Abstract
Purpose
Although controversial, obesity and underweight may have a negative impact on breast cancer outcome. However, the relationship between body mass index (BMI) and breast cancer outcomes according to tumor subtype and menopausal status remains unclear.
Methods
This study investigated the association between BMI and breast cancer outcome in stage I–III breast cancer patients. The relationships were further evaluated according to tumor subtype and menopausal status.
Results
A total of 5919 patients, 3475 (58.7%) hormone receptor (HR)(+) human epidermal growth factor receptor 2 (HER2)(–), 608 (10.3%) HR(+)HER2(+), 621 (10.5%) HR(–)HER2(+), and 1079 (18.2%) HR(–)HER2(–) were included. Underweight and obesity had a negative impact on relapse-free survival but did not affect overall survival. Importantly, the prognostic role of BMI was different according to tumor subtype and menopausal status. In HR(+)HER2(–) patients, underweight was associated with poor relapse-free survival and overall survival in pre-menopausal women. In contrast, obesity had negative impact on relapse-free survival and overall survival in HR(+)HER2(–) post-menopausal patients. Underweight may have a negative prognostic role in HR(+)HER2(+) patients. However, BMI did not impact the outcome of HR(–)HER2(+) and HR(–)HER2(–) patients.
Conclusions
The impact of BMI on breast cancer outcome was dependent on tumor subtype and menopausal status. In HR(+)HER2(–) patients, underweight and obesity had a negative prognostic role in pre-menopausal and post-menopausal women, respectively. These findings in Asian population should be further evaluated and compared in Western population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most common cancer in women and leads second in cancer-related deaths in women of developed countries [1]. The incidence of breast cancer is increasing in developing countries, including South Korea, which is probably due to nationwide cancer screening and westernization of lifestyle [2, 3]. Although the precise mechanisms are not well understood, obesity can increase the risk of breast cancer development by increasing circulating insulin, insulin-like growth factor (IGF), adipokines, and local production of estrogen [4, 5]. The relationship between obesity and breast cancer development is strong in post-menopausal women, while the evidence is less clear or protective in pre-menopausal women [4].
Many studies investigated the impact of body mass index (BMI) on breast cancer outcome. The results are inconsistent that some studies identified obesity as a negative prognostic factor [6, 7], while other studies revealed underweight as a poor prognostic factor [8, 9]. The negative impact of underweight seems to be more prominent in Asian population [8, 9]. In addition, there are controversial results on the prognostic role of BMI according to tumor subtype or menopausal status [10,11,12,13]. Heterogeneous study population, menopausal status, and tumor subtype may have affected such outcome.
This study was conducted in a homogeneous cohort of patients with early-stage (I–III) breast cancer who were treated with curative surgery followed by an adjuvant chemotherapy. The primary purpose of this study was to investigate the prognostic role of baseline BMI according to tumor subtype and menopausal status.
Patients and methods
Study population
This single-institute retrospective study included 5919 pathologically proven breast cancer patients who received curative surgery followed by an adjuvant chemotherapy at Seoul National University Hospital (SNUH, Seoul, South Korea) between January, 2000 and December, 2015. Primary treatments included radical mastectomy, modified radical mastectomy, and breast-conserving surgery with concomitant sentinel lymph node biopsy or axillary lymph node dissection. Patients received adjuvant radiotherapy and/or hormone in the discretion of treating physician. Eligible patients were identified from the electronic database of SNUH and medical charts were reviewed using the electronic medical record system of SNUH. The study protocol was reviewed and approved by the institutional review board of SNUH [H-1709-052-883]. As this study was retrospectively designed, informed consent was waived by the IRB. This study was carried out in accordance with the recommendations of the Declaration of Helsinki for biomedical research involving human subjects.
Measurement of baseline BMI
Body weight and height was measured on the day of admission for breast cancer surgery. Trained nurses measured weight to the nearest 0.1 kg and height to the nearest 0.1 cm. BMI was calculated by dividing weight in kilograms by the square of height in meters (kg/m2). Patients were classified according to BMI cut-offs proposed by World Health Organization (WHO) for Asian population [14]: underweight, BMI < 18.5 kg/m2; normal weight, 18.5–22.9; overweight, 23–24.9; obese, ≥ 25.
