Abstract
Purpose
Obesity is associated with tumor promoting pathways related to insulin resistance and chronic low-grade inflammation which have been linked to various disease states, including cancer. Many studies have focused on the relationship between obesity and increased estrogen production, which contributes to the pathogenesis of estrogen receptor-positive breast cancers. The link between obesity and other breast cancer subtypes, such as triple-negative breast cancer (TNBC) and Her2/neu+ (Her2+) breast cancer, is less clear. We hypothesize that obesity may be associated with the pathogenesis of specific breast cancer subtypes resulting in a different subtype distribution than normal weight women.
Methods
A single-institution, retrospective analysis of tumor characteristics of 848 patients diagnosed with primary operable breast cancer between 2000 and 2013 was performed to evaluate the association between BMI and clinical outcome. Patients were grouped based on their BMI at time of diagnosis stratified into three subgroups: normal weight (BMI = 18–24.9), overweight (BMI = 25–29.9), and obese (BMI > 30). The distribution of breast cancer subtypes across the three BMI subgroups was compared.
Results
Obese and overweight women were more likely to present with TNBC and normal weight women with Her2+ breast cancer (p = 0.008).
Conclusions
We demonstrated, for the first time, that breast cancer subtype distribution varied significantly according to BMI status. Our results suggested that obesity might activate molecular pathways other than the well-known obesity/estrogen circuit in the pathogenesis of breast cancer. Future studies are needed to understand the molecular mechanisms that drive the variation in subtype distribution across BMI subgroups.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the United States and the developed world, obesity rates have reached epidemic proportions; the majority (>60%) of the adult US population falls in the overweight and obese categories as determined by body mass index (BMI: 25–29.9 and >30, respectively) [1, 2]. The molecular links between obesity and cancer have been the subject of many studies [3, 4]. Increased adiposity is long known to be associated with pathways associated with insulin resistance and increased circulating estrogen levels. Both of which have tumor promoting activities for breast cancer. The relationship between increased adiposity and hormone receptor-positive breast cancer is well established [5]. It has been hypothesized that chronic, low-grade, systemic inflammatory state associated with obesity may promote triple-negative breast cancer (TNBC) tumorigenesis. However, the physiologic link is still being elucidated [6].
A recent population study has reported on the distribution of four molecular breast cancer subtypes [7, 8] approximated by the expression of three surrogate tumor markers: estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor (Her2) in the United States [9]. Either ER+ or PR+ breast cancers were defined as hormone receptor (HR)+ in that study. Overall, luminal A (HR+/Her2−) subtype comprised the majority (72.6%) of all diagnosed breast cancers. Basal subtype (HR−/Her2− or TNBC) represented 13% of all breast cancers, while luminal B (HR+/Her2+) represented 5% and Her2− enriched (HR−/Her2+) represented 10% of all breast cancers diagnosed in 2011. The distribution of these four breast cancer subtypes varied with age and race. Whether breast cancer subtype distribution also varied with other clinical variables such as body mass index (BMI) was unknown.
We hypothesize that breast cancer subtype distribution in overweight and obese women may differ from normal weight women. The association of BMI and various breast cancer subtypes has not been extensively explored as prior large population studies that were powered to address this topic often lacked complete tumor marker data [10]. Our objective was to examine this association in a large retrospective breast cancer patient cohort treated at a single institution.
Materials and methods
Study population
After obtaining Institutional Review Board approval, we identified all patients with primary operable breast cancer in our electronic medical record (EMR) treated between 1998 and 2013 at our institution. Patients with an ICD-9 diagnosis code of invasive breast cancer on at least two separate in-person visits and who underwent definitive surgery and were followed-up at our institution were included.
To be included for analysis, age at diagnosis, height, and weight from within 3 months of breast cancer diagnosis and postoperative clinical follow-up of greater than 30 days must be available. Clinical covariates collected also include self-reported race, diagnosis of diabetes mellitus, and the presence of cardiovascular co-morbidities (diabetes, coronary artery disease, and cerebrovascular disease). Patients were stratified by BMI into three groups: normal weight, overweight, or obese (BMI = 18.5–24.9, 25–29.9, and >30 kg/m2, respectively); BMI was calculated using the formula: weight in kg/height in m2.
