Abstract
Rationale
Aging is characterized by a decrease in N-methyl-D-aspartate receptors (NMDARs) in the hippocampus, which might be one of the factors involved in the age-dependent cognitive decline. D-Cycloserine (DCS), a partial agonist of the NMDAR glycine recognition site, could improve memory deficits associated to neurodegenerative disorders and cognitive deficits observed in normal aging.
Objectives and Methods
The aim of the present study was to explore whether DCS would reverse age-dependent memory deficits and decreases in NMDA receptor subunits (GluN1, GluN2A, and GluN2B) and the presynaptic protein synaptophysin in Wistar rats. We investigated the effects of pre-training infusions of DCS (10 μg/hemisphere) in the ventral hippocampus on two hippocampal-dependent learning tasks, the social transmission of food preference (STFP), and the Morris water maze (MWM).
Results
The results revealed that infusions of DCS administered before the acquisition sessions rescued deficits in the STFP retention and MWM reversal learning in old rats. DCS also significantly increased the hippocampal levels of synaptophysin in old rats, which correlated with STFP and MWM performance in all tests. Moreover, although the levels of the GluN1 subunit correlated with the MWM acquisition and reversal, DCS did not enhance the expression of such synaptic protein.
Conclusions
The present behavioral results support the role of DCS as a cognitive enhancer and suggest that enhancing the function of NMDARs and synaptic plasticity in the hippocampus may be related to improvement in social memory and spatial learning reversal in aged animals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The physiological process of aging involves a decline in brain function that causes a progressive cognitive loss (Yankner et al. 2008). Numerous studies indicate that the hippocampus (HPC) is an early target of age-related structural and physiological changes that may contribute to difficulties in learning and memory (Driscoll and Sutherland 2005; Rosenzweig and Barnes 2003; Stephens et al. 2011). Some of these changes are associated to hippocampal synaptic plasticity elements (Hajjar et al. 2013; Martin et al. 2000), such as the reduction of postsynaptic N-methyl-d-aspartate receptors (NMDARs) (Liu et al. 2008) and presynaptic proteins, such as synaptophysin, a synaptic vesicle glycoprotein linked to hippocampal connectivity (Smith et al. 2000) widely used as a marker of synaptic plasticity (Counts et al. 2008). The importance of the glutamatergic mechanisms has recently been emphasized as the altered expression of presynaptic and postsynaptic glutamatergic components (v.g. GluN2A and GluN2B subunits) has been proposed as a marker for age-related cognitive deficits (Ménard et al. 2015). Such data suggest that boosting the function of NMDARs and reducing age-dependent decreases in specific synaptic proteins in the HPC may be effective approaches to alleviate cognitive deficits in the elderly (Billard 2015; Kumar 2015). In this regard, the ventral hippocampus (vHPC), which plays a relevant role in relational memory both spatial or contextual (Ferbinteanu et al. 2003; Loureiro et al. 2012, Fanselow and Dong 2010; Kjelstrup et al. 2008) and non-spatial (Portero-Tresserra et al. 2014), may be a suitable target as it seems more vulnerable than the dorsal HPC (dHPC) to the age-associated decrease in NMDARs (Liu et al. 2008; Magnusson et al. 2006).
Many data indicate that d-cycloserine (DCS), a partial agonist at the glycine-binding site of the NMDARs (Monahan et al. 1989) that enhances receptor activation in the presence of glutamate (Norberg et al. 2008) and rapidly goes through the blood–brain barrier (Walker and Murdoch, 1957), may be considered an optimum approach to reduce cognitive impairments associated with normal aging. Thus, it has been shown in vitro that DCS facilitated NMDAR-dependent synaptic potentials and CA1 synaptic plasticity mechanisms, such as long-term potentiation (LTP) and long-term depression (LTD), which were significantly weakened in aged animals (Billard and Rouaud 2007). Moreover, DCS prevented the scopolamine-induced LTP suppression in the hippocampus of adult rats (Portero-Tresserra et al. 2014). In addition, intra-hippocampal infusions of DCS enhanced fear extinction and the expression of the NMDAR subunit GluN2B in the HPC of young rats (Ren et al. 2013), which may contribute to enhancing LTP and memory (Clayton et al. 2002). As for its behavioral effects when administered systemically in aged rats, DCS reduced hippocampal-dependent learning and memory deficits in tasks such as the Morris water maze (MWM) (Aura and Riekkinen 2000; Aura et al. 1998; Baxter et al. 1994; Riekkinen et al. 1997) and trace eyeblink conditioning (Thompson and Disterhoft 1997). DCS has also been shown to improve cognition in Alzheimer’s disease patients (Lin et al. 2014; Schwartz et al. 1996) and to enhance activity-dependent plasticity and accelerate the acquisition of two learning tasks in healthy young adults (Forsyth et al. 2015). Nevertheless, such data indicate that the behavioral and cellular effects of DCS intracerebral administration have been not previously studied in old subjects, thus limiting the comprehension of the specific brain areas in which this substance may act and the neurophysiological mechanisms involved in the rescue of age-related cognitive deficits.
In this context, the aim of the present study was to examine in aged rats whether DCS administered in the vHPC may recover relational memory deficits and increase NMDAR subunits and synaptophysin levels in the HPC. For this purpose, two hippocampal-dependent learning paradigms were used: the social transmission of food preference (STFP) and the MWM. STFP is a paradigm of social learning involving an ethologically meaningful test of olfactory memory, with no explicit spatial components (Bunsey and Eichenbaum 1995). It exhibits some of the key features of relational memory in that the information can be quickly acquired and the memory has to be expressed flexibly, that is, in a test situation very different from the circumstances of the original (Alvarez et al. 2001). Previous experiments (Carballo-Márquez et al. 2009) have confirmed the relevance of the vHPC in the STFP, as injections of the muscarinic receptor antagonist scopolamine into this region deteriorated memory, which was rescued by intra-vHPC DCS administration in young rats (Portero-Tresserra et al. 2014). Moreover, STFP seems to be an appropriate model for evaluating age-related deficits as demonstrated by the fact that old rats forget the socially transmitted food preference more rapidly than young rats (Countryman and Gold 2007). Indeed, olfaction-based tasks, such as STFP, are especially relevant in aging studies as smell loss disturbances are present in old subjects (Robitsek et al. 2008) and the formative stages of neurodegenerative diseases (Kovács 2004). Nevertheless, as far as we know, no previous studies evaluated the effects of cognitive enhancers in social olfactory learning in aging.
Although there is some controversy, the MWM (Morris et al. 1982) may be considered a relational learning paradigm in which the subject is required to learn complex spatial relationships of visual cues. In a relational version of this task, rats acquire and remember the location of an escape platform guided by a configuration of spatial cues surrounding the maze, entering to it from different starting points (Gerlai et al. 2002; Vorhees and Williams 2014). Interestingly, the MWM task may also be appropriate to assess cognitive flexibility using a reversal learning test (García-Brito et al. 2017). Although the dorsal HPC has extensively been related to MWM performance, the vHPC is also activated during MWM training (Snyder et al. 2009) contributing to general aspects of spatial processing (Ruediger et al. 2012). An age-associated decline in the MWM performance has been well established (Kennard and Woodruff-Pak 2011; Klencklen et al. 2012) in relation to alterations in the HPC, such as a reduction in the size of postsynaptic densities of perforant synapses in CA1 (Nicholson et al. 2004) and decrease in synaptophysin in CA3 (Smith et al. 2000).
In the present study, we analyzed the effects of DCS infusions into the vHPC in young (3 months) and old (24 months) rats to test whether the same administration protocol (dose and brain region) that had been effective in preventing scopolamine-induced cognitive deficits in adult rats would prevent age-associated deficits (Portero-Tresserra et al. 2014). In the STFP, the DCS treatment was administered before the acquisition (social interaction) and memory was assessed in a subsequent drug-free 72-h test. In the MWM, DCS was administered before each of the five acquisition sessions and memory was evaluated in a drug-free 72-h probe test and a subsequent reversal learning test. We also determined, using western blotting, the effects of DCS on the hippocampal expression of three main subunits of the NMDARs (GluN1, GluN2A, and GluN2B) and the synaptic vesicle protein synaptophysin.