Analysis of tumor subtype
Immunohistochemical (IHC) staining of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) was performed with formalin-fixed, paraffin-embedded tissue acquired from surgical specimen. HER2 FISH was performed in patients with HER2 IHC grade 2. Nuclear expression of tumor cells was interpreted as positive for ER and PR, while membrane staining of tumor cells was considered positive for HER2. ER and PR expression was categorized as positive when ≥ 1% of the tumor cells were stained [15]. Hormone receptor (HR) was defined positive when either ER or PR was expressed. HER2 status was defined positive if HER2 IHC was grade 3 or grade 2 with FISH positive [16]. Patients were categorized as ‘HR(+)HER2(–),’ ‘HR(+)HER2(+),’ ‘HR(–)HER2(+),’ or ‘HR(–)HER2(–).’
Statistical analysis
The primary objective of this study was to investigate the prognostic role of BMI on breast cancer outcome (relapse-free survival and overall survival) according to tumor subtype and menopausal status. Relapse-free survival was calculated from the date of operation to the first date of documented relapse. Data from patients who were free of relapse or who died in a cancer-free state were censored at the date of the last follow-up visit for relapse-free survival. Overall survival was defined as the time from the date of operation to the date of death. Categorical variables were compared with the Chi-square test, and continuous variables were compared with an independent-sample t test. Relapse-free survival and overall survival were calculated with the Kaplan–Meier method, and comparisons were made with log-rank tests. Hazard ratios (HR) of obesity or underweight were calculated using the Cox proportional hazard model. Baseline characteristics were adjusted by using a forward stepwise model including covariates that have a prognostic role: menopausal status (pre-menopausal vs. post-menopausal), tumor stage (I vs. II vs. III), and tumor subtype. Two-sided p values of < 0.05 were considered statistically significant. Statistical analysis was performed with SPSS software for Windows, version 21.0 (SPSS, Chicago, IL, USA).
Results
Patient characteristics according to baseline BMI
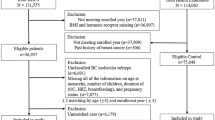
A total of 5919 patients were included in the present study (Fig. 1). Baseline characteristics are summarized in Table 1. Based on the cut-offs proposed by WHO for Asians, 196 patients (3.3%) were underweight (BMI < 18.5 kg/m2), 2833 patients (47.9%) were normal weight (18.5–22.9 kg/m2), 1332 patients (22.5%) were overweight (23–24.9 kg/m2), and 1558 patients (26.3%) were obese (≥ 25 kg/m2). Tumor stage was I in 1678 (28.3%) patients, II in 3442 (58.2%), and III in 776 (13.1%). According to the inclusion criteria, all patient received adjuvant chemotherapy. Obese patients had higher proportion of patients with age over 50, post-menopausal state, and higher tumor stage. Post-operation radiation therapy was less likely received in underweight patients probably due to relatively lower tumor stage. HR status and HER2 status was similar regardless of baseline BMI.
Prognostic implication of baseline BMI
After a median follow-up duration of 71 months, 764 recurrent events and 210 deaths have occurred. Underweight patients and obese patients had higher recurrence rate compared to normal weight patients (Fig. 2a). The 10-year relapse-free survival was 72.2% [95% confidence index (CI) 62.4–82.0%] for underweight patients (p = 0.005, vs. normal weight), 78.2% (95% CI 75.3–81.1%) for obese patients (p = 0.008, vs. normal weight), and 82.0% (95% CI 79.8–84.8%) for normal weight patients. In a multivariate analysis using Cox proportional hazard model, underweight was an independent negative prognostic factor for relapse-free survival. Underweight patients had significantly higher risk of recurrence compared with normal and overweight group (adjusted HR for relapse-free survival, 1.72; 95% CI 1.22–2.43; p = 0.002). In addition, obese patients had a tendency of higher recurrence compared to normal and overweight patients (adjusted HR for relapse-free survival, 1.16; 95% CI 0.99–1.36; p = 0.074). In the analysis of overall survival, obese patients had poor survival compared to normal weight patients. The 10-year overall survival of obese patients was 90.5% (95% CI 88.0–93.0%) compared to 94.4% (95% CI 93.0–95.8%) in normal weight patients (p = 0.002) (Fig. 2b). Underweight did not impact overall survival. In the multivariate analysis, neither underweight nor obese was a prognostic factor for overall survival.
