Abstract
In order to obtain accurate diagnosis, treatment and prognostication in many cardiac conditions, there is a need for assessment of left ventricular (LV) filling pressure. While systole depends on ejection function of LV, diastole and its disturbances influence filling function and pressures. The commonest condition that represents the latter is heart failure with preserved ejection fraction in which LV ejection is maintained, but diastole is disturbed and hence filling pressures are raised. Significant diastolic dysfunction results in raised LV end-diastolic pressure, mean left atrial (LA) pressure and pulmonary capillary wedge pressure, all referred to as LV filling pressures. Left and right heart catheterization has traditionally been used as the gold standard investigation for assessing these pressures. More recently, Doppler echocardiography has taken over such application because of its noninvasive nature and for being patient friendly. A number of indices are used to achieve accurate assessment of filling pressures including: LV pulsed-wave filling velocities (E/A ratio, E wave deceleration time), pulmonary venous flow (S wave and D wave), tissue Doppler imaging (E′ wave and E/E′ ratio) and LA volume index. LA longitudinal strain derived from speckle tracking echocardiography (STE) is also sensitive in estimating intracavitary pressures. It is angle-independent, thus overcomes Doppler limitations and provides highly reproducible measures of LA deformation. This review examines the application of various Doppler echocardiographic techniques in assessing LV filling pressures, in particular the emerging role of STE in assessing LA pressures in various conditions, e.g., HF, arterial hypertension and atrial fibrillation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background
Evaluation of left ventricular (LV) filling pressure is an essential step for accurate diagnosis, therapy and prognosis in patients with various cardiac diseases, particularly those with heart failure (HF). In recent years, increasing attention has been given to such patients particularly those with preserved ejection fraction (EF) (typically >50 %) and maintained LV cavity size, but still diagnosed with HF based on typical signs and symptoms in association with a diastolic dysfunction and a relevant structural heart disease (LV hypertrophy, left atrial enlargement) [1]. This subset of patients suffers from elevated morbidity and mortality [2, 3], not less than those with reduced LV EF [4].
Several studies have demonstrated that the incidence of HF with preserved ejection fraction (HFpEF) is increasing in the population and a significant proportion of hospitalized patients shows this variant of HF. They are mainly older women with a history of arterial hypertension [5], but obesity, CAD, diabetes mellitus, atrial fibrillation and dyslipidemia are also frequently met risk factors [6]. It has been suggested that these subjects might represent the largest cohort of patients with a cardiovascular disorder that is of substantial public health impact, who have not been systematically studied [7]. The pathophysiological mechanisms that convert these patients from asymptomatic to symptomatic HF remain undefined [8]. Furthermore, patients who show a LV systolic dysfunction frequently have some elements of diastolic dysfunction as well [9], thus adding to the relevance of accurately defining diastolic disturbances that are characteristics for HF in its two entities.
The diastolic dysfunction includes altered LV relaxation, reduced compliance with or without raised filling pressures [10]. Regardless of the etiology, an abnormality in diastolic function can result in an increase in LV end-diastolic pressure (LVEDP), mean left atrial (LA) pressure and pulmonary capillary wedge pressure (PCWP). All these pressures are commonly referred to as LV filling pressures. During diastole, there is a direct continuity between the pulmonary capillary bed, the pulmonary veins, the LA and the LV. Therefore, a pressure rise in the LV leads to an increase in LA and pulmonary capillary pressures and eventually pulmonary venous congestion and HF symptoms. Consequently, accurate assessment of diastolic function can be used to estimate LV filling pressure [11].
Invasive estimation of LV filling pressure
The invasive left and right heart catheterization has been considered the gold standard investigation for confirming or refuting the presence of high LV filling pressures with a LVEDP greater than 16 mmHg or a mean PCWP greater than 12 mmHg suggestive of diastolic dysfunction [12]. In particular, PCWP turned out to be directly associated with functional capacity and prognosis in patients with HF [13–15]. However, the cardiac catheterization is a technique that is not without risks, often poorly accessible and utilizable especially in the acute setting and outpatients services. Therefore, it would be desirable to restrict its use to already diagnosed patients who developed unexplained symptoms or signs.
Noninvasive estimation of LV filling pressure
Currently, echocardiography has been shown the only noninvasive investigation that allows accurate estimation of LV filling pressures as well as evaluating LV and LA pathophysiological changes that associate diastolic dysfunction. Several echocardiographic indices have been proposed for this purpose (Table 1).
Pulsed-wave Doppler mitral flow velocity
The most widespread echocardiographic technique for assessment of LV filling pressure is the pulsed-wave Doppler of mitral flow velocities. It includes the following variables: peak early diastolic flow velocity (E wave, referred to as rapid filling), peak late diastolic flow velocity (A wave, related to atrial contraction), E wave deceleration time (DT) and the total duration of the A wave (A dur). Normally with physiological filling pressures, a rapidly accelerating E wave is followed by a significantly smaller A wave with an E/A ratio greater than 1 and a DT between 160 and 240 ms. However, with normal aging, it is common for the E wave to be reduced due to a slowing of LV relaxation, resulting in equalization of early and late diastolic filling velocities in the sixth decade of life [16]. In patients with diastolic dysfunction, three different abnormal filling patterns are recognized: impaired relaxation (grade 1), characterized by a low E wave velocity and a high A wave velocity, causing E/A ratio to fall below 1 and E wave deceleration to prolong (>240 ms); pseudonormal pattern (grade 2), is associated with a normal appearance of the transmitral flow (E/A between 0.8 and 1.5, with normalized DT); restrictive pattern (grade 3), with marked elevation of E wave, low A wave, E/A usually greater than 2 and very shortened DT (<160 ms). LV filling is normally affected by preload, so the Valsalva maneuver represents an additional supplement for testing them: In patients with grade 2 pattern, the maneuver lowers LA pressure and unmasks impaired relaxation with E/A ratio <1. In subjects with early, still reversible, restrictive pattern, Valsalva maneuver can revert filling pattern to a pseudonormal or even impaired relaxation one. It should be remembered that in patients not in sinus rhythm, the A wave will be absent. Other conditions in which the A wave is not seen are the summation filling where a fusion between E and A waves occurs, which makes accurate identification of the predominant filling wave difficult. An example of the latter is patients with sinus tachycardia, prolonged PR interval and intraventricular dyssynchrony. It has been shown that restrictive LV filling pattern represents a powerful predictor of mortality, independent from LVEF and age, in patients with HF [2]. Moreover, studies have reported a strong correlation between DT and LV filling pressure, comparing Doppler mitral flow velocity and PCWP obtained by cardiac catheterization [17, 18], but only in patients with reduced EF [19].
