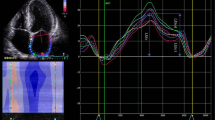
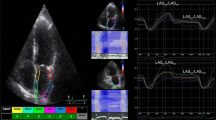
Abstract
Assessing left ventricular (LV) filling pressure (LVFP) is challenging in patients with coronary artery disease (CAD) and preserved LV ejection fraction (LVEF). We aimed to correlate left atrial strain (LAS) with two invasive complementary parameters of LVFP and compared its accuracy to other echocardiographic data to predict high LVFP. This cross-sectional, single-center study enrolled 81 outpatients with LVEF > 50% and significant CAD from a database. Near-simultaneous echocardiography and invasive measurements of both LV end-diastolic pressure (LVEDP) and LV pre-atrial contraction (pre-A) pressure were performed in each patient, based on the definition of LVEDP > 16 mmHg and LV pre-A > 12 mmHg as high LVFP. A moderate to strong correlation was observed between LAS reservoir (LASr), contractile strain, and LVEDP (r: 0.67 and 0.62, respectively; p < 0.001); the same was true for LV pre-A (r: 0.65 and 0.63, respectively; p < 0.001). LASr displayed good diagnostic performance to identify elevated LVFP, which was higher when compared to traditional parameters. Median value of LASr was higher for an isolated increase of LVEDP than for simultaneously high LV pre-A. The cutoff found to predict high LVFP was lower for LV pre-A than that one for LVEDP. In the current study, LASr did not provide an additional contribution to the 2016 diastolic function algorithm. LAS is a valuable tool for predicting LVFP in patients with CAD and preserved LVEF. The choice of LVEDP or LV pre-A as the representative marker of LVFP leads to different cutoffs to predict high pressures. The best strategy for adding this tool to a multiparametric algorithm requires further investigation.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Evaluation of left ventricular (LV) filling pressure (LVFP) has been performed through both invasive and noninvasive methods, routinely by echocardiography [1]. Some challenging scenarios include heart failure with preserved ejection fraction (HFPEF) and coronary artery disease (CAD). HFPEF represents at least half of the patients with this syndrome [2]. CAD is an important cause of mortality worldwide [3]. Left atrial strain (LAS) has been described as a supplementary marker of LVFP and a useful tool for grading diastolic dysfunction (DD) in patients with preserved LVEF [4, 5].
The 2016 American Society of Echocardiography (ASE) and European Association of Cardiovascular Imaging (EACVI) guidelines [6] for LV diastolic function evaluation recommended key parameters—left atrial volume index (LAVI), tricuspid regurgitation (TR) peak velocity, mitral annulus velocity (e′) and ratio E/e′—in an algorithm to predict LVFP. Some studies have revealed a modest diagnostic performance in normal LVEF [7, 8]. The EACVI recommendation for imaging in HFPEF [9] has included LAS reservoir (LASr) to replace any missing parameter.
Actually, the widespread term “filling pressure” refers to different pressures such as the mean capillary wedge pressure (PCWP), mean left atrial pressure (LAP), mean LV diastolic pressure, LV pre-atrial contraction pressure (LV pre-A), and LV end diastolic pressure (LVEDP). These pressures are not necessarily interchangeable, and their elevation may occur in different stages of the cardiac disease [10, 11].
We aimed to evaluate the correlation of LAS with two different and complementary invasive parameters of LVFP in each patient with significant CAD and preserved LVEF: LVEDP and LV pre-A. In addition, we compared the LAS diagnostic accuracy with conventional echocardiographic parameters.
Methods
Study population
This cross-sectional, single-center study was conducted at Dante Pazzanese Institute of Cardiology (São Paulo, Brazil), a specialized cardiovascular center. A total of 141 outpatients referred for diagnostic left-sided heart catheterization were retrospectively reviewed from a database. Inclusion criteria were defined as: appropriate register of LVFP invasive measurements, sinus rhythm, LVEF > 50%, significant CAD (defined by invasive angiography as > 50% stenosis of the left main coronary artery, > 70% in a major coronary vessel), and no intravenous fluid administration within 24 h prior to the test. Patients with arrhythmia, paced rhythm, prosthetic valve, or more than mild valvopathy, complex congenital heart disease, interatrial septal aneurysm, primary myocardial disease, pericardial diseases, ventricular septal defect, or an inappropriate acoustic window would be excluded. The study was approved by the Institutional Ethics Committee. All patients had provided written informed consent at the moment of recruitment.
