Abstract
Background
Left ventricular diastolic function is an important prognostic marker in acute coronary syndrome. However, classification of the dysfunction grade using isolated echocardiographic parameters remains difficult. Therefore, it is necessary to combine multiple data in diagnostic algorithms. The purpose of this study was to evaluate the capacity of left atrial strain (LAS) components to classify left ventricular diastolic dysfunction (DD) grade.
Methods
Cross-sectional study with 109 consecutive patients admitted to the emergency room with acute coronary syndrome. Patients were referred for echocardiographic evaluation within 72 h. Mean values of LAS, corresponding to three phases of atrial function (reservoir, conduit and contraction), were obtained by speckle-tracking echocardiography. Patients were divided according to the diastolic dysfunction grade for later association with the LAS.
Results
The three LAS components showed moderate correlation with most diastolic variables (left atrial volume index, E/e′ ratio and e′ wave). In addition, there was related reduction of the LAS, which was inversely proportional to the DD grade (p < 0.05). LAS was effective for the identification of patients with DD grade III [area under the curve (AUC) for the reservoir = 0.99; conduit AUC = 0.89; contraction AUC = 0.99) and also those with DD grade II or III (reservoir AUC = 0.94; conduit AUC = 0.92; contraction AUC = 0.80].
Conclusions
LAS alone presented excellent capacity to classify DD in patients with acute coronary syndrome and may represent an additional tool for this purpose.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acute coronary syndrome (ACS) remains one of the major causes of morbidity and mortality around the world [1, 2]. LV diastolic function is known to be one of the parameters that changes earlier during acute ischemia and the identification of diastolic dysfunction (DD) is an important prognostic marker in this disease [3,4,5,6].
Nevertheless, the assessment of diastolic function remains challenging, especially in ACS patients. Besides, according to the last published guidelines, a high percentage of patients is still classified as indeterminate diastolic function [7, 8].
As a consequence of DD, patients with ACS can increase LV filling pressures, which will be transmitted to left atrium (LA), leading to pulmonary congestion symptoms, as well as LA remodeling and dysfunction [9].
Introduction of two-dimensional strain has allowed the measurement of LA myocardial deformation, providing a direct measure of all atrial function components [10, 11]. Recent studies have shown significant correlation of the LAS with pulmonary capillary wedge pressure [12] and LV end-diastolic pressure [13,14,15,16]. Other studies have already shown the utility of LAS to categorize DD in a population without significant coronary artery disease [17, 18].
However, there are no studies evaluating LAS in the setting of ACS, applying the last recommendations for the evaluation of LV diastolic function as a Ref. [7].
Therefore, the main objective of this study was to evaluate the capacity of LAS components (reservoir, conduit and contraction) to classify LV DD grade according to the last guidelines, in patients admitted to a tertiary hospital with ACS.
Methods
Study design
This is a cross-sectional study that prospectively evaluated consecutive patients admitted to the emergency unit of Dante Pazzanese Institute of Cardiology, Sao Paulo, Brazil, presenting ACS with non-ST segment elevation. Patients underwent transthoracic echocardiogram within 72 h after admission.
Patients
Patients were required to meet the following inclusion criteria for participating in the study: diagnosis of acute myocardial infarction (AMI) with non-ST segment elevation or unstable angina (UA) of moderate–high risk according to the GRACE score [19], > 18 years of age and no clinical history of atrial fibrillation/flutter, valve prosthesis or intracardiac devices (cardiac defibrillator or pacemaker).
AMI diagnosis was defined by transient and documented elevation of CK-MB mass or troponin, by the presence of clinical symptoms, and/or typical electrocardiographic alterations, according to universal AMI definitions [20]. UA was defined by early-onset angina, progressive or at rest, with or without ischemic alterations at electrocardiogram (EKG), and with no elevation of serum levels of myocardial necrosis markers [2].
Exclusion criteria were the following: significant valvopathy (mitral regurgitation with grade more than mild, mitral stenosis of any grade and any aortic valvulopathy with grade more than mild), ACS due to a secondary cause (for instance, anemia or sepsis), inadequate acoustic window, diagnostic changes after patient inclusion in the study or the presence of frequent arrhythmias during the test.