Pulmonary venous flow
Another parameter, used to integrate LV filling velocities in assessing severity of diastolic dysfunction, is the pulmonary venous flow. Four main measurements are used: peak systolic velocity (S), peak diastolic velocity (D), peak atrial reversal flow velocity (PVa) and PVa duration (A dur). Normally, pulmonary venous flow goes from the PV to the LA in systolic and early diastole, with a small component reversing back during atrial contraction, in late diastole. When the LV and, consequently, LA pressure increase, most of the anterograde flow occurs in diastole with a reduction in S velocity, which becomes smaller than D velocity, thus reversing the S/D ratio. Furthermore, it has been demonstrated that a PVa greater than 35 cm/s with a duration longer than A dur by 30 ms or more, is predictive of raised LVEDP greater than 15 mmHg [20, 21]. It must be mentioned that pulmonary venous flow recordings are not so easy to obtain in all patients though, because of habitus and operator experience. It may be also affected by other factors including significant mitral regurgitation and atrial fibrillation.
Tissue Doppler imaging
Tissue Doppler imaging (TDI) has been widely used as an accurate tool for the assessment of LV diastolic function noninvasively. It is obtained from the apical four- or two-chamber view by placing the pulsed-wave sample at the junction between LV wall and the mitral annulus, in septal (or medial) and lateral position. Three waves are displayed in each cardiac cycle: systolic wave (S′), early diastolic wave (E′) and atrial contraction wave, at the end of diastole (A′). Normally, the lateral velocity is higher than the septal one, but the two values can be averaged. E′ represents the main TDI parameter, and it is considered normal if the septal one is ≥8 cm/s and the lateral one ≥10 cm/s. If diastolic function is normal, E′ is higher than A′ and their ratio, like the transmitral flow velocities, decreases in mild dysfunction (E′/A′ < 1), but E′ continues to decrease with worsening segmental myocardial function. It must be remembered that in patients with normal filling pressures, E′ determines transmitral flow pattern, and in patients with raised filling pressures, the E′ wave remains small with respect to the spectral velocity [22]. E′ has been shown to correlate with Tau, an invasive index of myocardial relaxation which is obtained from heart catheterization [23].
In addition to absolute measurement of E′, the E/E′ ratio may be used to predict LV filling pressures and has been shown to correlate with PCWP in a large variety of cardiac conditions [24–29]. In normal LV relaxation and normal LV filling pressure, both E and E′ are elevated. With impaired relaxation and normal LV pressures, both E and E′ are depressed, and in subjects with impaired relaxation and elevated filling pressures, E is high but E′ is reduced. An E/E′ ratio less than 8 is considered normal, while a ratio of 13 or greater represents an evidence of diastolic dysfunction. Values between 8 and 13 represent a gray zone in which more investigations are necessary, including mitral flow E/A ratio and its reduction with Valsalva maneuver, DT, pulmonary venous reversal flow duration compared with spectral A wave duration, as described above [30]. Dini et al. have proposed a model for noninvasive estimation of LV filling pressure using all these parameters, in patients with LV dysfunction and a wide range of EF. Averaged E/E′ ratio ≥13 or E wave deceleration time <150 ms was closely associated with elevated LV filling pressures. Alternatively, prediction of PCWP > 15 mmHg requires E/E′ ratio between 8 and 15, LA volume index (another parameter that reflects LV filling pressures, as explained later) >40 ml/m2 or difference in duration of pulmonary venous and mitral flow at atrial contraction >30 ms and estimated pulmonary artery systolic pressure >35 mmHg [31]. Nevertheless, the E/E′ ratio accuracy is still uncertain in patients with advanced HF and extensive LV remodeling, which frequently are not included in the studies. In fact, it has been demonstrated that, in advance decompensated HF, this parameter is less reliable in predicting filling pressures than previously reported in studies, especially with very low cardiac output and elevated LV volumes. Moreover, it is strongly influenced by the presence of cardiac resynchronization therapy or other treatments, like inotropic medications which may have independent effects on mitral annular motion [32]. It is well known how several conditions may distort the velocity of the E′ value and of the E/E′ ratio: mitral annular calcifications, surgical rings, prosthetic valves, several mitral regurgitation, atrioventricular or intraventricular conduction delay.
LA volume index
Transthoracic echocardiogram is the gold standard approach for assessing LA size. LA volume, and in particular LA volume index (LA volume indexed to body surface area), is a more robust marker of cardiovascular events over and above LA area or diameter [33]. The American Society of Echocardiography recommends the measurement of LA volumes by ellipsoid model and Simpson’s method in four- and two-chamber apical views [34]. A LA volume index >34 mL/m2 is considered abnormal and represents an independent predictor of mortality, HF, atrial fibrillation and ischemic stroke [35].
LA volume index has been shown also to correlate with the other parameters of diastolic function [36]. However, it must be remembered that while E/E′ ratio is an instant measurement of LV filling pressure, LA volume index reflects the cumulative effect of the filling pressures over time. Therefore, since LA enlargement is seen in several conditions, even in the absence of ventricular disease (atrial flutter or fibrillation, mitral valve diseases, anemia and other high-output states, etc.), it has a low specificity. Therefore, it should always be considered in conjunction with patient’s clinical status, other chambers’ volume and Doppler parameters of LV relaxation [30].
Speckle tracking echocardiography: left atrial strain
Speckle tracking echocardiography (STE) is a new non-Doppler-based method for the objective quantification of myocardial deformation from standard bidimensional data acquisitions, which presents significant feasibility and reproducibility [37–40]. In contrast to Doppler-derived indexes previously analyzed, STE has several advantages being angle-independent and less affected by reverberations, side lobes and dropout artifacts. Although STE was originally developed to study ventricular function, it has been recently used to evaluate atrial chambers function as well: The atrial longitudinal strain represents an excellent parameter, useful for analysis of LA function in several conditions.
STE measurements
Two-dimensional strain imaging is an echocardiographic technique that utilizes standard B-mode images for speckle tracking analysis. The speckle pattern (acoustic backscatter generated by the reflected ultrasound beam) is followed frame-by-frame, identifying the best matching area by a statistical approach. The displacement of this pattern is considered to follow myocardial movement, and every change between speckles is evaluated as a myocardial deformation [41].