Echocardiographic imaging acquisition and analysis
Echocardiographic images were acquired from GE Healthcare equipment, and analysis was performed on EchoPAC PC software Version 204 by an expert echocardiographer. Patients were evaluated in left lateral decubitus as the initial position. Standard exams were performed according to current recommendations [12] at the lab cath just before coronary angiography. LVEF and LAVI by Simpson´s method, transmitral peak early (E) and late atrial flow (A) velocities, septal and lateral mitral annular velocities, and TR peak velocity were measured and averaged over three cardiac cycles. Dedicated views were recorded for LV and LA analyses.
LAS reservoir (LASr), conduit (LAScd), contractile (LASct), and LV global longitudinal strain (GLS), were measured by two-dimensional speckle tracking echocardiography technique using a frame rate from 40–80/s. Biplanar calculation of LAS was based on the ASE/EACVI standardization document [13]. As recommended by this task force, the endocardial border was manually traced and the regions of interest were adjusted to cover the myocardium (Fig. 1) according to previously described steps for nondedicated software [14]. These results were displayed on the following tables.
Besides, more recently, novel dedicated LA tracking tool has been available in the software, so that an additional biplanar analysis of LAS was also performed for all cases (Fig. 2). This dedicated tool provided automated recognition of the chamber based on initial landmarks (two points to mark mitral ring and one point for LA roof). Diastolic function analysis was performed according to the 2016 ASE/EACVI guidelines [6]. Pulmonary vein Doppler was also acquired and analyzed.
Echocardiographic recordings were analyzed while blinded to invasive hemodynamic data.
Cardiac catheterization and invasive measurements
Left heart catheterization was performed in the supine position, without intravenous sedation, immediately after echocardiogram, using a 5 or 6 F catheter with femoral or radial arterial access. LV pressures and simultaneous electrocardiographic recordings were taken during an expiratory pause, before contrast injection, using a hemodynamic polygraph (SP12H-TEB—Brazilian Electronic Technology). A fluid-filled catheter was used and transducers were leveled at the midaxillary line “zero” in the fluid column. A minimum of five consecutive heartbeat cycles were averaged to obtain LVEDP and LV pre-A. LVEDP > 16 mmHg [15] and LV pre-A > 12 mmHg [16] were defined as high LVFP.
Statistical analysis
Normality was tested using Kolmogorov-Smirnov with Lilliefors correction. Continuous variables were provided as mean ± standard deviation for parametric variables or median and interquartile range for nonparametric variables. Categorical data were expressed as absolute numbers and percentages. Comparison of continuous variables used T-Student and Wilcoxon tests. For categorical variables, Chi-squared test was performed. Correlations of continuous variables were made using two-tailed test Pearson or Spearman method as appropriate. Receiver-operating characteristic (ROC) analysis was carried out to assess the diagnostic ability of echocardiographic parameters to identify elevated LVFP. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and overall accuracy were calculated using standard definitions to predict high LVFP.
Reproducibility of strain measurements was tested through intraclass correlation coefficient (ICC) based on a subset of 16 exams randomly selected to be reevaluated by another experienced operator. In addition, Bland-Altman analysis was performed to evaluate the agreement between LV-software and LA-software measurements of LAS for the entire study sample.
The association of LVEDP and LV pre-A with dichotomous variables was evaluated using binary logistic regression, assuming odds ratio (OR) as the main analysis.
Statistical analysis was performed using IBM SPSS Statistics version 20 (SPSS Inc., IL, USA) software. A p-value < 0.05 was considered statistically significant.
Results
We selected a final sample of 81 patients. From the initial database, 22 did not meet the inclusion criteria due to inappropriate trace recordings of LVFP invasive measurements, and 17 presented reduced LVEF. Arrhythmia and more than mild valvopathy excluded 12 patients. A subset of 9 patients was excluded due to poor acoustic image quality.
Baseline clinical characteristics of the study population are summarized in Table 1.
Coronary angiography was requested for outpatients investigating CAD under elective conditions. Abnormal findings in noninvasive ischemic testing were noted in 41 (50.6%) patients. Prior acute coronary syndrome more than 30 days before was reported in 18 (22.2%) subjects. Symptoms were the only reason for angiogram in 22 (27.2%) patients.
