Abstract
Echocardiographic assessment of left ventricular (LV) filling pressures is performed using a multi-parametric algorithm. Left atrial (LA) strain was recently found to accurately classify the degree of diastolic dysfunction. We hypothesized that LA strain could be used as a stand-alone marker and sought to identify and test a cutoff, which would accurately detect elevated LV pressures. We studied 76 patients with a spectrum of LV function who underwent same-day echocardiogram and invasive left-heart catheterization. Speckle tracking was used to measure peak LA strain. The protocol involved a retrospective derivation group (N = 26) and an independent prospective validation cohort (N = 50) to derive and then test a peak LA strain cutoff which would identify pre-A-wave LV diastolic pressure > 15 mmHg. The guidelines-based assessment of filling pressures and peak LA strain were compared side-by-side against invasive hemodynamic data. In the derivation cohort, receiver-operating characteristic analysis showed area under curve of 0.76 and a peak LA strain cutoff < 20% was identified as optimal to detect elevated filling pressure. In the validation cohort, peak LA strain demonstrated better agreement with the invasive reference (81%) than the guidelines algorithm (72%). The improvement in classification using LA strain compared to the guidelines was more pronounced in subjects with normal LV function (91% versus 81%). In summary, the use of a peak LA strain to estimate elevated LV filling pressures is more accurate than the current guidelines. Incorporation of LA strain into the non-invasive assessment of LV diastolic function may improve the detection of elevated filling pressures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The noninvasive estimation of left ventricular (LV) filling pressures using echocardiography based criteria is frequently employed in the clinical diagnosis of heart failure. The recently published 2016 guidelines for the assessment of LV diastolic function [1] advocate a more concise approach to identifying patients with elevated filling pressures when compared to the 2009 version [2]. The primary revision to the guidelines update was to streamline the use of four variables into a single algorithm to characterize LV pressures. A subsequent multicenter study validated these guidelines against invasively determined LV filling pressures, demonstrating an overall accuracy of 87% [3]. While the 2016 algorithm has been simplified from previous iterations, it still employs multiple parameters, and cannot solve the diagnostic quandary of “indeterminate” status for a select group of patients whose data does not neatly fulfill the algorithm.
Left atrial (LA) strain during the reservoir phase is a marker of LA dysfunction, and has been shown to be reduced in the setting of diastolic LV dysfunction [4]. A growing number of studies have demonstrated that the progressive LA dysfunction implicated in a number of disease states, including heart failure with preserved EF, likely reflects the interplay of elevated LV filling pressures that eventuates LA dysfunction and leads to reduced peak LA strain. Our recent study showed that peak LA reservoir strain can be used to accurately categorize the severity of diastolic dysfunction [4]. While previous studies have shown that peak LA strain correlates well with LV end-diastolic pressure, as well as pulmonary capillary wedge pressure, no discrete cutoff of LA reservoir strain has been prospectively examined for clinical use [5, 6]. We sought to both identify and prospectively test a distinct peak LA reservoir strain cutoff as a stand-alone echocardiographic parameter to identify patients with elevated LV filling pressures using the gold-standard of invasive hemodynamic assessment.
Methods
Patients and study design
We prospectively studied a total of 76 patients referred for a clinically indicated left heart catheterization (including chest pain, acute coronary syndrome excluding ST-elevation myocardial infarction, transcatheter aortic valve replacement, preoperative evaluation, history of ventricular arrhythmia/cardiac arrest) with invasive hemodynamic assessment who also underwent transthoracic 2D echocardiography just prior to catheterization. Patients with atrial fibrillation or flutter, ≥ mild mitral or aortic valve regurgitation, mitral stenosis, heart transplant, sinus tachycardia, ≥ moderate pericardial effusion, poor image quality that would preclude adequate speckle tracking and prosthetic valves were excluded. The University of Chicago Medical Center Institutional Review Board approved the study protocol, and informed consent was obtained in each patient.
The study included a derivation of a single peak LA strain cutoff to identify elevated LV filling pressure (> 15 mmHg), which was then applied to an independent series of consecutive patients as a validation cohort, with comparison to the guidelines-based assessment of elevated LA pressure.
The derivation group of 26 patients (Table 1) was selected to ensure representation of a spectrum of LV ejection fraction (normal, mild, moderate and severely reduced by ASE guidelines) [7]. Pre-A-wave LV diastolic pressure was determined from invasive hemodynamic tracings optimized for scale. A receiver-operating curve (ROC) analysis was performed to identify the optimal peak LA strain cutoff, which would identify patients with LV filling pressure > 15 mmHg.