For speckle tracking analysis, LA apical four- and two-chamber view images are obtained using conventional two-dimensional gray scale echocardiography, with an emphasis on the distinction between myocardium and extracardiac structures, during a brief breath hold and with a stable ECG recording. Three consecutive heart cycles are recorded and averaged. The recommended frame rate is set between 60 and 80 frames per second [37]. Recordings are processed using an acoustic-tracking software (Echo Pac, GE, USA), allowing off-line semi-automated analysis of speckle-based strain.
The LA endocardium surface is manually traced in both four- and two-chamber views by a point-and-click approach. An epicardial surface tracing is automatically generated by the system, and a region of interest (ROI) is identified. To trace the ROI in the discontinuities of LA wall, corresponding to pulmonary veins and LA appendage, the direction of LA endocardial and epicardial surfaces at the junction with these structures is extrapolated. The ROI can be manually adjusted in width and shape; then, the software divides it into six segments, and the resulting tracking quality for each segment is automatically scored as either acceptable or nonacceptable, with the possibility of further additional corrections. Segments, in which no adequate images quality can be obtained, are rejected by the software and automatically excluded from the analysis. In patients with adequate image quality, a total of 12 segments are then analyzed [37], and for each of these segments, the software generates the longitudinal strain curves together with a mean curve of all segments that reflects the pathophysiology of LA function.
Since the LA fills and stretches during the reservoir phase, the atrial strain increases, reaching a positive peak at the end of atrial filling, before the opening of the mitral valve. After opening of the valve, LA empties quickly and its volume falls so the strain initially decreases, up to a plateau corresponding to the phase of diastasis; then, it is followed by a second positive peak, less than the first, which corresponds to the period preceding atrial contraction (present only if patients are in sinus rhythm) and finally by a negative peak after the atrial contraction [37].
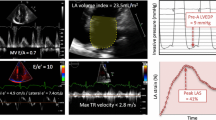
Peak atrial longitudinal strain (PALS) is measured at the end of reservoir phase, and peak atrial contraction strain (PACS) is measured just before the start of active atrial contractile phase (Fig. 1); the values obtained in four- and two-chamber views are then averaged (four- and two-chamber average PALS and PACS, respectively).
Measurement of peak atrial longitudinal strain (PALS) at the end of reservoir phase and peak atrial contraction strain (PACS) before the start of atrial systole. The dashed curve is representative of the average atrial longitudinal strain during the cardiac cycle [65]
LA contraction strain index (CSI), representing in percentual values the contribution of the LA active contraction to the LV filling phase, can be calculated as: \(({\text{global}}\,{\text{PACS}}/{\text{global}}\,{\text{PALS}}) \times 100\). The time to peak longitudinal strain (TPLS) is also measured as the average of all 12 segments (global TPLS) and by separately averaging values observed in the two apical views (four- and two-chamber average TPLS) [42].
In the current ASE/EAE consensus [43], two techniques have been proposed to quantify atrial deformation by STE, regarding the measurement of peak atrial strain. They differ only by choice of frame from which processing software starts: The first takes the QRS onset as the reference point and measures the positive peak atrial longitudinal strain, corresponding to atrial reservoir. The second uses the P wave as the reference point, thus enabling the measurement of a first negative peak atrial longitudinal strain, corresponding to atrial systole, and a second positive peak atrial strain, corresponding to LA conduit function, and their sum (Fig. 2).
Two different techniques and reference points (QRS, above, and P wave, below) proposed in ASE/EAE consensus for the measurement of global atrial deformation by STE [43]
Estimation of LV filling pressures
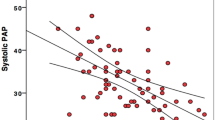
LA longitudinal strain has been proposed as an alternative approach for LV filling pressure assessment (Fig. 3). PALS has been shown to have excellent sensitivity and specificity in predicting a PCWP of 18 mmHg or above in patients with HF, despite having no relationship between mean E/E′ ratio and PCWP. In particular, a PALS cutoff value lower than 15.1 % results in the highest diagnostic accuracy [44]. Recently, a new study [45] explored the correlation between LA longitudinal function by STE, Doppler E/E′ ratio and invasive measurements of LVEDP in patients, stratified for different values of EF. Between all echocardiographic parameters analyzed, global PALS, with a cutoff value of 18.0 %, had the greatest diagnostic accuracy in predicting a LVEDP above 12 mmHg. In subjects with preserved (>55 %) or mildly reduced (45–54 %) LV EF, mean E/E′ ratio had good correlation with LVEDP, while in those with moderate (30–44 %) and severe (<30 %) LV EF, E/E′ ratio poorly correlated with LV filling pressure. In contrast, global PALS showed a strong correlation in all four groups of patients, even with low EF. The potential mechanism for the inverse relationship between global PALS value and high LV filling pressure could be explained by the principle that LVEDP is the afterload of LA function; hence, LVEDP is elevated, the LA could be mechanically stressed, and its reservoir function reduced, hence inducing a progressive LA dilation [46–48].
Furthermore, it has been demonstrated that LA strain at the end of ventricular systole is closely related not only to LVEDP, but also to NT-pro-BNP plasma levels [49]: Natriuretic peptides and in particular BNP (brain or B-type natriuretic peptide) and his aminoterminal prohormone, are produced by atrial and ventricular cardiomyocytes in response to mechanical stress. Plasma concentration of BNP is well correlated with LVEDP in patients with LV hypertrophy and systolic or diastolic HF [50, 51], so a combination of this parameter and LA strain could be used to increase the accuracy of noninvasive diagnostics of patients with HF.
LA stiffness index
The presence of atrial fibrosis [42, 52–54] and other ultrastructural alterations, often secondary to a chronic increase in LV filling pressure, likely influences the LA distensibility, increasing LA stiffness, and this finally could represent an anatomical substrate for the development of atrial arrhythmias [55] and a major risk of HF [56, 57]. Stiffness is defined as “the force required to displace a passive spring a unit length.” Physiologically, it is the change in pressure required to increase the volume of a passive container a unit amount. The units of strain in physiology are mmHg/mL [58]. The ratio of invasively measured PCWP and left atrial systolic strain is used to estimate LA stiffness. Alternatively, E/E′ ratio (related to LVEDP) can be used instead of PCWP in conjunction with atrial strain as a noninvasive measure of the same parameter. LA stiffness index is elevated in patients with diastolic dysfunction and is highest in subjects with HF [59].
Clinical implication of LA longitudinal strain
In the context of HF, a reduced atrial strain has been demonstrated to differentiate between subjects with diastolic dysfunction from those who already suffer from HFpEF [59]. In addition, it has been observed that, in patients with new-onset symptoms of HF, LA dysfunction is frequently present which could represent the primum movens of the clinical manifestations of HFpEF. Therefore, evaluation of LA strain in patients with new-onset dyspnea may be extremely useful for a correct diagnosis of HF and for the demonstration of a possible reversibility of LA dysfunction and symptoms with therapy [60]. In addition, LA strain has a strong negative correlation with NYHA class [61, 62].