Left main coronary artery disease was reported in 7 (8.6%) patients. Of the remaining group, 47 (58%) had single-vessel disease, 19 (23.5%) presented double-vessel disease, and 8 (9.9%) three-vessel disease. In 43 (53%) cases, the left anterior descending artery presented significant stenosis.
There was no significant difference in regular oral medication use between normal and high LVFP groups. Prevalence of cardiovascular risk factors was also similar as well as heart rate and blood pressure at the time of echocardiographic evaluation and catheterization.
LVEDP was normal in 41 patients (group A) and > 16 mmHg in 40 patients (group B). From this last group, 27 patients presented simultaneously LV pre-A > 12 mmHg (mean 15.8 ± 3.3 mmHg), while 13 had isolated high LVEDP. Mean LVEDP and LV pre-A in group A were 12.3 ± 2.9 mmHg and 8.2 ± 2.8 mmHg respectively; in group B, they were 20.6 ± 3.1 mmHg and 14 ± 3.8 mmHg, respectively.
Echocardiographic profile
Echocardiographic parameters are described in Table 2. Median values of LASr (Fig. 3) in normal LVFP [39.78 (38.55–41.86)] were higher than in isolated high LVEDP [34.41 (30.88–38.13); p < 0.001] and in high LV pre-A [23.69 (21.97–26.68); p < 0.001].
Correlation of LAS and traditional echocardiographic variables with LVFP
LASr and LASct revealed a moderate to strong correlation with LVEDP and LV pre-A (Table 3). The scatter diagrams of LAS are exhibited in Fig. 4. E/e′ average, TR peak velocity, and LAVI were also correlated with LVFP. TR peak evaluation was not feasible (none/trace) in 18 (22%) cases.
A binary logistic regression analysis was performed to study the variables LAVI > 34 ml/m², LASr < 26%, and E/e′ average > 14 as predictors of LVEDP and LV pre-A VE. The model containing these 3 variables was significant for predicting LVEDP [X² (3) = 24.99; p < 0.001; Nagelkerke R² = 0.35] and LV pre-A [X² (3) = 39.87; p < 0.001; Nagelkerke R² = 0.54]. In this model, LASr was the only significant predictor for LVEDP (OR 7.1; 95% CI 1.7–29.3; p = 0.007) and LV pre-A (OR 18.2; 95% CI 4.5–73.4; p < 0.001).
Diagnostic accuracy of traditional echocardiographic parameters to predict elevated LVFP
E/e′ average, TR peak velocity, and LAVI, as single variables, demonstrated high specificity and lower sensitivity (Table 4). On ROC analysis, these parameters revealed a good ability to identify high LVEDP (E/e′: area under the curve—AUC 0.78; TR peak velocity: AUC 0.77, for feasible evaluations; LAVI: AUC 0.71) and LV pre-A (E/e′: AUC 0.83; TR peak velocity: AUC 0.75 for feasible evaluations; LAVI: 0.70).
Based on the 2016 ASE/EACVI guidelines, the multiparametric algorithm classified 14 patients with normal diastolic function, 55 with grade I DD, and 11 with grade II DD. No case was defined as grade III DD. In one case, the grade of DD was classified as indeterminate. Excluding the indeterminate case, sensitivity, specificity, PPV, NPV, and accuracy for elevated LVEDP were 26.3%, 97.6%, 90.9%, 59.4% and 63.8%, respectively; for high LV pre-A, 37.5%, 96.4%, 81.8%, 78.3% and 78.8%, respectively.
Diagnostic accuracy of LAS to predict high LVFP
LAS presented powerful diagnostic performance for predicting high LVFP (Fig. 5). On ROC analysis, AUC for LASr was superior compared with E/e′ (difference AUC of 0.13 and 0.11 for LVEDP and LV pre-A, respectively; p < 0.001) and LAVI (difference AUC of 0.20 and 0.24 for LVEDP and LV pre-A, respectively; p < 0.001).
For LASr, LAScd and LASct, AUC values were 0.91, 0.81, and 0.85 to identify high LVEDP and 0.94, 0.84 and 0.91 to diagnose high LV pre-A (Fig. 6). LASr < 35% demonstrated 87% sensitivity and 88% specificity for LVEDP > 16 mmHg. On the other hand, LASr < 26% displayed 73% sensitivity and 93% specificity for LV pre-A > 12 mmHg. For a different cutoff, LASr < 23% showed 48% sensitivity and 98% specificity. For LASct, the cut-off 14% displayed 74% sensitivity and 85% specificity to identify elevated LV pre-A. LASct cut-off 15.4% demonstrated 73% sensitivity and 83% specificity for high LVEDP.