Materials and methods
Subjects
For this experiment, 57 male Wistar rats from our laboratory’s breeding stock (Prolabor, Charles River Laboratories, Abresle, France) were used. To reliably increase lifespan and avoid the development of tumors and other health problems (Speakman and Mitchell 2011), 25 old animals (age = 23–24 months; weight = 499.88 g, SD = 66.48) were kept under conditions of caloric restriction (CR) from the age of 4 months with approximately a 30% reduction of total food intake (18–20 g/day) under free access. In addition, as it has been suggested that lifetime diets may have an impact on spatial learning, as shown by a different pattern of age-related cognitive decline across lifespan (Adams et al. 2008; Ménard et al. 2014), a set of ten old rats (age = 23–24 months; weight = 815.52 g, SD = 83.2) fed ad libitum (AL) was added to control the effect of the diet on behavioral performance. Old animals were pair-housed from the beginning of the experiment. Procedures for housing are explained in detail elsewhere (Portero-Tresserra et al. 2013b). Moreover, 22 young rats (age = 3–4 months; weight = 370.9 g, SD = 25.79) and an additional set of 28 young rats (mean age = 2 months; mean weight = 253.17 g, SD = 27.71), serving as demonstrator subjects in the STFP task, were used. Rat-chow pellets (Scientific Animal Food and Engineering, Augy, France) were provided AL to young and to old rats except during habituation, acquisition, and test sessions of the STFP task, in which all the rats were submitted to a food restriction schedule (12 g/day to maintain body weight at 85% of their initial weight). Schematic representation showing the schedule of the behavioral and biochemical procedures is shown in Fig. 1.
Surgery
All the rats, except the young subjects, underwent stereotaxic implantation of bilateral chronic guide cannulae into the vHPC: AP − 5.0 mm, ML ± 5.0 mm, and DV − 6.8 mm (Paxinos and Watson 1997) under anesthesia with 150 mg/kg of Imalgene ketamine chlorhydrate (Merial, Lyon, France) and 0.08 mg/kg Rompun xylazine (Bayer, Barcelona, Spain) (see Portero-Tresserra et al. 2014). After surgery, the rats were returned to their home cages for 10 days before behavioral training began.
Microinfusion procedure
Two days previous to training (one time/day), rats were adapted to a mock infusion protocol (no solutions injected) in order to minimize any stress associated with the procedure. The rats were administered 10 μg/hemisphere of DCS (Sigma-Aldrich, Madrid, Spain) or phosphate-buffered saline (PBS, 0.1 M, pH 7.4) infusions in the vHPC 20 min before the STFP interaction session and the five MWM acquisition sessions. The solutions were infused bilaterally in a volume of 0.5 μl/hemisphere for 2 min (plus 1 min to allow for diffusion) following procedures described previously (Portero-Tresserra et al. 2013b).
The injection parameters were selected on the basis of previous studies in which 10 μg/hemisphere of DCS in the vHPC reversed deficits produced by scopolamine in STFP memory consolidation (Portero-Tresserra et al. 2014). The same dose of DCS also produced beneficial effects on memory when injected in other brain regions such as the dorsal hippocampus (Ohno and Watanabe 1996), the basolateral amygdala (BLA) (Espejo et al. 2016; Portero-Tresserra et al. 2013a), or the prelimbic cortex (PLC) (Portero-Tresserra et al. 2013b; Villarejo-Rodríguez et al. 2010). The time of drug administration was based on previous studies in which DCS was administered in different brain areas 20 min prior to behavioral memory tasks showing cognitive impairment reversion (Portero-Tresserra et al. 2013b; Villarejo-Rodríguez et al. 2013).
Behavioral procedure
Social transmission of food preference
Habituation
In the STFP task, all the rats were habituated, trained, and tested in their own cages following the procedures described elsewhere (Portero-Tresserra et al. 2013b). In essence, after 5 days of food restriction, prior to surgery, observers and demonstrators were habituated during 3 days to eating powdered chow (Scientific Animal Food and Engineering, Augy, France) from glass jars to minimize neophobia. The observers were habituated for 2 h on the first day, 1 h the second day, and 45 min the third day, and the demonstrators were habituated for 45 min for each of the 3 days. For the observers, a one 45-min rehabituation session was repeated 9 days after surgery. In all the sessions, the mean g of regular ground food eaten was recorded. After the rehabituation session, animals were food-restricted for 1 day before the training–testing sessions began.
Acquisition and test
The STFP training followed procedures explained elsewhere (Portero-Tresserra et al. 2013b). The task began when a demonstrator rat was allowed to eat food flavored with 2.2% cocoa (Oxfam Fairtrade, Gent, Belgium) or 1% cinnamon (Carmencita, Alicante, Spain) for 30 min. The observers received a bilateral intra-vHPC infusion of PBS (VEH) or DCS 20 min before the 30-min social interaction session (acquisition). The 45-min STFP test was performed 72 h after acquisition, and a preference score (percentage of trained food) was calculated as follows: 100 × (weight of trained food eaten/weight of all food eaten). The number of times each observer sniffed the muzzle, body or anogenital region of the demonstrator, fighting, and grooming (acquisition) and the number of jar climbs (test) were recorded (JVC, Everio Model GZ-X900). To rule out olfactory alterations, an additional olfactory perception test (described in Portero-Tresserra et al. 2013b) was conducted on a sample of subjects (n = 7 per group).
Morris water maze
Acquisition
Five days after finishing the STFP procedures, all the rats performed the MWM task. The maze consisted of an elevated black-colored circular pool (2 m diameter; 60 cm above the floor) with water maintained at 22 ± 2 °C. The pool was placed in the middle of a dark room and surrounded by black curtains forming a circular enclosure (2.4 m in diameter). A submerged circular platform (11 cm in diameter; 2 cm below the water surface) was placed in the middle of the southeast (SE) quadrant. The location of the platform could only be encoded relative to distal visual landmarks surrounding the MWM. In all the sessions, the swim paths of the animals were recorded using a video camera connected to a computer running tracking-software (Smart Video Tracking System, Version 2.5, Panlab, Barcelona, Spain). The task acquisition sessions consisted of four daily trials (average intertrial interval, ITI, 120 s) for five consecutive days. Twenty minutes before each acquisition session, the rats were infused intra-HPC with DCS or PBS. In each trial, the rats were placed in the pool with their noses pointing toward the wall at one of the starting points (randomly N, S, E, or W), and they were required to find the platform whose position remained stable across trials and days in the SE quadrant. If the animal failed to find the platform within 90 s, it was manually guided to the platform, and after 15 s, it was removed from the pool. When a rat found the platform, it was left there for 15 s and then removed from the pool. The latency to find the hidden platform, path length, swim speed, and thigmotaxis (percentage of time spent near the maze walls) was recorded.
Probe test and reversal learning
A drug-free single probe trial was conducted 72 h after the last acquisition session. The platform was removed from the pool, and rats were allowed to swim freely for 60 s starting from the E cardinal point, and the percentage of time spent in the target quadrant, platform crossings, swim speed, thigmotaxis, and path length were recorded. Immediately after, a reversal learning protocol was conducted. Firstly, the experimenter guided the rat to the platform located at the opposite quadrant (NW) of the original acquisition. After 15 s, the rat was removed from the pool and three trials (average ITI 120 s) were carried out, with the platform remaining in the NW quadrant. Latency to find the hidden platform, swim speed, thigmotaxis, and path length was recorded.
