels, usually due to industrial exposure, cause acute symptoms that require urgent treatment. This article focuses on a different type of exposure: low-level chronic exposure from environmental sources. Such exposure may appear outwardly asymptomatic, but is insidiously vasculotoxic, and there is increasing high-quality evidence that enhancing excretion of these metals through chelation may be beneficial.
Although toxic metals such as lead and cadmium have been implicated in the pathogenesis of several medical disorders such as cardiovascular disease, chronic kidney disease, and diabetes [2–6], orthodox medical practitioners have rarely employed toxic metal chelation as secondary prevention of atherosclerotic disease. Before 2012, there was insufficient evidence to support or refute the use of chelation in the management of cardiovascular disease and there had been legitimate concerns about the safety of intravenous chelating agents such as ethylene diamine tetraacetic acid (edetate) and its salts [7].
The Trial to Assess Chelation Therapy (TACT) marked a watershed moment in chelation therapy for cardiovascular disease. TACT was designed and conducted to fill the gap in knowledge about the efficacy and safety of chelation therapy in a post-myocardial infarction, secondary prevention population. The findings were unexpectedly positive, showing a reduction in a combined cardiovascular endpoint. This finding opened a new chapter in the discussion about the role of toxic metals in pathogenesis of cardiovascular disease and the possible protective effects from chelating toxic metals in persons who have known cardiovascular disease. This article reviews the role of toxic metals in the development of atherosclerotic cardiovascular disease and the body of evidence supporting chelation therapy for the prevention of cardiovascular disease in a post-myocardial infarction population.
Toxic Metals and Cardiovascular Disease
In the scientific literature, the nomenclature of metals that are known to do harm to the human body is varied, often named heavy, toxic, or xenobiotic metals. For the purposes of this paper, we will refer to these metals as toxic metals. Four of them, arsenic, lead, cadmium, and mercury are in the top ten of the most recent Toxic Substances and Disease Registry Priority List of Hazardous Substances (ATSDR) published by the Centers for Disease Control and Prevention (CDC) [8]. Although there is scientific evidence linking each to cardiovascular disease, the epidemiologic evidence is strongest for lead and cadmium, two metals effectively chelated by edetate disodium [9•].
Lead
Lead is the most abundant of the toxic metals and ranks second on the 2015 priority list of hazardous materials [8]. According to the Environmental Protection Agency (EPA), lead can be found in common household products, including lead-based paint, drinking water pipes, ceramics, and some cosmetics. Lead is also found in solder, batteries, ammunition, and gasoline additives [10]. Although federal, state, and local regulations have helped to reduce the level of lead exposure, exposure to lead persists from lead-based paint and contamination of soil and ground water. A recent example is the Flint, Michigan lead crisis in which a change in water supply led to increasing levels of lead in the city’s water, in association with delayed recognition of same [11]. The problem of excess lead in water is not limited to Flint, as over 5300 water systems in all 50 states of the USA and serving approximately 18 million Americans have also been shown to have excess lead levels [12]. Underscoring the relative lack of knowledge about chronic lead burden and cardiovascular disease in adults, the lead crisis in Flint was discovered by a pediatrician and the efforts at mitigation have focused on children [11, 13].
Once absorbed, lead accumulates in the blood (largely erythrocytes), soft tissues, and bones [14]. In the erythrocytes and soft tissues, the half-life of lead is 35 and 40 days, respectively. However, in bones, with a half-life of about 30 years, absorbed lead may last a lifetime. Unfortunately, the blood lead load only reflects lead exposure within the last 3–5 weeks and does not correlate with chronic lead exposure [14]. Thus, the attempts to mitigate the lead poisoning in Flint and other cities with bottled water simply allows blood lead levels to fall, while body lead burden likely remains unchanged.
The health effects of lead exposure has been extensively studied, and lead has been shown to be associated with increased cardiovascular disease and all-cause mortality [5, 15, 16, 17•, 18, 19]. An analysis from the National Health and Nutrition Examination Survey (NHANES) data from 1976 to 1980 and 1988 to 1994 showed that elevated blood lead levels including those below 10 μg/dl (previously the US cut-off for acceptable levels [20]) were associated with increased risk of all cause and cardiovascular disease mortality [15, 16]. The strongest epidemiologic evidence supporting the association of directly measured total lead burden and mortality comes from the Veterans Affairs Normative Aging Study in which participants in the highest tertile of bone (patella) lead had a fivefold increase in cardiovascular mortality risk, eightfold increase in ischemic heart disease mortality, and an 86% increase in all-cause mortality compared to those in the lowest tertile after accounting for other confounders [14, 17•]. Exposure to lead is also associated with an increased prevalence of cardiovascular disease risk factors such as hypertension [21–23] and peripheral arterial disease [24, 25]. There has also been described an association between atherosclerosis and lead concentrations [26, 27].