Tumor pathology data collected included tumor size, grade (Nottingham histologic score), lymphovascular invasion (LVI), receptor status (ER, PR and Her2), and nodal status. We classified breast cancer into four main molecular subtypes based on receptor status as determined by immunohistochemical (IHC) staining: (1) HR+ Her2−; (2) HR−Her2+; (3) HR+ Her2+; and (4) ER−, PR−, and Her2− or TNBC [9, 11]. Outcomes, including overall survival (OS), disease-free survival (DFS), local-regional recurrence (LRR), and distant metastasis, were ascertained based on records within the EMR and by use of the SSN Death Certificate Index. Length of follow-up was determined by duration from the date of breast cancer diagnosis to the last follow-up date listed in their EMR as of December 31, 2016. Disease status of each patient was classified as no evidence of disease (NED), alive with disease (AWD), death of disease (DOD), death from other causes (DOO), or death from cause unknown (DCU).
Statistical analyses
Association between BMI, other clinical and pathologic covariates, and disease status was performed using Fisher’s exact or χ 2 test. A Cochran–Mantel–Haenszel χ 2 test was used to evaluate for conditional independence. P-values less than or equal to 0.05 were accepted as statistically significant.
Univariate and multivariate survival analyses for OS and DFS were performed using Kaplan–Meier and Cox-proportional hazard models. Forward–backward stepwise regression was used to determine independent covariates contributing to the final survival models on multivariate analysis.
Results
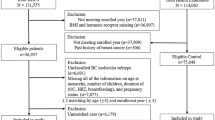
A total of 4776 women with diagnoses of breast cancer were initially identified from the EMR; 848 women fulfilled all inclusion criteria and were included in the final analysis. The clinical characteristics of our study cohort as stratified by three BMI categories (normal, overweight, and obese) are summarized in Table 1. Patients were evenly distributed among the three BMI subgroups. Tumor size, the presence of lymphovascular invasion and nodal stage, and extracapsular extension did not differ between the three BMI groups. We did note that overweight and obese patients were older than normal weight patients (p = 0.03).
Obesity was strongly associated with race in our patient cohort (p < 0.001). Overall, 77.1% of normal weight women were non-black, while only 49.0% of obese women were non-black. In black patients, only 11.3% were normal weight, while 47.4% were obese. Women with higher BMI were also more likely to present with tumors of higher tumor grade (p = 0.03). As expected, women who were overweight or obese were more likely to present with diabetes and/or cardiovascular co-morbidities such as coronary artery disease (CAD) (p < 0.001 and p = 0.03, respectively).
Overall, the distribution of breast cancer subtypes across our patient cohort is consistent with those previously reported [9], with HR+/Her2− tumors being the most common (52.5%), followed by Her2+ breast cancer (30.9%, HR− and HR+ combined) and TNBC (14%) (Table 1). When we evaluated the distribution of breast cancer subtypes across the three BMI subgroups, we noted that obese women were more likely to present with HR+ Her2− breast cancer as expected (58.1%). However, obese women and overweight women were also more likely to present with TNBC, as compared with normal weight women (13.9, 18.5 and 10.2%, respectively). Interestingly, normal weight women were more likely to present with Her2+ tumors than overweight and obese women: 36.6 versus 30.7 and 26.8% (HR− and HR+ combined), respectively. The difference in the distribution of breast cancer subtypes across the three BMI strata was statistically significant (p < 0.008).
As TNBC is known to be more common in black women [12], our results may merely be attributed to the fact that there were more black women in the overweight and obese patient subgroups. To exclude this possibility, we performed a Cochran–Mantel–Haenszel test, examining the distribution of tumor receptor subtypes among women of different BMI, stratified by race. Our results demonstrated that there was a statistically significant association between breast cancer subtypes and BMI independent of race, (Table 2, M2 = 13.4, df = 6, p = 0.04).