This study was approved by the Research Ethics Committee of the institution and was registered under the number 09202912.0.0000.5462. All patients read and signed a written informed consent about the project, to be able to participate in the study.
The investigators had full access to all data in the study and take responsibility for its integrity and the data analysis.
Echocardiogram
Echocardiographic images were acquired using Vivid E9 or Vivid S6 (GE Healthcare, Horten, Norway) equipment. All exams were performed before any percutaneous intervention and within 72 h after admission. All exams were acquired and analyzed by a single operator, in accordance with the European Association of Cardiovascular Imaging (EACVI) guidelines [21].
For the purpose of LV diastolic function evaluation, we considered all patients in this study as having some structural heart disease, as at least one of the following were observed in all cases: LVEF < 50%, LV hypertrophy, regional wall motion abnormalities, significant coronary artery lesion or ischaemic cardiomyopathy. Therefore, diastolic function was evaluated accordingly, based on the 2016 recommendations [7]. Diastolic function was then categorized into four groups: grade I, grade II, grade III and indeterminate grade, taking into account early–late ventricular filling velocities (E/A) ratio, E/e′ ratio, tricuspid regurgitation velocity, and LA volume index (LAVI). In patients with indeterminate grade of diastolic dysfunction and depressed LV ejection fraction (LVEF), the systolic/diastolic pulmonary venous flow velocity ratio was used.
Left atrial strain evaluation
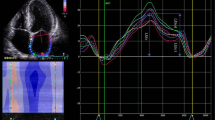
LA function was evaluated by means of two-dimensional strain measurements obtained by speckle-tracking echocardiography (STE) technique. The following steps were performed using the EchoPAC software (GE Healthcare, Horten, Norway): (1) marking the systolic event (opening and closure of the aortic valve) by an aortic flow pulsatile Doppler, (2) manual tracing of LA endocardial border, (3) evaluating proper approval and tracing of the six segments. If two or more segments were not approved, step 2 had to be performed again. Otherwise, analysis was approved and the software generated a strain average curve. (4) Using the strain average curve, built from the beginning of QRS complex, we have obtained LASs and LASa, representing the LA reservoir and contraction functions, respectively. The conduit function was calculated by the difference between these two values (Fig. 1). (5) This procedure was performed in LA-focused four-chamber and two-chamber apical windows. Care was taken to ensure a good EKG visualization, especially regarding the P wave, that was used to determine the atrial booster function.
LA strain by the STE technique. For analysis purposes, the curve representing the average from the strain of six LA segments is used. This method uses QRS as reference. LASs4cand LASs2c positive peak in the four and two-chamber apical window, representing the ventricular systole period and corresponding to LASR. LASa4cand LASa2c point relative to the p wave of the electrocardiogram in the four and two-chamber apical windows and associated with LASCT. LASCD is obtained by the difference between LASR and LASCT
The following variables were obtained by calculating the average strain values in the four and two-chamber apical windows: global left atrial strain of reservoir (LASR), global left atrial strain of conduit (LASCD) and global left atrial strain of contraction (LASCT).
Statistical analysis
Categorical variables were demonstrated as absolute numbers and percentages. These data were analyzed using Fisher’s exact test or Chi-square test, whichever was deemed appropriate.
Continuous variables were demonstrated using mean and standard deviation or median and interquartile intervals. For the comparison of continuous variables between DD groups, Kruskal–Wallis test or Jonckheere–Terpstra test (when the variables between groups were expected to be ordered) were used.
Spearman correlation was used to evaluate the correlation between LAS variables and LV DD variables.
ROC (Receiver Operating Characteristics) curves were created to evaluate the performance of LAS components to categorize the DD grade and to identify patients with E/e′ ratio > 14. Cut-offs were determined using Youden index.
Intra- and inter-observer reproducibilities were assessed in 20 randomly selected subjects, by calculation of Lin’s concordance correlation coefficient.
The statistical significance was defined as p < 0.05.