The validation cohort consisted of prospectively enrolled, consecutive series of 50 patients (50% female, age 61 ± 12 years, 68% with normal LV function). Each patient was categorized as having either normal or elevated LV filling pressure using the above LA strain cutoff obtained in the derivation cohort. The accuracy of this classification was tested against the invasive pre-A-wave LV diastolic pressure as the reference standard. In parallel, the accuracy of the 2016 guidelines algorithm was determined in the same patients against the same invasive reference standard for comparison. Subsequently, these comparisons were repeated for two subgroups of patients with normal (EF ≥ 50%) and reduced LV function, in order to determine the accuracy of this methodology in these subgroups.
Echocardiographic imaging and analysis
All echocardiograms were performed using commercial equipment (Philips EPIQ imaging system). Digital loops were stored and analyzed offline (Xcelera, Philips Healthcare). All traditional echocardiographic and LA strain measurements were performed by a board-certified echocardiographer blinded to invasive pressure data. Volumetric analysis of the LA and LV were performed using standard methodology recommended by the recent chamber quantification guidelines [7]. Pulsed-wave Doppler of the mitral inflow at the level of valve leaflet tips was used to measure the peak early (E-wave) and late (A-wave) diastolic flow velocities and calculate the E/A ratio. In addition, pulsed-wave tissue Doppler imaging was performed with the sample volume at the lateral and septal mitral annulus to obtain average peak longitudinal early diastolic annular (e′) velocity, which was used to calculate E/e′ ratio. Peak velocity of the tricuspid regurgitant jet was determined by continuous wave Doppler. The 2016 guideline document on echocardiographic assessment of diastolic function was utilized to determine LA pressures (normal, abnormal or indeterminate) using the recommended algorithm from the above four parameters: LA volume, E/A ratio, E/e′ ratio and peak velocity of the tricuspid regurgitant jet [1].
2D speckle tracking software (Epsilon, EchoInsight, Ann Arbor, MI) was used to trace the LA endocardial border in the apical 4-chamber view, as recommended by the EACVI/ASE/Industry Task Force to standardize deformation imaging [8] while taking care to exclude the LA appendage and pulmonary veins from the LA cavity, and a composite LA longitudinal reservoir strain curve throughout the cardiac cycle was generated (Video 1). This curve was comprised of six individual atrial segments. If more than one atrial segment had to be excluded from analysis because of suboptimal visualization and tracking (approximately 12% of patients in both the derivation/validation cohort), a different loop was selected to ensure as complete analysis as possible for each subject. The reference point for zero strain was set at LV end-diastole (i.e. beginning of the QRS complex in the EKG). Peak strain value was derived from the composite LA strain curve.
Invasive LV pressure measurements
Left heart catheterization was performed according to standard procedure by an interventional cardiologist blinded to echocardiographic data. Invasive LV pressure measurements were performed using a 6F pigtail catheter (Impulse™, Boston Scientific, Marlborough, MA) placed in the left ventricle. A fluid-filled transducer was balanced prior to the measurements with the zero level at the mid-axillary line. Continuous pressure tracings were acquired over 3 consecutive respiratory cycles. Pre-A-wave LV diastolic pressure, which best reflects the mean LA pressure was determined at end expiration and considered elevated if > 15 mmHg.
Reproducibility analysis
Reproducibility analysis included repeated measurements of peak LA strain in a randomly selected subgroup of 20 patients. These measurements were performed by two independent readers blinded to each other’s measurements and also to the invasive LV EDP pressure classifications, who analyzed the same cardiac cycle in each patient to eliminate the effects of intrinsic beat-to-beat variations. Measurement variability was expressed in terms of absolute difference between repeated measurements in percent of their mean value and intraclass correlation coefficients.
