Abstract
Purpose of Review
Epidemiologic and experimental evidence support that exposure to moderate-to-high arsenic (As) is a cardiovascular disease (CVD) risk factor. Little is known, however, on the cardiovascular effects of low water As exposure (< 10 μg/L) through diet, particularly rice. The goal is to summarize the evidence on As and CVD and the research needs at low levels of exposure.
Recent Findings
Studies of populations in Taiwan, Chile, and Bangladesh have consistently shown that high water As (> 100 μg/L) constitutes a CVD risk factor. In experimental studies, chronic inorganic As in drinking water increased atherosclerotic lesions in mice. Cohort studies at low-to-moderate levels of exposure (< 100 μg/L) based on biomarkers or individual water As measures in American Indian from rural communities and in Whites and Hispanics from Colorado found higher risk of CVD incidence and mortality, particularly coronary heart disease (CHD) among those with higher arsenic exposure.
Summary
A major limitation of existent dose-response meta-analyses is the limited number of studies in populations exposed to water As at levels < 10 μg/L. Measuring metals, in particular arsenic, in general populations with comprehensive assessment of clinical cardiovascular disease can inform on the cardiovascular role of low-level arsenic and contribute to CVD prevention and control in general populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Increasing evidence indicates that metals and metalloids are risk factors for clinical cardiovascular disease (CVD) [1,2,3,4,5]. However, evidence from population-based prospective cohort studies, which are the basis for CVD prevention and control programs in the USA, is lacking. This is particularly true for arsenic (As), for which most of the evidence comes from populations exposed to high water levels internationally (generally > 100 μg/l) [6,7,8,9] and to moderate water levels in rural areas in the USA (generally 10–100 μg/l) [10••, 11••]. The significant findings in those international and rural US studies, the relevance of dietary As exposure, and experimental evidence showing increased atherosclerosis even at relatively low levels [12] support the need to investigate the association between As and CVD in populations living in urban settings. Prospective cohort studies in general populations with data on subclinical and clinical CVD, wide geographical coverage across US regions, multi-ethnic inclusiveness, and comprehensive characterization of CVD risk factors, covering traditional, environmental, and social risk factors (e.g., air pollution) are needed. Indeed, while low-level As exposure is widespread, very few studies have characterized the potential cardiovascular effects at those low levels.
Methods
In this review, we summarized a large body of literature on the role of arsenic and CVD with a particular focus on the evidence available at low-moderate levels of exposure. We first review the sources of As exposure at low levels and the role of cooking and eating practices across different ethnics groups in general populations. Second, we discuss the evidence on As as a CVD risk factor, the relevant mechanisms, and the shape of the dose-response. Third, we discuss particular challenges when conducting research at low-level As exposure, in particular the challenge of interpreting total As and some As species in the presence of seafood intake and how to address this challenge. Finally, we discuss the research needs for general populations and present an overall conclusion. The manuscripts identified for this review have been compiled over many years by the study investigators through systematic reviews and ongoing systematic searches of the literature using both free text and indexed terms including arsenic, arsenite, arsenate, methylated arsenic species, arsenic poisoning, cardiovascular disease, coronary heart disease, stroke, and other terms.
Sources of Arsenic Exposure in General Populations
Inorganic As (iAs) is a potent [13] toxic and carcinogenic metalloid widespread in the environment. It is found in water, soil, food, and air. Groundwater contaminated with iAs affects populations worldwide, including 5 million people in the USA with water above the EPA standard (10 μg/l) [14]. In populations exposed to water As < 10 μg/l, diet contributes to up to 85% of iAs exposure [15, 16•]. Arsenic enters the food chain from contaminated soil and water, industrial contamination, and past use of As pesticides [16•, 17,18,19]. Rice is a major source of iAs and dimethylarsinate (DMA) because it accumulates in the grain (Fig. 1) [20,21,22,23,24,25]. USA-grown rice is particularly high in As. In a US population, consuming ½ cup of cooked rice was estimated to be equivalent to drinking 1 L of water at 10 μg/l [26]. Fruit juice, especially apple, pear, and grape concentrates, can have relatively high iAs levels [16•, 27]. Poultry was a source of iAs and other species before the recent ban of As-based drugs in poultry production (chicken in 2013, turkey in 2015) [28,29,30, 31•]. Foods that contain lower levels but are consumed in high quantities such as wheat and non-rice cereals can also contribute to exposure [16•]. Seafood is generally low in iAs but contains high levels of arsenobetaine, arsenosugars, and arsenolipids, which have low toxicity but complicate exposure assessment [32,33,34]. Air pollution can also contribute to iAs exposure, although it has been less studied [35,36,37]. Given the complexity of exposure sources, epidemiologic studies of low-chronic As exposure must rely on established biomarkers and sensitive methods such as urinary arsenic with low limits of detection.
Cooking and eating preferences differ greatly by racial/ethnic groups and can impact arsenic exposure. It is estimated that rice and rice products contribute to 80.4%, 64.2%, and 41.7% of dietary As (excluding seafood) for Asian Americans, non-Mexican-American Hispanics, and non-Hispanic Whites, respectively; while cereals contribute to 1.8%, 9.0%, and 25.0% [27]. Asians also have the highest dietary As exposure (0.11 μg/kg/day) followed by non-Mexican-American Hispanics (0.07), Mexican-Americans (0.06), and non-Hispanic Blacks and Whites (0.05) [38•]. In our pilot in the Multi-Ethnic Study of Atherosclerosis (MESA), Chinese-Americans and Hispanics were disproportionately exposed to As compared to Blacks and Whites. Little is known, however, about the long-term health effects of dietary As and if some racial/ethnic groups are disproportionately affected. Studies with racial/ethnic diversity, for instance MESA, are ideal to assess the long-term cardiovascular effects of low-chronic As exposure, primarily from food.
Evidence on Arsenic as a CVD Risk Factor
Epidemiologic Evidence
Studies of populations in Taiwan [8, 39, 40], Chile [6], and Bangladesh [7, 41] have consistently shown that high water As (> 100 μg/l) constitutes a CVD risk factor (Fig. 2). Occupational studies that account for healthy worker effects support this finding [42], as well as experimental studies showing that chronic iAs in drinking water increased lesions covering the aortic intima compared to unexposed mice [12, 43, 44]. Of note, iAs concentrations as low as 10 μg/l increase atherosclerotic lesion in apolipoprotein E knock-out (apoE−/−) mice (Fig. 3) [45••]. These data suggest that humans might be susceptible to even lower As concentrations, considering that mice methylate and excrete As at much higher rate than humans [46]. Early epidemiologic CVD research of low-to-moderate As (< 100 μg/l) was limited by ecological designs [47, 48], inconsistent findings [9, 49], or lack of statistical power [7]. In contrast, cohort studies based on biomarkers or individual water As measures in rural American Indian communities from Arizona, Oklahoma, and North and South Dakota (Strong Heart Study [SHS]) [11••] and in White and Hispanic communities from rural Colorado (San Luis Valley Diabetes Study [SLVDS]) [10••] found statistically significant associations with CVD incidence and mortality, particularly coronary heart disease (CHD). In the SHS, the association of As with incident CVD was attenuated after adjustment for hypertension and diabetes, supporting the idea that some of these risk factors may link As and CVD. In experimental and epidemiological studies, As at moderate-to-high levels has also been associated with subclinical outcomes including carotid intima media thickness (CIMT) [50, 51], plaque score [52••], and CVD risk factors such as hypertension [53,54,55], diabetes [56, 57], and electrocardiographic abnormalities (prolonged QT-interval) [58,59,60], although the findings are not entirely consistent.