Cadmium
Like lead, cadmium is another substance of concern ranked by ATSDR (number 7) [8]. The major sources of cadmium are from mining and smelting of cadmium ores, combustion of fossil fuels such as coal, waste incineration, and disposal, manufacture or application of phosphate fertilizers, and manufacture and disposal of rechargeable nickel-cadmium batteries [28]. From these sources, cadmium can be released into water, soil, or air. In the air, cadmium may exist in a vaporized form and can be transported over significant distances and deposited in the soil or water. In the soil, cadmium competes with other mineral nutrients such as calcium and magnesium at absorption sites within the transport systems of plants [29]. Cadmium’s uptake in plant roots is facilitated by a transmembrane carrier and is dependent on the soil concentrations of cadmium as well as other factors such as temperature, soil PH, and the concentrations of other elements [30]. Cadmium, therefore, is readily taken up by green leafy plants such as tobacco, spinach, and kale [28]. Consequently, cadmium exposure occurs both by inhalation (as seen with cigarette smoking) and by ingestion of these cadmium-rich foods [5]. The association between cigarette smoking and cadmium exposure has been shown to extend to secondhand smoking [31, 32]. Close to half of the inhaled cadmium and about 5% of ingested cadmium is absorbed and stored in the kidneys and other tissues including the adrenals, testes, placenta, and bones. Although cadmium is filtered in the urine, it is almost entirely reabsorbed in the renal tubules. Therefore, the half-life of cadmium is very long; up to 38 years [5], similar to that of lead in the bone.
Cadmium exposure is associated with increased risk of coronary artery disease, peripheral artery disease, stroke, and mortality from all cause and cardiovascular disease [2, 27–30, 33, 34]. The epidemiological evidence linking cadmium to peripheral artery disease is robust. A 2004 analysis of the 1999–2000 NHANES showed that persons in the highest quartile of blood cadmium had nearly three times the odds of having peripheral artery disease compared with those in the first quartile (OR 2.82, 95%CI 1.36–5.85) [24]. This NHANES analysis also demonstrated an effect modification of the association of blood levels with cadmium and peripheral artery disease by smoking [24]. Urinary cadmium, a marker of long-term cadmium exposure, is associated with incident peripheral artery disease (HR for highest versus lowest tertile was 1.96, 95%CI 1.32–2.81) [35].
Linking Toxic Metals to Atherosclerosis
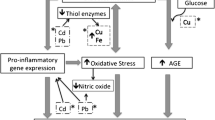
The pathophysiologic mechanisms linking toxic metals to cardiovascular disease are incompletely understood but appear to be linked to oxidative stress [5]. Many metals are thought to generate toxic vascular environments through the intracellular accumulation of reactive oxygen species (ROS). Metals can form bonds with several enzyme systems responsible for detoxifying ROS such as glutathione, depleting them and promoting accumulation of ROS and free oxygen radicals. These free radicals are promoters of atherogenesis through many mechanisms, including LDL peroxidation, one of the early events in development of atherosclerosis. In addition to the above, each metal also has idiosyncratic effects. For instance, lead exposure has been linked to DNA hypomethylation in the COLIA2 gene which codes for the alpha 2 chain of type I collagen [36]. Lead, and to a lesser extent cadmium, interacts with cellular calcium, competing with it for transport systems and modifying its intracellular distribution [37, 38]. There is evidence that both lead and cadmium are substitutes for calcium in calmodulin-dependent reactions [38]. These interactions are thought to promote vascular remodeling and may contribute to vascular disease [37]. Cadmium has been linked to apoptosis, abnormal glucose metabolism, and reduced energy production in cardiomyocytes [39].
Chelation Treatment for Chronic, Low-Level, Metal Toxicity
Edetate and its salts, first patented by Ferdinand Munz nearly a century ago [40] is one of the most used chelating agents. Edetate chelates lead, cadmium, and a handful of other metals [9•]. Thus, it has been used for industrial metal toxicity such as lead poisoning [41]. A recent study showed that edetate disodium-based infusions increased the urinary excretion of lead and cadmium by 3700 and 750%, respectively [9•]. An older study also showed similar marked increases in the excretion of both lead and cadmium within 24 hours of an EDTA-based infusion [42].