Results
A total of 132 patients were screened for the study. However, 14 patients were excluded during the image acquisition process, 7 during the offline echocardiographic analysis and 2 due to change in diagnosis during hospitalization. Therefore, the group effectively comprised of 109 patients. The mean time between hospital admission and echocardiography acquisition was 26.9 ± 15 h.
Table 1 shows the behavior of the clinical variables according to DD groups. Among the patients included, the clinical diagnosis of AMI and multi-vessel lesions were the most prevalent in all DD groups. Age presented a significant difference between DD groups. Renal failure and high levels of troponin and CK-MB were more frequently in the DD grade III.
Patients with DD grade III presented the worst echocardiographic parameters, except for isovolumetric relaxation time (IVRT) and color M-mode mitral inflow propagation velocity (Table 2). LVEF was similar between DD grade I and II groups. However, patients with DD grade III and indeterminate presented a lower value compared to others groups (32% ± 18 and 48% ± 16, respectively; p < 0.001).
All atrial strain values presented moderate correlation with most parameters used to define LV diastolic function excepted the E wave deceleration time, pulmonary venous flow parameters, IVRT and PASP (Online Resource 1).
LASR, LASCD and LASCT showed a decrease proportional to DD worsening (Table 3, Fig. 2). LASCT was the component presenting the highest relative decrease when values of DD grade I group were compared to DD grade III group (from 13.6 to 3.3%, a 75.7% reduction; p < 0.001). The relative decrease of other components was 65.7 and 56% for the reservoir and conduit functions, respectively (p < 0.001). The indeterminate group showed similar LAS values as DD grade II group.
Distribution of values for left atrial strain components according to the diastolic dysfunction group. LASR left atrial strain of reservoir, LASCD left atrial strain of conduit, LASCT left atrial strain of contraction, DD diastolic dysfunction. LASR non-significant p value in DD grade II vs. indeterminate; p < 0.05 in the remaining analysis. LASCD non-significant p value in DD grade II vs. III, DD grade II vs. indeterminate and DD grade III vs. indeterminate; p < 0.05 in the remaining analysis. LASCT non-significant p value in DD grade I vs. grade II and DD grade II vs. indeterminate; p < 0.05 in the remaining analysis
When patients were divided into two groups according to the increase of LV filling pressures, differences between atrial function components became more evident, with LASR, LASCD and LASCT worsening in the group presenting higher LV filling pressures (p < 0.001 for all comparisons—please refer to Table 3).
ROC curves were created to evaluate the LAS capacity to categorize the DD grade (Fig. 3). LAS was particularly useful to identify patients with DD grade III. For this purpose, cut-offs of 14.1% for LASR and 5.8% for LASCT had, individually, 100% sensitivity, 97% specificity and area under curve (AUC) value was 0.99 (Table 4).
ROC curves to evaluate performance of left atrial strain components to classify diastolic dysfunction. LASR left atrial strain of reservoir, LASCD left atrial of conduit, LASCT left atrial strain of contraction, DD diastolic dysfunction. Please refer to the AUC values in Table 5ja
In addition to its importance in identifying patients with DD grade III, it also plays a major role when it comes to identify patients with increased LV filling pressures (DD grade II and III). From the three components, LASR had the best performance, with 85% sensitivity and 90% specificity for a 21% cut-off. The LASCD had a good performance too, with 85% sensitivity and 83% specificity for a 9.8% cut-off (Table 4).
The ROC curve analysis was also used to evaluate the LAS capacity to identify patients with E/e′ ratio higher than 14 (Fig. 4). In this study, LASCD lower than 9.8% presented 84% sensitivity, 79% specificity and 0.88 AUC to identify patients with E/e′ ratio higher than 14. LASR also had a good performance, with AUC equal to 0.87 (Table 5).
Analysis of LAS intraobserver/interobserver variability demonstrated an intraclass correlation coefficient of 0.98/0.96, 0.95/0.93 and 0.88/0.91 to reservoir, conduit and contraction strain, respectively.