Prognostic implication of baseline BMI according to tumor subtype and menopausal status
We next evaluated whether the prognostic role of baseline BMI is different according to tumor subtype and menopausal status. In HR(+)HER2(–) patients, underweight and obese patients had poor survival outcome (Supplement Fig. 1). In the multivariate analysis, underweight was associated with poor relapse-free survival (adjusted HR versus normal and overweight, 1.80; 95% CI 1.11–2.91; p = 0.017) and overall survival (adjusted HR versus normal and overweight, 2.77; 95% CI 1.09–7.04; p = 0.032). Although obesity did not impact relapse-free survival, it had a tendency of poor overall survival (adjusted HR versus normal and overweight, 1.55; 95% CI 0.98–2.47; p = 0.063).
In HR(+)HER2(+) patients, underweight was associated with higher recurrence (10-year relapse-free survival of 46.6% vs. 84.6%, p < 0.001) (Supplement Fig. 1c) but did not impact overall survival (Supplement Fig. 1d). Multivariate analysis revealed underweight as an independent negative prognostic factor for relapse-free survival in HR(+)HER2(+) patients (adjusted HR vs. non-underweight, 4.54; 95% CI 2.05–10.03; p < 0.001). Obesity did not impact outcome of HR(+)HER2 patients. In addition, neither underweight nor obese affected survival in hormone receptor negative [HR(–)HER2(+) or HR(–)HER2(–)] patients.
The impact of BMI on breast cancer outcome was also affected by menopausal status. In pre-menopausal patients, underweight was associated with higher recurrence but did not impact overall survival (Supplement Fig. 2a, b). Multivariate analysis revealed underweight as an independent negative prognostic factor for relapse-free survival in pre-menopausal patients (adjusted HR versus non-underweight, 1.92; 95% CI 1.33–2.78; p < 0.001). In post-menopausal patients, obesity was associated with poor relapse-free survival (10-year relapse-free survival of 73.5% vs. 80.6% in normal weight, p = 0.010) (Supplement Fig. 2c) and overall survival (10-year overall survival of 87.3% vs. 93.2% in normal weight, p = 0.026) (Supplement Fig. 2d). In the multivariate analysis, obesity was an independent negative prognostic factor for relapse-free survival (adjusted HR versus non-obese, 1.35; 95% CI 1.08–1.69; p = 0.009) but not for overall survival.
Prognostic implication of baseline BMI incorporating tumor subtype and menopausal status
To comprehensively analyze the prognostic role of baseline BMI, we performed subgroup analysis incorporating tumor subtype and menopausal status. In HR(+)HER2(–) patients, prognostic role of baseline BMI was different according to menopausal status (Fig. 3). Underweight was associated with negative outcome in HR(+)HER2(–) pre-menopausal women (Fig. 3a, b). Multivariate analysis revealed underweight as an independent negative prognostic factor for relapse-free survival (adjusted HR versus non-underweight, 1.90; 95% CI 1.16–3.12; p = 0.011) and overall survival (adjusted HR for overall survival versus non-underweight, 2.99; 95% CI 1.17–7.63; p = 0.022) in pre-menopausal HR(+)HER2(–) patients. By contrast, obesity was a negative prognostic factor in HR(+)HER2(–) post-menopausal women (Fig. 3c, d). Obesity was an independent negative prognostic factor for relapse-free survival (adjusted HR vs. non-obese, 1.53; 95% CI 1.10–2.12; p = 0.012) and overall survival (adjusted HR versus non-obese, 2.09; 95% CI 1.10 – 3.97; p = 0.024) in post-menopausal HR(+)HER2(–) patients.
In HR(+)HER2(+) patients, underweight was associated with higher recurrence regardless of menopausal status (Supplement Fig. 3a, c). However, these data need to be interpreted carefully as only 16 patients were underweighted in pre-menopausal group and five patients were underweighted in post-menopausal group. Moreover, underweight did not impact overall survival in pre-menopausal or post-menopausal women with HR(+)HER2(+) (Supplement Fig. 3b, d).
In patients with HR(–)HER2(+) or HR(–)HER2(–), BMI did not impact relapse-free survival or overall survival in pre-menopausal patients nor post-menopausal patients.