Low arsenic concentrations increase the size of atherosclerotic plaques dose-dependently. ApoE<sup>−/−</sup > mice were given tap water or 10–200 ppb arsenic in the drinking water for 13 weeks. Plaque was quantified in the aortic arch (a) or aortic sinus (b) after oil red O staining and imaging. Statistical significance from control is represented as follows: *p < .05; **p < .01; ***p < .001; ****p < .0001. (Figure reproduced from Makhani K et al., Using the Apolipoprotein E Knock-Out Mouse Model to Define Atherosclerotic Plaque Changes Induced by Low Dose Arsenic Toxicol Sci. 2018;166(1):213–218, with permission from Oxford University Press).
Mechanistic Evidence
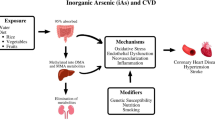
The health effects of As may occur via numerous pathophysiological pathways (Fig. 4), influenced by exposure levels, genetic variants, As metabolism, and nutritional status [58, 61]. Elevated pro-inflammatory cytokines and markers of oxidative stress were detected in plasma, serum, and atherosclerotic lesions of As-treated vs. untreated mice [44]. In mouse models, As interferes with cholesterol homeostasis and functions of macrophage and induces upregulation of inflammatory signaling, enhanced oxidative stress, activation of nuclear factor-κB, and inhibition of NO availability [62,63,64,65,66,67]. These effects can promote proliferation of endothelial cells and smooth muscle cells, cell adhesion, platelet aggregation, and arterial vasoconstriction [64, 68, 69]. Many of these mechanisms have been evaluated at high levels, although in a recent relatively small study in a population from New Hampshire (n = 415) exposed mostly to water As < 10 μg/L, urinary As was associated with 15-F2t-Isoprostane, a biomarker of oxidative stress [70••]. Neovascularization, angiogenesis, and vessel remodeling have been shown at As levels even below the current water standard [68, 71, 72]. Relatively specific effects of As exposure in animal experiments are cardiac electrophysiology changes, specifically QT prolongation, a risk factor for sudden cardiac death [60, 73, 74]. Prolonged QT-interval is also a common secondary effect of As trioxide, a treatment for promyelocytic leukemia [75,76,77], consistent with epidemiologic findings [60]. Toxic effects of As may also be mediated through epigenetic mechanisms, including DNA methylation (DNAm) [78,79,80,81]. In addition to experimental studies, human studies with markers of these pathways are available and have been associated with CVD outcomes, providing the opportunity to assess relevant mechanisms of low-chronic As in an epidemiological setting.
Dose-response meta-analysis showing pooled log-linear and non-linear relative risks of incident overall cardiovascular disease, coronary heart disease, and stroke in relation to estimated water arsenic. Pooled linear (red) and non-linear (blue) relative risks of CVD endpoints (overall CVD, CHD, and stroke, stratified by studies of incidence and mortality) were estimated for drinking water arsenic concentrations in reference to 10 μg/l. Dashed lines correspond to pooled relative risks, and shaded regions correspond to the 95% confidence intervals of the pooled relative risks. Log-linear associations were estimated from models with log-transformed estimated water arsenic concentrations. Non-linear associations were estimated from models with restricted cubic splines of log-transformed estimated water arsenic concentrations with knots at the 10th, 50th, and 90th percentiles of log-transformed arsenic (exact knot locations vary by model; for CHD incidence, knots were placed at 5.1, 20.5, and 58.7 μg/l). A rug plot along the x-axis provides the median estimated water arsenic concentrations included in each model. (Reproduced from: Moon KA et al. A dose-response meta-analysis of chronic arsenic exposure and incident cardiovascular disease. Int J Epidemiol. 2017;46 (6):1924–1939, with permission from Oxford University Press)
Shape of the Dose-Response
The shape of the dose-response across low-moderate and high As levels with CVD is uncertain but critical for As risk assessment [82••]. In the SHS, urine As levels > 10 μg/g creatinine (or 10 μg/L in a sensitivity analysis standardized by specific gravity) were associated with higher risk of CVD incidence and mortality [11••]. Below 10 μg/g, however, the shape was inconsistent, showing a potential linear association for incident total CVD and stroke but a possible threshold for incident CHD, although for all outcomes, the confidence intervals were consistent with multiple shapes. In a dose-response meta-analysis of prospective studies of As and CVD, the pooled relative risks (95%CI) for a twofold increase in As levels were 1.11 (1.05, 1.17) (number of studies = 4) and 1.16 (1.07, 1.26) (n = 6) for CHD incidence and mortality, respectively [82••]. There was no evidence of non-linearity using flexible splines, although a non-linear dose-response could not be discarded due to low power (Fig. 4). The major limitation of the existing evidence is the lack of data below 10 μg/L in either water or urine. Ongoing measures of arsenic at low levels in the Multi-Ethnic Study of Atherosclerosis (MESA) can help address this gap and inform on the shape of the association with CVD at low chronic As exposure levels.
Important Aspects to Consider when Conducing as Research at Low Levels
Arsenic Exposure Assessment in the Presence of Seafood
Measuring As at low levels requires highly sensitive methods. For example, the limit of detection (LOD) for iAs species in NHANES 2003–2010 was 10 times higher [31•] than the current LOD in the Trace Metals Core Laboratory at Columbia University (0.1 μg/L), resulting in a large proportion of the population being undetectable for several of the species (e.g., > 90% had undetectable iAs levels in NHANES [31•]). High sensitivity is thus critical for a successful study at low As levels.
Seafood intake represents another major challenge to assess As exposure in general populations. When seafood intake is low, the sum of iAs, MMA, and DMA in urine reflects As intake from drinking water, other dietary sources like rice and other sources (e.g. air pollution), and is an accepted biomarker of iAs exposure (Fig. 5) [83,84,85]. Rice may also contain DMA in addition to iAs, which is also excreted through urine. Seafood, including fish, shellfish, and seaweed are important sources of organic arsenicals (arsenobetaine, arsenosugars, and arsenolipids); however, these species have low toxicity [33, 86,87,88]. Arsenobetaine (which can be simultaneously measured with iAs, MMA and DMA) is rapidly cleared from the blood stream, excreted unchanged via the kidneys and contributes to total urine As [89,90,91]. In murine models, iAs, MMA, and DMA, but not arsenobetaine, increased atherosclerotic lesions [92••]. Seaweed, mollusks (e.g., scallops, mussels), and fatty fishes are rich in arsenosugars and/or arsenolipids that are metabolized to As species such as DMA and dimethylated thiol As species [29, 30, 32, 33]. Therefore, in populations with moderate-high fish intake, the sum of iAs, MMA, and DMA in urine cannot be used as a biomarker of iAs intake.