Statistical analysis
Continuous variables were expressed as mean ± SD, and categorical variables as numbers and percentages. Receiver operating characteristic (ROC) curve was constructed for peak LA strain values spanning the entire range of this variable, and area under curve (AUC) was obtained to assess its diagnostic performance. A threshold was then selected for peak LA strain to distinguish normal from elevated LV EDP, by maximizing the agreement with the invasive hemodynamic measurements in the derivation cohort (maximal overall accuracy). This optimal threshold was then applied to LA strain data obtained in the validation cohort to test the accuracy of this approach for detection of elevated filling pressures. Sensitivity, specificity, positive and negative predictive values (PPV, NPV) and overall accuracy were calculated from the numbers of true/false positive/negative classifications, using standard definitions. Contingency tables of normal and elevated pressure values by both echocardiographic techniques (LA strain and the guidelines based algorithm) and the invasive reference technique were created to evaluate inter-technique agreement, which was tested using kappa statistics (GraphPad software, Inc). The calculated kappa coefficients were judged as follows: 0–0.2 low, 0.21–0.4 moderate, 0.41–0.6 substantial, 0.61–0.8 good, and > 0.8 excellent. In addition, the significance of the difference in the frequency of discordant determinations between the two sets of echo parameters and the invasive reference was tested using Chi square tests for ratios.
Results
Of 89 total subjects who initially fit the inclusion criteria, 9 (10%) were excluded for poor image quality, either referring to image acquisition which failed to capture the totality of the left atrial geometry (mostly incomplete contours of the LA roof) or image quality which precluded speckle-tracking. Another 4 subjects were excluded on the basis of mitral regurgitation (≥ mild) alone. In the remaining patients, frame rate was 54 ± 9 Hz. Inter-reader variability was 13.5 ± 8% with an intra-class correlation of 0.89.
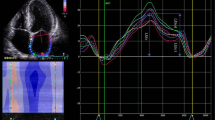
Figures 1, 2 and 3 depict the variations in the determination of pre-A-wave LV diastolic pressure by the different techniques. Figure 1 shows an example of data from a patient in whom both the guidelines algorithm and LA strain analysis resulted in normal LV EDP, in agreement with the invasive pressure measurement. Similarly, Fig. 2 shows an example of another patient in whom both methodologies determined that LV EDP was elevated, also in agreement with the invasive reference technique. In contrast, Fig. 3 shows an example where the guidelines algorithm was unable to determine whether LV EDP was normal or elevated, while LA strain analysis indicated a normal LV EDP, which was in concordance with the invasive measurement.
Example of data obtained in a patient in whom both the 2016 ASE Diastolic Guidelines algorithm (left and middle) and left atrial strain (LAS) analysis (bottom right, LAS < 20%) resulted in elevated LVEDP, both in agreement with the invasive pressure measurement (top right, LVEDP > 15 mmHg). Abbreviations as in Fig. 1
Example of data obtained in a patient in whom the 2016 ASE Diastolic Guidelines algorithm (left and middle) was unable to assess the degree of tricuspid regurgitation (TR, middle bottom), resulting in an “indeterminate” classification. In contrast, left atrial strain (LAS) analysis (bottom right, LAS > 20%) depicted normal LVEDP, in agreement with the invasive pressure measurement (top right, LVEDP < 15 mmHg). Abbreviations as in Fig. 1
Within the derivation group (Table 1), the mean EF was 47 ± 16%, with a balance of normal and elevated pre-A-wave LV diastolic pressure represented (mean 13.4 ± 7.6 mmHg; median 15 mmHg; range 1–30). The mean peak LA strain was 19.5 ± 9.7%. ROC analysis resulted in an AUC value of 0.76, reflecting good diagnostic performance. A cutoff value of < 20% was able to most accurately identify patients with LV EDP > 15 mmHg (Fig. 4).
Using the 2016 guidelines in the validation cohort, 3/50 (6%) of patients were categorized as having indeterminate filling pressures and were excluded from further analysis. In the remaining 47 subjects in the validation cohort, 26% had elevated pre-A-wave LV diastolic pressure. Using peak LA strain cutoff of 20%, we accurately determined filling pressure status in agreement with invasive measurements in 38 subjects (81%). In contrast, the 2016 guidelines demonstrated agreement with the invasive reference in 34 subjects (72%). The kappa coefficient for LA strain was 0.482, showing substantial agreement with the reference technique, which was significantly better than that of the 2016 guidelines with kappa coefficient of 0.302, indicating only moderate agreement. The sensitivity, specificity and accuracy of the LA strain cutoff were all higher than those of the 2016 guidelines (Table 2).