Discussion
Echocardiogram is a very valuable test for risk stratification of patients with non-ST segment elevation ACS [3,4,5,6]. LV diastolic function undergoes early modifications depending on the ischemic cascade and myocardial ischemia, which influences all diastole phases. For instance, active relaxation is early delayed. Furthermore, LV loses its compliance, which varies depending on the extension of the infarction and ventricular remodeling.
Several diastolic function parameters have shown prognostic value after an acute coronary event. Division of patients into groups according to DD grade is difficult as it requires combined use of several echocardiographic parameters in a diagnostic algorithm [22]. Recently, EACVI published a new guideline, reviewing this algorithm for DD categorization using mostly E/A and E/e′ ratio, tricuspid reflow velocity, pulmonary venous flow-derived variables, and LAVI [7]. However, it remains a challenge to determine DD in many ACS patients, mainly because of the difficulty in acquiring all parameters associated to LV diastolic function. For instance, in this study, it was possible to evaluate peak tricuspid regurgitation velocity and pulmonary venous flow only in 64 and 82% patients, respectively.
Although LA is directly involved in DD physiopathology [23], few studies have been conducted to analyze phasic atrial function for this purpose [17, 18]. LAS might be a promising tool for this goal and, possibly, a parameter to be added in the algorithm to categorize LV DD.
LASR has been the most studied component of LA function in all clinical scenarios [24,25,26]. The study conducted by Singh et al. evaluated retrospectively LASR capacity to categorize DD in 90 patients, and the authors concluded that LASR has an excellent discriminatory capacity for that purpose [17]. However, unlike the present study, patients in this group presented preserved LVEF and no evidence of coronary artery disease. It is well known that evaluation of the diastolic function can be more challenging in coronary heart disease patients than in those with no evidence of coronary obstructive disease [7]. We sought to analyze atrial function components and verify association between atrial function and DD analysis, in a population with ACS and high percentage compromise of LV systolic function.
LASR and LASCT play a significant role in determining patients with DD grade III. This fact could be physiologically explained because these groups present increased LV diastolic pressure which is transmitted to the LA. The increased pressure regimen within the LA ends up causing progressive atrial remodeling in such a way that in later stages, it causes significant damage to the additional dilation capacity to receive flow from pulmonary veins during ventricular systole (LASR) and to the intrinsic contractile capacity (LASCT).
LASR and LASCD were the components presenting the highest capacity to differentiate patients with DD grade II and III from DD grade I, and also to diagnose patients with E/e′ ratio higher than 14, which is widely used as an echocardiographic sign of increased ventricular filling pressures [5, 27]. Indeed, this would be an important applicability of the LAS, i.e., identifying those patients with higher degree of DD associated with elevated LV filling pressures. Comparing to the two other LA function components, LASCT was the one that presented the lowest discriminatory capacity for this purpose. This weak performance of LASCT can be due to the compensatory increase of LA contractibility during DD early stages, in such a way that failure of intrinsic atrial contraction would eventually occur only in more advanced DD phases. Besides, it could be explained by the fact that we evaluated patients during the acute phase of the coronary disease. In this setting, it is possible that diastolic pressures and E/e′ ratio had a sudden increase, but there was not enough time for a decrease in atrial contraction, as such decrease also depends the atrial myocardial reserve.
Cameli et al. recruited 80 stable patients with sinus rhythm to explore the correlation between LAS, Doppler E/e′ ratio, and invasive measurements of LV end-diastolic pressure, in patients stratified for different grade of systolic function [15]. Regardless of the LVEF, LASR showed a strong correlation in all groups of patients (0.87 AUC for prediction of LV end-diastolic pressure > 12 mmHg). Although the study demonstrated results similar to ours, they did not evaluate the LASCD, which was the atrial component that best correlated with the E/e′ ratio > 14.
We believe that the use of LAS is a promising tool to classify LV diastolic dysfunction and might improve the accuracy of the EACVI algorithm for the detection of patients with elevated LV filling pressures. For instance, if we used LAS cut-off found in our study, we would be able to reclassify 71% of patients in the indeterminate group to the grade II or III DD group (with elevated LV end-diastolic pressure). This fact is in accordance with a recent study by Lancellotti et al. that compared the accuracy of the new EACVI guidelines [7] with the previous (2009) recommendations for grading DD. Even though a better performance of the new algorithm was observed, the accuracy was still moderate, with an AUC of 0.78 [22].