Tissue collection and processing
Upon completion of all the behavioral tests (24 h after the MWM reversal learning session), a cohort of old and young rats (Old-VEH, n = 5; Old-DCS, n = 5; Young-VEH, n = 6; Young-DCS, n = 6) were intracardially perfused and their brains were post-fixed following procedures explained elsewhere (Carballo-Márquez et al. 2009). Coronal 40-μm sections were processed for acetylcholinesterase histochemistry, essentially as described earlier (Paxinos and Watson 1997). The sections were examined under a light microscope (Olympus BX 41; Olympus Optical CO, LTD, Tokyo, Japan), and microphotographs of the cannulae placements were taken using a digital camera (Olympus DP70). Also, 24 h after finishing the behavioral tests, another sample of old and young rats (Old-VEH, n = 6; Old-DCS, n = 6; Young-VEH, n = 5; Young-DCS, n = 3) were decapitated, their brains removed rapidly, and the HPC dissected on ice, weighed, frozen, and stored at − 80 °C to perform western blot analyses.
Semi-quantitative western blot analyses
Hippocampal samples (see “Tissue collection and processing”) were collected in lysis buffer (25 mM Tris-HCl, 150 mM NaCl, 0.5% sodium deoxycholate, 0.1% SDS, 1% NP-40, pH 7.6) at different time-points. Lysates were homogenated with pellet pestle (Sigma-Aldrich Corp., Madrid, Spain), sonicated, and total protein amount quantified by BCA assay (Pierce Chemical Co.). Equal amounts of protein (20 μg/well) of each sample were resolved in SDS-PAGE and transferred to nitrocellulose membrane (Whatman, Dassel, Germany) at the same time using a Criterion Blotter (Bio-Rad; Hercules, CA, USA) at 100 V for 1 h. Membranes were blocked with 5% non-fat dry milk in Tris-buffered saline (TBS; 75 mM NaCl, 1.5 mM KCl, 12.4 mM Tris, pH 7.4) for 1 h at 20–25 °C and incubated overnight with the corresponding primary antibody diluted in 5% (w/v) bovine serum albumin (BSA): monoclonal mouse anti-NMDAR1 (BD Pharmingen, USA), rabbit polyclonal anti-NMDAR2A (Chemicon International), anti-NMDAR2B (Chemicon International), monoclonal mouse anti-synaptophysin (Sigma, USA), and mouse anti-β-tubulin (Becton-Dickinson, Franklin Lakes, NJ, USA). After several washes with TBS 0.1% Tween 20, membranes were incubated for 1 h with an appropriate secondary antibody conjugated with horseradish peroxidase (1: 3000): anti-mouse-HRP (Dako Denmark, Glostrup, Denmark) and anti-rabbit-HRP (Invitrogen Corp., USA). All the samples to be compared were processed at the same time, simultaneously transferred into a membrane, and incubated with the same antibody dilution. Blots were developed using a chemiluminescent mix 1:1 (0.5 M luminol, 79.2 mM p-coumaric acid, 1 M Tris-HCl; pH 8.5) and (8.8 M hydrogen peroxide, 1 M Tris-HCl; pH 8.5), and visualized using a GeneGnome HR chemiluminescence detection system coupled to a CCD camera (Syngene; Cambridge, UK). The apparent molecular weight of proteins was determined by calibrating the blots with pre-stained molecular weight markers (All Blue; Pierce Chemical Co., USA). Chemiluminescence signals of the obtained bands were all within the linear range of the imaging system and were not saturated. Densitometry was carried out using ImageJ software (National Institute of Health, Bethesda, MD, USA). The total content of each specific protein was assayed after membrane stripping (0.1 mM glycine; pH 2.3) for 1 h at 20–25 °C, blocked again, and incubated with corresponding primary antibody.
Data analysis
All statistical analyses were performed using SPSS v23 software (IBM Corporation). To control the effects of the old rats’ diet on behavioral performance, preliminary ANOVAs were carried out. In the STFP, Diet (CR or AL) and Drug (VEH or DCS) factors, as the independent variables, and percentage of trained food, as the dependent variable, were considered. In the MWM, the fact that very few of the old rats from the AL-fed group had reached this phase of the experiment (n = 5) did not allow for a subdivision into DCS and VEH, and consequently, all the AL old rats were assigned to a VEH group (see “Effects of the old rats’ diet on behavioral performance”). Therefore, the Diet factor (CR or AL) was the independent variable and latency in finding the hidden platform throughout five acquisition sessions (each session: four-trial average score) the dependent variable.
After such a preclusive analysis, ANOVA analysis, followed by post hoc contrasts (multiple comparisons were performed with the Bonferroni correction), assessed the effects of Age (Old or Young) and Drug (VEH or DCS) on STFP and MWM performance. In the STFP, the dependent variables were percentage of trained food, total food eaten, and jar climbs (which evaluated the motivation to eat and explore, respectively). In addition, a one-sample t test against a constant (50) was used for each group to determine whether the percentage of trained food eaten was different from the chance level (50%). Further ANOVAs were carried out to evaluate whether all the animals had similar opportunities of learning considering the following dependent variables of the interaction session: sniffs of the demonstrator’s muzzle, body, anogenital region, fightings, and grooming. Moreover, regular food (mean g of food eaten during the last rehabituation session prior to training) and new food (mean g of total food eaten, trained + untrained, during the test) were also analyzed to study possible neophobic effects. Regarding the olfactory perception test, an additional ANOVA was carried out with latency in finding the buried cookie as the dependent variable. In the MWM, the dependent variables were the latency in finding the hidden platform in the acquisition and reversal and the percentage of time spent in the target quadrant in the probe test. In addition, a one-sample t test against a constant (25) was used for each group in order to evaluate whether the percentage of time spent in the target quadrant was different from the chance level (25%). Finally, swim speed, path length, platform crossings, and thigmotaxis were considered as dependent variables to control motor and emotional alterations. P values less than 0.05 were considered to be significant.
ANOVA was carried out to assess the effects of DCS treatment on protein levels in the HPC, with Age (Old or Young) and Drug (VEH or DCS) as the independent variables and the percentage of NMDAR subunits (GluN1, GluN2A, and GluN2B) and synaptohysin as the dependent variables. In order to analyze relationships between protein levels and behavior, Pearson’s correlations were made between GluN1 and synaptophysin and the performance during STFP memory test, MWM acquisition, test, and reversal.
Results
Histology and final sample
Histological analysis of the sections processed with acetylcholinesterase histochemistry showed that the cannula tips were located bilaterally in the vHPC, within the area delimited by CA3 and CA1 (Fig. 2a) along coordinates from 4.52 to 5.80 mm posterior to bregma (Fig. 2b) according to the stereotaxic atlas (Paxinos and Watson 1997). Seven rats were excluded from behavioral data analyses due to several problems: technical complications during drug infusion (n = 2), misplaced cannulae (n = 3), or on the grounds of being considered outliers in some behavioral measures (n = 2). Additionally, two old rats died after surgery and four more prior to the MWM task.
a Photomicrograph (magnification ×10) of acetylcholinesterase histochemistry at the level of the ventral hippocampus showing the cannula tracks of a representative subject. CA cornu ammonis, DG dentate gyrus. b Cannula tip placements (microinfusors extending 1 mm below) for Old-VEH (white circles), Old-DCS (black circles), Young-VEH (white triangles), and Young-DCS (black triangles) groups
Behavioral testing
Effects of the old rats’ diet on behavioral performance
In the STFP task, no differences in the percentage of trained food eaten between both groups of old rats (AL, CR) were found (Diet factor: F[1,29] = 0.641, P = 0.431; Diet × Drug factor: F[1,29] = 0.034, P = 0.856), and DCS improved the performance of old animals irrespectively of type of diet (Drug factor: F[1,29] = 11.631, P = 0.002). Therefore, in the subsequent analyses (see “Social transmission of food preference and olfactory perception test”), all the old rats were pooled into two groups (VEH and DCS). In the MWM, both aged VEH group (AL and CR) showed a decrease in latencies in finding the hidden platform along the five sessions (F[4,56] = 3.611, P = 0.011), although the Old-AL-VEH rats exhibited longer latencies (F[1,14] = 6.011, P = 0.028). Such a finding led us to exclude AL subjects from subsequent analysis (see “Morris water maze”) (data shown in Table 1).