Reproducibility of LAS measurements
Two observers analyzed LAS in 16 cases randomly selected. Interobserver ICCs were 0.94 (95% CI 0.84–0.97), 0.93 (95% CI 0.80–0.97), and 0.84 (95% CI 0.60–0.94) for LASr, LAScd, and LASct, respectively. The corresponding intraobserver ICCs were 0.98 (95% CI 0.94–0.99), 0.96 (CI 95% 0.90–0.99) and 0.85 (95% CI 0.63–0.95).
Potential added value of LAS to the 2016 ASE/EACVI diastolic function algorithm
Incorporating LASr into the 2016 ASE/EACVI recommendation to substitute any missing additional parameter [17] did not improve its accuracy. E/e′ and LAVI were feasible in all cases in our sample. In 17 of 18 cases of missing TR peak velocity, E/e′ average and LAVI were both normal or both positive, so including a new parameter would not have changed algorithm conclusion.
Adding LASr to LAVI in the algorithm so that the parameter could be positive in case of LAVI > 34 ml/m² or LASr < 23% also did not change algorithm performance, due to the low rate of positivity of the other parameters. The isolated positive “LA criteria” was not sufficient to alter the algorithm conclusion.
Agreement of nondedicated and dedicated LA tracking tool for strain analysis
LAS measurements with both nondedicated (LV software) and dedicated (LA software) tools were similar (Table 5). Bland-Altman analysis plots are provided in Fig. 7.
Discussion
Our study could provide some insight into a challenging group, that has been studied across a heterogeneous spectrum of patients [7, 18].
Simultaneous analysis of both LVEDP and pre-A provides an incremental comprehension of diastolic function evolution. LVEDP data allow the analysis of the earliest pressure change in the initial stages of diastolic dysfunction. The isolated increase in LVEDP should not be assumed as diagnostic of HFPEF [19]. Although LVEDP is a metric of LV compliance, there is a pitfall in assuming this measurement to be synonym of mean pulmonary artery wedge pressure [20]. In addition, LV pre-A pressure includes a better surrogate parameter for LAP, which is a fundamental substrate for dyspnea in HFPEF. Of note, LVEDP was elevated in all patients with increased LV pre-A, but this pressure was normal in one third of the cases with high LVEDP in our study. The choice of the reference parameter for LVFP impacts the cutoff used to predict high pressures, as revealed by the lower value found when LV pre-A was the standard. The median value of LASr (34.4%) described for an isolated high LVEDP is near to a possible normal range and much higher to the one found for simultaneously high LVEDP and LV pre-A (23.7%). This reinforces that LV pre-A is a better surrogate of LAP and its increase is more related to a worse left atrial function. As previously reported by Braunwald et al. [10], in our sample, even when LVEDP is higher than 20 mmHg, the mean LAP (represented by LV pre-A) could be normal.
The near-simultaneous echocardiographic and hemodynamic data establish a potential better correlation of measurements, since variations in heart rate, blood pressure and volume status become insignificant. Hummel et al. [8] acquired echocardiographic data simultaneously with invasive study in supine position. Kasner et al. [21] performed an echocardiogram 3 to 5 h after invasive measurements. Reddy et al. [22] performed echocardiographic analysis at a median interval range of 6 days after hemodynamic study, with a possible limitation on parameter correlation.
Most patients presented reduced LV GLS (< 18% in absolute values), reinforcing that normal LVEF does not necessarily imply normal systolic function. However, no structural echocardiographic differences were found between the groups of normal or high LVFP in our study. Mean LV GLS also did not show any difference between these groups and the CAD profile was similar. Nonetheless, there was a higher prevalence of low LV GLS in the high LV pre-A group, although not statistically significant, which may can be related to sample size. The similar prevalence of cardiovascular risk factors in patients with normal or high LVFP does not imply a coincident evolutive stage, control, and magnitude of diseases, with a possible impact on diastolic function. Diabetes mellitus, for instance, independently predicts poorer LASr and LASct due to advanced glycated end products [23]. In addition, the grade of fibrosis in LA may be associated with a worsening of its function [24], although these data are not available for our sample.