Complementary and alternative medicine practitioners have embraced the use of metal chelation for the treatment of cardiovascular disease, particularly with edetate and its salts (disodium and calcium disodium, most prominently), for many years. In contrast, the traditional medical community has shunned this practice. The reasons for chelation therapy being unpopular among orthodox medical practitioners arise from the poor evidence base prior to the TACT trial, concerns about the safety of edetate-based chelation therapy, and general suspicion and distrust of alternative medicine. The safety concerns regarding edetate disodium have been addressed by the FDA. They reviewed their database encompassing over 30 years of use and reported four cases of hypocalcemia-induced mortality following definite edetate disodium infusion in a timeframe when hundreds of thousands, perhaps millions of infusions had been administered in the USA [43, 44].
Design and Results of TACT
Before the initiation of TACT, there had been five randomized trials examining the efficacy of EDTA-based chelation therapy [45–49]. Overall, these were small trials testing surrogate endpoints with generally negative results; but, even in aggregate, they could not exclude a small to moderate benefit of therapy. A Cochrane review concluded that there was “insufficient evidence” in support or against the use of chelation therapy to improve outcomes of persons with atherosclerotic cardiovascular disease [50]. Despite the results of the RCTs discussed above, chelation therapy continued to be used by US adults [44] for a variety of indications, not all cardiovascular. TACT, developed in response to a Request for Applications from the National Institutes of Health, was designed under the Food and Drug Administration (FDA) Investigational New Drug (IND) listing as a pivotal trial of edetate disodium therapy to finally understand whether edetate disodium was harmful or ineffective (expected outcome), or safe and effective. To date, TACT remains unique as the only pivotal trial of edetate disodium therapy for coronary disease.
TACT was a multicenter randomized double-blind clinical trial with a 2 × 2 factorial design sponsored by both the National Center for Complementary and Alternative Medicine (NCCAM) and the National Heart Lung and Blood Institute (NHLBI) [51]. This design was chosen to control for the potential confounder of high doses of oral vitamins and minerals commonly used in the chelation practices [52]. Thus, participants were randomly assigned to one of four groups:
-
IV chelation plus oral vitamins (active/active)
-
IV chelation plus oral placebo (active/placebo)
-
IV placebo plus oral vitamins (placebo/active)
-
IV placebo plus oral placebo (placebo/placebo)
The chelation group received up to 3 g of Na2EDTA (based on weight and renal function) in addition to 7 g of ascorbic acid, 2 g of magnesium chloride, 840 mg of sodium bicarbonate, 250 mg of pantothenic acid, 100 mg of thiamine, 100 mg of pyridoxine, 2500 U of unfractionated heparin, 2 meq of potassium chloride, 100 mg of procaine hydrochloride and sterile water to make up 500 ml of infusion. The placebo infusion consisted of 500 ml of normal saline and 1.2% dextrose [53••]. Participants received 30 weekly infusions followed by 10 maintenance infusions that were 2 to 8 weeks apart. Those randomized to multivitamins received a 28-component high oral dose combination of vitamins and minerals [51]. Participants in TACT were 50 years and older with a previous myocardial infarction ≥6 weeks before enrollment. Persons with renal impairment (serum creatinine >2 mg/dl) or those unable to tolerate 500 ml of chelation on a weekly basis were excluded [51].
The study protocol strongly suggested to investigators that post-MI patients receive evidence-based cardiac therapies, so that rather than chelation as alternative medicine, TACT really tested chelation as add-on therapy post-MI. Thus, 83% of the study population had undergone a prior revascularization, 91% were on an anticoagulant or anti-platelet, 73% on a statin, and the median LDL was 89 (67–115) mg/dL [53••].
The primary outcome was a composite of all-cause mortality, myocardial infarction or coronary revascularization, stroke, and hospitalization for angina. The secondary outcomes included a composite of cardiovascular mortality, myocardial infarction, or stroke. There were also pre-specified subgroup analyses including participants with diabetes and anterior MI [51]. Participants were followed for up to 5 years (with a median of 55 months) [53••].
The trial took 9 years to complete. There were 55,222 infusions of active or placebo chelation delivered. The Kaplan-Meier 5-year estimate for the primary endpoint in the chelation group (i.e., active/active plus active placebo) was 32.8% (95%CI, 29.1–36.5%) compared with 38.5% (95%CI, 34.6–42.3%) in the placebo group (placebo/active and placebo/placebo) with a HR of 0.82 (95%CI0.69–0.99; p = 0.035; see Fig. 1), meeting the statistical goals of the trial [53••]. Compared to the placebo group, participants in the chelation group had lower frequencies of myocardial infarction (6 vs. 8%) and coronary revascularizations (15 vs. 18%); however, the individual effect sizes did not achieve statistical significance [53••]. This was expected, as the study was not powered to assess for these individual outcomes [53••], but for the aggregate primary endpoint.