Discussion
As body fat affects estrogen production, the effect of BMI on breast cancer outcome may be different among menopausal status and tumor subtype. This study evaluated the prognostic role of baseline BMI in homogeneous cohort of breast cancer patients treated with curative surgery followed by an adjuvant chemotherapy. We revealed that the impact of BMI is different according to tumor subtype and menopausal status.
In the present study, underweight was an independent negative prognostic factor for relapse-free survival and obese patients had a tendency of poor relapse-free survival. However, underweight and obesity was not associated with overall survival. Important finding of the present study is that the impact of BMI on breast cancer outcome is different according to menopausal status and tumor subtype. In pre-menopausal women, underweight had a negative impact on relapse-free survival while obesity did not impact breast cancer outcome. By contrast, in post-menopausal women, obesity was a negative prognostic factor for relapse-free survival but underweight did not impact outcome. The prognostic role of baseline BMI was not only affected by menopausal status but also by tumor subtype. Neither underweight nor obese affected outcome in hormone receptor negative breast cancer patients [HR(–)HER2(+) or HR(–)HER2(–)]. However, in HR(+)HER2(–) patients, underweight had a negative impact on outcome (both relapse-free survival and overall survival) and obesity tended to have a negative impact on overall survival. In HR(+)HER2(+) patients, underweight patients had a tendency of poor relapse-free survival.
As the impact of BMI on breast cancer outcome was dependent on menopausal status and tumor subtype, we next incorporated menopausal status and tumor subtype to evaluate the prognostic role of BMI on breast cancer outcome. In HR(+)HER2(–) patients, the impact of BMI was different according to menopausal status. Underweight was associated with poor outcome (both relapse-free survival and overall survival) in pre-menopausal women with HR(+)HER2(–) tumor. In contrast, obesity had a negative impact (both relapse-free survival and overall survival) in post-menopausal women with HR(+)HER2(–) tumor.
This study revealed that the prognostic role of BMI was dependent on tumor subtype and menopausal status. The negative impact of obesity in hormone-positive breast cancer was previously reported [12]. From the study results of three adjuvant trials coordinated by the Eastern Cooperative Oncology Group (ECOG), obesity was associated with inferior outcomes in HR(+)HER2(–) patients but not in HER2(+) or triple-negative patients [12]. However, the study did not evaluate the impact of underweight nor performed subgroup analysis according to menopausal status. In a single-center study performed in Japan, high BMI (≥ 25.8 kg/m2) and low BMI (< 21.2 kg/m2) was associated with poor survival in 410 hormone-positive breast cancer patients [9]. Increased estrogen exposure is a risk factor of developing hormone-positive breast cancer [17, 18]. The correlation between obesity and breast cancer development is complex that obesity may have detrimental effect in post-menopausal women but may have protective role in pre-menopausal women [4]. Likewise, in the present study, obesity was associated with poor survival in post-menopausal women with HR(+)HER2(–) tumor while underweight was associated with poor survival in pre-menopausal women with HR(+)HER2(–) tumor. As obesity elevates circulating insulin, IGF, adipokines level, and local synthesis of estrogen, increased estrogen production by adipose tissue in post-menopausal state may have affected negative prognostic role of obesity [4, 5, 13, 19]. Although alteration of immune system by undernutrition and protective role of mammary adipocytes has been proposed, it is not known why underweight affects breast cancer outcome [8, 20]. There may be an ethnic difference as two studies which showed negative prognostic role of underweight were performed in Asian population [8, 9]. However, our results show that underweight is associated with poor outcome only in HR(+)HER2(–) pre-menopausal women. The higher proportion of pre-menopausal women in Asian breast cancer patients may have affected such findings in previous reports [21, 22].