Arsenic exposure, metabolism, and urine biomarkers. Other sources of arsenic (occupational settings and air pollution) are not shown. Urine arsenic species commonly measured in epidemiologic studies are marked in blue. Red arrows reflect how adjusting for arsenobetaine and extracting model residuals can control the contribution of seafood arsenicals to DMA and total arsenic. (Adapted from Jones MR et al. Estimation of Inorganic Arsenic Exposure in populations with frequent seafood intake: evidence from MESA and NHANES. AJE 2016;184 (8):590–602, with permission from Oxford University Press)
To address this important problem, which may explain the scarce number of epidemiologic As studies in general populations, we have developed and validated a method that estimates As exposure not derived from seafood by regressing iAs, MMA, and DMA on arsenobetaine and extracting the arsenobetaine-independent model residuals. The method results and validation have been published [93•]. The residuals reflect As not explained by arsenobetaine and likely by seafood intake. The method of residuals to obtain adjusted estimates has been extensively used in the literature [94,95,96,97,98,99,100]. To have As levels that represent concentrations after removing the impact of seafood, we added the mean of the corresponding As species (iAs, MMA, or DMA estimated from participants with low arsenobetaine [< 1 μg/L]) to the residuals, assuming that As not derived from seafood is similar in participants with low and high arsenobetaine [93•]. To validate that estimated biomarkers reflect iAs exposure but not seafood intake, we compared urine As levels by seafood and rice intake data from a FFQ and n-3 fatty acids. Self-reported seafood intake (Table 1), estimated n-3 fatty acids, and measured n-3 fatty acids were positively associated with the original urine As biomarkers but no longer associated with the estimated ones [93•]. The associations with self-reported rice intake, however, remained similar (Table 1). We replicated these findings for DMA in NHANES. These preliminary findings in MESA and the replication in NHANES support that the residual-based method estimates As exposure and metabolism for each participant not due to exposure from seafood, and therefore can be used to investigate the health effects of low-level As in populations with frequent seafood intake.
Understanding Determinants of As Metabolism and their Influence on CVD
The toxicity of As depends on its metabolism, a process that is influenced by genetic determinants and relies on folate-dependent one-carbon metabolism (OCM) [101,102,103,104,105]. After exposure to iAs (arsenite and arsenate), As is methylated in the body by As (III) methyltransferase (AS3MT) to mono and dimethylated arsenicals (MMA and DMA) using s-adenosylmethionine (SAM) as the methyl donor [106, 107]. Mice lacking As3MT cannot methylate arsenic [108]. DMA, MMA, and iAs are excreted in the urine, with half-lives ranging from 2 days for DMA to 38 days for iAs [87]. SAM synthesis is dependent on OCM and is inhibited by homocysteine, a sensitive biomarker of OCM nutritional status, collectively reflecting the status of folate and other B vitamins required for OCM [109]. Moreover, nutritional manipulation of OCM has been shown to increase As methylation and to lower blood As concentrations [102, 110]. Other factors that influence As methylation efficiency include age, sex, smoking, and As exposure levels [106, 111,112,113]. The relative proportions of iAs, MMA, and DMA in urine in respect to their sum (iAs%, MMA%, and DMA%) serve as biomarkers of As metabolism. Patterns of As metabolism in urine that reflect inefficient methylation (e.g., higher MMA% vs. lower DMA%) have been related to higher risk of CVD [114,115,116]. Deletion of As3MT protects apoE−/− mice from arsenic-induced atherosclerosis [92••]. Candidate gene association studies [117,118,119,120,121], GWAS data from Bangladesh [103], and Metabochip data from the SHS [122] show that variants in AS3MT are strong predictors of As metabolic patterns in urine. AS3MT genetic variants are also associated with As toxicity [104]. Metabochip data, which provides fine mapping coverage of AS3MT, GWAS data, and plasma homocysteine—all available in MESA—will allow for a comprehensive assessment of genetic and nutritional factors that influence As metabolism and interact with As exposure on CVD outcomes.
Research Needs in General Populations
Little is known about the health effects of dietary, geographical, and racial/ethnic disparities in As exposure in the USA. Regarding geographical variability, As in community water systems in L.A. is relatively high compared to other cities (although still < 10 μg/L) (Table 2) [123]. In suburban areas of LA, St. Paul, and Chicago, private wells are also used as a source of drinking water (sometimes > 10 μg/L) [124]. In a pilot study in MESA, participants from LA had higher median As levels compared to other cities for most ethnic groups, supporting that water As in LA contributes to As exposure. Other Western US cities are affected similarly to LA [93•]. Findings from MESA can be relevant for general populations in the USA and other countries.
The extensive existing MESA resources, moreover, can be leveraged for As research, including genomic (GWAS and Metabochip), epigenomic (Illumina 450 K), OCM nutritional status (plasma homocysteine), dietary data (food frequency questionnaire (FFQ)), social and environmental factors (air pollution, geocoding, neighborhood characteristics), CVD risk factors (smoking, blood pressure, glycemia, lipids), and pathophysiological mechanisms (e.g., markers of inflammation, electrocadiographic findings) together with the well-characterized subclinical and clinical outcomes during continuous follow-up. Adding As exposure and metabolism data to MESA will allow us to evaluate a potentially important, modifiable CVD risk factor along with other outcomes relevant for As, including lung and kidney disease, and potentially cancer. The multi-element analytical technique to be used for urine As, moreover, facilitate the assessment of metal mixtures including metals related to CVD such as cadmium [125, 126], nickel, and tungsten [5].
Conclusions
The assessment of metals, in particular arsenic, in high-quality prospective cohort studies of vascular outcomes can provide relevant information for clinical, environmental, nutritional, and occupational CVD prevention programs. This is particularly of high need for arsenic, given the EPA’s Integrated Risk Information System (IRIS) ongoing arsenic risk assessment and the lack of data at low levels of exposure. From a clinical perspective, this type of research can impact clinical strategies for CVD risk reduction, including the identification of individuals at risk of As exposure because they use private wells for drinking or rely on As-rich diets (e.g., celiac disease patients, certain racial/ethnic groups). From a risk assessment perspective, the US EPA has so far based the As drinking water standard on quantitative estimates of As cancer effects. An updated risk assessment is ongoing. The 2013 National Research Council committee on iAs recommended that the EPA give priority to the evaluation of CVD [127]. While there is strong evidence at As levels > 10 μg/L, data < 10 μg/L remain insufficient [9, 61, 82••]. These data are critical for informing decisions regarding water As standards, which are currently highly variable: the WHO and the US EPA standard is 10 μg/L while New Jersey’s standard is 5 μg/L (the lowest in the USA). In addition, while the need to regulate As in food is agreed upon, the lack of data on health effects of dietary As makes regulation difficult [16•, 128, 129]. All current legislative actions for As in food are non-binding (e.g., As standards in juice and rice baby products are at different legislative stages). Assessing the dose-response relationship between As and CVD at low chronic As exposure, understanding mechanisms, and identifying environmental, genetic, and nutrition susceptibility factors can inform policies to protect the general population as well as susceptible subgroups.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Lee C-H, Wu S-B, Hong C-H, Liao WT, Wu CY, Chen GS, et al. Aberrant cell proliferation by enhanced mitochondrial biogenesis via mtTFA in arsenical skin cancers. Am J Pathol. 2011;178(5):2066–76. https://doi.org/10.1016/j.ajpath.2011.01.056.
Lamas GA, Navas-Acien A, Mark DB, Lee KL. Heavy metals, cardiovascular disease, and the unexpected benefits of chelation therapy. J Am Coll Cardiol. 2016;67(20):2411–8. https://doi.org/10.1016/j.jacc.2016.02.066.
Bhatnagar A. Environmental cardiology: studying mechanistic links between pollution and heart disease. Circ Res. 2006;99(7):692–705. https://doi.org/10.1161/01.RES.0000243586.99701.cf.
Navas-Acien A, Guallar E, Silbergeld EK, Rothenberg SJ. Lead exposure and cardiovascular disease--a systematic review. Environ Health Perspect. 2007;115(3):472–82. https://doi.org/10.1289/ehp.9785.