Kaplan-Meier estimates of the primary composite endpoint, EDTA chelation therapy vs. placebo in the overall participant population (a) and in participants with diabetes (b). EDTA ethylene diamine tetraacetic acid. The primary endpoint was a composite of death from any cause, reinfarction, stroke, coronary revascularization, or hospitalization for angina. Reproduced with permission from [52] and [54]
When the factorial groups were compared, patients receiving both chelation and vitamins (active/active) were 26% less likely to experience the primary outcome than those receiving IV placebo and oral placebo (placebo/placebo) (HR 0.74 95%CI 0.57, 0.95, p = 0.016) [52]. This corresponded to a 5-year number needed to treat (NNT) of 12 [52]. This compared favorably with the 5-year NNT for statin therapy [54].
In the TACT trial, the edetate disodium-based infusions exhibited an excellent safety profile as the frequency of non-endpoint serious adverse events (other than mortality) were very low and similar (statistically identical) in both groups. Among the non-severe adverse events, hypocalcemia occurred more frequently in the chelation arm than in the placebo arm (6.2 vs. 3.5%; p = 0.008). This is somewhat consistent with the reported adverse effects that predated TACT in which no serious adverse effects were noted but hypocalcemia was more common in the chelation groups [55]. Of the 839 participants, about 67% completed all 40 infusions and nearly 80% (77%) completed 30 or more infusions. Similar completions rates were seen in the placebo infusion arm (64 and 75%, respectively) [53••].
Perhaps the most interesting results from TACT were found in the pre-specified subgroup of patients with diabetes. There were 633 participants with diabetes mellitus (322 edetate and 311 placebo). There was a 41% (HR 0.59, 95%CI 0.44–0.79, p = 0.0002) reduction in the composite endpoint (Table 1) with a corresponding 5-year NNT of 6.5 [56••]. For the secondary endpoints, participants with diabetes demonstrated a 43% reduction in all-cause mortality (p = 0.011) and a 52% reduction in recurrent MIs (p = 0.015).
There are some clear differences between the former trials assessing the effects of chelation therapy and TACT, which may account for TACT’s success. TACT had a larger sample size, almost six times the number of study participants in the other trials combined thus increasing the power to detect smaller differences. Likewise, the length of follow-up in TACT (median of 55 months) far exceeded that of any of the previous studies (longest reported follow-up duration of 12 months) [50]. TACT also had twice the number of chelation infusion cycles (40 vs. about 20 in most of the earlier trials). Unlike the previous trials, TACT did not use surrogate outcomes but employed “hard” clinical outcomes similar to those used in other major cardiovascular trials [57, 58].
Edetate Disodium Therapy and Diabetes
Admittedly, the reasons why participants with diabetes had the greatest benefits from chelation therapy were unclear; however, persons with diabetes tend to have greater co-morbidity such as hypertension, chronic kidney disease, dyslipidemia, and tend to have more severe atherosclerosis. While these may have contributed to the unexpected findings, there is biologic plausibility that the effects of chelation therapy observed in the TACT diabetic population could be due to prevention of diabetic vascular complications through mechanisms that involve a reduction of oxidative stress. These potential mechanisms may involve alterations in the creation or metabolism of advanced glycation end-products (AGEs). AGEs, which are non-enzymatically modified proteins or lipids (or in some cases, nucleic acids) generated in the presence of reducing sugars like glucose, which activate toxic cytokines through the receptor for AGEs (RAGE) [59] and may be causative for some vascular complications of diabetes [60, 61]. Transition metals including iron and copper have been implicated in the development of AGEs and the consequent oxidative complications they bring [62]. AGE inhibitors and AGE breakers may even act on AGEs through their chelation effects [62, 63]. Therefore, it is a plausible hypothesis, albeit emphatically unproven, that chelation with edetate disodium therapy may protect against the long-term vascular complications of diabetes through mitigating the accumulation of AGEs.
TACT results were initially greeted with skepticism and harsh criticism [64–66]. The criticisms surrounding TACT’s positive results surround the 17% withdrawal of consent observed in the trial and other issues involving the conduct of the trial including differential follow-up rates. As pointed out in an editorial (not written by the authors), since consent withdrawal was greater in the placebo group, the bias would be against chelation therapy if indeed there was one [67]. The other issues surrounding the study’s conduct have been addressed extensively [53••].