Social transmission of food preference and olfactory perception test
The final sample consisted of four groups: Old-VEH, n = 15; Old-DCS, n = 14; Young-VEH, n = 10; and Young-DCS, n = 10. The results revealed that DCS had different effects depending on the age of rats (interaction Age × Drug: F[1,45] = 8.357, P = 0.006) (Fig. 3). Thus, the untreated old rats showed the lower percentage of trained food eaten (performance similar to chance level, t(14) = 2.077, P = 0.067). In contrast, the DCS-treated old rats showed a performance not different from that of the young rats (treated or untreated) and significantly better than the Old-VEH rats (P = 0.001). Moreover, Old-VEH animals differed from Young-VEH group (P = 0.001). Accordingly, the old rats that received DCS performed above chance level (t(13) = 11.840, P < 0.0001), similarly to both groups of young rats (t(9) = 5.713, P = 0.001, t(9) = 3.862, P = 0.004). The analysis of the social interaction variables revealed that the old rats had performed fewer sniffs of the demonstrator’s anogenital region (F[1,45] = 6.214, P = 0.016), but not of any of the other body regions or other behaviors recorded (all F’s < 1.5, all P’s > 0.05). The neophobia analysis demonstrated that the old rats ate less regular food in the rehabituation session compared with the young rats (F[1,45] = 12.869, P = 0.001). However, performance was not related to deficits in olfactory sensitivity due to DCS since no differences were observed in the latency in finding the buried cookie due to drug infusion (all P > 0.05). Moreover, no differences were found due to Age or Drug on food consumption and jar climbs during the 72-h test (all F’s < 1.2, all P’s > 0.05).
Percentage of trained food eaten (± SE) in the STFP test for each group. Untreated old rats expressed a significant poor memory of the task compared to DCS-treated old rats and untreated young rats; **P < 0.01. The dotted line represents chance level; only the Old-VEH group showed a performance similar to chance
Morris water maze
The final sample was made up of the following four groups: Old-VEH n = 11, Old-DCS n = 9, Young-VEH n = 10, Young-DCS n = 10). The results in the MWM task revealed that all groups progressively reduced their latency to locate the platform during acquisition (Session factor: F[4,144] = 20.431, P < 0.0001), although old rats showed a poorer performance than young animals (Age factor: F[1,36] = 17.238, P < 0.0001). However, DCS did not affect the acquisition performance (Drug factor: F[1,36] = 2.631, P = 0.114; and interaction Age × Drug: F[1,36] = 0.405, P = 0.529) (Fig. 4a). As for the control variables speed and thigmotaxis, analysis revealed that the young rats swum faster and spent less time at the walls than the old rats, and that the administration of DCS decreased swim speed and thigmotaxis regardless of Age (Fig. 4b, c) (statistical analyses shown in Table 2).
MWM acquisition (5 days of training, four trials/day). a Latency in finding the hidden platform (± SE). b Thigmotaxis measured as the percentage of time spent in the walls (± SE). c Swim speed (± SE). Young animals showed a decrease in thigmotaxis and swim speed compared to old animals (age factor). DCS administration decreased swim speed and thigmotaxis regardless of age (drug factor)
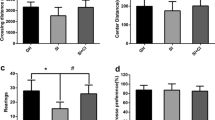
During the 72-h probe memory test (Fig. 5a), although the old untreated rats exhibited a lower percentage of time in the target quadrant, their performance did not differ from the remaining groups, as factors Age (F[1,36] = 0.910, P = 0.346), Drug (F[1,36] = 1.442, P = 0.238), and Age × Drug (F[1,36] = 1.695, P = 0.201) were not statistically significant. That is, all groups remembered the location of the platform, since they remained in the target quadrat more time than just at chance level, i.e., spent more time in the target than in the other quadrants, throughout the whole test (all t’s > 4.4, P’s < 0.05). However, when we examined performance during the last 30 s of the session, which may display a more stable behavior, only DCS-treated rats did so (Old-VEH: t(10) = 1.499, P = 0.165; Old-DCS: t(8) = 2.834, P = 0.022; Young-VEH: t(9) = 1.596, P = 0.145; Young-DCS: t(9) = 3.793, P = 0.004) (Fig. 5b). Regarding the analysis of the control variables during the probe trial, although it has been shown that Old-VEH group presented more thigmotaxic behavior than Old-DCS (Fig. 5c) (P = 0.003), there were no significant differences in the variable speed (Fig. 5d) (statistical analysis shown in Table 2).
MWM probe test (one trial). a Percentage of time spent in the target quadrant (± SE) during the whole test. b Percentage of time spent in the target quadrant (± SE) during the last part of the test. Asterisk shows statistically significant differences from chance level in time spent in the target for each group. *P < 0.05; **P < 0.01. c Thigmotaxis measured as the percentage of time spent in the walls (± SE) during the whole test. The Old-VEH group showed more thigmotaxic behavior than the Old-DCS group (**P < 0.01). d Swim speed (± SE) during the whole test
In the reversal learning, statistically significant effects of the factors Age (F[1,36] = 14.619, P = 0.001) and Age × Drug (F[1,36] = 4.615, P = 0.038) were found, suggesting that the Old-VEH group exhibited impaired cognitive flexibility, as the latency (average of the three trials of the test) in finding the new location of the platform was longer than the young groups (P < 0.001). Nevertheless, Old-DCS group presented a better performance during the reversal learning since their latencies in finding the new location were shorter than the Old-VEH group (P = 0.015) and did not differ to the ones of young subjects (Fig. 6a). Regarding the thigmotaxis response (Fig. 6b), aged rats showed higher thigmotaxic behavior during the whole reversal session. However, DCS administration did no modify this variable and no between-group differences in swim speed (Fig. 6c) were observed (statistical analyses shown in Table 2).
MWM reversal learning (one three-trial session). a Latency in locating the hidden platform (± SE). b Thigmotaxis measured as the percentage of time spent in the walls (± SE). c Swim speed (± SE). Old-VEH rats showed a worse performance (i.e., they needed significantly more time to find the platform; asterisk shows statistically significant between-group differences. *P < 0.05; ***P < 0.001. No significant differences were found in any of the control variables
Protein levels in the hippocampus
The main analysis revealed a significant effect of the Age × Drug factor on synaptophysin protein levels (F[1,20] = 6.695, P = 0.02). The contrast analyses showed that Old-VEH expressed lower levels of synaptophysin than Old-DCS (P = 0.008). Furthermore, the analysis of subunit GluN1 levels showed that the interaction Age × Drug (F[1,20] = 4.092, P = 0.06) tended to be statistically significant and no statistically significant effects were observed in any of the other NMDAR subunits (all F’s < 1.5, all P’s > 0.4) (Fig. 7).
a Representative western blot showing GluN1 subunit, synaptophysin, and β-tubulin expression in HPC from two animals of each experimental group. Integrated density of the bands obtained for b GluN1, c synaptophysin, d GluN2A, and e GluN2B was standardized by β-tubulin and represented as a percentage (± SE) of the control condition Young-VEH. Untreated old rats showed lower protein levels, although the only significant difference was found in synaptophysin when compared to DCS-treated old rats (**P < 0.01)
Pearson correlation analyses performed with the subsample of subjects used for the western blot analyses (Old-VEH, n = 6; Old-DCS, n = 6; Young-VEH, n = 5; Young-DCS, n = 3) showed significant negative correlations between the levels of synaptophysin and the latency in locating the platform during the last session of MWM acquisition (r = − 0.585, P = 0.0071) (Fig. 8a) and the reversal MWM learning (r = − 0.663, P = 0.001) (Fig. 8c), while positive correlations were found between the time spent in the target quadrant during the MWM memory test (r = 0.524; P = 0.018) (Fig. 8b) and the percentage of trained food in the STFP (r = 0.577; P = 0.008) (Fig. 8d). The levels of GluN1 subunit also showed significant negative correlations with the latency in finding the platform during the last session of MWM acquisition (r = − 0.557; P = 0.01) (Fig. 8e) and the MWM reversal learning (r = − 0.442; P = 0.05) (Fig. 8f).