Figure 5 shows plots of individual data points of both peak LA strain (panel A) and LA volume index (LAVi) (panel B) against LV EDP with the dashed lines showing abnormality cutoffs for both variables, and thus dividing the plot area into 4 quadrants: two where the echocardiographic parameters agree with the invasive pressure measurements (true positives and negatives), and the other two where they do not (false positives and negatives). Peak LA strain showed good agreement with LV EDP (Fig. 5a) with a small number of points in the bottom left quadrant (n = 5; 10%), reflecting reduced strain despite normal pressure (false positives), and in the top right quadrant (n = 4; 8%), reflecting normal strain despite elevated pressure (false negatives). In contrast, LA volume (Fig. 5b) showed a larger number of points in the top left quadrant (false positives), reflecting enlarged volumes despite normal pressure (n = 15; 30%), while the number of points in the bottom right quadrant (n = 4; 8%), reflecting normal volumes despite elevated pressure (false negatives), was identical to the number of false negatives for LA strain.
a Scatter plot of all subjects comparing Peak LA strain (y-axis) and LV EDP (x-axis). Dotted lines denote the clinical cutoffs we employed to identify decreased LA strain (< 20%) and elevated LV filling pressure (LV EDP > 15 mmHg). b Scatter plot of all subjects comparing Peak LAVi (y-axis) and LV EDP (x-axis). Dotted lines denote the clinical cutoffs we employed to identify increased LAVi (> 34 mL/m2) and elevated LV filling pressure (LV EDP > 15 mmHg). See text for details. TN, TP true negative and true positive quadrants, FN, FP false negative and false positive quadrants
When the validation cohort was limited to subjects with normal LV function (> 55%), the agreement with the invasive reference improved to 91% for peak LA strain with a kappa coefficient of 0.711 indicating good agreement. The use of LA strain in the normal LV EF group resulted in 2 false negatives and one false positive detection of elevated LV EDP. While the 2016 guidelines performance was higher in this subgroup than in the entire validation cohort, with an agreement of 81% with the invasive reference, the kappa coefficient was 0.451, indicating substantial agreement only. Furthermore, the sensitivity, specificity, PPV and NPV of LA strain were higher than those of the guidelines algorithm in this subset of patients (Fig. 6). In subjects with reduced LV EF, the 2016 guidelines and LA strain did not perform as well, though agreement for LA strain with the invasive reference was higher than that observed for the guidelines algorithm (Table 2).
Contingency tables of agreement between the 2016 ASE Diastolic Guidelines (left) and left atrial strain analysis with a peak strain cutoff < 20% (right) for the identification of patients with normal and elevated filling pressures. Tables include the entire validation cohort (top), as well as subgroups of patients with normal left ventricular ejection fraction (EF; middle) and low EF (bottom). See text for details
Moreover, while the subjects for whom the guidelines categorized LV filling pressures as “indeterminate” were excluded from analysis of inter-technique agreement, we were able to obtain peak LA strain values in all cases, and two of the three patients were accurately categorized using peak LA strain as having normal LV EDP. Finally, in an order to estimate the added value of LA reservoir strain by adding it to the guidelines algorithm, accuracy analysis was performed for the combination of the guidelines algorithm with LA strain. We found that all accuracy metrics were identical to those obtained using LA strain alone.
Discussion
To our knowledge, this is the first study to test a discrete peak LA reservoir strain cutoff as a single parameter for use in the commonly encountered clinical question of whether elevated filling pressures are present against an invasive gold standard reference. Our previous report [4] described an exploratory retrospective study that established semi-qualitative relationship between diastolic dysfunction and LA reservoir strain. In contrast, the current report describes the results of a prospective validation study with an invasive quantitative reference standard.
Our results demonstrate that the use of a sole echo-derived peak LA reservoir strain cutoff value can accurately categorize subjects as having normal or elevated LV filling pressures. Furthermore, this approach showed better agreement with invasively determined pressures when compared to the 2016 guidelines. This incremental increase in accurate categorization was more apparent in the subjects with preserved LV function, in whom the accuracy of LA strain based noninvasive pressure assessment exceeded 90%.