Subjects who undergo heart transplant (HTX) are strictly monitored with periodically invasive measurements of PCWP. Recently, in pediatric HTX patients, PALS resulted to be a great noninvasive surrogate of cardiac catheterization, better than E/E′ ratio, with a good reproducibility [63]. Moreover, it has been demonstrated how, in HTX with bicaval technique (in which the right atrium is whole donor tissue and LA is from both donor and recipient tissue), LA strain is reduced related to high PCWP and elevated LA volume [64].
Studies have already shown early progressive reduction in LA strain in hypertensive patients [65] with diastolic dysfunction even before the appearance of LA enlargement and other structural alteration [66]. This adverse pattern is more evident in nondipper hypertensive patients (that is subjects in which daytime ambulatory systolic and diastolic blood pressure do not decrease by at least 10 % during the night) [67] and may reflect the raised LV pressure secondary to the systemic pressure overload that leads to increased LA stiffness during ventricular systole and to an abnormal LA–LV coupling [68], although other factors may also be involved. Similar aspect could be demonstrated in diabetic patients and even more in subjects with coexisting hypertension and diabetes [69–71]. Whether renin-angiotensin system (RAS) inhibitors have positive effect on LA myocardium in these hypertensive subjects is still unknown; however, patients treated with this class of drugs usually show improved atrial strain even after few months of therapy, whereas standard LA echocardiographic parameters (e.g., LA volumes, ejection fraction, active and passive emptying fraction) and other LV diastolic indices do not change [72].
LA strain has an important role also in predicting LV filling pressures and atrial dysfunction in ischemic and nonischemic cardiomyopathies. In patients with hypertrophic cardiomyopathy (HCM), LA is essential in maintaining an optimal cardiac output despite reduced LV compliance and elevated filling pressures, and undergoes important remodeling. LA function progressively decreases, and typical HF symptoms begin to appear. LA strain has been shown to be reduced in HCM, related to LV dysfunction [61, 73], and moreover allows to distinguish HCM from the nonpathological athlete’s heart in which LA deformation parameters result similar to healthy controls [74]. Also in dilated cardiomyopathy (DCM), it is possible to find raised LVEDP due to diastolic and systolic LV dysfunction and consequently increased LA volume and pressures. PALS results to be particularly reduced in patients with idiopathic compared to ischemic DCM and closely associated with functional capacity during exercise [75]. LA strain is also an independent predictor of impaired LVEDP in these patients [76]. Lastly, LA myocardial dysfunction analysis by STE allows to differentiate restrictive cardiomyopathy (RCM), in which LA strain at septal segments is significantly reduced, from constrictive pericarditis, where the decreasing is placed mostly at superior and lateral segments [77]. In patients with cardiac amyloidosis, LA strain is impaired and, in particular, has resulted to be lower in those subjects with late gadolinium enhancement at atrial level showing a correlation with adverse atrial remodeling [78].
LVEDP results to be raised also in aortic stenosis (AS) where chronic afterload determines structural and functional LA changes. In these patients, all LA strain parameters are markedly reduced [79] and PACS is particularly affected by the severity of AS and LV diastolic function [80]. LA dysfunction is already observed in asymptomatic patients, and the reduction in strain is more pronounced in those who will develop symptoms during follow-up, besides normal LA volume [81]. Furthermore, patients who undergo transcatheter aortic valve implantation (TAVI) show, in addition to a reduction in LA volume, a significant increase in PALS and PACS at 3-month follow-up, proving a reverse cavity remodeling [82].
Acute myocardial infarction (AMI) determines both systolic and diastolic LV dysfunction in surviving patients. In particular, the latter has been correlated with mortality and morbidity independently from systolic function [83], so in this setting, LA strain represents an important parameter, in addition to conventional ones, to detect subjects who are more likely to develop chronic HF. PALS decreases with reducing systolic and diastolic function in patients with AMI with ST elevation (STEMI) treating with percutaneous coronary intervention [84] and tends to increase in patients who undertake cardiac rehabilitation [85]. Also in AMI with non-ST segment elevation (NSTEMI), LA strain has demonstrated an essential prognostic role, being associated with the outcome in acute setting [86] and representing a valuable predictor of cardiovascular events over conventional echocardiographic parameters during follow-up [87].
Limitations and future perspectives
The measurement of LA strain, although feasible in most cases, needs adequate apical views and operator skills. A dedicated software for strain analysis has not been released yet, and currently, the software for LV is applied to the study of LA pattern strain. Moreover, different software uses various algorithms of analysis that could generate biases in comparisons between studies. The difficulty in obtaining the effective shape of LA in an accurate way, should as well as the possible contamination by signal components arising from structures placed around LA, also should be noticed. In patients with nonsinus rhythm is required an averaged measurement of almost five consecutive beats.
STE application is rapidly increasing. Development of dedicated and homogenous software and implementation of 3D-STE methods will allow an optimal and diffuse application of strain parameters. STE requires only a dedicated workstation: In addition to current use in diagnosis and prognostication, results of semi-automated analysis could be obtained and applied at the same time in acute settings like in hemodynamics laboratory and operating room, helping the decision-making process on the patient.
Conclusion
Noninvasive estimation of LV filling pressure is an essential step in the assessment of diastolic dysfunction in patients with HF, particularly in those with preserved EF. Most widespread echocardiographic indexes used for this purpose have limitations. The novel STE-derived parameter, left atrial longitudinal atrial strain, strongly correlates with invasive measurement of LV filling pressure and therefore could be easily utilized, in addition to the conventional parameters. Moreover, its ability of quantitative deformation measurements accurately reflects the degree of structural alterations, myocardial fibrosis and chamber stiffness.