Significant relationships between protein levels and behavior. Pearson correlation analyses between a synaptophysin levels and performance in the last MWM acquisition session, b the probe memory test, c reversal learning, and d the STFP memory test. Pearson correlation analyses between e GluN1 subunit levels and performance in the last MWM acquisition session and f reversal learning
Discussion
The main objectives of the present research were to determine whether the administration of DCS may rescue age-induced memory impairments in two hippocampal-dependent tasks, STFP and MWM, and increase the hippocampal expression of NMDAR subunits and the presynaptic marker synaptophysin, a protein related to synaptic plasticity and memory (Smith et al. 2000). The results demonstrated that pre-acquisition injections of DCS into the vHPC in old rats improved STFP memory and MWM reversal learning, both tested 72 h after task acquisition, and the levels of synaptophysin. In aged rats, DCS injections also tended to increase the levels of GluN1, the NMDAR subunit in which the glycine binding site is located (Kalia et al. 2008), correlating with MWM performance in acquisition and reversal learning.
Regarding the STFP, our results agree with a previous study (Countryman and Gold 2007) showing that old rats exhibited more rapid forgetfulness after STFP training than young rats. In our study, the vehicle old rats exhibited a lower percentage of trained food eaten, with a performance that was not significantly different from the chance level, indicating poorer task retention, in contrast with the old rats administered with DCS and also young rats. However, although intra-vHPC DCS counteracted STFP deficits in aged rats, DCS treatment did not enhance memory in young rats, as in a previous study in which DCS ameliorated scopolamine-induced STFP deficits but did not improve memory in scopolamine-untreated animals (Portero-Tresserra et al. 2014). All these data suggest that DCS may act as a memory enhancer in subjects showing mnemonic deficits (induced by several factors such as cholinergic dysfunction, aging etc.) but may not exert a substantial effect in normal or young subjects (as we will discuss below).
The facilitative effects of DCS on STFP in old animals cannot be attributed to non-mnemonic variables such as changes in social interaction, motivation to eat, or motor behavior, as DCS did not modify any of these variables, with the exception of anogenital sniffs, which is less relevant than the muzzle to transmit food preference. With regard to neophobia, although aged rats ate less ground food than young rats in the rehabituation session, both groups showed a similar intake during the test, which suggests that old rats did not show a neophobic reaction to the flavored food. As for olfactory perception, the administration of DCS did not modify latency in the test, although old rats, regardless of the substance injected, took longer to find the buried cookie than young rats, which may be attributed to a possible age-related decrease in olfactory sensitivity (Kraemer and Apfelbach 2004). Taking into account all the above aspects, STFP would seem to be a suitable learning model for the study of age-related relational memory deficits and, therefore, appropriate for analyzing the properties of cognitive-enhancing compounds.
The findings in the MWM task revealed that all groups gradually reduced latency to locate the platform during acquisition, which is an indicator of spatial learning (Gallagher et al. 1993). Nevertheless, old rats swam more slowly than young rats and showed more thigmotatic behavior, as has also been reported in other studies (Brothers et al. 2013; Novier et al. 2013). In the 72-h memory test, all groups spent a long time in the target quadrant and performed above the chance level when the whole session was analyzed, indicating that all the animals had correctly learned and/or recalled the platform location. However, DCS rats showed a positive effect in the last 30 s of the session, where animals may display a more stable behavior, and such result has been interpreted earlier as prevention of the extinction process (in this trial, the platform is removed), thus strengthening memory retention (Portero-Tresserra et al. 2013b).
In MWM reversal learning, DCS improved performance in aged rats as they showed significantly shorter latencies than the untreated old rats. Furthermore, the latency in locating the platform in the new position shown by Old-DCS rats was similar to that of young rats, suggesting that the administration of DCS into the vHPC may enhance cognitive flexibility in old animals, as previously found with systemic DCS administration (Riekkinen et al. 1998). During reversal learning, there were no significant differences in the swim speed between old and young rats, which suggests that the longer latencies shown by untreated old rats cannot be attributable to motor disabilities, but rather to learning difficulties, thus supporting the possibility of cognitive rigidity (Nieves-Martinez et al. 2012). With regard to thigmotaxic behavior, a measure indicative of fearfulness (Von Lubitz et al. 1993) and/or poor search strategy (Brothers et al. 2013), old rats spent longer swimming near the walls during the acquisition, test, and reversal learning, as has already been reported (Burger et al. 2007; Brothers et al. 2013). Such a stress response may have prevented them from adopting an accurate spatial search strategy (Anderson et al. 2014). Therefore, we cannot reject the possibility that DCS enhanced memory in old rats by reducing stress levels, which agrees with the observation that systemic DCS is capable of alleviating stress-induced difficulties in learning and memory (Waddell et al. 2010; Yamamoto et al. 2008). In this context, unpublished data from our laboratory showed that DCS injections administered to old rats before an open file test slightly increased the percentage of time in the center of the apparatus, which could be interpreted as an anxiolytic-like effect. However, such effects were not maintained in the reversal learning, as DCS enhanced the performance in old animals without affecting thigmotaxic behavior, making it difficult to draw a clear conclusion.
As discussed above, the effects of DCS in old rats were more marked in the STFP than in the MWM memory test. A possible explanation for the different findings from both tasks is that, although the vHPC is a critical region for STFP, as has previously been demonstrated (Carballo-Márquez et al. 2009; Portero-Tresserra et al. 2014), it may not be the most sensitive area involved in MWM consolidation (Ferbinteanu et al. 2003). Indeed, this task has been linked to the dHPC as it seems to be particularly related to spatial or contextual learning (Bannerman et al. 2004; Fanselow and Dong 2010; Moser and Moser 1998). Furthermore, early research in young rats showed that DCS in the dHPC improved spatial working memory deficits, induced by the blockade of glutamatergic or muscarinic receptors (Kishi et al. 1998; Ohno and Watanabe 1996). Nonetheless, the vHPC seems to be more susceptible to the age-associated decrease in NMDARs (Magnusson et al. 2006), which have also been related to spatial memory (Ferbinteanu et al. 2003; Loureiro et al. 2012; Ruediger et al. 2012) as place fields have been found in this hippocampal portion (Fanselow and Dong 2010; Kjelstrup et al. 2008). However, lesion studies showed that damage restricted to dHPC produced spatial learning impairments on tasks like the MWM and, in contrast, lesions of the vHPC are more associated with changes in emotional aspects, such as anxiety. Specifically, dHPC has a greater involvement in spatial working memory than the vHPC, being NMDAR activation in the dorsal portion essential to spatial processing (Bannerman et al. 2002; McHugh et al. 2008). Therefore, one may expect that if DCS had been injected in the dHPC in old subjects, a greater positive effect would have been found in the MWM acquisition and probe tests. Another possible explanation should take into account the pattern of administration of the DCS in both tasks, as it was acutely administered prior to a single STFP acquisition and before each of the five MWM sessions. Previous studies reported that DCS systemic chronic administration may lead to desensitization and, consequently, to lower responsiveness to the drug (Quartermain et al. 1994), with a possible decrease in its capacity to act as a cognitive enhancer (Mickley et al. 2012). However, as the main effect in MWM performance was detected in the reversal training, i.e., in the final phase of the task, this would not seem to be the most likely interpretation.
Regarding the effects of the old animals’ lifelong diet, it may be suggested that the potential positive effects of DCS in old rats have been masked, to some extent, by the potential cognitive benefits provided by CR. Indeed, although its effects on cognition are far from being certain (Gallagher et al. 2011), CR is regarded as a method to extend lifespan and protect against age-related degenerative processes (Speakman and Mitchell 2011; Guo et al. 2015), as it has been shown to attenuate age-related deficits in HPC-dependent learning tasks (Adams et al. 2008; Carter et al. 2009). In our experiment, however, the performance in STFP was similar between CR and AL old rats, although a certain beneficial effect was suggested in MWM learning. Therefore, the facilitative effect of DCS found both in STFP memory and MWM reversal learning in old rats may have added to the possible protective effect of CR. This hypothesis is currently being studied in our laboratory.