There are several limitations in this study. First, the study was conducted in a single institution at South Korea; thus all patients in this cohort were Asian so we used BMI cut-offs proposed by WHO for Asian population [14]. Asians tend to have higher total and central adiposity for a given BMI compared to Western populations [23]. Validation of our results in Western population is warranted. Second limitation is that we did not perform multiple hypothesis comparison. As body fat affects hormonal status, we hypothesized that the effect of BMI may be different among menopausal status and tumor subtype. We did not perform multiple hypothesis comparison as the standard treatments and prognosis is different according to each breast cancer subtype. Including all subtype as a single disease will rather cause a mixed effect. However, careful interpretation is needed. Lastly, we did not have data on other parameters that could reflect body habitus (such as waist circumference, fat distribution, diet, physical activity, and smoking) as the study was designed retrospectively and was analyzed using an established cohort. Likewise, only three covariates were included in the multivariate analysis. Comprehensive analysis on the impact of BMI and lifestyle factors on breast cancer outcome is needed. In addition, the effect of body weight and nutrition modification on breast cancer outcome needs to be evaluated. In Women’s Intervention Nutrition Study (WINS) trial, a lifestyle intervention reducing dietary fat intake and body weight was associated with improved relapse-free survival in breast cancer patients [24]. However, adoption of a diet high in vegetables, fruit, and fiber and low in fat did not reduce breast cancer outcome in Women’s Healthy Eating and Living (WHEL) Randomized Trial [25]. Incorporation of menopausal status and tumor subtype may be important in studies identifying the role of life style or body weight modification.
Conclusion
We have revealed that underweight and obesity is associated with poor relapse-free survival in breast cancer patients treated with surgery followed by an adjuvant chemotherapy. In addition, the effect of BMI on breast cancer survival was different according to menopausal status and tumor subtype. Underweight was associated with poor survival in pre-menopausal women with HR(+)HER2(–) breast cancer. In contrast, obesity had a negative impact in post-menopausal women with HR(+)HER2(–) breast cancer. In HR(+)HER2(+) patients, underweight may have a negative impact on relapse-free survival. Lastly, BMI did not impact the outcome of HR(–) tumors. Patients at risk might be advised to modify their weight accordingly.
REFERENCES
Torre LA, Bray F, Siegel RL et al (2015) Global cancer statistics, 2012. CA Cancer J Clin 65(2):87–108. https://doi.org/10.3322/caac.21262
Althuis MD, Dozier JM, Anderson WF et al (2005) Global trends in breast cancer incidence and mortality 1973-1997. Int J Epidemiol 34(2):405–412. https://doi.org/10.1093/ije/dyh414
Jung KW, Won YJ, Kong HJ et al (2018) Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2015. Cancer Res Treat 50(2):303–316. https://doi.org/10.4143/crt.2018.143
Renehan AG, Tyson M, Egger M et al (2008) Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 371(9612):569–578. https://doi.org/10.1016/S0140-6736(08)60269-X
Renehan AG, Roberts DL, Dive C (2008) Obesity and cancer: pathophysiological and biological mechanisms. Arch Physiol Biochem 114(1):71–83. https://doi.org/10.1080/13813450801954303
Ewertz M, Jensen MB, Gunnarsdottir KA et al (2011) Effect of obesity on prognosis after early-stage breast cancer. J Clin Oncol 29(1):25–31. https://doi.org/10.1200/JCO.2010.29.7614
Chan DS, Vieira AR, Aune D et al (2014) Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol 25(10):1901–1914. https://doi.org/10.1093/annonc/mdu042
Moon HG, Han W, Noh DY (2009) Underweight and breast cancer recurrence and death: a report from the Korean Breast Cancer Society. J Clin Oncol 27(35):5899–5905. https://doi.org/10.1200/JCO.2009.22.4436
Kawai M, Minami Y, Nishino Y et al (2012) Body mass index and survival after breast cancer diagnosis in Japanese women. BMC Cancer 12:149. https://doi.org/10.1186/1471-2407-12-149
Jeon YW, Kang SH, Park MH et al (2015) Relationship between body mass index and the expression of hormone receptors or human epidermal growth factor receptor 2 with respect to breast cancer survival. BMC Cancer 15:865. https://doi.org/10.1186/s12885-015-1879-4