One of the compelling advantages of peak LA strain as a clinical tool is that, with adequate image quality, it is a feasible “snapshot” of LA function reflected in the single discrete value it provides. This is in contrast to the major limitation of the current guidelines, which relates to the multiple variables required for the comprehensive conventional assessment of filling pressures and how frequently encountered conditions may hinder the evaluation of all necessary parameters. Theoretically, due to the technique inherent to its measurement, peak LA reservoir strain can overcome some of these scenarios in which acquisition of Doppler parameters are complex or equivocal. This includes situations like mitral annular calcification, the presence of mitral prostheses, or tachycardia, all of which may obscure or interfere with the ability to discern a medial or lateral e′ value; insufficient or incomplete interrogation of tricuspid regurgitation jets; or patients for whom the available algorithm classifies as indeterminate. By inclusion criteria, our study mandated feasible LA strain tracings for analysis; we estimate feasibility of LA strain to be over 90% based upon our previous work [4]. The view required for LA strain analysis is the commonly acquired apical 4-chamber view, with optimization of the LA axis (Video 1). Our study indicates that LA strain is a high feasibility marker, utilizing a frequently used view for acquisition, measurable in every patient with adequate image quality, and furthermore these measurements never lead to indeterminate classification of filling pressures.
There is a growing body of data supporting the clinical application of LA reservoir strain as a diagnostic and prognostic marker across a spectrum of cardiovascular disease, including atrial fibrillation, heart failure with preserved EF, mitral valve disorders, and categorization of diastolic dysfunction [4, 9,10,11,12,13]. It has been proposed that peak LA strain provides incremental insight beyond typical measures of remodeling, namely LA enlargement, a finding which in itself accompanies a number of cardiovascular diseases [9, 14]. Indeed, our results support this notion, as LAVi > 34 mL/m2 was able to identify subjects with elevated LV filling pressures, but at the cost of a higher false positive rate compared to peak LA strain (Fig. 5b).
Why alterations in peak LA reservoir strain may result from elevated LV filling pressures can be understood if we examine the interaction between the left atrium and the left ventricle throughout the cardiac cycle. As LV pressures and/or stiffness increase, the left atrium is chronically exposed to higher pressures that lead to dilatation. These transmitted pressures may blunt the compliance of the left atrium, impairing atrial relaxation and thereby reducing the ability of the atrium to act as a reservoir in ventricular systole. Several recent studies suggested that, in the setting of increased LV pressures, LA function is already compromised before the LA starts to dilate, and this blunted atrial function may be demonstrated by reduced LA reservoir strain in patients with normally-sized atria [15,16,17]. Often, the clinical puzzle of determining elevated filling pressures is influenced by the degree of LV dysfunction, as there is frequently a higher suspicion of elevated LVEDP in patients with reduced LV EF. However, in these patients with reduced LV function, the presence and extent of LA dysfunction is more likely to be preexisting and significant, as well. This phenomenon was reflected in our findings, in which peak LA strain was less accurate in characterizing LV filling pressures in patients with LV dysfunction than in patients with normal systolic function. This seems to illustrate that in the longstanding and marked LA dysfunction which occurs at baseline in the setting of LV dysfunction, LA strain is perhaps a less reliable index.
However, the converse of this finding was evident in the improved performance of LA reservoir strain in the cohort with preserved LV EF. Although elevated filling pressures in the setting of normal LV function may be less frequently suspected, accurate diagnosis of this condition is of increasing importance given the growing epidemic of heart failure with preserved EF, in which patients are symptomatic and LA dysfunction has been implicated as a major contributor to symptomatology [9, 18]. Furthermore, the previously demonstrated relationship between progressive diastolic dysfunction and worsening LA strain demonstrated by our group suggests that with preserved EF, the associated alterations in LA strain may provide timely reflections of dynamic changes in filling pressures and concomitant cardiac mechanics [4].
It is noteworthy that in our study group, the accuracy of the guidelines recommended algorithm was lower (72%) than that recently reported by Andersen and colleagues (87%) [3]. This disparity likely stems from the differences in the percentage of patients with normal versus reduced LV EF between the two studies, since in both studies, LV function was shown to affect the accuracy of the guidelines algorithm. Importantly, however, LA strain determination of elevated filling pressure was more accurate than the guidelines algorithm in both subgroups stratified by LV EF.
The argument that LA dysfunction simply “mirrors” LV processes has been challenged by a recent study, the results of which demonstrated that while changes in LA reservoir and conduit function were associated with LV function, LA booster function was not [19]. There are components of LA strain which are unique reflections of the LA-LV coupling, and with further research, we may be better equipped to utilize this index in common clinical scenarios where atrial enlargement exists, and diastolic dysfunction is suspected.