Among the 27 patients with simultaneously high LVEDP and LV pre-A, only 3 patients had normal geometry, wall motion score index, and LV GLS. Some HFPEF trials [25, 26] have reported normal diastolic function in one-third of the sample. However, this classification was not based on the most recent guidelines, which include the essential concept of myocardial disease as indicative of DD.
Correlation of LAS and LVFP
In a sample of patients with CAD and preserved LVEF, LASr and LASct provided a powerful diagnostic ability to predict LVFP based on hemodynamic data of LVEDP and LV pre-A.
LASr performance was superior to that of conventional parameters for diastolic function evaluation as previously reported. In patients with preserved LVEF, Hummel et al. [8] described AUCs to predict PCWP for E/e′ > 15, TR peak velocity > 2.8 m/s, LAVI > 34 ml/m² and multiparametric ASE/EACVI algorithm of 0.59, 0.69, 0.76 and 0.75, respectively. In multivariable linear regression analysis, only IVRT and LASr remained independently associated with this invasive measurement. Correlation of E/e′ with invasive measurement, mainly in preserved LVEF, has been described as weak to moderate [27, 28]. Our findings have corroborated lower sensitivity and higher specificity of the individual parameters E/e′, LAVI, and TR peak velocity in predicting LVFP [18]. Singh et al. [29] have previously reported the usefulness of LASr as a sole parameter for categorizing patients as having normal or high LFVP.
A potential contribution of adding LASr to the 2016 ASE/EACVI multiparametric algorithm is related to the sensitivity of the other parameters. The cutoff of E/e′ average > 14, for instance, has revealed better specificity than sensitivity in predicting high LVFP, mainly for patients with preserved LVEF [18]. Furthermore, many patients present intermediate E/e′ values and remain in the gray zone of 8–14 [27], as revealed by 70% of false negatives for E/e′ > 14 in patients with high LV pre-A in our sample.
Defining optimal cutoffs for key parameters demands an appropriate balance between sensitivity, specificity and prognostic value. Morris et al. described an association of LASr < 23% with dyspnea, worse functional class and PCWP > 15 mmHg, even for normal LAVI. In our sample, this value was similar to the median of the high LV pre-A group, but had low sensitivity and high specificity to predict LVFP. None of our patients were classified as having grade III diastolic dysfunction, which can be related to less advanced stages of LA dysfunction and less reduced LAS values [30]. Additionally, studies with higher LVFP have shown lower cutoffs for LAS, as revealed by Inoue et al. in a group in which 8% had PCWP > 30 mmHg. None of our patients presented LV pre-A > 30 mmHg and only 4 cases > 20 mmHg. Moreover, the exclusion of patients with atrial fibrillation and more than mild mitral regurgitation in our study may have influenced higher cutoffs. Inoue et al. [31] reported LASr < 20% in the subgroup of atrial fibrillation regardless of LVFP.
Notwithstanding the good correlation of LASr with LVFP, using it as a stand-alone index may be questionable. Since the normal range is wide, patients with diastolic function can have normal LARs and normal subjects can present decreased strain values due to loading conditions [32].
Echo software and technical aspects of strain analysis
Commercially available software was utilized in our analysis, which has been widely used to define the normal value range of LAS [33]. There has been good correlation demonstrated with other vendors, mainly after the technical standardization initiative [34]. However, the grade of correlation between LAS and invasive pressure measurements may still vary according to the software used [31].
Reproducibility of LAS measurements based on nondedicated and dedicated software
Specific tracking tools for LA provided interchangeable mean measurement results for strain compared to nondedicated software. A previous study [35] based on the same vendor also showed that the choice of software did not significantly impact strain values, but presented a better intra and interobserver correlation. It is reasonable to believe that automatized tools may favor the routine use of new techniques, mainly outside of a research setting.
Limitations
This was a single-center study with a relatively limited number of patients in a reference center with a profile of severe cardiovascular diseases. A single commercially available software was used to perform echo analysis, which may limit generalizability. Despite the use of two parameters for LVFP evaluation, PCWP data were not available, since right circulation was not studied invasively. Although fluid-filled transducers have often been used [16, 18], high-fidelity catheters could have provided better accuracy for pressure measurements. Ultrasound enhancing agents were not used in order to improve TR jet signal.