Conclusions
According to the 2016 AHA Heart Disease and Stroke Statistics, approximately 200,000 recurrent myocardial infarctions occur and there are over 100,000 deaths from myocardial infarction per year [68]. Data from epidemiologic studies suggest that within 5 years of a myocardial infarction, 36% of men and 42% of women aged 45 years and older will die [68]. With over 11 billion USD in yearly costs, myocardial infarction is not only one of the leading causes of death but is also one of the most expensive illnesses [68]. Diabetes is a risk factor for recurrent myocardial infarction and death [69]. These data emphasize the enormity of the public health burden associated with recurrent myocardial infarction, particularly among those with diabetes. The results of TACT, which showed a modest but significant reduction in risk among persons with a prior MI and a more robust benefit among patients with diabetes provide impetus to further investigate chelation therapy for cardiovascular disease. Unsurprisingly, with the findings of TACT, chelation therapy for the management of stable ischemic heart disease has been upgraded from a class III (no benefit) to class IIb (benefit uncertain; treatment may be considered) [70].
Spurred by the findings of TACT, particularly the strong protective effect of chelation among persons with diabetes mellitus [53••, 56••], the National Institutes of Health has funded TACT2 for $37 million. This second, replicative study will definitively inform the scientific and lay community as to whether chelation and excretion of vasculotoxic metals will confirm (or refute) the very positive findings in post-MI patients with diabetes. In the meantime, the TACT2 study team has just enrolled the first few of 1200 patients. We are still looking for enrolling sites interested in this potentially disruptive pharmacotherapy.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Sears ME, Sears ME. Chelation: harnessing and enhancing heavy metal detoxification—a review. Sci World J Sci World J. 2013;2013(2013):e219840.
Barregard L, Sallsten G, Fagerberg B, Borné Y, Persson M, Hedblad B, et al. Blood cadmium levels and incident cardiovascular events during follow-up in a population-based cohort of Swedish adults: the Malmö Diet and Cancer study. Environ Health Perspect. 2015;30.
Fagerberg B, Barregard L, Sallsten G, Forsgard N, Ostling G, Persson M, et al. Cadmium exposure and atherosclerotic carotid plaques—results from the Malmö diet and Cancer study. Environ Res. 2015;136:67–74.
Menke A, Guallar E, Cowie CC. Metals in urine and diabetes in U.S. adults. Diabetes. 2016;65(1):164–71.
Solenkova NV, Newman JD, Berger JS, Thurston G, Hochman JS, Lamas GA. Metal pollutants and cardiovascular disease: mechanisms and consequences of exposure. Am Heart J. 2014;168(6):812–22.
Lin J-L, Lin-Tan D-T, Hsu K-H, Yu C-C. Environmental lead exposure and progression of chronic renal diseases in patients without diabetes. N Engl J Med. 2003;348(4):277–86.
Centers for Disease Control and Prevention (CDC). Deaths associated with hypocalcemia from chelation therapy—Texas, Pennsylvania, and Oregon, 2003–2005. MMWR Morb Mortal Wkly Rep. 2006;55(8):204–7.
Agency for Toxic Substances and Diseases Registry. 2015 Priority list of hazardous substances [Internet]. 2015 [cited 2016 Apr 4]. Available from: http://www.atsdr.cdc.gov/spl/resources/atsdr_2015_spl_detailed_data_table.pdf
• Arenas I, Navas-Acien A, Lamas G. Enhanced vasculotoxic metal excretion in post-myocardial infarction patients receiving edetate disodium-based infusion. J Am Coll Cardiol. 2016;67(13_S):2125. Study demonstrating that edetate disodium increases urinary excretion of heavy metals in a post-myocardial infarction population.
US EPA O. Learn about lead [Internet]. [cited 2016 Apr 5]. Available from: https://www.epa.gov/lead/learn-about-lead#found
Hanna-Attisha M, LaChance J, Sadler RC, Champney Schnepp A. Elevated blood lead levels in children associated with the Flint drinking water crisis: a spatial analysis of risk and public health response. Am J Public Health. 2015;106(2):283–90.
Erik Olson, Kristi Pullen Fedinick. What’s in your water? Flint and beyond [Internet]. Natural Resources Defense Council; 2016 [cited 2016 Aug 8]. Available from: https://www.nrdc.org/resources/whats-your-water-flint-and-beyond
Laura Ungar. Lawmakers urge the EPA to reduce its standard for lead in drinking water. USA TODAY [Internet]. 2016 [cited 2016 Jul 12]; Available from: http://www.usatoday.com/story/news/2016/06/30/lawmakers-urge-epa-reduce-its-standard-lead-drinking-water/86576032/
Papanikolaou NC, Hatzidaki EG, Belivanis S, Tzanakakis GN, Tsatsakis AM. Lead toxicity update. A brief review. Med Sci Monit Int Med J Exp Clin Res. 2005;11(10):RA329–36.