DCS was unable to improve behavioral performance in young animals in any of both tasks; one possible explanation is that the enhancing effects may be limited for young or healthy subjects. This is consistent with previous studies showing a facilitative effect of DCS in animals with cognitive impairment but not in young or control animals. For example, Ohno and Watanabe (1996) showed that intra-HPC administration of DCS did not improve working memory performance in young rats. However, they reported an enhancing effect of DCS when it was administered after local infusion of scopolamine into the HPC. Similarly, it has previously been reported that intra-HPC DCS administration in scopolamine-treated animals ameliorated relational memory deficits but did not improve memory in untreated animals (Portero-Tresserra et al. 2014). An alternative non-exclusive explanation could be that a potential ceiling effect may be observed in young animals, which have not increase performance because they may have already reached the highest score that can be achieved on the tests. This is, as the tasks have a limited difficulty, the most highly functioning subjects that will show the highest possible preference for the trained food (around 80%) in STFP or the shortest latency/most time in target quadrant in the MWM. As for the memory processes that DCS could enhance, in our research, we can determine that learning and consolidation are potentiated in old rats. However, early studies showed that intrahippocampal DCS injected before memory tests reversed working memory deficits in a spatial task, which were induced by cholinergic and glutamatergic antagonists (Kishi et al. 1998; Ohno and Watanabe 1996; Ohno et al. 1997). Such findings provide evidence that NMDAR-mediated neurotransmission in the hippocampus may have a significant role in the regulation of memory processes, at least in spatial memory. Therefore, we could expect a similar effect in retrieval of the memory tasks used in the present research, especially in the spatial MWM paradigm, but unfortunately, as far as we know, there are no reports on the effects of pre-test DCS in old subjects.
DCS injections enhanced the hippocampal expression of synaptophysin in old animals, suggesting that one of the mechanisms through which DCS may recover age-dependent cognitive deficits is related to the increased expression of this protein. Indeed, during learning and memory processes, synaptic inputs activate NMDARs leading to an increased expression of proteins, such as synaptophysin, which are essential for neurotransmission in hippocampal neurons (Weimer and Jorgensen 2003) and necessary for synaptic remodeling (Martin et al. 2000). Furthermore, the performance in both behavioral tasks correlated with the HPC levels of synaptophysin, showing that the higher the protein level, the better the cognitive performance. Similar results have been obtained with another compound, peptide 021, derived from the ciliary neurotrophic factor, which was able to rescue cognitive impairment and synaptophysin levels in the hippocampus in aged rats (Bolognin et al. 2014).
As for the NMDAR subunits, which are essential for the expression of synaptic plasticity involved in learning and memory, some reports indicate that the cognitive impairment observed in aged animals is related to the decrease in the number of postsynaptic NMDARs in the HPC (Le Jeune et al. 1996; Rosenzweig and Barnes 2003; Shi et al. 2007). In our research, although DCS in old rats did not significantly increase GluN1subunit expression, such levels correlated with MWM acquisition and reversal learning, likewise synaptophysin, in accordance with data showing that aged rats with intact spatial memory had postsynaptic ionotropic glutamate receptor levels similar to those of young animals, in contrast to memory-impaired aged rats (Ménard et al. 2014, 2015). Regarding the other subunits, GluN2A and GluN2B levels were unaffected by age or treatment in contrast with recent data (Brim et al. 2013; Ren et al. 2013). The lack of strong effects on NMDAR subunits may be attributable, at least in part, to the fact that CR or learning itself (Cercato et al. 2017) may restore age-dependent decreases in synaptic proteins, such as synaptophysin (Singh et al. 2015), and NMDAR subunits in the hippocampal formation (Adams et al. 2008; Monti et al. 2004; Newton et al. 2008; Shi et al. 2007), thus masking a possible beneficial effect of DCS on NMDA subunits.
In summary, our results add further evidence to the role of DCS as a cognitive enhancer in animals with cognitive deficits, indicating that boosting the function of NMDARs in the vHPC may improve memory in aged rats and that a possible underlying mechanism may be the promotion of synaptic plasticity (e.g., synaptophysin levels). The present results confirm that the positive modulation of glutamatergic transmission, acting on the glycine site of NMDARs, may be a suitable strategy for the study of the neural mechanisms responsible for age-related cognitive decline and provide an effective approach to test new treatments aimed to alleviate memory deficits.
References
Adams MM, Shi L, Linville MC, Forbes ME, Long AB, Bennett C, Newton IG, Carter CS, Sonntag WE, Riddle DR, Brunso-Bechtold JK (2008) Caloric restriction and age affect synaptic proteins in hippocampal CA3 and spatial learning ability. Exp Neurol 211:141–149
Alvarez P, Lipton PA, Melrose R, Eichenbaum H (2001) Differential effects of damage within the hippocampal region on memory for a natural, nonspatial odor-odor association. Learn Mem 8(2):79–86
Anderson RM, Birnie AK, Koblesky NK, Romig-Martin SA, Radley JJ (2014) Adrenocortical status predicts the degree of age-related deficits in prefrontal structural plasticity and working memory. J Neurosci 34:8387–8397
Aura J, Riekkinen M, Riekkinen P (1998) Tetrahydroaminoacridine and D-cycloserine stimulate acquisition of water maze spatial navigation in aged rats. Eur J Pharmacol 342:15–20
Aura J, Riekkinen P (2000) Pre-training blocks the improving effect of tetrahydroaminoacridine and D-cycloserine on spatial navigation performance in aged rats. Eur J Pharmacol 390:313–318
Bannerman DM, Deacon RMJ, Offen S, Friswell J, Grubb M, Rawlins JNP (2002) Double dissociation of function within the hippocampus: spatial memory and hyponeophagia. Behav Neurosci 116(5):884
Bannerman DM, Rawlins JNP, McHugh SB, Deacon RMJ, Yee BK, Bast T et al (2004) Regional dissociations within the hippocampus-memory and anxiety. Neurosci Biobehav Rev 28:273–283
Baxter MG, Lanthorn TH, Frick KM, Golski S, Wan RQ, Olton DS (1994) D-Cycloserine, a novel cognitive enhancer, improves spatial memory in aged rats. Neurobiol Aging 15:207–213
Billard J, Rouaud E (2007) Deficit of NMDA receptor activation in CA1 hippocampal area of aged rats is rescued by D-cycloserine. Eur J Neurosci 25(8):2260–2268
Billard JM (2015) d-Serine in the aging hippocampus. J Pharm Biomed Anal 116:18–24
Bolognin S, Buffelli M, Puoliväli J, Iqbal K (2014) Rescue of cognitive-aging by administration of a neurogenic and/or neurotrophic compound. Neurobiol Aging 35:2134–2146
Brim BL, Haskell R, Awedikian R, Ellinwood NM, Jin L, Kumar A, Foster TC, Magnusson KR (2013) Memory in aged mice is rescued by enhanced expression of the GluN2B subunit of the NMDA receptor. Behav Brain Res 238:211–226
Brothers HM, Bardou I, Hopp SC, Kaercher RM, Corona AW, Fenn AM, Godbout JP, Wenk GL (2013) Riluzole partially rescues age-associated, but not LPS-induced, loss of glutamate transporters and spatial memory. J NeuroImmune Pharmacol 8:1098–1105