Conclusions
LAS revealed a moderate to strong correlation with invasive measurements of LVFP and may be a useful adjunct parameter to identify elevated pressures in patients with CAD and preserved LVEF. The choice of LVEDP or LV pre-A as the representative parameter of LVFP leads to different cutoffs to predict high pressures. The best strategy to add LASr to a multiparametric algorithm for the evaluation of LVFP demands further investigation.
References
Nagueh SF (2020) Left ventricular diastolic function understanding pathophysiology, diagnosis, and prognosis with echocardiography. JACC Cardiovasc Imaging 13:228–244
Owan TE, Hodge DO, Herges RM et al (2006) Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 355:251–259. https://doi.org/10.1056/NEJMoa052256
Vos T, Lim SS, Abbafati C et al (2020) Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of Disease Study 2019. Lancet 396:1204–1222
Morris DA, Belyavskiy E, Aravind-Kumar R et al (2018) Potential usefulness and clinical relevance of adding left atrial strain to left atrial volume index in the detection of left ventricular diastolic dysfunction. JACC Cardiovasc Imaging 11:1405–1415. https://doi.org/10.1016/j.jcmg.2017.07.029
Singh A, Addetia K, Maffessanti F et al (2017) LA strain for categorization of LV diastolic dysfunction. JACC Cardiovasc Imaging 10:735–743. https://doi.org/10.1016/j.jcmg.2016.08.014
Nagueh SF, Smiseth OA, Appleton CP et al (2016) Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 29:277–314. https://doi.org/10.1016/j.echo.2016.01.011
Balaney B, Medvedofsky D, Mediratta A et al (2018) Invasive validation of the echocardiographic assessment of left ventricular filling pressures using the 2016 diastolic guidelines: head-to-head comparison with the 2009 guidelines. J Am Soc Echocardiogr 31:79–88. https://doi.org/10.1016/j.echo.2017.09.002
Hummel YM, Liu LCY, Lam CSP et al (2017) Echocardiographic estimation of left ventricular and pulmonary pressures in patients with heart failure and preserved ejection fraction: a study utilizing simultaneous echocardiography and invasive measurements. Eur J Heart Fail 19:1651–1660
Smiseth OA, Morris DA, Cardim N et al (2022) Multimodality imaging in patients with heart failure and preserved ejection fraction: an expert consensus document of the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 23:e34–e61
Braunwald E, Frahm CJ (1961) Studies on Starling´s law of the heart IV. Observations on the hemodynamic functions of the left atrium in man. Circulation 3:566–571
Peverill RE (2015) Left ventricular filling pressure (s)—ambiguous and misleading terminology, best abandoned. Int J Cardiol 191:110–113
Lang RM, Badano LP, Mor-Avi V et al (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the american society of echocardiography and the european association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging 16:233–271. https://doi.org/10.1093/ehjci/jev014
Badano LP, Kolias TJ, Muraru D et al (2018) Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: a consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging 19:591–600
Haji K, Wong C, Wright L et al (2019) Left atrial strain performance and its application in clinical practice. JACC Cardiovasc Imaging 12:1093–1101
Sato K, Grant ADM, Negishi K et al (2017) Reliability of updated left ventricular diastolic function recommendations in predicting elevated left ventricular filling pressure and prognosis. Am Heart J 189:28–39. https://doi.org/10.1016/j.ahj.2017.03.022
Andersen OS, Smiseth OA, Dokainish H et al (2017) Estimating left ventricular filling pressure by echocardiography. J Am Coll Cardiol 69:1937–1948. https://doi.org/10.1016/j.jacc.2017.01.058
Popescu BA, Beladan CC, Nagueh SF, Smiseth OA (2022) How to assess left ventricular filling pressures by echocardiography in clinical practice. Eur Heart J Cardiovasc Imaging 23:1127–1129
Lancellotti P, Galderisi M, Edvardsen T et al (2017) Echo-Doppler estimation of left ventricular filling pressure: results of the multicentre EACVI Euro-Filling study. Eur Heart J Cardiovasc Imaging 18:961–968. https://doi.org/10.1093/ehjci/jex067
Oh JK, Miranda WR, Kane GC (2023) Diagnosis of heart failure with preserved ejection fraction relies on detection of increased diastolic filling pressure, but how? J Am Heart Assoc 12:e028867