It is important to note that we do not advocate for the routine usurpation of LV filling pressure assessment by LA reservoir strain alone, because of the limited size of our study. However, we do feel that it has potential for final adjudication of filling pressures in subjects with normal LV function. In fact, this idea has been recently explored by Morris et al. in a study suggested that adding LA strain to LAVi in may be helpful in the diagnosis of diastolic dysfunction on patients with preserved LV EF [20]. Some scenarios that come to mind, where LS strain may indeed be useful include indeterminate diastolic function, or patients in whom the inability to assess the major diastolic parameters is limited, e.g. lack of/incomplete tricuspid regurgitation jet, tachycardia obscuring mitral annular tissue Doppler tracing, etc. It would be interesting to examine the outcomes of LA strain values in a population of patients with “indeterminate diastolic function”, particularly if invasive filling pressures are available.
Limitations
There are limitations to note in our study. This was a single center study that excluded patients with poor quality images, atrial fibrillation, or severe mitral regurgitation; both of these factors may limit the generalizability of results. Also, the invasive and non-invasive measurements of filling pressure were not obtained simultaneously, although we tried to limit the interval between these measurements to no more than a few hours. We also employed ultrasound equipment from a single vendor for measurement of peak LA strain, and thus our reported threshold of LA strain for determination of filling pressures may be vendor-specific. However, the ongoing efforts that are underway to unify and standardize strain imaging techniques, which until now have focused on ventricular strain, will aid in integrating strain measurement for clinical use across a spectrum of laboratories and vendors [8].
Conclusions
Our results suggest that there is a role for LA reservoir strain in the noninvasive assessment of elevated left heart filling pressures, with particularly promising results in patients with normal EF. These results suggest that the addition of LA strain to the clinical guidelines may be beneficial for clinical decision making.
Abbreviations
- AUC:
-
Area under curve
- EDP:
-
End-diastolic pressure
- EF:
-
Ejection fraction
- LA:
-
Left atrial
- LAVi:
-
Left atrial volume index
- LV:
-
Left ventricular
- NPV:
-
Negative predictive value
- PPV:
-
Positive predictive value
- ROC:
-
Receiver-operating characteristic
References
Nagueh SF, Smiseth OA, Appleton CP, Byrd BF III, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Popescu BA, Waggoner AD (2016) Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr 29:277–314
Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A (2009) Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr 22:107–133
Andersen OS, Smiseth OA, Dokainish H, Abudiab MM, Schutt RC, Kumar A, Sato K, Harb S, Gude E, Remme EW, Andreassen AK, Ha JW, Xu J, Klein AL, Nagueh SF (2017) Estimating left ventricular filling pressure by echocardiography. J Am Coll Cardiol 69:1937–1948
Singh A, Addetia K, Maffessanti F, Mor-Avi V, Lang RM (2017) LA strain categorization of LV diastolic dysfunction. JACC Cardiovasc Imaging 10:735–743
Hewing B, Theres L, Spethmann S, Stangl K, Dreger H, Knebel F (2017) Left atrial strain predicts hemodynamic parameters in cardiovascular patients. Echocardiography 34:1170–1178
Kurt M, Wang J, Torre-Amione G, Nagueh SF (2009) Left atrial function in diastolic heart failure. Circulation 2:10–15
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr 28:1–39 e14
Voigt JU, Pedrizzetti G, Lysyansky P, Marwick TH, Houle H, Baumann R, Pedri S, Ito Y, Abe Y, Metz S, Song JH, Hamilton J, Sengupta PP, Kolias TJ, d’Hooge J, Aurigemma GP, Thomas JD, Badano LP (2015) Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/industry task force to standardize deformation imaging. J Am Soc Echocardiogr 28:183–193
Freed BH, Daruwalla V, Cheng JY, Aguilar FG, Beussink L, Choi A, Klein DA, Dixon D, Baldridge A, Rasmussen-Torvik LJ, Maganti K, Shah SJ (2016) Prognostic utility and clinical significance of cardiac mechanics in heart failure with preserved ejection fraction: importance of left atrial strain. Circ Cardiovasc Imaging 9:e004521