Niraula S, Ocana A, Ennis M et al (2012) Body size and breast cancer prognosis in relation to hormone receptor and menopausal status: a meta-analysis. Breast Cancer Res Treat 134(2):769–781. https://doi.org/10.1007/s10549-012-2073-x
Sparano JA, Wang M, Zhao F et al (2012) Obesity at diagnosis is associated with inferior outcomes in hormone receptor-positive operable breast cancer. Cancer 118(23):5937–5946. https://doi.org/10.1002/cncr.27527
Protani M, Coory M, Martin JH (2010) Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Res Treat 123(3):627–635. https://doi.org/10.1007/s10549-010-0990-0
WHO (2000) The Asia-Pacific perspective: redefining obesity and its treatment. Health Communications Australia, Sydney
Hammond ME, Hayes DF, Dowsett M et al (2010) American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 28(16):2784–2795. https://doi.org/10.1200/JCO.2009.25.6529
Wolff AC, Hammond ME, Hicks DG et al (2013) Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 31(31):3997–4013. https://doi.org/10.1200/JCO.2013.50.9984
Petracci E, Decarli A, Schairer C et al (2011) Risk factor modification and projections of absolute breast cancer risk. J Natl Cancer Inst 103(13):1037–1048. https://doi.org/10.1093/jnci/djr172
Nelson ER, Chang CY, McDonnell DP (2014) Cholesterol and breast cancer pathophysiology. Trends Endocrinol Metab 25(12):649–655. https://doi.org/10.1016/j.tem.2014.10.001
Rose DP, Vona-Davis L (2010) Interaction between menopausal status and obesity in affecting breast cancer risk. Maturitas 66(1):33–38. https://doi.org/10.1016/j.maturitas.2010.01.019
Cunningham-Rundles S, McNeeley DF, Moon A (2005) Mechanisms of nutrient modulation of the immune response. J Allergy Clin Immunol 115(6):1119–1128. https://doi.org/10.1016/j.jaci.2005.04.036
Min SY, Kim Z, Hur MH et al (2016) The basic facts of Korean Breast Cancer in 2013: results of a Nationwide Survey and Breast Cancer Registry Database. J Breast Cancer 19(1):1–7. https://doi.org/10.4048/jbc.2016.19.1.1
Kan Z, Ding Y, Kim J et al (2018) Multi-omics profiling of younger Asian breast cancers reveals distinctive molecular signatures. Nat Commun 9(1):1725. https://doi.org/10.1038/s41467-018-04129-4
Ramachandran A, Chamukuttan S, Shetty SA et al (2012) Obesity in Asia—is it different from rest of the world. Diabetes Metab Res Rev 28(Suppl 2):47–51. https://doi.org/10.1002/dmrr.2353
Chlebowski RT, Blackburn GL, Thomson CA et al (2006) Dietary fat reduction and breast cancer outcome: interim efficacy results from the Women’s Intervention Nutrition Study. J Natl Cancer Inst 98(24):1767–1776. https://doi.org/10.1093/jnci/djj494
Pierce JP, Natarajan L, Caan BJ et al (2007) Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: the Women’s Healthy Eating and Living (WHEL) randomized trial. JAMA 298(3):289–298. https://doi.org/10.1001/jama.298.3.289
Funding
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (Grant No. HC17C0043).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
The study protocol was reviewed and approved by the institutional review board of SNUH [H-1709-052-883]. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
As this study was retrospectively designed, informed consent was waived by the IRB of Seoul National University Hospital.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10549_2019_5249_MOESM1_ESM.tif
Supplement Figure 1. Prognostic role of BMI according to tumor subtype. (1A: HR(+)HER2(-), relapse-free survival; 1B: HR(+)HER2(-), overall survival; 1C: HR(+)HER2(+), relapse-free survival; 1D: HR(+)HER2(+), overall survival). Supplementary material 1 (TIFF 889 kb)
10549_2019_5249_MOESM2_ESM.tif
Supplement Figure 2. Prognostic role of BMI according to menopause status. (2A: pre-menopausal, relapse-free survival; 2B: pre-menopausal, overall survival; 2C: post-menopausal, relapse-free survival; 2D: post-menopausal, overall survival). Supplementary material 2 (TIFF 894 kb)
10549_2019_5249_MOESM3_ESM.tif
Supplement Figure 3. Prognostic role of BMI in HR(+)HER2(+) patients stratified by menopause status. (3A: pre-menopausal, relapse-free survival; 3B: pre-menopausal, overall survival; 3C: post-menopausal, relapse-free survival; 3D: post-menopausal, overall survival). Supplementary material 3 (TIFF 877 kb)
Rights and permissions
About this article
Cite this article
Kim, J.Y., Lee, DW., Lee, KH. et al. Prognostic role of body mass index is different according to menopausal status and tumor subtype in breast cancer patients. Breast Cancer Res Treat 176, 453–460 (2019). https://doi.org/10.1007/s10549-019-05249-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-019-05249-1