Rahimtoola SH, Ehsani A, Sinno MZ et al (1975) Left atrial transport function in myocardial infarction. Importance of its booster pump function. Am J Med 59:686–694
Kasner M, Westermann D, Steendijk P et al (2007) Utility of Doppler echocardiography and tissue doppler imaging in the estimation of diastolic function in heart failure with normal ejection fraction: a comparative doppler-conductance catheterization study. Circulation 116:637–647
Reddy YNV, Obokata M, Egbe A et al (2019) Left atrial strain and compliance in the diagnostic evaluation of heart failure with preserved ejection fraction. Eur Heart J Cardiovasc Imaging 21:891–900
Georgievska-Ismail L, Zafirovska P, Hristovski Z (2016) Evaluation of the role of left atrial strain using two-dimensional speckle tracking echocardiography in patients with diabetes mellitus and heart failure with preserved left ventricular ejection fraction. Diab Vasc Dis Res 13:384–394
Kuppahally SS, Akoum N, Burgon NS et al (2010) Left atrial strain and strain rate in patients with paroxysmal and persistent atrial fibrillation: relationship to left atrial structural remodeling detected by delayed-enhancement MRI. Circ Cardiovasc Imaging 3:231–239
Zile MR, Gottdiener JS, Hetzel SJ et al (2011) Prevalence and significance of alterations in cardiac structure and function in patients with heart failure and a preserved ejection fraction. Circulation 124:2491–2501
Shah A, Shah S, Anand IS et al (2014) Cardiac structure and function in heart failure with preserved ejection fraction. Circ Heart Fail 7:104–115
Ommen SR, Nishimura RA, Appleton CP et al (2000) Clinical utility of Doppler echocardiography and tissue Doppler Imaging in the estimation of left ventricular filling pressures: a comparative simultaneos Doppler-catheterization study. Circulation 102:1788–1794. https://doi.org/10.1161/01.CIR.102.15.1788
Nauta JF, Hummel YM, Meer P, van der et al (2018) Correlation with invasive left ventricular filling pressures and prognostic relevance of the echocardiographic diastolic parameters used in the 2016 ESC heart failure guidelines and in the 2016 ASE/EACVI recommendations. Eur J Heart Fail 20:1303–1311
Singh A, Medvedofsky D, Mediratta A et al (2019) Peak left atrial strain as a single measure for the non-invasive assessment of left ventricular filling pressures. Int Cardiovasc Forum J 5:23–32
Thomas L, Marwick TH, Popescu BA et al (2019) Left atrial structure and function, and left ventricular diastolic dysfunction: JACC state-of-the-art review. J Am Coll Cardiol 73:1961–1977
Inoue K, Khan FH, Remme EW et al (2022) Determinants of left atrial reservoir and pump strain and use of atrial strain for evaluation of left ventricular filling pressure. Eur Heart J Cardiovasc Imaging 23:61–70
Nagueh SF, Khan SU (2023) Left atrial strain for assessment of left ventricular diastolic function: focus on populations with normal LVEF. JACC Cardiovasc Imaging 16:691–707
Pathan F, D´Elia N, Nolan MT et al (2017) Normal ranges of left atrial strain by speckle-tracking echocardiography: a systematic review and meta-analysis. J Am Soc Echocardiogr 30:59–70
Yang H, Marwick TH, Fukuda N et al (2015) Improvement in strain concordance between two major vendors after the strain standardization initiative. J Am Soc Echocardiogr 28:642–648
Mirea O, Duchenne J, Voigt JU (2022) Comparison between nondedicated and novel dedicated tracking tool for right ventricular and left atrial strain. J Am Soc Echocardiogr 35:419–425
Author information
Authors and Affiliations
Contributions
GN: Echocardiographic analysis, manuscript writing and reviewing, figures preparing. AAC: Echocardiographic analysis, manuscript reviewing. JEA: Data reviewing, manuscript writing and reviewing. NSSS: Echocardiographic analysis, manuscript writing and reviewing. AAV: Data reviewing, manuscript reviewing. SLNB: Hemodynamic data analysis, manuscript reviewing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nishida, G., Calvilho Junior, A.A., Assef, J.E. et al. Left atrial strain as a predictor of left ventricular filling pressures in coronary artery disease with preserved ejection fraction: a comprehensive study with left ventricular end-diastolic and pre-atrial contraction pressures. Int J Cardiovasc Imaging 39, 2193–2204 (2023). https://doi.org/10.1007/s10554-023-02938-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-023-02938-3