Nigra AE, Ruiz-Hernandez A, Redon J, Navas-Acien A, Tellez-Plaza M. Environmental metals and cardiovascular disease in adults: a systematic review beyond lead and cadmium. Curr Environ Heal Reports. 2016;3(4):416–33. https://doi.org/10.1007/s40572-016-0117-9.
Yuan Y, Marshall G, Ferreccio C, Steinmaus C, Selvin S, Liaw J, et al. Acute myocardial infarction mortality in comparison with lung and bladder cancer mortality in arsenic-exposed region II of Chile from 1950 to 2000. Am J Epidemiol. 2007;166(12):1381–91. https://doi.org/10.1093/aje/kwm238.
Chen Y, Graziano JH, Parvez F, Liu M, Slavkovich V, Kalra T, et al. Arsenic exposure from drinking water and mortality from cardiovascular disease in Bangladesh: prospective cohort study. BMJ. 2011;342(7806):d2431. https://doi.org/10.1136/bmj.d2431.
Chen CJ, Chiou HY, Chiang MH, Lin LJ, Tai TY. Dose-response relationship between ischemic heart disease mortality and long-term arsenic exposure. Arterioscler Thromb Vasc Biol. 1996;16(4):504–10 http://www.ncbi.nlm.nih.gov/pubmed/8624771. Accessed 4/11/2019.
Moon K, Guallar E, Navas-Acien A. Arsenic exposure and cardiovascular disease: an updated systematic review. Curr Atheroscler Rep. 2012;14(6):542–55. https://doi.org/10.1007/s11883-012-0280-x.
•• James KA, Byers T, Hokanson JE, Meliker JR, Zerbe GO, Marshall JA. Association between lifetime exposure to inorganic arsenic in drinking water and coronary heart disease in Colorado residents. Environ Health Perspect. 2015;123(2):128–34. https://doi.org/10.1289/ehp.1307839 One of the few US propsective studies on arsenic and incident cardiovascular disease.
•• Moon KA, Guallar E, Umans JG, et al. Association between exposure to low to moderate arsenic levels and incident cardiovascular disease. A prospective cohort study. Ann Intern Med. 2013;159(10):649–59. https://doi.org/10.7326/0003-4819-159-10-201311190-00719 One of the few US prospective studies on arsenic and incident cardiovascular disease.
Lemaire M, Lemarié CA, Molina MF, Schiffrin EL, Lehoux S, Mann KK. Exposure to moderate arsenic concentrations increases atherosclerosis in ApoE−/− mouse model. Toxicol Sci. 2011;122(1):211–21. https://doi.org/10.1093/toxsci/kfr097.
Smith AH, Hopenhayn-Rich C, Bates MN, Goeden HM, Hertz-Picciotto I, Duggan HM, et al. Cancer risks from arsenic in drinking water. Environ Health Perspect. 1992;97:259–67. https://doi.org/10.1289/ehp.9297259.
EPA 816-R-13-003. Fiscal year 2011 drinking water and ground water statistics. http://water.epa.gov/scitech/datait/databases/drink/sdwisfed/howtoaccessdata.cfm. Published 2013. Accessed 4/11/2019.
Kurzius-Spencer M, Burgess JL, Harris RB, Hartz V, Roberge J, Huang S, et al. Contribution of diet to aggregate arsenic exposures—an analysis across populations. J Expo Sci Environ Epidemiol. 2014;24(2):156–62. https://doi.org/10.1038/jes.2013.37.
• Cubadda F, Jackson BP, Cottingham KL, Van Horne YO, Kurzius-Spencer M. Human exposure to dietary inorganic arsenic and other arsenic species: state of knowledge, gaps and uncertainties. Sci Total Environ. 2017;579:1228–39. https://doi.org/10.1016/j.scitotenv.2016.11.108 Study reporting on dietary sources of inorganic arsenic.
Nachman KE, Graham JP, Price LB, Silbergeld EK. Arsenic: a roadblock to potential animal waste management solutions. Environ Health Perspect. 2005;113(9):1123–4. https://doi.org/10.1289/ehp.7834.
Navas-Acien A, Nachman KE. Public health responses to arsenic in rice and other foods. JAMA Intern Med. 2013;173(15):1395–6. https://doi.org/10.1001/jamainternmed.2013.6405.
Yao L, Huang L, He Z, Zhou C, Lu W, Bai C. Delivery of roxarsone via chicken diet→chicken→chicken manure→soil→rice plant. Sci Total Environ. 2016;566-567:1152–8. https://doi.org/10.1016/j.scitotenv.2016.05.157.
Meharg AA, Williams PN, Adomako E, Lawgali YY, Deacon C, Villada A, et al. Geographical variation in Total and inorganic arsenic content of polished (White) rice. Environ Sci Technol. 2009;43(5):1612–7. https://doi.org/10.1021/es802612a.
Chen Y, Moore KL, Miller AJ, McGrath SP, Ma JF, Zhao F-J. The role of nodes in arsenic storage and distribution in rice. J Exp Bot. 2015;66(13):3717–24. https://doi.org/10.1093/jxb/erv164.
Carey A-M, Lombi E, Donner E, de Jonge MD, Punshon T, Jackson BP, et al. A review of recent developments in the speciation and location of arsenic and selenium in rice grain. Anal Bioanal Chem. 2012;402(10):3275–86. https://doi.org/10.1007/s00216-011-5579-x.
Robinson GR, Larkins P, Boughton CJ, Reed BW, Sibrell PL. Assessment of contamination from arsenical pesticide use on orchards in the Great Valley region, Virginia and West Virginia, USA. J Environ Qual. 2007;36(3):654–63. https://doi.org/10.2134/jeq2006.0413.
Tariba B. Metals in wine--impact on wine quality and health outcomes. Biol Trace Elem Res. 2011;144(1–3):143–56. https://doi.org/10.1007/s12011-011-9052-7.
Wilson D, Hooper C, Shi X. Arsenic and lead in juice: apple, citrus, and apple-base. J Environ Health. 2012;75(5):14–20 quiz 44. http://www.ncbi.nlm.nih.gov/pubmed/23270108. Accessed 4/11/2019.
Gilbert-Diamond D, Cottingham KL, Gruber JF, Punshon T, Sayarath V, Gandolfi AJ, et al. Rice consumption contributes to arsenic exposure in US women. Proc Natl Acad Sci. 2011;108(51):20656–60. https://doi.org/10.1073/pnas.1109127108.
Xue J, Zartarian V, Wang S-W, Liu SV, Georgopoulos P. Probabilistic modeling of dietary arsenic exposure and dose and evaluation with 2003-2004 NHANES data. Environ Health Perspect. 2010;118(3):345–50. https://doi.org/10.1289/ehp.0901205.
Nachman KE, Baron PA, Raber G, Francesconi KA, Navas-Acien A, Love DC. Roxarsone, inorganic arsenic, and other arsenic species in chicken: a U.S.-based market basket sample. Environ Health Perspect. 2013;121(7):818–24. https://doi.org/10.1289/ehp.1206245.
Nachman KE, Love DC, Baron PA, Nigra AE, Murko M, Raber G, et al. Nitarsone, inorganic arsenic, and other arsenic species in Turkey meat: exposure and risk assessment based on a 2014 U.S. market basket sample. Environ Health Perspect. 2017;125(3):363–9. https://doi.org/10.1289/EHP225.