Therefore, accordingly to our data, there is an important association between LV diastolic dysfunction and left atrial performance, and the use of LAS for atrial evaluation may be helpful. However, further studies would be needed to assess whether the incorporation of atrial strain may actually improve the algorithm accuracy and also if atrial function implies in an incremental prognostic information.
Limitations
This paper presents the limitations that are inherent to every observational, cross-sectional unicentric study. Moreover, the relatively small number of patients could also represent a limitation, especially for the DD grade III group of our sample, as this was the group with the smallest number of subjects.
Evaluation of LAS by two-dimensional strain is not a routine part of echocardiographic tests yet, especially the bedside emergency procedures. Additionally, as it is a new technique, there is no commercially available software for specific LA evaluation. Thereby, as in most reported studies, we used a software designed for LV strain evaluation, and adapted it for atrial analysis.
Evaluation of left atrial volume was performed by two-dimensional method. Due to the complex LA geometry, studies have shown that its measurement by three-dimensional method is most accurate. Nevertheless, our institution did not have portable equipment with three-dimensional technology able to perform this type of test at bedside.
This study used only echocardiographic parameters to evaluate LV diastolic function. The absence of an invasive pulmonary capillary wedge pressure measurement is a limitation. Yet, our primary goal was to compare LAS data with traditional echocardiographic data to verify the actual performance of LAS on DD categorization. Nonetheless, we acknowledge that another study comparing LAS with invasive assessment of LV filling pressures is needed.
Conclusions
Based on the results found in our study, we can conclude that LAS presents excellent diagnostic capacity to categorize DD grade in patients with ACS, using the last EACVI guidelines as reference. The three components of left atrial function presented their best performance in identifying patients with DD grade III and patients with increased LV filling pressures.
References
Sanchis-Gomar F, Perez-Quilis C, Leischik R, et al. Epidemiology of coronary heart disease and acute coronary syndrome. Ann Transl Med. 2016;4:256–66.
Roffi M, Patrono C, Collet J-P, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2016;37:267–315.
Heyndrickx GR, Baig H, Nellens P, et al. Depression of regional blood flow and wall thickening after brief coronary occlusions. Am J Physiol. 1978;234:H653–9.
Cerisano G, Bolognese L, Carrabba N, et al. Doppler-derived mitral deceleration time: an early strong predictor of left ventricular remodeling after reperfused anterior acute myocardial infarction. Circulation. 1999;99:230–6 (American Heart Association, Inc).
Nagueh SFMD, Middleton KJ, Kopelen HA, et al. Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol. 1997;30:1527–33.
Hillis GS, Møller JE, Pellikka PA. Noninvasive estimation of left ventricular filling pressure by e/é is a powerful predictor of survival after acute myocardial infarction. ACC Curr J Rev. 2004;13:42.
Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2016;17:1321–60.
Almeida JG, Fontes-Carvalho R, Sampaio F, et al. Impact of the 2016 ASE/EACVI recommendations on the prevalence of diastolic dysfunction in the general population. Eur Heart J Cardiovasc Imaging. 2017;289:194.
Casaclang-Verzosa G, Gersh BJ, Tsang TSM. Structural and functional remodeling of the left atrium. J Am Coll Cardiol. 2008;51:1–11.
Leong DP, Penhall A, Perry R, et al. Speckle-tracking strain of the left atrium: a transoesophageal echocardiographic validation study. Eur Heart J Cardiovasc Imaging. 2013;14:898–905.
Sirbu C, Herbots L, Dhooge J, et al. Feasibility of strain and strain rate imaging for the assessment of regional left atrial deformation: a study in normal subjects. Eur J Echocardiogr. 2006;7:199–208.
Cameli M, Lisi M, Mondillo S, et al. Left atrial longitudinal strain by speckle tracking echocardiography correlates well with left ventricular filling pressures in patients with heart failure. Cardiovasc Ultrasound BioMed Cent. 2010;8:14.