Bunsey M, Eichenbaum H (1995) Selective damage to the hippocampal region blocks long-term retention of a natural and nonspatial stimulus-stimulus association. Hippocampus 5:546–556
Burger C, López MC, Feller JA, Baker HV, Muzyczka N, Mandel RJ (2007) Changes in transcription within the CA1 field of the hippocampus are associated with age-related spatial learning impairments. Neurobiol Learn Mem 87:21–41
Carballo-Márquez A, Vale-Martínez A, Guillazo-Blanch G, Martí-Nicolovius M (2009) Muscarinic transmission in the basolateral amygdala is necessary for the acquisition of socially transmitted food preferences in rats. Neurobiol Learn Mem 91:98–101
Carter CS, Leeuwenburgh C, Daniels M, Foster TC (2009) Influence of calorie restriction on measures of age-related cognitive decline: role of increased physical activity. J Gerontol A Biol Sci Med Sci 64:850–859
Cercato MC, Vázquez CA, Kornisiuk E, Aguirre AI, Colettis N, Snitcofsky M, Baez MV (2017) GluN1 and GluN2A NMDA receptor subunits increase in the hippocampus during memory consolidation in the rat. Front Behav Neurosci 10:242
Clayton DA, Mesches MH, Alvarez E, Bickford PC, Browning MD (2002) A hippocampal NR2B deficit can mimic age-related changes in long-term potentiation and spatial learning in the Fischer 344 rat. J Neurosci 22:3628–3637
Countryman RA, Gold PE (2007) Rapid forgetting of social transmission of food preferences in aged rats: relationship to hippocampal CREB activation. Learn Mem 14:350–358
Counts SE, Perez SE, Mufson EJ (2008) Galanin in Alzheimer’s disease: neuroinhibitory or neuroprotective? Cell Mol Life Sci 65:1842–1853
Driscoll I, Sutherland RJ (2005) The aging hippocampus: navigating between rat and human experiments. Rev Neurosci 16:87–121
Espejo PJ, Ortiz V, Martijena ID, Molina VA (2016) Stress-induced resistance to the fear memory labilization/reconsolidation process. Involvement of the basolateral amygdala complex. Neuropharmacology 109:349–356
Fanselow MS, Dong HW (2010) Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 65:7–19
Ferbinteanu J, Ray C, McDonald RJ (2003) Both dorsal and ventral hippocampus contribute to spatial learning in Long–Evans rats. Neurosci Lett 345:131–135
Forsyth JK, Bachman P, Mathalon DH, Roach BJ, Asarnow RF (2015) Augmenting NMDA receptor signaling boosts experience-dependent neuroplasticity in the adult human brain. Proc Natl Acad Sci U S A 112:15331–15336
Gallagher M, Burwell R, Burchinal M (1993) Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav Neurosci 107:618–626
Gallagher M, Stocker AM, Koh MT (2011) Mindspan: lessons from rat models of neurocognitive aging. ILAR J 52:32–40
García-Brito S, Morgado-Bernal I, Biosca-Simon N, Segura-Torres P (2017) Intracranial self-stimulation also facilitates learning in a visual discrimination task in the Morris water maze in rats. Behav Brain Res 317:360–366
Gerlai RT, McNamara A, Williams S, Phillips HS (2002) Hippocampal dysfunction and behavioral deficit in the water maze in mice: an unresolved issue? Brain Res Bull 57(1):3–9
Guo J, Bakshi V, Lin AL (2015) Early shifts of brain metabolism by caloric restriction preserve white matter integrity and long-term memory in aging mice. Front Aging Neurosci 7:213
Hajjar T, Goh YM, Rajion MA, Vidyadaran S, Li TA, Ebrahimi M (2013) Alterations in neuronal morphology and synaptophysin expression in the rat brain as a result of changes in dietary n-6: n-3 fatty acid ratios. Lipids Health Dis 12:113
Kalia LV, Kalia SK, Salter MW (2008) NMDA receptors in clinical neurology: excitatory times ahead. Lancet Neurol 7:742–755
Kennard J, Woodruff-Pak DS (2011) Age sensitivity of behavioral tests and brain substrates of normal aging in mice. Front Aging Neurosci 3:9
Kishi A, Ohno M, Watanabe S (1998) Concurrent activation of hippocampal glycine and polyamine sites of the N-methyl-D-aspartate receptor synergistically reverses working memory deficits in rats. Neurosci Lett 257:131–134
Kjelstrup KB, Solstad T, Brun VH, Hafting T, Leutgeb S, Witter MP, Moser EI, Moser MB (2008) Finite scale of spatial representation in the hippocampus. Science 321:140–143
Klencklen G, Després O, Dufour A (2012) What do we know about aging and spatial cognition? Reviews and perspectives. Ageing Res Rev 11(1):123–135
Kovács T (2004) Mechanisms of olfactory dysfunction in aging and neurodegenerative disorders. Ageing Res Rev 3:215–232
Kraemer S, Apfelbach R (2004) Olfactory sensitivity, learning and cognition in young adult and aged male Wistar rats. Physiol Behav 81:435–442
Kumar A (2015) NMDA receptor function during senescence: implication on cognitive performance. Front Neurosci 9:473
Le Jeune H, Cécyre D, Rowe W, Meaney MJ, Quirion R (1996) Ionotropic glutamate receptor subtypes in the aged memory-impaired and unimpaired Long-Evans rat. Neuroscience 74:349–363
Lin CH, Huang YJ, Lin CJ, Lane HY, Tsai GE (2014) NMDA neurotransmission dysfunction in mild cognitive impairment and Alzheimer’s disease. Curr Pharm Des 20:5169–5179
Liu P, Smith PF, Darlington CL (2008) Glutamate receptor subunits expression in memory-associated brain structures: regional variations and effects of aging. Synapse 62:834–841
Loureiro M, Lecourtier L, Engeln M, Lopez J, Cosquer B, Geiger K, Kelche C, Cassel JC, Pereira de Vasconcelos A (2012) The ventral hippocampus is necessary for expressing a spatial memory. Brain Struct Funct 217:93–106
Magnusson KR, Kresge D, Supon J (2006) Differential effects of aging on NMDA receptors in the intermediate versus the dorsal hippocampus. Neurobiol Aging 27:324–333
Martin SJ, Grimwood PD, Morris RGM (2000) Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci 23:649–711
McHugh SB, Niewoehner B, Rawlins JNP, Bannerman DM (2008) Dorsal hippocampal N-methyl-D-aspartate receptors underlie spatial working memory performance during non-matching to place testing on the T-maze. Behav Brain Res 186(1):41–47
Ménard C, Quirion R, Bouchard S, Ferland G, Gaudreau P (2014) Glutamatergic signaling and low prodynorphin expression are associated with intact memory and reduced anxiety in rat models of healthy aging. Front Aging Neurosci 6:81
Ménard C, Quirion R, Vigneault E, Bouchard S, Ferland G, El Mestikawy S et al (2015) Glutamate presynaptic vesicular transporter and postsynaptic receptor levels correlate with spatial memory status in aging rat models. Neurobiol Aging 36:1471–1482
Mickley GA, Remus JL, Ramos L, Wilson GN, Biesan OR, Ketchesin KD (2012) Acute, but not chronic, exposure to d-cycloserine facilitates extinction and modulates spontaneous recovery of a conditioned taste aversion. Physiol Behav 105:417–427
Monahan JB, Handelmann GE, Hood WF, Cordi AA (1989) D-Cycloserine, a positive modulator of the N-methyl-D-aspartate receptor, enhances performance of learning tasks in rats. Pharmacol Biochem Behav 34:649–653
Monti B, Virgili M, Contestabile A (2004) Alterations of markers related to synaptic function in aging rat brain, in normal conditions or under conditions of long-term dietary manipulation. Neurochem Int 44:579–584
Morris RG, Garrud P, Rawlins JN, O’Keefe J (1982) Place navigation impaired in rats with hippocampal lesions. Nature 297:681–683
Moser MB, Moser EI (1998) Functional differentiation in the hippocampus. Hippocampus 8:608–619
Newton IG, Forbes ME, Linville MC, Pang H, Tucker EW, Riddle DR, Brunso-Bechtold JK (2008) Effects of aging and caloric restriction on dentate gyrus synapses and glutamate receptor subunits. Neurobiol Aging 29:1308–1318
Nicholson DA, Yoshida R, Berry RW, Gallagher M, Geinisman Y (2004) Reduction in size of perforated postsynaptic densities in hippocampal axospinous synapses and age-related spatial learning impairments. J Neurosci 24:7648–7653