Liu Q, Peng H, Lu X, Zuidhof MJ, Li X-F, Le XC. Arsenic species in chicken breast: temporal variations of metabolites, elimination kinetics, and residual concentrations. Environ Health Perspect. 2016;124(8):1174–81. https://doi.org/10.1289/ehp.1510530.
• Nigra AE, Nachman KE, Love DC, Grau-Perez M, Navas-Acien A. Poultry consumption and arsenic exposure in the U.S. population. Environ Health Perspect. 2017;125(3):370–7. https://doi.org/10.1289/EHP351 Study showing that poultry was a source of inorganic arsenic before the banning of arsenical drugs in poultry production.
Taylor V, Goodale B, Raab A, Schwerdtle T, Reimer K, Conklin S, et al. Human exposure to organic arsenic species from seafood. Sci Total Environ. 2017;580:266–82. https://doi.org/10.1016/j.scitotenv.2016.12.113.
Francesconi KA, Edmonds JS. Arsenic and marine organisms. Adv Inorg Chem. 1996;44:147–89. https://doi.org/10.1016/S0898-8838(08)60130-0.
Francesconi KA, Kuehnelt D. Determination of arsenic species: a critical review of methods and applications, 2000-2003. Analyst. 2004;129(5):373–95. https://doi.org/10.1039/b401321m.
Wai K-M, Wu S, Li X, Jaffe DA, Perry KD. Global atmospheric transport and source-receptor relationships for arsenic. Environ Sci Technol. 2016;50(7):3714–20. https://doi.org/10.1021/acs.est.5b05549.
Wilson WE. The relationship between daily cardiovascular mortality and daily ambient concentrations of particulate pollutants (sulfur, arsenic, selenium, and mercury) and daily source contributions from coal power plants and smelters individually, combined, and wit. J Air Waste Manag Assoc. 2015;65(5):599–610. https://doi.org/10.1080/10962247.2015.1033067.
Franklin M, Koutrakis P, Schwartz P. The role of particle composition on the association between PM2.5 and mortality. Epidemiology. 2008;19(5):680–9 http://www.ncbi.nlm.nih.gov/pubmed/18714438. Accessed 4/11/2019.
• Awata H, Linder S, Mitchell LE, Delclos GL. Association of Dietary Intake and Biomarker Levels of Arsenic, Cadmium, Lead, and Mercury among Asian Populations in the United States: NHANES 2011–2012. Environ Health Perspect. 2016;125(3):314–23. https://doi.org/10.1289/EHP28 Study showing that dietary inorganic arsenic exposure is particularly important for Asian populations in the US.
Chiou HY, Huang WI, Su CL, Chang SF, Hsu YH, Chen CJ. Dose-response relationship between prevalence of cerebrovascular disease and ingested inorganic arsenic. Stroke. 1997;28(9):1717–23 http://www.ncbi.nlm.nih.gov/pubmed/9303014. Accessed 4/11/2019.
Tseng C-H, Chong C-K, Tseng C-P, Hsueh YM, Chiou HY, Tseng CC, et al. Long-term arsenic exposure and ischemic heart disease in arseniasis-hyperendemic villages in Taiwan. Toxicol Lett. 2003;137(1–2):15–21 http://www.ncbi.nlm.nih.gov/pubmed/12505429. Accessed 4/11/2019.
Sohel N, Persson LA, Rahman M, Streatfield PK, Yunus M, Ekström EC, et al. Arsenic in drinking water and adult mortality: a population-based cohort study in rural Bangladesh. Epidemiology. 2009;20(6):824–30. https://doi.org/10.1097/EDE.0b013e3181bb56ec.
Hertz-Picciotto I, Arrighi HM, Hu SW. Does arsenic exposure increase the risk for circulatory disease? Am J Epidemiol. 2000;151(2):174–81 http://www.ncbi.nlm.nih.gov/pubmed/10645820. Accessed 4/11/2019.
Bunderson M, Brooks DM, Walker DL, Rosenfeld ME, Coffin JD, Beall HD. Arsenic exposure exacerbates atherosclerotic plaque formation and increases nitrotyrosine and leukotriene biosynthesis. Toxicol Appl Pharmacol. 2004;201(1):32–9. https://doi.org/10.1016/j.taap.2004.04.008.
Srivastava S, Vladykovskaya EN, Haberzettl P, Sithu SD, D’Souza SE, States JC. Arsenic exacerbates atherosclerotic lesion formation and inflammation in ApoE−/− mice. Toxicol Appl Pharmacol. 2009;241(1):90–100. https://doi.org/10.1016/j.taap.2009.08.004.
•• Makhani K, Chiavatti C, Plourde D, et al. Using the apolipoprotein E knock-out mouse model to define atherosclerotic plaque changes induced by low dose arsenic. Toxicol Sci. 2018;166(1):213–8. https://doi.org/10.1093/toxsci/kfy201 Animal experiment showing that low-level arsenic exposure can induce atherosclerotic plaque and that there is a monotonic dose-response.
Vahter M. Species differences in the metabolism of arsenic compounds. Appl Organomet Chem. 1994;8(3):175–82. https://doi.org/10.1002/aoc.590080304.
Medrano MAJ, Boix R, Pastor-Barriuso R, et al. Arsenic in public water supplies and cardiovascular mortality in Spain. Environ Res. 2010;110(5):448–54. https://doi.org/10.1016/j.envres.2009.10.002.
Engel RR, Smith AH. Arsenic in drinking water and mortality from vascular disease: an ecologic analysis in 30 counties in the United States. Arch Environ Heal An Int J. 1994;49(5):418–27. https://doi.org/10.1080/00039896.1994.9954996.
Navas-Acien A, Sharrett AR, Silbergeld EK, Schwartz BS, Nachman KE, Burke TA, et al. Arsenic exposure and cardiovascular disease: a systematic review of the epidemiologic evidence. Am J Epidemiol. 2005;162(11):1037–49. https://doi.org/10.1093/aje/kwi330.
Osorio-Yáñez C, Ayllon-Vergara JC, Aguilar-Madrid G, Arreola-Mendoza L, Hernández-Castellanos E, Barrera-Hernández A, et al. Carotid intima-media thickness and plasma asymmetric dimethylarginine in Mexican children exposed to inorganic arsenic. Environ Health Perspect. 2013;121(9):1090–6. https://doi.org/10.1289/ehp.1205994.
Wang C-H, Jeng J-S, Yip P-K, Chen CL, Hsu LI, Hsueh YM, et al. Biological gradient between long-term arsenic exposure and carotid atherosclerosis. Circulation. 2002;105(15):1804–9 http://www.ncbi.nlm.nih.gov/pubmed/11956123. Accessed 4/11/2019.
•• Mateen FJ, Grau-Perez M, Pollak JS, et al. Chronic arsenic exposure and risk of carotid artery disease: the strong heart study. Environ Res. 2017;157:127–34. https://doi.org/10.1016/j.envres.2017.05.020 Observational study showing that low-moderate arsenic exposure is associated with carotid artery disease in a US population.
Abhyankar LN, Jones MR, Guallar E, Navas-Acien A. Arsenic exposure and hypertension: a systematic review. Environ Health Perspect. 2012;120(4):494–500. https://doi.org/10.1289/ehp.1103988.
Hall EM, Acevedo J, López FG, Cortés S, Ferreccio C, Smith AH, et al. Hypertension among adults exposed to drinking water arsenic in northern Chile. Environ Res. 2017;153:99–105. https://doi.org/10.1016/j.envres.2016.11.016.