Menke A, Muntner P, Batuman V, Silbergeld EK, Guallar E. Blood lead below 0.48 micromol/L (10 microg/dL) and mortality among US adults. Circulation. 2006;114(13):1388–94.
Lustberg M, Silbergeld E. Blood lead levels and mortality. Arch Intern Med. 2002;162(21):2443–9.
• Weisskopf MG, Jain N, Nie H, Sparrow D, Vokonas P, Schwartz J, et al. A prospective study of bone lead concentration and death from all causes, cardiovascular diseases, and cancer in the Department of Veterans Affairs Normative Aging Study. Circulation. 2009;120(12):1056–64. Demonstrated an association between chronic lead exposure and mortality from all cause and cardiovascular disease.
Cosselman KE, Navas-Acien A, Kaufman JD. Environmental factors in cardiovascular disease. Nat Rev Cardiol. 2015;12(11):627–42.
Lamas GA, Navas-Acien A, Mark DB, Lee KL. Heavy metals, cardiovascular disease, and the unexpected benefits of chelation therapy. J Am Coll Cardiol. 2016;67(20):2411–8.
CDC. Adult Blood Lead Epidemiology and Surveillance (ABLES): program description: NIOSH Workplace safety and health topic [Internet]. [cited 2016 Aug 1]. Available from: http://www.cdc.gov/niosh/topics/ables/description.html
Nash D, Magder L, Lustberg M, et al. BLood lead, blood pressure, and hypertension in perimenopausal and postmenopausal women. JAMA. 2003;289(12):1523–32.
Pirkle JL, Schwartz J, Landis JR, Harlan WR. The relationship between blood lead levels and blood pressure and its cardiovascular risk implications. Am J Epidemiol. 1985;121(2):246–58.
Harlan WR, Landis JR, Schmouder RL, Goldstein NG, Harlan LC. Blood lead and blood pressure. Relationship in the adolescent and adult US population. JAMA. 1985;253(4):530–4.
Navas-Acien A, Selvin E, Sharrett AR, Calderon-Aranda E, Silbergeld E, Guallar E. Lead, cadmium, smoking, and increased risk of peripheral arterial disease. Circulation. 2004;109(25):3196–201.
Nawrot TS, Staessen JA. Low-level environmental exposure to lead unmasked as silent killer. Circulation. 2006;114(13):1347–9.
Voors AW, Shuman MS, Johnson WD. Additive statistical effects of cadmium and lead on heart-related disease in a North Carolina autopsy series. Arch Environ Health. 1982;37(2):98–102.
Revis NW, Zinsmeister AR, Bull R. Atherosclerosis and hypertension induction by lead and cadmium ions: an effect prevented by calcium ion. Proc Natl Acad Sci U S A. 1981;78(10):6494–8.
ATSDR. Public Health Statement: cadmium [Internet]. [cited 2016 Jun 1]. Available from: http://www.atsdr.cdc.gov/phs/phs.asp?id=46&tid=15
Hasan SA, Fariduddin Q, Ali B, Hayat S, Ahmad A. Cadmium: toxicity and tolerance in plants. J Environ Biol Acad Environ Biol India. 2009;30(2):165–74.
Benavides MP, Gallego SM, Tomaro ML. Cadmium toxicity in plants. Braz J Plant Physioiogy. 2005;17(1):21–34.
Richter PA, Bishop EE, Wang J, Swahn MH. Tobacco smoke exposure and levels of urinary metals in the U.S. youth and adult population: the National Health and Nutrition Examination Survey (NHANES) 1999–2004. Int J Environ Res Public Health. 2009;6(7):1930–46.
Jung SY, Kim S, Lee K, Kim JY, Bae WK, Lee K, et al. Association between secondhand smoke exposure and blood lead and cadmium concentration in community dwelling women: the fifth Korea National Health and Nutrition Examination Survey (2010–2012). BMJ Open. 2015;5(7):e008218.
Tellez-Plaza M, Jones MR, Dominguez-Lucas A, Guallar E, Navas-Acien A. Cadmium exposure and clinical cardiovascular disease: a systematic review. Curr Atheroscler Rep. 2013;15(10):356.