Nieves-Martinez E, Haynes K, Childers SR, Sonntag WE, Nicolle MM (2012) Muscarinic receptor/G-protein coupling is reduced in the dorsomedial striatum of cognitively impaired aged rats. Behav Brain Res 227:258–264
Norberg MM, Krystal JH, Tolin DF (2008) A meta-analysis of D-cycloserine and the facilitation of fear extinction and exposure therapy. Biol Psychiatry 63:1118–1126
Novier A, Van Skike CE, Diaz-Granados JL, Mittleman G, Matthews DB (2013) Acute alcohol produces ataxia and cognitive impairments in aged animals: a comparison between young adult and aged rats. Alcohol Clin Exp Res 29:1–8
Ohno M, Watanabe S (1996) D-Cycloserine, a glycine site agonist, reverses working memory failure by hippocampal muscarinic receptor blockade in rats. Eur J Pharmacol 318:267–271
Ohno M, Kobayashi M, Kishi A, Watanabe S (1997) Working memory failure by combined blockade of muscarinic and β‐adrenergic transmission in the rat hippocampus. Neuroreport 8(7):1571–1575
Paxinos G, Watson C (1997) The rat brain in stereotaxic coordinates. San Diego Acad. Press
Portero-Tresserra M, Cristóbal-Narváez P, Martí-Nicolovius M, Guillazo-Blanch G, Vale-Martínez A (2013b) D-Cycloserine in prelimbic cortex reverses scopolamine-induced deficits in olfactory memory in rats. PLoS One 8:e70584
Portero-Tresserra M, Del Olmo N, Martí-Nicolovius M, Guillazo-Blanch G, Vale-Martínez A (2014) D-Cycloserine prevents relational memory deficits and suppression of long-term potentiation induced by scopolamine in the hippocampus. Eur Neuropsychopharmacol 24:1798–1807
Portero-Tresserra M, Martí-Nicolovius M, Guillazo-Blanch G, Boadas-Vaello P, Vale-Martínez A (2013a) D-Cycloserine in the basolateral amygdala prevents extinction and enhances reconsolidation of odor-reward associative learning in rats. Neurobiol Learn Mem 100:1–11
Quartermain D, Mower J, Rafferty MF, Herting RL, Lanthorn TH (1994) Acute but not chronic activation of the NMDA-coupled glycine receptor with D-cycloserine facilitates learning and retention. Eur J Pharmacol 257:7–12
Ren J, Li X, Zhang X, Li M, Wang Y, Ma Y (2013) The effects of intra-hippocampal microinfusion of D-cycloserine on fear extinction, and the expression of NMDA receptor subunit NR2B and neurogenesis in the hippocampus in rats. Prog Neuro-Psychopharmacol Biol Psychiatry 44:257–264
Riekkinen M, Kemppainen S, Riekkinen P (1997) Effects of stimulation of alpha 1-adrenergic and NMDA/glycine-B receptors on learning defects in aged rats. Psychopharmacology 131(1):49–56
Riekkinen P, Ikonen S, Riekkinen M (1998) D-Cycloserine, a partial NMDA receptor-associated glycine-B site agonist, enhances reversal learning, but a cholinesterase inhibitor and nicotine has no effect. Neuroreport 9:3647–3651
Robitsek RJ, Fortin NJ, Koh MT, Gallagher M, Eichenbaum H (2008) Cognitive aging: a common decline of episodic recollection and spatial memory in rats. J Neurosci 28:8945–8954
Rosenzweig ES, Barnes CA (2003) Impact of aging on hippocampal function: plasticity, network dynamics, and cognition
Ruediger S, Spirig D, Donato F, Caroni P (2012) Goal-oriented searching mediated by ventral hippocampus early in trial-and-error learning. Nat Neurosci 15:1563–1571
Schwartz BL, Hashtroudi S, Herting RL, Schwartz P, Deutsch SI (1996) d-Cycloserine enhances implicit memory in Alzheimer patients. Neurology 46:420–424
Shi L, Adams MM, Linville MC, Newton IG, Forbes ME, Long AB, Riddle DR, Brunso-Bechtold JK (2007) Caloric restriction eliminates the aging-related decline in NMDA and AMPA receptor subunits in the rat hippocampus and induces homeostasis. Exp Neurol 206:70–79
Singh R, Manchanda S, Kaur T, Kumar S, Lakhanpal D, Lakhman SS, Kaur G (2015) Middle age onset short-term intermittent fasting dietary restriction prevents brain function impairments in male Wistar rats. Biogerontology 16:775–788
Smith TD, Adams MM, Gallagher M, Morrison JH, Rapp PR (2000) Circuit-specific alterations in hippocampal synaptophysin immunoreactivity predict spatial learning impairment in aged rats. J Neurosci 20:6587–6593
Snyder JS, Radik R, Wojtowicz JM, Cameron HA (2009) Anatomical gradients of adult neurogenesis and activity: young neurons in the ventral dentate gyrus are activated by water maze training. Hippocampus 19:360–370
Speakman JR, Mitchell SE (2011) Caloric restriction. Mol Asp Med 32:159–221
Stephens ML, Quintero JE, Pomerleau F, Huettl P, Gerhardt GA (2011) Age-related changes in glutamate release in the CA3 and dentate gyrus of the rat hippocampus. Neurobiol Aging 32:811–820
Thompson LT, Disterhoft JF (1997) Age- and dose-dependent facilitation of associative eyeblink conditioning by D-cycloserine in rabbits. Behav Neurosci 111:1303–1312
Villarejo-Rodríguez I, Vale-Martínez A, Guillazo-Blanch G, Martí-Nicolovius M (2010) d-Cycloserine in prelimbic cortex enhances relearning of an odor-reward associative task. Behav Brain Res 213(1):113–116
Villarejo-Rodríguez I, Boadas-Vaello P, Portero-Tresserra M, Vale-Martínez A, Martí-Nicolovius M, Guillazo-Blanch G (2013) Learning deficits in an odor reward-task induced by parafascicular thalamic lesions are ameliorated by pretraining d-cycloserine in the prelimbic cortex. Behav Brain Res 238:289–292
Von Lubitz DK, Paul IA, Bartus RT, Jacobson KA (1993) Effects of chronic administration of adenosine A1 receptor agonist and antagonist on spatial learning and memory. Eur J Pharmacol 249:271–280
Vorhees CV, Williams MT (2014) Assessing spatial learning and memory in rodents. ILAR J 55:310–332
Waddell J, Mallimo E, Shors T (2010) D-Cycloserine reverses the detrimental effects of stress on learning in females and enhances retention in males. Neurobiol Learn Mem 93:31–36
Weimer RM, Jorgensen EM (2003) Controversies in synaptic vesicle exocytosis. J Cell Sci 116:3661–3666
Yamamoto S, Morinobu S, Fuchikami M, Kurata A, Kozuru T, Yamawaki S (2008) Effects of single prolonged stress and D-cycloserine on contextual fear extinction and hippocampal NMDA receptor expression in a rat model of PTSD. Neuropsychopharmacology 33:2108–2116
Yankner BA, Lu T, Loerch P (2008) The aging brain. Annu Rev Pathol 3:41–66
Walker WC, Murdoch JM (1957) Cycloserine in the treatment of pulmonary tuberculosis. A report on toxicity. Tubercle, Lond 38:297
Acknowledgements
This work was supported by funds from the Ministerio de Economía y Competitividad (PSI2014-52660-R). The authors thank Dr. Jose Aguilera for his advice in conducting and interpreting the western blots data and Mr. Gerald-Patrick Fannon for his support with the English-language editing.
Author information
Authors and Affiliations
Contributions
MPT, MMN, GGB, and AVM designed the experiments and wrote the manuscript. MPT, MMN, MTG, and AC conducted the experiments. MPT and MMN analyzed the data. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
All procedures were carried out in compliance with the European Community Council Directive for care and use of laboratory animals (86/609/ECC) and with the Generalitat de Catalunya’s authorization (DOGC 2450 7/ 8/1997, DARP protocol number 3046).
Conflict of interest statement
All authors have contributed to the work and agree with the findings. This work has not been published before nor is it being considered for publication in another journal. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Rights and permissions
About this article
Cite this article
Portero-Tresserra, M., Martí-Nicolovius, M., Tarrés-Gatius, M. et al. Intra-hippocampal d-cycloserine rescues decreased social memory, spatial learning reversal, and synaptophysin levels in aged rats. Psychopharmacology 235, 1463–1477 (2018). https://doi.org/10.1007/s00213-018-4858-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-018-4858-z