Jiang J, Liu M, Parvez F, Wang B, Wu F, Eunus M, et al. Association between arsenic exposure from drinking water and longitudinal change in blood pressure among HEALS cohort participants. Environ Health Perspect. 2015;123(8):806–12. https://doi.org/10.1289/ehp.1409004.
Maull EA, Ahsan H, Edwards J, Longnecker MP, Navas-Acien A, Pi J, et al. Evaluation of the association between arsenic and diabetes: a National Toxicology Program workshop review. Environ Health Perspect. 2012;120(12):1658–70. https://doi.org/10.1289/ehp.1104579.
Kuo C-C, Moon K, Thayer KA, Navas-Acien A. Environmental chemicals and type 2 diabetes: an updated systematic review of the epidemiologic evidence. Curr Diab Rep. 2013;13(6):831–49. https://doi.org/10.1007/s11892-013-0432-6.
Wu F, Molinaro P, Chen Y. Arsenic exposure and subclinical endpoints of cardiovascular diseases. Curr Environ Heal Rep. 2014;1(2):148–62. https://doi.org/10.1007/s40572-014-0011-2.
Wang C-H, Chen C-L, Hsiao CK, Chiang FT, Hsu LI, Chiou HY, et al. Arsenic-induced QT dispersion is associated with atherosclerotic diseases and predicts long-term cardiovascular mortality in subjects with previous exposure to arsenic: a 17-year follow-up study. Cardiovasc Toxicol. 2010;10(1):17–26. https://doi.org/10.1007/s12012-009-9059-x.
Mordukhovich I, Wright RO, Amarasiriwardena C, et al. Association between low-level environmental arsenic exposure and QT interval duration in a general population study. Am J Epidemiol. 2009;170(6):739–46. https://doi.org/10.1093/aje/kwp191.
Cosselman KE, Navas-Acien A, Kaufman JD. Environmental factors in cardiovascular disease. Nat Rev Cardiol. 2015;12(11):627–42. https://doi.org/10.1038/nrcardio.2015.152.
Lemaire M, Negro Silva LF, Lemarié CA, Bolt AM, Flores Molina M, Krohn RM, et al. Arsenic exposure increases monocyte adhesion to the vascular endothelium, a pro-atherogenic mechanism. PLoS One. 2015;10(9):e0136592. https://doi.org/10.1371/journal.pone.0136592.
Padovani AMS, Molina MF, Mann KK. Inhibition of liver x receptor/retinoid X receptor-mediated transcription contributes to the proatherogenic effects of arsenic in macrophages in vitro. Arterioscler Thromb Vasc Biol. 2010;30(6):1228–36. https://doi.org/10.1161/ATVBAHA.110.205500.
Barchowsky A, Dudek EJ, Treadwell MD, Wetterhahn KE. Arsenic induces oxidant stress and NF-kappa B activation in cultured aortic endothelial cells. Free Radic Biol Med. 1996;21(6):783–90 http://www.ncbi.nlm.nih.gov/pubmed/8902524. Accessed 4/11/2019.
Zhang C, Ferrari R, Beezhold K, Stearns-Reider K, D'Amore A, Haschak M, et al. Arsenic promotes NF-Κb-mediated fibroblast dysfunction and matrix remodeling to impair muscle stem cell function. Stem Cells. 2016;34(3):732–42. https://doi.org/10.1002/stem.2232.
Wang L, Kou M-C, Weng C-Y, Hu L-W, Wang Y-J, Wu M-J. Arsenic modulates heme oxygenase-1, interleukin-6, and vascular endothelial growth factor expression in endothelial cells: roles of ROS, NF-κB, and MAPK pathways. Arch Toxicol. 2012;86(6):879–96. https://doi.org/10.1007/s00204-012-0845-z.
Barchowsky A, Klei LR, Dudek EJ, Swartz HM, James PE. Stimulation of reactive oxygen, but not reactive nitrogen species, in vascular endothelial cells exposed to low levels of arsenite. Free Radic Biol Med. 1999;27(11–12):1405–12 http://www.ncbi.nlm.nih.gov/pubmed/10641735. Accessed 4/11/2019.
States JC, Srivastava S, Chen Y, Barchowsky A. Arsenic and cardiovascular disease. Toxicol Sci. 2009;107(2):312–23. https://doi.org/10.1093/toxsci/kfn236.
Kumagai Y, Pi J. Molecular basis for arsenic-induced alteration in nitric oxide production and oxidative stress: implication of endothelial dysfunction. Toxicol Appl Pharmacol. 2004;198(3):450–7. https://doi.org/10.1016/j.taap.2003.10.031.
•• Farzan SF, Howe CG, Zens MS, et al. Urine arsenic and arsenic metabolites in U.S. adults and biomarkers of inflammation, oxidative stress, and endothelial dysfunction: a cross-sectional study. Environ Health Perspect. 2017;125(12):127002. https://doi.org/10.1289/EHP2062 Study showing that arsenic exposure is associated with biomarkers of inflammation, oxidative stress and endothelial dysfunction in a US population.
Soucy NV, Klei LR, Mayka DD, Barchowsky A. Signaling pathways for arsenic-stimulated vascular endothelial growth factor-a expression in primary vascular smooth muscle cells. Chem Res Toxicol. 2004;17(4):555–63. https://doi.org/10.1021/tx034193q.
Soucy NV, Mayka D, Klei LR, Nemec AA, Bauer JA, Barchowsky A. Neovascularization and angiogenic gene expression following chronic arsenic exposure in mice. Cardiovasc Toxicol. 2005;5(1):29–41 http://www.ncbi.nlm.nih.gov/pubmed/15738583. Accessed 4/11/2019.
Chen Y, Wu F, Parvez F, Ahmed A, Eunus M, McClintock TR, et al. Arsenic exposure from drinking water and QT-interval prolongation: results from the health effects of arsenic longitudinal study. Environ Health Perspect. 2013;121(4):427–32. https://doi.org/10.1289/ehp.1205197.
Mumford JL, Wu K, Xia Y, Kwok R, Yang Z, Foster J, et al. Chronic arsenic exposure and cardiac repolarization abnormalities with QT interval prolongation in a population-based study. Environ Health Perspect. 2007;115(5):690–4. https://doi.org/10.1289/ehp.9686.
Siu C-W, Au W-Y, Yung C, Kumana CR, Lau CP, Kwong YL, et al. Effects of oral arsenic trioxide therapy on QT intervals in patients with acute promyelocytic leukemia: implications for long-term cardiac safety. Blood. 2006;108(1):103–6. https://doi.org/10.1182/blood-2006-01-0054.
Barbey JT, Pezzullo JC, Soignet SL. Effect of arsenic trioxide on QT interval in patients with advanced malignancies. J Clin Oncol. 2003;21(19):3609–15. https://doi.org/10.1200/JCO.2003.10.009.
Ohnishi K, Yoshida H, Shigeno K, Nakamura S, Fujisawa S, Naito K, et al. Prolongation of the QT interval and ventricular tachycardia in patients treated with arsenic trioxide for acute promyelocytic leukemia. Ann Intern Med. 2000;133(11):881–5 http://www.ncbi.nlm.nih.gov/pubmed/11103058. Accessed 4/11/2019.
Koestler DC, Avissar-Whiting M, Houseman EA, Karagas MR, Marsit CJ. Differential DNA methylation in umbilical cord blood of infants exposed to low levels of arsenic in utero. Environ Health Perspect. 2013;121(8):971–7. https://doi.org/10.1289/ehp.1205925.