Hsiao S-H, Chiou K-R, Porter TR, et al. Left Atrial parameters in the estimation of left ventricular filling pressure and prognosis in patients with acute coronary syndrome. Am J Cardiol. 2011;107:1117–24.
Wakami K, Ohte N, Asada K, et al. Correlation between left ventricular end-diastolic pressure and peak left atrial wall strain during left ventricular systole. J Am Soc Echocardiogr. 2009;22:847–51.
Cameli M, Sparla S, Losito M, et al. Correlation of left atrial strain and doppler measurements with invasive measurement of left ventricular end-diastolic pressure in patients stratified for different values of ejection fraction. Echocardiography. 2015;33:398–405 (5 ed).
Kurt M, Tanboga IH, Aksakal E, et al. Relation of left ventricular end-diastolic pressure and N-terminal pro-brain natriuretic peptide level with left atrial deformation parameters. Eur Heart J Cardiovasc Imaging. 2012;13:524–30.
Singh A, Addetia K, Maffessanti F, et al. LA Strain for categorization of LV diastolic dysfunction. JACC Cardiovascr Imaging. 2017;10:735–43.
Morris DA, Belyavskiy E, Aravind-Kumar R, et al. Potential usefulness and clinical relevance of adding left atrial strain to left atrial volume index in the detection of left ventricular diastolic dysfunction. JACC Cardiovasc Imaging. 2018;11(10):1405–15.
Granger CB. Predictors of hospital mortality in the global registry of acute coronary events. Arch Intern Med. 2003;163:2345.
Thygesen K, Chaitman BR, White HD, et al. Third universal definition of myocardial infarction. Eur Heart J. 2012;33(20):2551–67.
Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. European Heart J Cardiovascr Imaging. 2015;16:233–71.
Lancellotti P, Galderisi M, Edvardsen T, et al. Echo-Doppler estimation of left ventricular filling pressure: results of the multicentre EACVI Euro-Filling study. Eur Heart J Cardiovasc Imaging. 2017;18:961–8.
Ho SY, McCarthy KP, Faletra FF. Anatomy of the left atrium for interventional echocardiography. Eur J Echocardiogr. 2011;12:i11–5.
Vieira MJ, Teixeira R, Gonçalves L, et al. Left atrial mechanics: echocardiographic assessment and clinical implications. J Am Soc Echocardiogr. 2014;27:463–78.
Liu Y-T, Li R-J, Fang F, et al. Left atrial function assessed by tissue doppler imaging as a new predictor of cardiac events after non-ST-elevation acute coronary syndrome. Echocardiography. 2012;29:785–92 (Blackwell Publishing Inc).
Donal E, Behagel A, Feneon D. Value of left atrial strain: a highly promising field of investigation. Eur Heart J Cardiovasc Imaging. 2015;16:356–7.
Galderisi M, Rapacciuolo A, Esposito R, et al. Site-dependency of the E/e’ ratio in predicting invasive left ventricular filling pressure in patients with suspected or ascertained coronary artery disease. Eur Heart J Cardiovasc Imaging. 2013;14:555–61.
Acknowledgements
We would like to thank the Statistics and Epidemiology Laboratory of Dante Pazzanese Institute of Cardiology, especially João Ítalo Dias França MSc, a professional who contributed significantly to the analyses performed in this study.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
David Le Bihan and Rodrigo Barretto have been granted speaker honoraria from GE Vingmed (Brazil). Rafael M. Fernandes, Andrea A. Vilela, Elizabete S. Santos, Jorge E. Assef, Simone Rolim Fontes Pedra, Amanda G.M.R Sousa and Ari Timerman have no conflicts of interest.
Human rights statements and informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later revisions. Informed consent was obtained from all patients for being included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fernandes, R.M., Le Bihan, D., Vilela, A.A. et al. Association between left atrial strain and left ventricular diastolic function in patients with acute coronary syndrome. J Echocardiogr 17, 138–146 (2019). https://doi.org/10.1007/s12574-018-0403-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12574-018-0403-7