Larsson SC, Wolk A. Urinary cadmium and mortality from all causes, cancer and cardiovascular disease in the general population: systematic review and meta-analysis of cohort studies. Int J Epidemiol. 2015;20.
Tellez-Plaza M, Guallar E, Fabsitz RR, Howard BV, Umans JG, Francesconi KA, et al. Cadmium exposure and incident peripheral arterial disease. Circ Cardiovasc Qual Outcomes. 2013;6(6):626–33.
Hanna CW, Bloom MS, Robinson WP, Kim D, Parsons PJ, vom Saal FS, et al. DNA methylation changes in whole blood is associated with exposure to the environmental contaminants, mercury, lead, cadmium and bisphenol A, in women undergoing ovarian stimulation for IVF. Hum Reprod Oxf Engl. 2012;27(5):1401–10.
Vaziri ND. Mechanisms of lead-induced hypertension and cardiovascular disease. Am J Physiol - Heart Circ Physiol. 2008;295(2):H454–65.
Habermann E, Crowell K, Janicki P. Lead and other metals can substitute for Ca2+ in calmodulin. Arch Toxicol. 1983;54(1):61–70.
Chen C, Zhang S, Liu Z, Tian Y, Sun Q. Cadmium toxicity induces ER stress and apoptosis via impairing energy homoeostasis in cardiomyocytes. Biosci Rep [Internet]. 2015 [cited 2016 May 24];35(3). Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4613727/
Lewin MR. Chelation therapy for cardiovascular disease. Review and commentary. Tex Heart Inst J. 1997;24(2):81–9.
Klaassen CD. Heavy metals and heavy metal antagonists. In: Goodman L, Gilman A, editors. The pharmacological basis of therapeutics. New York: McGraw Hill, Medical Publishing Division; 2006.
Waters RS, Bryden NA, Patterson KY, Veillon C, Anderson RA. EDTA chelation effects on urinary losses of cadmium, calcium, chromium, cobalt, copper, lead, magnesium, and zinc. Biol Trace Elem Res. 2001;83(3):207–21.
US Food and Drug Administration. Postmarket Drug safety information for patients and providers—questions and answers on edetate disodium [Internet]. [cited 2016 Jul 4]. Available from: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrug SafetyInformationforPatientsandProviders/ucm113738.htm
Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Rep. 2008;12:1–23.
Knudtson ML, Wyse DG, Galbraith PD, Brant R, Hildebrand K, Paterson D, et al. Chelation therapy for ischemic heart disease: a randomized controlled trial. JAMA. 2002;287(4):481–6.
Guldager B, Jelnes R, Jørgensen SJ, Nielsen JS, Klaerke A, Mogensen K, et al. EDTA treatment of intermittent claudication—a double-blind, placebo-controlled study. J Intern Med. 1992;231(3):261–7.
Sloth-Nielsen J, Guldager B, Mouritzen C, Lund EB, Egeblad M, Nørregaard O, et al. Arteriographic findings in EDTA chelation therapy on peripheral arteriosclerosis. Am J Surg. 1991;162(2):122–5.
Olszewer E, Sabbag FC, Carter JP. A pilot double-blind study of sodium-magnesium EDTA in peripheral vascular disease. J Natl Med Assoc. 1990;82(3):173–7.
van Rij AM, Solomon C, Packer SG, Hopkins WG. Chelation therapy for intermittent claudication. A double-blind, randomized, controlled trial. Circulation. 1994;90(3):1194–9.
Villarruz MV, Dans A, Tan F. Chelation therapy for atherosclerotic cardiovascular disease. Cochrane Database Syst Rev. 2002;4:CD002785.
Lamas GA, Goertz C, Boineau R, Mark DB, Rozema T, Nahin RL, et al. Design of the Trial to Assess Chelation Therapy (TACT). Am Heart J. 2012;163(1):7–12.
Lamas GA, Boineau R, Goertz C, Mark DB, Rosenberg Y, Stylianou M, et al. EDTA chelation therapy alone and in combination with oral high-dose multivitamins and minerals for coronary disease: the factorial group results of the Trial to Assess Chelation Therapy. Am Heart J. 2014;168(1):37–44.e5.
•• Lamas GA, Goertz C, Boineau R, Mark DB, Rozema T, Nahin R, et al. Effect of disodium EDTA chelation regimen on cardiovascular events in patients with previous myocardial infarction: the TACT randomized trial. JAMA. 2013;309(12):1241–50. Largest trial of chelation therapy for the prevention of cardiovascular disease-related outcomes in a post-myocardial infarction population. Study’s findings have focused a discussion on the role of toxic metals in the pathogenesis of atherosclerotic CVD and have changed current guidelines on the use of chelation therapy in post-myocardial infarction patients.