References
McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, Guidelines ESCCfP (2012) ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 33(14):1787–1847. doi:10.1093/eurheartj/ehs104
Meta-analysis Research Group in Echocardiography Heart Failure Collaborators, Doughty RN, Klein AL, Poppe KK, Gamble GD, Dini FL, Moller JE, Quintana M, Yu CM, Whalley GA (2008) Independence of restrictive filling pattern and LV ejection fraction with mortality in heart failure: an individual patient meta-analysis. Eur J Heart Fail 10(8):786–792. doi:10.1016/j.ejheart.2008.06.005
Redfield MM, Jacobsen SJ, Burnett JC Jr, Mahoney DW, Bailey KR, Rodeheffer RJ (2003) Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA 289(2):194–202
Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP (2006) Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med 355(3):260–269. doi:10.1056/NEJMoa051530
Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM (2006) Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 355(3):251–259. doi:10.1056/NEJMoa052256
Lee DS, Gona P, Vasan RS, Larson MG, Benjamin EJ, Wang TJ, Tu JV, Levy D (2009) Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: insights from the Framingham Heart Study of the National Heart, Lung, and Blood Institute. Circulation 119(24):3070–3077. doi:10.1161/CIRCULATIONAHA.108.815944
Sweitzer NK, Stevenson LW (2000) Diastolic heart failure: miles to go before we sleep. Am J Med 109(8):683–685
Melenovsky V, Borlaug BA, Rosen B, Hay I, Ferruci L, Morell CH, Lakatta EG, Najjar SS, Kass DA (2007) Cardiovascular features of heart failure with preserved ejection fraction versus nonfailing hypertensive left ventricular hypertrophy in the urban Baltimore community: the role of atrial remodeling/dysfunction. J Am Coll Cardiol 49(2):198–207. doi:10.1016/j.jacc.2006.08.050
Kane GC, Karon BL, Mahoney DW, Redfield MM, Roger VL, Burnett JC Jr, Jacobsen SJ, Rodeheffer RJ (2011) Progression of left ventricular diastolic dysfunction and risk of heart failure. JAMA 306(8):856–863. doi:10.1001/jama.2011.1201
Nichimura RA, Tajik AJ (1997) Evaluation of diastolic filling of left ventricle in health and disease: Doppler echocardiography is the clinician’s Rosetta stone. J Am Coll Cardiol 30:8–18
Mottram PM, Marwick TH (2005) Assessment of diastolic function: what the general cardiologist needs to know. Heart 91(5):681–695. doi:10.1136/hrt.2003.029413
Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite-Moreira AF, Borbely A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL (2007) How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J 28(20):2539–2550. doi:10.1093/eurheartj/ehm037
Keogh AM, Baron DW, Hickie JB (1990) Prognostic guides in patients with idiopathic or ischemic dilated cardiomyopathy assessed for cardiac transplantation. Am J Cardiol 65(13):903–908
Stevenson WG, Stevenson LW, Middlekauff HR, Fonarow GC, Hamilton MA, Woo MA, Saxon LA, Natterson PD, Steimle A, Walden JA et al (1995) Improving survival for patients with advanced heart failure: a study of 737 consecutive patients. J Am Coll Cardiol 26(6):1417–1423
Haskell RJ, French WJ (1988) Accuracy of left atrial and pulmonary artery wedge pressure in pure mitral regurgitation in predicting left ventricular end-diastolic pressure. Am J Cardiol 61(1):136–141
Yamada H, Klein AL (2010) Diastology 2010: clinical approach to diastolic heart failure. J Echocardiogr 8(3):65–79. doi:10.1007/s12574-010-0055-8
Giannuzzi P, Imparato A, Temporelli PL, de Vito F, Silva PL, Scapellato F, Giordano A (1994) Doppler-derived mitral deceleration time of early filling as a strong predictor of pulmonary capillary wedge pressure in postinfarction patients with left ventricular systolic dysfunction. J Am Coll Cardiol 23(7):1630–1637
Pozzoli M, Capomolla S, Pinna G, Cobelli F, Tavazzi L (1996) Doppler echocardiography reliably predicts pulmonary artery wedge pressure in patients with chronic heart failure with and without mitral regurgitation. J Am Coll Cardiol 27(4):883–893
Yamamoto K, Nishimura RA, Chaliki HP, Appleton CP, Holmes DR Jr, Redfield MM (1997) Determination of left ventricular filling pressure by Doppler echocardiography in patients with coronary artery disease: critical role of left ventricular systolic function. J Am Coll Cardiol 30(7):1819–1826
Rossvoll O, Hatle LK (1993) Pulmonary venous flow velocities recorded by transthoracic Doppler ultrasound: relation to left ventricular diastolic pressures. J Am Coll Cardiol 21(7):1687–1696
Appleton CP, Galloway JM, Gonzales MS, Gaballa M, Bansight MA (1993) Estimation of left ventricular filling pressures using two-dimensional and Doppler echocardiography in adult patients with cardiac disease. Additional value of analyzing left atrial size, left atrial ejection fraction and the difference in duration of pulmonary venous and mitral flow velocity at atrial contraction. J Am Coll Cardiol 22:1972–1982
Dini FL (2012) Diastolic dysfunction: improved understanding using emerging imaging techniques. Heart Metab 57:13–17
Sohn DW, Chai IH, Lee DJ, Kim HC, Kim HS, Oh BH, Lee MM, Park YB, Choi YS, Seo JD, Lee YW (1997) Assessment of mitral annulus velocity by Doppler tissue imaging in the evaluation of left ventricular diastolic function. J Am Coll Cardiol 30(2):474–480
Nagueh SF, Middleton KJ, Kopelen HA, Zoghbi WA, Quinones MA (1997) Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol 30(6):1527–1533
Nagueh SF, Mikati I, Kopelen HA, Middleton KJ, Quinones MA, Zoghbi WA (1998) Doppler estimation of left ventricular filling pressure in sinus tachycardia. A new application of tissue Doppler imaging. Circulation 98(16):1644–1650
Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, Tajik AJ (2000) Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: a comparative simultaneous Doppler-catheterization study. Circulation 102(15):1788–1794. doi:10.1161/01.cir.102.15.1788