Reichard JF, Schnekenburger M, Puga A. Long term low-dose arsenic exposure induces loss of DNA methylation. Biochem Biophys Res Commun. 2007;352(1):188–92. https://doi.org/10.1016/j.bbrc.2006.11.001.
Bailey KA, Fry RC. Arsenic-associated changes to the epigenome: what are the functional consequences? Curr Environ Heal Rep. 2014;1:22–34. https://doi.org/10.1007/s40572-013-0002-8.
Argos M. Arsenic exposure and epigenetic alterations: recent findings based on the Illumina 450K DNA methylation array. Curr Environ Heal Rep. 2015;2(2):137–44. https://doi.org/10.1007/s40572-015-0052-1.
•• Moon KA, Oberoi S, Barchowsky A, et al. A dose-response meta-analysis of chronic arsenic exposure and incident cardiovascular disease. Int J Epidemiol. 2017;46(6):1924–39. https://doi.org/10.1093/ije/dyx202 Dose-response meta-analysis on arsenic exposure and cardiovascular disease showing that evidence is needed at low-levels of arsenic exposure.
Ahsan H, Perrin M, Rahman A, et al. Associations between drinking water and urinary arsenic levels and skin lesions in Bangladesh. J Occup Environ Med. 2000;42(12):1195–201 http://www.ncbi.nlm.nih.gov/pubmed/11125683. Accessed 4/11/2019.
Calderon RL, Hudgens E, Le XC, Schreinemachers D, Thomas DJ. Excretion of arsenic in urine as a function of exposure to arsenic in drinking water. Environ Health Perspect. 1999;107(8):663–7. https://doi.org/10.1289/ehp.99107663.
Pellizzari ED, Clayton CA. Assessing the measurement precision of various arsenic forms and arsenic exposure in the National Human Exposure Assessment Survey (NHEXAS). Environ Health Perspect. 2006;114(2):220–7. https://doi.org/10.1289/ehp.8104.
Cannon J, Edmonds J, Francesconi K, Raston CL, Saunders JB, Skelton BW, et al. Isolation, crystal structure and synthesis of arsenobetaine, a constituent of the western rock lobster, the dusky shark, and some samples of human urine. Aust J Chem. 1981;34(4):787. https://doi.org/10.1071/CH9810787.
Cullen WR, Reimer KJ. Arsenic speciation in the environment. Chem Rev. 1989;89(4):713–64. https://doi.org/10.1021/cr00094a002.
Edmonds JS, Francesconi KA, Cannon JR, Raston CL, Skelton BW, White AH. Isolation, crystal structure and synthesis of arsenobetaine, the arsenical constituent of the western rock lobster panulirus longipes cygnus George. Tetrahedron Lett. 1977;18(18):1543–6. https://doi.org/10.1016/S0040-4039(01)93098-9.
Francesconi KA, Tanggaar R, McKenzie CJ, Goessler W. Arsenic metabolites in human urine after ingestion of an arsenosugar. Clin Chem. 2002;48(1):92–101 http://www.ncbi.nlm.nih.gov/pubmed/11751543. Accessed 4/11/2019.
Brown RM, Newton D, Pickford CJ, Sherlock JC. Human metabolism of arsenobetaine ingested with fish. Hum Exp Toxicol. 1990;9(1):41–6. https://doi.org/10.1177/096032719000900109.
Le XC, Cullen WR, Reimer KJ. Human urinary arsenic excretion after one-time ingestion of seaweed, crab, and shrimp. Clin Chem. 1994;40(4):617–24 http://www.ncbi.nlm.nih.gov/pubmed/8149620. Accessed 4/11/2019.
•• Silva N, LF LM, Lemarié CA, et al. Effects of inorganic arsenic, methylated arsenicals, and Arsenobetaine on atherosclerosis in the apoE−/− mouse model and the role of As3mt-mediated methylation. Environ Health Perspect. 2017;125(7):077001. https://doi.org/10.1289/EHP806 Animal experimental study showing that inorganic and methylated arsenic especies but not arsenobetaine can induce atherosclerosis.
• Jones MR, Tellez-Plaza M, Vaidya D, Grau M, Francesconi KA, Goessler W, et al. Estimation of inorganic arsenic exposure in populations with frequent seafood intake: evidence from MESA and NHANES. Am J Epidemiol. 2016;184(8):590–602. https://doi.org/10.1093/aje/kww097 Study showing the complexities of interpreting urinary arsenic species at low levels of exposure in the presence of seafood arsenic and validating a method to correct for seafood arsenicals.
Hu FB, Stampfer MJ, Rimm E, Ascherio A, Rosner BA, Spiegelman D, et al. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol. 1999;149(6):531–40 http://www.ncbi.nlm.nih.gov/pubmed/10084242. Accessed 4/11/2019.
Michels KB, Bingham SA, Luben R, Welch AA, Day NE. The effect of correlated measurement error in multivariate models of diet. Am J Epidemiol. 2004;160(1):59–67. https://doi.org/10.1093/aje/kwh169.
Rhee JJ, Cho E, Willett WC. Energy adjustment of nutrient intakes is preferable to adjustment using body weight and physical activity in epidemiological analyses. Public Health Nutr. 2014;17(5):1054–60. https://doi.org/10.1017/S1368980013001390.
Slager SL, Iturria SJ. Genome-wide linkage analysis of systolic blood pressure: a comparison of two approaches to phenotype definition. BMC Genet. 2003;4(Suppl 1):S13. https://doi.org/10.1186/1471-2156-4-S1-S13.
Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. 1986;124(1):17–27 http://www.ncbi.nlm.nih.gov/pubmed/3521261. Accessed 4/11/2019.
Willett WC. Nutritional epidemiology. 2nd ed. New York: Oxford University Press; 1998.
Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65(4 Suppl):1220S–8S; discussion 1229S-1231S. https://doi.org/10.1093/ajcn/65.4.1220S.
Steinmaus C, Yuan Y, Kalman D, Atallah R, Smith AH. Intraindividual variability in arsenic methylation in a U.S. population. Cancer Epidemiol Biomark Prev. 2005;14(4):919–24. https://doi.org/10.1158/1055-9965.EPI-04-0277.
Gamble MV, Liu X, Ahsan H, Pilsner JR, Ilievski V, Slavkovich V, et al. Folate and arsenic metabolism: a double-blind, placebo-controlled folic acid-supplementation trial in Bangladesh. Am J Clin Nutr. 2006;84(5):1093–101. https://doi.org/10.1093/ajcn/84.5.1093.
Pierce BL, Kibriya MG, Tong L, Jasmine F, Argos M, Roy S, et al. Genome-wide association study identifies chromosome 10q24.32 variants associated with arsenic metabolism and toxicity phenotypes in Bangladesh. PLoS Genet. 2012;8(2):e1002522. https://doi.org/10.1371/journal.pgen.1002522.
Pierce BL, Tong L, Argos M, Gao J, Jasmine F, Roy S, et al. Arsenic metabolism efficiency has a causal role in arsenic toxicity: Mendelian randomization and gene-environment interaction. Int J Epidemiol. 2013;42(6):1862–71. https://doi.org/10.1093/ije/dyt182.
Vahter M, Concha G. Role of metabolism in arsenic toxicity. Pharmacol Toxicol. 2001;89(1):1–5 http://www.ncbi.nlm.nih.gov/pubmed/11484904. Accessed 4/11/2019.
Vahter M. Mechanisms of arsenic biotransformation. Toxicology. 2002;181-182:211–7 http://www.ncbi.nlm.nih.gov/pubmed/12505313. Accessed 4/11/2019.