Obokata M, Negishi K, Kurosawa K, Tateno R, Tange S, Arai M, Amano M, Kurabayashi M (2014) Left atrial strain provides incremental value for embolism risk stratification over CHA(2)DS(2)-VASc score and indicates prognostic impact in patients with atrial fibrillation. J Am Soc Echocardiogr 27:709–716
Yasuda R, Murata M, Roberts R, Tokuda H, Minakata Y, Suzuki K, Tsuruta H, Kimura T, Nishiyama N, Fukumoto K, Aizawa Y, Tanimoto K, Takatsuki S, Abe T, Fukuda K (2015) Left atrial strain is a powerful predictor of atrial fibrillation recurrence after catheter ablation: study of a heterogeneous population with sinus rhythm or atrial fibrillation. Eur Heart J Cardiovasc Imaging 16:1008–1014
Santos AB, Roca GQ, Claggett B, Sweitzer NK, Shah SJ, Anand IS, Fang JC, Zile MR, Pitt B, Solomon SD, Shah AM (2016) Prognostic relevance of left atrial dysfunction in heart failure with preserved ejection fraction. Circ Heart Fail 9:e002763
Yang LT, Liu YW, Shih JY, Li YH, Tsai LM, Luo CY, Tsai WC (2015) Predictive value of left atrial deformation on prognosis in severe primary mitral regurgitation. J Am Soc Echocardiogr 28:1309–1317
Huynh QL, Kalam K, Iannaccone A, Negishi K, Thomas L, Marwick TH (2015) Functional and anatomic responses of the left atrium to change in estimated left ventricular filling pressure. J Am Soc Echocardiogr 28:1428–1433
O’Connor K, Magne J, Rosca M, Pierard LA, Lancellotti P (2011) Left atrial function and remodelling in aortic stenosis. Eur J Echocardiogr 12:299–305
Morris DA, Takeuchi M, Krisper M, Kohncke C, Bekfani T, Carstensen T, Hassfeld S, Dorenkamp M, Otani K, Takigiku K, Izumi C, Yuda S, Sakata K, Ohte N, Tanabe K, Osmanoglou E, Kuhnle Y, Dungen HD, Nakatani S, Otsuji Y, Haverkamp W, Boldt LH (2015) Normal values and clinical relevance of left atrial myocardial function analysed by speckle-tracking echocardiography: multicentre study. Eur Heart J Cardiovasc Imaging 16:364–372
Pessoa-Amorim G, Mancio J, Vouga L, Ribeiro J, Gama V, Bettencourt N, Fontes-Carvalho R (2018) Impaired left atrial strain as a predictor of new-onset atrial fibrillation after aortic valve replacement independently of left atrial size. Rev Esp Cardiol 71:466–476
von Roeder M, Rommel KP, Kowallick JT, Blazek S, Besler C, Fengler K, Lotz J, Hasenfuss G, Lucke C, Gutberlet M, Schuler G, Schuster A, Lurz P (2017) Influence of left atrial function on exercise capacity and left ventricular function in patients with heart failure and preserved ejection fraction. Circ Cardiovasc Imaging 10:e005467
Ramkumar S, Yang H, Wang Y, Nolan M, Negishi T, Negishi K, Marwick TH (2017) Association of the active and passive components of left atrial deformation with left ventricular function. J Am Soc Echocardiogr 30:659–666
Morris DA, Belyavskiy E, Aravind-Kumar R, Kropf M, Frydas A, Braunauer K, Marquez E, Krisper M, Lindhorst R, Osmanoglou E, Boldt LH, Blaschke F, Haverkamp W, Tschope C, Edelmann F, Pieske B, Pieske-Kraigher E (2017) Potential usefulness and clinical relevance of adding left atrial strain to left atrial volume index in the detection of left ventricular diastolic dysfunction. JACC Cardiovasc Imaging. https://doi.org/10.1016/j.jcmg.2017.07.029
Acknowledgements
AS was supported by funding from the NIH T32 Training Grant (#5T32HL7381).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial disclosures or conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Video 1
. Speckle tracking analysis of left atrial strain. After left atrial boundary is traced manually (left), it is automatically tracked throughout the cardiac cycle, and longitudinal strain is calculated for each consecutive frame in 6 atrial segments. Strain over time is displayed as 6 time curves, color coded the same way as the segmental boundaries to facilitate evaluation of the boundary tracking in each segment (top right). (MOV 2988 KB)
Rights and permissions
About this article
Cite this article
Singh, A., Medvedofsky, D., Mediratta, A. et al. Peak left atrial strain as a single measure for the non-invasive assessment of left ventricular filling pressures. Int J Cardiovasc Imaging 35, 23–32 (2019). https://doi.org/10.1007/s10554-018-1425-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-018-1425-y