Univariate analyses demonstrated that many variables including age at diagnosis, Black race, larger tumor size, higher tumor grade, presence of lymphovascular invasion, higher nodal stage, presence of extracapsular extension, HR+ HR+ subtype and the presence of diabetes, CAD, chronic obstructive pulmonary disease, and presence of local recurrence and metastases were associated with worse overall survival. Interestingly, of the three BMI subgroups, only the overweight subgroup was associated with worse disease-free survival along with black race, larger tumor size, higher tumor grade, presence of lymphovascular invasion, higher nodal stage, presence of extracapsular extension, HR− Her2+ subtype, TNBC subtype, the use of mastectomy as initial surgery type, and the presence of local and distant recurrence (Table 3).
On multivariate analysis using a cox-proportional hazard model (Table 4), we found that black race, larger tumor size, TNBC subtype, and the presence of at least one co-morbidity were associated with worse OS. Breast cancer subtype and BMI were not associated with OS or disease-free survival (DFS). In addition, our analysis demonstrated that larger tumor size, higher tumor grade, and the presence of extracapsular extension were independent predictors of worse DFS.
Discussion
Obesity has emerged as an important prognostic factor in breast cancer. A prospective study of over 500,000 women revealed a stepwise increase in worsening prognosis and increased mortality with each successive increase in BMI [13]. While this association has been repeatedly shown, the relationship between obesity and specific breast cancer characteristics, such as hormone receptor status and molecular subtype, is less clear [14]. Given the rising rate in obesity and its association with breast cancer risk and worse outcome, we are interested in understanding if there is a significant association between obesity and the pathogenesis of breast cancer, specifically if overweight or obese women may be more prone to develop breast cancer of a specific subtype.
Our results demonstrated, for the first time, that breast cancer subtype distribution varied significantly according to BMI status. Normal weight women were more likely to present with Her2+ breast cancer, while overweight and obese women were more likely to present with TNBC and, as expected, HR+ Her2− breast cancer. Our study was feasible due to the unique availability of receptor status including Her2, which has been consistently reported since 1990s in our patient cohort even before the 2005 landmark study that demonstrated the benefit of trastuzumab in early-stage breast cancer [15]. This dataset allowed us to ascertain the distribution of breast cancer subtype in a large patient cohort diagnosed and treated at a single institution between 1998 and 2013. Prior studies have examined the relationship between obesity and breast cancer subtype, but receptor status was not consistently available rendering analyses limited to a small number of patients [10].
Our results demonstrating a distinct distribution of breast cancer subtypes in overweight and obese women may be attributed to the metabolic consequences of obesity on breast cancer pathogenesis [16–18]. The association between obesity, insulin resistance, and hormone receptor-positive breast cancer has been well established [19]. The association between obesity and TNBC remains unclear except that obesityinduced chronic inflammatory state may activate molecular pathways that favor the pathogenesis of TNBC. Our finding that the rate of Her2+ breast cancer subtype was higher in normal weight than in overweight and obese women was indeed surprising and warrants further investigation.
One of the limitations of this study included the retrospective design and reliance on BMI to assess obesity. While easy to ascertain from epidemiologic data, BMI is an imperfect measurement of obesity and is unable to discriminate between different body compositions, notably patients with increased central adiposity. By grouping all obese patients into one BMI category (BMI > 30), we may have lost the ability to discriminate between the potentially greater impact of fat mass among patients with BMIs of 40 and beyond as demonstrated by Neuhauser et al. [20]. Another limitation of our study is that we have excluded breast cancer patients who did not have surgery at our institution.