Nagueh SF, Lakkis NM, Middleton KJ, Spencer WH 3rd, Zoghbi WA, Quinones MA (1999) Doppler estimation of left ventricular filling pressures in patients with hypertrophic cardiomyopathy. Circulation 99(2):254–261
Diwan A, McCulloch M, Lawrie GM, Reardon MJ, Nagueh SF (2005) Doppler estimation of left ventricular filling pressures in patients with mitral valve disease. Circulation 111(24):3281–3289. doi:10.1161/CIRCULATIONAHA.104.508812
Garcia MJ, Ares MA, Asher C, Rodriguez L, Vandervoort P, Thomas JD (1997) An index of early left ventricular filling that combined with pulsed Doppler peak E velocity may estimate capillary wedge pressure. J Am Coll Cardiol 29(2):448–454
Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelisa A (2009) Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr 10(2):165–193. doi:10.1093/ejechocard/jep007
Dini FL, Ballo P, Badano L, Barbier P, Chella P, Conti U, De Tommasi SM, Galderisi M, Ghio S, Magagnini E, Pieroni A, Rossi A, Rusconi C, Temporelli PL (2010) Validation of an echo-Doppler decision model to predict left ventricular filling pressure in patients with heart failure independently of ejection fraction. Eur J Echocardiogr 11(8):703–710. doi:10.1093/ejechocard/jeq047
Mullens W, Borowski AG, Curtin RJ, Thomas JD, Tang WH (2009) Tissue Doppler imaging in the estimation of intracardiac filling pressure in decompensated patients with advanced systolic heart failure. Circulation 119(1):62–70. doi:10.1161/CIRCULATIONAHA.108.779223
Tsang TS, Abhayaratna WP, Barnes ME, Miyasaka Y, Gersh BJ, Bailey KR, Cha SS, Seward JB (2006) Prediction of cardiovascular outcomes with left atrial size: Is volume superior to area or diameter? J Am Coll Cardiol 47(5):1018–1023. doi:10.1016/j.jacc.2005.08.077
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 28(1):1–39. doi:10.1016/j.echo.2014.10.003
Abhayaratna WP, Seward JB, Appleton CP, Douglas PS, Oh JK, Tajik AJ, Tsang TS (2006) Left atrial size: physiologic determinants and clinical applications. J Am Coll Cardiol 47(12):2357–2363. doi:10.1016/j.jacc.2006.02.048
Tsang TS, Barnes ME, Gersh BJ, Bailey KR, Seward JB (2002) Left atrial volume as a morphophysiologic expression of left ventricular diastolic dysfunction and relation to cardiovascular risk burden. Am J Cardiol 90(12):1284–1289
Cameli M, Caputo M, Mondillo S, Ballo P, Palmerini E, Lisi M, Marino E, Galderisi M (2009) Feasibility and reference values of left atrial longitudinal strain imaging by two-dimensional speckle tracking. Cardiovasc Ultrasound 7:6. doi:10.1186/1476-7120-7-6
Vianna-Pinton R, Moreno CA, Baxter CM, Lee KS, Tsang TS, Appleton CP (2009) Two-dimensional speckle-tracking echocardiography of the left atrium: feasibility and regional contraction and relaxation differences in normal subjects. J Am Soc Echocardiogr 22(3):299–305. doi:10.1016/j.echo.2008.12.017
Kim DG, Lee KJ, Lee S, Jeong SY, Lee YS, Choi YJ, Yoon HS, Kim JH, Jeong KT, Park SC, Park M (2009) Feasibility of two-dimensional global longitudinal strain and strain rate imaging for the assessment of left atrial function: a study in subjects with a low probability of cardiovascular disease and normal exercise capacity. Echocardiography 26(10):1179–1187. doi:10.1111/j.1540-8175.2009.00955.x
Saraiva RM, Demirkol S, Buakhamsri A, Greenberg N, Popovic ZB, Thomas JD, Klein AL (2010) Left atrial strain measured by two-dimensional speckle tracking represents a new tool to evaluate left atrial function. J Am Soc Echocardiogr 23(2):172–180. doi:10.1016/j.echo.2009.11.003
Mondillo S, Galderisi M, Mele D, Cameli M, Lomoriello VS, Zaca V, Ballo P, D’Andrea A, Muraru D, Losi M, Agricola E, D’Errico A, Buralli S, Sciomer S, Nistri S, Badano L, Echocardiography Study Group Of The Italian Society Of Cardiology (2011) Speckle-tracking echocardiography: a new technique for assessing myocardial function. J Ultrasound Med 30(1):71–83
Cameli M, Lisi M, Giacomin E, Caputo M, Navarri R, Malandrino A, Ballo P, Agricola E, Mondillo S (2011) Chronic mitral regurgitation: left atrial deformation analysis by two-dimensional speckle tracking echocardiography. Echocardiography 28(3):327–334. doi:10.1111/j.1540-8175.2010.01329.x
Mor-Avi V, Lang RM, Badano LP, Belohlavek M, Cardim NM, Derumeaux G, Galderisi M, Marwick T, Nagueh SF, Sengupta PP, Sicari R, Smiseth OA, Smulevitz B, Takeuchi M, Thomas JD, Vannan M, Voigt JU, Zamorano JL (2011) Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. Eur J Echocardiogr 12(3):167–205. doi:10.1093/ejechocard/jer021
Cameli M, Lisi M, Mondillo S, Padeletti M, Ballo P, Tsioulpas C, Bernazzali S, Maccherini M (2010) Left atrial longitudinal strain by speckle tracking echocardiography correlates well with left ventricular filling pressures in patients with heart failure. Cardiovasc Ultrasound 8:14. doi:10.1186/1476-7120-8-14
Cameli M, Sparla S, Losito M, Righini FM, Menci D, Lisi M, D’Ascenzi F, Focardi M, Favilli R, Pierli C, Fineschi M, Mondillo S (2015) Correlation of left atrial strain and Doppler measurements with invasive measurement of left ventricular end diastolic pressure in patients stratified for different values of ejection fraction. Echocardiography. doi:10.1111/echo.13094
Gottdiener JS, Kitzman DW, Aurigemma GP, Arnold AM, Manolio TA (2006) Left atrial volume, geometry, and function in systolic and diastolic heart failure of persons > or =65 years of age (the Cardiovascular Health Study). Am J Cardiol 97(1):83–89. doi:10.1016/j.amjcard.2005.07.126
Miyoshi H, Oishi Y, Mizuguchi Y, Iuchi A, Nagase N, Ara N, Oki T (2013) Early predictors of alterations in left atrial structure and function related to left ventricular dysfunction in asymptomatic patients with hypertension. J Am Soc Hypertens 7(3):206–215. doi:10.1016/j.jash.2013.02.001