Costa J, Borges M, David C, Vaz Carneiro A. Efficacy of lipid lowering drug treatment for diabetic and non-diabetic patients: meta-analysis of randomised controlled trials. BMJ. 2006;332(7550):1115–24.
Seely DMR, Wu P, Mills EJ. EDTA chelation therapy for cardiovascular disease: a systematic review. BMC Cardiovasc Disord. 2005;5:32.
•• Escolar E, Lamas GA, Mark DB, Boineau R, Goertz C, Rosenberg Y, et al. The effect of an EDTA-based chelation regimen on patients with diabetes mellitus and prior myocardial infarction in the Trial to Assess Chelation Therapy (TACT). Circ Cardiovasc Qual Outcomes. 2013 Nov 19;CIRCOUTCOMES.113.000663. This study showed significant reduction in the primary outcome among participants with diabetes mellitus in the TACT trial. The findings of this study form the basis for the TACT 2 trial which seeks to reproduce the findings of TACT in a post-myocardial infarction population with established diabetes mellitus.
Ridker PM, Danielson E, Fonseca FAH, Genest J, Gotto AMJ, Kastelein JJP, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195–207.
A study of Evacetrapib in high-risk vascular disease—Full Text View—ClinicalTrials.gov [Internet]. Clinicaltrials.gov. [cited 2016 Aug 10]. Available from: https://clinicaltrials.gov/ct2/show/NCT01687998
Schmidt AM, Vianna M, Gerlach M, Brett J, Ryan J, Kao J, et al. Isolation and characterization of two binding proteins for advanced glycosylation end products from bovine lung which are present on the endothelial cell surface. J Biol Chem. 1992;267(21):14987–97.
Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products sparking the development of diabetic vascular injury. Circulation. 2006;114(6):597–605.
Goh S-Y, Cooper ME. The role of advanced glycation end products in progression and complications of diabetes. J Clin Endocrinol Metab. 2008;93(4):1143–52.
Price DL, Rhett PM, Thorpe SR, Baynes JW. Chelating activity of advanced glycation end-product inhibitors. J Biol Chem. 2001;276(52):48967–72.
Nagai R, Murray DB, Metz TO, Baynes JW. Chelation: a fundamental mechanism of action of AGE inhibitors, AGE breakers, and other inhibitors of diabetes complications. Diabetes. 2012;61(3):549–59.
Atwood KC, Woeckner E, Baratz RS, Sampson WI. Why the NIH Trial to Assess Chelation Therapy (TACT) should be abandoned. Medscape J Med. 2008;10(5):115.
Nissen SE. Concerns about reliability in the Trial to Assess Chelation Therapy (TACT). JAMA. 2013;309(12):1293–4.
Callaway E. Chelation-therapy heart trial draws fire. Nature. 2012;491(7424):313–5.
Kaul S. Are concerns about reliability in the trial to assess chelation therapy fair grounds for a hasty dismissal? An alternative perspective. Circ Cardiovasc Qual Outcomes. 2014;7(1):5–7.
Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 2016;133(4):e38–360.
Malmberg K, Yusuf S, Gerstein HC, Brown J, Zhao F, Hunt D, et al. Impact of diabetes on long-term prognosis in patients with unstable angina and non-Q-wave myocardial infarction: results of the OASIS (Organization to Assess Strategies for Ischemic Syndromes) Registry. Circulation. 2000;102(9):1014–9.
Fihn SD, Blankenship JC, Alexander KP, Bittl JA, Byrne JG, Fletcher BJ, et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for thoracic surgery, preventive cardiovascular nurses association, society for cardiovascular angiography and interventions, and society of thoracic surgeons. J Am Coll Cardiol. 2014;64(18):1929–49.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Ehimen C. Aneni and Esteban Escolar declare that they have no conflict of interest.
Gervasio A. Lamas declares grant support from the NIH for the Trial to Assess Chelation Therapy (TACT).
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Nonstatin Drugs
Rights and permissions
About this article
Cite this article
Aneni, E.C., Escolar, E. & Lamas, G.A. Chronic Toxic Metal Exposure and Cardiovascular Disease: Mechanisms of Risk and Emerging Role of Chelation Therapy. Curr Atheroscler Rep 18, 81 (2016). https://doi.org/10.1007/s11883-016-0631-0
Published:
DOI: https://doi.org/10.1007/s11883-016-0631-0