In summary, our results demonstrated that overweight and obese women were more likely to present with TNBC, an aggressive breast cancer subtype, compared to normal weight women. Furthermore, our results suggested that normal weight women were more likely to present with Her2+ breast cancer compared to overweight and obese women. Future studies are needed to understand the molecular mechanisms that drive this subtype distribution differences across BMI subgroups. Ongoing studies leveraging large-scale, multi-omics analyses may shed light on the genetic and epigenetic interactions that underlie the pathophysiologic association between body weight and specific breast cancer subtypes.
References
Flegal KM, Carroll MD, Kit BK, Ogden CL (2012) Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA 307:491–497
Ogden CL, Carroll MD, Kit BK, Flegal KM (2014) Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA 311:806–814
Lorincz AM, Sukumar S (2006) Molecular links between obesity and breast cancer. Endocr Relat Cancer 13:279–292
Hopkins BD, Goncalves MD, Cantley LC (2016) Obesity and cancer mechanisms: cancer metabolism. J Clin Oncol 34:4277–4283
Jiralerspong S, Goodwin PJ (2016) Obesity and breast cancer prognosis: evidence, challenges, and opportunities. J Clin Oncol 34:4203–4216
Pierobon M, Frankenfeld CL (2013) Obesity as a risk factor for triple-negative breast cancers: a systematic review and meta-analysis. Breast Cancer Res Treat 137:307–314
Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA et al (2000) Molecular portraits of human breast tumours. Nature 406:747–752
Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H et al (2001) Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 98:10869–10874
Kohler BA, Sherman RL, Howlader N, Jemal A, Ryerson AB, Henry KA (2015) Annual report to the Nation on the Status of Cancer, 1975–2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. J Natl Cancer Inst 107(6):djv048. doi:10.1093/jnci/djv048
Morimoto LM, White E, Chen Z, Chlebowski RT, Hays J, Kuller L et al (2002) Obesity, body size, and risk of postmenopausal breast cancer: the Women’s Health Initiative (United States). Cancer Causes Control 13:741–751
Onitilo AA, Engel JM, Greenlee RT, Mukesh BN (2009) Breast cancer subtypes based on ER/PR and Her2 expression: comparison of clinicopathologic features and survival. Clin Med Res 7:4–13
Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K et al (2006) Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 295:2492–2502
Calle EE, Kaaks R (2004) Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer 4:579–591
Gershuni VM, Ahima RS, Tchou J (2016) Obesity and breast cancer: a complex relationship. Curr Surg Rep 4:1–9
Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE Jr, Davidson NE et al (2005) Trastuzumab plus adjuvant chemotherapy for operable HER2− positive breast cancer. N Engl J Med 353:1673–1684
Harvie M, Hooper L, Howell AH (2003) Central obesity and breast cancer risk: a systematic review. Obes Rev 4:157–173
Ladoire S, Dalban C, Roche H, Spielmann M, Fumoleau P, Levy C et al (2014) Effect of obesity on disease-free and overall survival in node-positive breast cancer patients in a large French population: a pooled analysis of two randomised trials. Eur J Cancer 50:506–516
Jung SY, Hursting SD, Guindani M, Vitolins MZ, Paskett E, Chang S (2014) Bioavailable insulin-like growth factor-I inversely related to weight gain in postmenopausal women regardless of exogenous estrogen. Cancer Epidemiol Biomark Prev 23:534–544
Rose DP, Vona-Davis L (2010) Interaction between menopausal status and obesity in affecting breast cancer risk. Maturitas 66:33–38
Neuhouser ML, Aragaki AK, Prentice RL, Manson JE, Chlebowski R, Carty CL et al (2015) Overweight, obesity, and postmenopausal invasive breast cancer risk: a secondary analysis of the women’s health initiative randomized clinical trials. JAMA Oncol 1:611–621
Acknowledgements
We would like to thank the Penn Data Store for their assistance in assembling the information used in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Rights and permissions
About this article
Cite this article
Gershuni, V., Li, Y.R., Williams, A.D. et al. Breast cancer subtype distribution is different in normal weight, overweight, and obese women. Breast Cancer Res Treat 163, 375–381 (2017). https://doi.org/10.1007/s10549-017-4192-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-017-4192-x