Machino-Ohtsuka T, Seo Y, Tada H, Ishizu T, Machino T, Yamasaki H, Igarashi M, Xu D, Sekiguchi Y, Aonuma K (2011) Left atrial stiffness relates to left ventricular diastolic dysfunction and recurrence after pulmonary vein isolation for atrial fibrillation. J Cardiovasc Electrophysiol 22(9):999–1006. doi:10.1111/j.1540-8167.2011.02049.x
Kurt M, Tanboga IH, Aksakal E, Kaya A, Isik T, Ekinci M, Bilen E (2012) Relation of left ventricular end-diastolic pressure and N-terminal pro-brain natriuretic peptide level with left atrial deformation parameters. Eur Heart J Cardiovasc Imaging 13(6):524–530. doi:10.1093/ejechocard/jer283
Haug C, Metzele A, Kochs M, Hombach V, Grunert A (1993) Plasma brain natriuretic peptide and atrial natriuretic peptide concentrations correlate with left ventricular end-diastolic pressure. Clin Cardiol 16(7):553–557
Kazanegra R, Cheng V, Garcia A, Krishnaswamy P, Gardetto N, Clopton P, Maisel A (2001) A rapid test for B-type natriuretic peptide correlates with falling wedge pressures in patients treated for decompensated heart failure: a pilot study. J Card Fail 7(1):21–29. doi:10.1054/jcaf.2001.23355
Cameli M, Lisi M, Righini FM, Massoni A, Natali BM, Focardi M, Tacchini D, Geyer A, Curci V, Di Tommaso C, Lisi G, Maccherini M, Chiavarelli M, Massetti M, Tanganelli P, Mondillo S (2013) Usefulness of atrial deformation analysis to predict left atrial fibrosis and endocardial thickness in patients undergoing mitral valve operations for severe mitral regurgitation secondary to mitral valve prolapse. Am J Cardiol 111(4):595–601. doi:10.1016/j.amjcard.2012.10.049
Longobardo L, Todaro MC, Zito C, Piccione MC, Di Bella G, Oreto L, Khandheria BK, Carerj S (2014) Role of imaging in assessment of atrial fibrosis in patients with atrial fibrillation: state-of-the-art review. Eur Heart J Cardiovasc Imaging 15(1):1–5. doi:10.1093/ehjci/jet116
Cameli M, Lisi M, Righini FM, Mondillo S (2012) Novel echocardiographic techniques to assess left atrial size, anatomy and function. Cardiovasc Ultrasound 10:4. doi:10.1186/1476-7120-10-4
Cameli M, Lisi M, Righini FM, Focardi M, Alfieri O, Mondillo S (2012) Left atrial speckle tracking analysis in patients with mitral insufficiency and history of paroxysmal atrial fibrillation. Int J Cardiovasc Imaging 28(7):1663–1670. doi:10.1007/s10554-011-9987-y
Cameli M, Lisi M, Focardi M, Reccia R, Natali BM, Sparla S, Mondillo S (2012) Left atrial deformation analysis by speckle tracking echocardiography for prediction of cardiovascular outcomes. Am J Cardiol 110(2):264–269. doi:10.1016/j.amjcard.2012.03.022
Iio C, Inoue K, Nishimura K, Fujii A, Nagai T, Suzuki J, Okura T, Higaki J, Ogimoto A (2015) Characteristics of left atrial deformation parameters and their prognostic impact in patients with pathological left ventricular hypertrophy: analysis by speckle tracking echocardiography. Echocardiography. doi:10.1111/echo.12961
Appleton CP, Kovács SJ (2009) The role of left atrial function in diastolic heart failure. Circ Cardiovasc Imaging 2:6–9. doi:10.1161/CIRCIMAGING.108.845503
Kurt M, Wang J, Torre-Amione G, Nagueh SF (2009) Left atrial function in diastolic heart failure. Circ Cardiovasc Imaging 2(1):10–15. doi:10.1161/CIRCIMAGING.108.813071
Sanchis L, Gabrielli L, Andrea R, Falces C, Duchateau N, Perez-Villa F, Bijnens B, Sitges M (2015) Left atrial dysfunction relates to symptom onset in patients with heart failure and preserved left ventricular ejection fraction. Eur Heart J Cardiovasc Imaging 16(1):62–67. doi:10.1093/ehjci/jeu165
Rosca M, Popescu BA, Beladan CC, Calin A, Muraru D, Popa EC, Lancellotti P, Enache R, Coman IM, Jurcut R, Ghionea M, Ginghina C (2010) Left atrial dysfunction as a correlate of heart failure symptoms in hypertrophic cardiomyopathy. J Am Soc Echocardiogr 23(10):1090–1098. doi:10.1016/j.echo.2010.07.016
Ahmed MK, Soliman MA, Reda AA, Abd El-Ghani RS (2015) Assessment of left atrial deformation properties by speckle tracking in patients with systolic heart failure. Egypt Heart J 67(3):199–208. doi:10.1016/j.ehj.2014.07.002
Yeh J, Aiyagari R, Gajarski RJ, Zamberlan MC, Lu JC (2015) Left atrial deformation predicts pulmonary capillary wedge pressure in pediatric heart transplant recipients. Echocardiography 32(3):535–540. doi:10.1111/echo.12679
Bech-Hanssen O, Pergola V, Al-Admawi M, Fadel BM, Di Salvo G (2015) Atrial function in heart transplant recipients operated with the bicaval technique. Scand Cardiovasc J 14:1–10. doi:10.3109/14017431.2015.1091946
Cameli M, Ciccone MM, Maiello M, Modesti PA, Muiesan ML, Scicchitano P, Novo S, Palmiero P, Saba PS, Pedrinelli R (2014) Speckle tracking analysis: a new tool for left atrial function analysis in systemic hypertension: an overview. J Cardiovasc Med. doi:10.2459/JCM.0000000000000073
Hassanin N (2015) Left atrium function in systemic hypertension. Int J Sci Res (IJSR) 4(4):1813–1816
Açar G, Bulut M, Arslan K, Alizade E, Ozkan B, Alici G, Tanboga IH, Yazicioglu MV, Akcakoyun M, Esen AM (2013) Comparison of left atrial mechanical function in nondipper versus dipper hypertensive patients: a speckle tracking study. Echocardiography 30(2):164–170. doi:10.1111/echo.12023
Miyoshi H, Oishi Y, Mizuguchi Y, Iuchi A, Nagase N, Ara N, Oki T (2013) Effect of an increase in left ventricular pressure overload on left atrial-left ventricular coupling in patients with hypertension: a two-dimensional speckle tracking echocardiographic study. Echocardiography 30(6):658–666. doi:10.1111/echo.12117
Mondillo S, Cameli M, Caputo ML, Lisi M, Palmerini E, Padeletti M, Ballo P (2011) Early detection of left atrial strain abnormalities by speckle-tracking in hypertensive and diabetic patients with normal left atrial size. J Am Soc Echocardiogr 24(8):898–908. doi:10.1016/j.echo.2011.04.014