Hughes MF, Edwards BC, Herbin-Davis KM, Saunders J, Styblo M, Thomas DJ. Arsenic (+3 oxidation state) methyltransferase genotype affects steady-state distribution and clearance of arsenic in arsenate-treated mice. Toxicol Appl Pharmacol. 2010;249(3):217–23. https://doi.org/10.1016/j.taap.2010.09.017.
Drobna Z, Naranmandura H, Kubachka KM, Edwards BC, Herbin-Davis K, Styblo M, et al. Disruption of the arsenic (+3 oxidation state) methyltransferase gene in the mouse alters the phenotype for methylation of arsenic and affects distribution and retention of orally administered arsenate. Chem Res Toxicol. 2009;22(10):1713–20. https://doi.org/10.1021/tx900179r.
Mason JB. Biomarkers of nutrient exposure and status in one-carbon (methyl) metabolism. J Nutr 2003;133 Suppl(3):941S–947S. doi:https://doi.org/10.1093/jn/133.3.941S.
Gamble MV, Liu X, Slavkovich V, Pilsner JR, Ilievski V, Factor-Litvak P, et al. Folic acid supplementation lowers blood arsenic. Am J Clin Nutr. 2007;86(4):1202–9. https://doi.org/10.1093/ajcn/86.4.1202.
Steinmaus C, Carrigan K, Kalman D, Atallah R, Yuan Y, Smith AH. Dietary intake and arsenic methylation in a U.S. population. Environ Health Perspect. 2005;113(9):1153–9. https://doi.org/10.1289/ehp.7907.
Navas-Acien A, Umans JG, Howard BV, Goessler W, Francesconi KA, Crainiceanu CM, et al. Urine arsenic concentrations and species excretion patterns in American Indian communities over a 10-year period: the Strong Heart Study. Environ Health Perspect. 2009;117(9):1428–33. https://doi.org/10.1289/ehp.0800509.
Hsueh Y-M, Ko Y-F, Huang Y-K, Chen HW, Chiou HY, Huang YL, et al. Determinants of inorganic arsenic methylation capability among residents of the Lanyang Basin, Taiwan: arsenic and selenium exposure and alcohol consumption. Toxicol Lett. 2003;137(1–2):49–63 http://www.ncbi.nlm.nih.gov/pubmed/12505432. Accessed 4/11/2019.
Chen Y, Wu F, Liu M, Parvez F, Slavkovich V, Eunus M, et al. A prospective study of arsenic exposure, arsenic methylation capacity, and risk of cardiovascular disease in Bangladesh. Environ Health Perspect. 2013;121(7):832–8. https://doi.org/10.1289/ehp.1205797.
Huang Y-L, Hsueh Y-M, Huang Y-K, Yip P-K, Yang M-H, Chen C-J. Urinary arsenic methylation capability and carotid atherosclerosis risk in subjects living in arsenicosis-hyperendemic areas in southwestern Taiwan. Sci Total Environ. 2009;407(8):2608–14. https://doi.org/10.1016/j.scitotenv.2008.12.061.
Chen Y, Wu F, Graziano JH, Parvez F, Liu M, Paul RR, et al. Arsenic exposure from drinking water, arsenic methylation capacity, and carotid intima-media thickness in Bangladesh. Am J Epidemiol. 2013;178(3):372–81. https://doi.org/10.1093/aje/kwt001.
Agusa T, Iwata H, Fujihara J, Kunito T, Takeshita H, Minh TB, et al. Genetic polymorphisms in glutathione S-transferase (GST) superfamily and arsenic metabolism in residents of the Red River Delta, Vietnam. Toxicol Appl Pharmacol. 2010;242(3):352–62. https://doi.org/10.1016/j.taap.2009.11.007.
Chung C-J, Hsueh Y-M, Bai C-H, Huang YK, Huang YL, Yang MH, et al. Polymorphisms in arsenic metabolism genes, urinary arsenic methylation profile and cancer. Cancer Causes Control. 2009;20(9):1653–61. https://doi.org/10.1007/s10552-009-9413-0.
Engström K, Vahter M, Mlakar SJ, Concha G, Nermell B, Raqib R, et al. Polymorphisms in arsenic(+III oxidation state) methyltransferase (AS3MT) predict gene expression of AS3MT as well as arsenic metabolism. Environ Health Perspect. 2011;119(2):182–8. https://doi.org/10.1289/ehp.1002471.
Gomez-Rubio P, Meza-Montenegro MM, Cantu-Soto E, Klimecki WT. Genetic association between intronic variants in AS3MT and arsenic methylation efficiency is focused on a large linkage disequilibrium cluster in chromosome 10. J Appl Toxicol. 2010;30(3):260–70. https://doi.org/10.1002/jat.1492.
Lindberg A-L, Kumar R, Goessler W, Thirumaran R, Gurzau E, Koppova K, et al. Metabolism of low-dose inorganic arsenic in a central European population: influence of sex and genetic polymorphisms. Environ Health Perspect. 2007;115(7):1081–6. https://doi.org/10.1289/ehp.10026.
Balakrishnan P, Vaidya D, Franceschini N, Voruganti VS, Gribble MO, Haack K, et al. Association of cardiometabolic genes with arsenic metabolism biomarkers in American Indian communities: the Strong Heart Family Study (SHFS). Environ Health Perspect. 2017;125(1):15–22. https://doi.org/10.1289/EHP251.
EWG. National drinking water database.
Water quality data. www.waterqualitydata.us/portal/. Accessed April 2 2017.
Tellez-Plaza M, Guallar E, Howard BV, Umans JG, Francesconi KA, Goessler W, et al. Cadmium exposure and incident cardiovascular disease. Epidemiology. 2013;24(3):421–9. https://doi.org/10.1097/EDE.0b013e31828b0631.
Tellez-Plaza M, Jones MR, Dominguez-Lucas A, Guallar E, Navas-Acien A. Cadmium exposure and clinical cardiovascular disease: a systematic review. Curr Atheroscler Rep. 2013;15(10):356. https://doi.org/10.1007/s11883-013-0356-2.
National Academy of Sciences. Critical aspects of EPA’s IRIS assessment of inorganic arsenic. Washington, D.C.: National Academies Press; 2013. https://doi.org/10.17226/18594.
Nachman KE, Ginsberg GL, Miller MD, Murray CJ, Nigra AE, Pendergrast CB. Mitigating dietary arsenic exposure: current status in the United States and recommendations for an improved path forward. Sci Total Environ. 2017;581-582:221–36. https://doi.org/10.1016/j.scitotenv.2016.12.112.
FDA. Arsenic in rice and rice products.
Acknowledgments
The authors thank Anne E. Nigra for her support with this manuscript, in particular Figure 1 and the section on Sources of Arsenic Exposure in General Populations.
Funding
This work was supported in part by grants from the National Institutes of Health (R01ES028758, R01ES025216, P42ES010349, P30ES009089).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Koren Mann reports grants from Canadian Institute of Health Research, during the conduct of the study. Ana Navas-Acien, Tiffany R. Sanchez, and Miranda R. Jones each declare no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Environmental Epidemiology
Rights and permissions
About this article
Cite this article
Navas-Acien, A., Sanchez, T.R., Mann, K. et al. Arsenic Exposure and Cardiovascular Disease: Evidence Needed to Inform the Dose-Response at Low Levels. Curr Epidemiol Rep 6, 81–92 (2019). https://doi.org/10.1007/s40471-019-00186-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40471-019-00186-5