Muranaka A, Yuda S, Tsuchihashi K, Hashimoto A, Nakata T, Miura T, Tsuzuki M, Wakabayashi C, Watanabe N, Shimamoto K (2009) Quantitative assessment of left ventricular and left atrial functions by strain rate imaging in diabetic patients with and without hypertension. Echocardiography 26(3):262–271. doi:10.1111/j.1540-8175.2008.00805.x
Liu Y, Wang K, Su D, Cong T, Cheng Y, Zhang Y, Wu J, Sun Y, Shang Z, Liu J, Zhong L, Zou L, Chitian C, Zhang X, Jiang Y (2014) Noninvasive assessment of left atrial phasic function in patients with hypertension and diabetes using two-dimensional speckle tracking and volumetric parameters. Echocardiography 31(6):727–735. doi:10.1111/echo.12492
Dimitroula H, Damvopoulou E, Giannakoulas G, Dalamanga E, Dimitroulas T, Sarafidis PA, Styliadis H, Hatzitolios A, Karvounis H, Parcharidis G (2010) Effects of renin-angiotensin system inhibition on left atrial function of hypertensive patients: an echocardiographic tissue deformation imaging study. Am J Hypertens 23(5):556–561. doi:10.1038/ajh.2010.4
Tigen K, Sunbul M, Karaahmet T, Dundar C, Ozben B, Guler A, Cincin A, Bulut M, Sari I, Basaran Y (2014) Left ventricular and atrial functions in hypertrophic cardiomyopathy patients with very high LVOT gradient: a speckle tracking echocardiographic study. Echocardiography 31(7):833–841. doi:10.1111/echo.12482
Gabrielli L, Enriquez A, Cordova S, Yanez F, Godoy I, Corbalan R (2012) Assessment of left atrial function in hypertrophic cardiomyopathy and athlete’s heart: a left atrial myocardial deformation study. Echocardiography 29(8):943–949. doi:10.1111/j.1540-8175.2012.01719.x
D’Andrea A, Caso P, Romano S, Scarafile R, Cuomo S, Salerno G, Riegler L, Limongelli G, Di Salvo G, Romano M, Liccardo B, Iengo R, Ascione L, Del Viscovo L, Calabro P, Calabro R (2009) Association between left atrial myocardial function and exercise capacity in patients with either idiopathic or ischemic dilated cardiomyopathy: a two-dimensional speckle strain study. Int J Cardiol 132(3):354–363. doi:10.1016/j.ijcard.2007.11.102
Guler A, Tigen KM, Dundar C, Karaahmet T, Karabay CY, Aung SM, Akgun T, Bulut M, Kirma C (2014) Left atrial deformation and nonischemic dilated cardiomyopathy. A 2D speckle-tracking imaging study. Herz 39(2):251–257. doi:10.1007/s00059-013-3817-z
Liu S, Ma C, Ren W, Zhang J, Li N, Yang J, Zhang Y, Qiao W (2015) Regional left atrial function differentiation in patients with constrictive pericarditis and restrictive cardiomyopathy: a study using speckle tracking echocardiography. Int J Cardiovasc Imaging 31(8):1529–1536. doi:10.1007/s10554-015-0726-7
Di Bella G, Minutoli F, Madaffari A, Mazzeo A, Russo M, Donato R, Zito C, Aquaro GD, Piccione MC, Pedri S, Vita G, Pingitore A, Carerj S (2014) Left atrial function in cardiac amyloidosis. J Cardiovasc Med. doi:10.2459/JCM.0000000000000188
O’Connor K, Magne J, Rosca M, Pierard LA, Lancellotti P (2011) Left atrial function and remodelling in aortic stenosis. Eur J Echocardiogr 12(4):299–305. doi:10.1093/ejechocard/jer006
O’Connor K, Magne J, Rosca M, Pierard LA, Lancellotti P (2010) Impact of aortic valve stenosis on left atrial phasic function. Am J Cardiol 106(8):1157–1162. doi:10.1016/j.amjcard.2010.06.029
Todaro MC, Carerj S, Khandheria B, Cusma-Piccione M, La Carrubba S, Antonini-Canterin F, Pugliatti P, Di Bello V, Oreto G, Di Bella G, Zito C (2015) Usefulness of atrial function for risk stratification in asymptomatic severe aortic stenosis. J Cardiol. doi:10.1016/j.jjcc.2015.04.010
D’Andrea A, Padalino R, Cocchia R, Di Palma E, Riegler L, Scarafile R, Rossi G, Bianchi R, Tartaglione D, Cappelli Bigazzi M, Calabro P, Citro R, Bossone E, Calabro R, Russo MG (2015) Effects of transcatheter aortic valve implantation on left ventricular and left atrial morphology and function. Echocardiography 32(6):928–936. doi:10.1111/echo.12808
Oh JK, Ding ZP, Gersh BJ, Bailey KR, Tajik AJ (1992) Restrictive left ventricular diastolic filling identifies patients with heart failure after acute myocardial infarction. J Am Soc Echocardiogr 5(5):497–503
Dogan C, Ozdemir N, Hatipoglu S, Bakal RB, Omaygenc MO, Dindar B, Candan O, Emiroglu MY, Kaymaz C (2013) Relation of left atrial peak systolic strain with left ventricular diastolic dysfunction and brain natriuretic peptide level in patients presenting with ST-elevation myocardial infarction. Cardiovasc Ultrasound 11:24. doi:10.1186/1476-7120-11-24
Acar RD, Bulut M, Ergun S, Yesin M, Akcakoyun M (2015) Evaluation of the effect of cardiac rehabilitation on left atrial and left ventricular function and its relationship with changes in arterial stiffness in patients with acute myocardial infarction. Echocardiography 32(3):443–447. doi:10.1111/echo.12701
Ersboll M, Andersen MJ, Valeur N, Mogensen UM, Fahkri Y, Thune JJ, Moller JE, Hassager C, Sogaard P, Kober L (2014) Early diastolic strain rate in relation to systolic and diastolic function and prognosis in acute myocardial infarction: a two-dimensional speckle-tracking study. Eur Heart J 35(10):648–656. doi:10.1093/eurheartj/eht179
Shao C, Zhu J, Chen J, Xu W (2015) Independent prognostic value of left atrial function by two-dimensional speckle tracking imaging in patients with non-ST-segment-elevation acute myocardial infarction. BMC Cardiovasc Disord 15:145. doi:10.1186/s12872-015-0135-9
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Drs. Cameli, Mandoli, Loiacono, Dini, Henein and Mondillo have no conflicts of interest or financial ties to disclose.
Rights and permissions
About this article
Cite this article
Cameli, M., Mandoli, G.E., Loiacono, F. et al. Left atrial strain: a new parameter for assessment of left ventricular filling pressure. Heart Fail Rev 21, 65–76 (2016). https://doi.org/10.1007/s10741-015-9520-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-015-9520-9