Abstract
Aluminum is a ubiquitous metal that causes multiple brain pathologies such as, cognitive dysfunction and Alzheimer’s disease like symptoms. Exposure to aluminum through drinking water is responsible for hampering learning and memory. This study aimed to compare (1) the time-dependent effect of aluminum exposure (keeping total exposure of 5850 mg/kg same) in two durations, 30 and 45 days, and (2) to compare post-exposure self-recovery effect after 20 days in both (30 and 45 days exposure) groups. Rats were given 130 and 195 mg/kg of AlCl3·6H2O for 45 and 30 days respectively, to see the time-dependent exposure effect. At the end of exposure, rats were given distilled water and allowed to self-recover for 20 days to study the recovery. Expression levels of synaptic genes (Syp, SNAP25, Nrxn1/2, PSD95, Shank1/2, Homer1, CamkIV, Nrg1/2 and Kalrn) were measured using qPCR and compared in the exposure and recovery groups. Cellular morphology of the rat brain cortex and hippocampus was also investigated. Damage in lipid and protein profile was measured by employing FTIR. Results showed downregulation of mRNA expression of synaptic genes, plaques deposition, disorganization in lipid and protein profile by increasing membrane fluidity, and disorder and alteration of protein secondary structure after both exposure periods. However, better improvement/recovery in these parameters were observed in recovery group of 30 days aluminum exposure compared to 45 days aluminum exposure group. Taken together, these results suggested that short-term exposure resulted in better restoration of lipid and protein profile after time-dependent exposure of aluminum than prolonged exposure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aluminum (Al) is one of the most widely present metal on earth crust [1] and a known neurotoxicant even at low concentrations [2]. It can be consumed through foods as fruits and vegetables (estimated 10 mg/day), commercially processed foods (95 mg/day) [3] and drinking water (< 0.2 mg/L) [4] and also from sources as cooking utensils, pharmaceutical products and antiperspirants [5]. Al in drinking water contribute to only 1% of daily intake [6], but it is more bioavailable [7] owing to its uncomplexed nature and can cross blood brain barrier by replacing iron through iron transport protein transferrin [8]. Moreover, Al also binds to citrate through L-glutamate/cysteine exchanger transporter [9], thus leading to its accumulation in brain tissues. This accumulation has been associated with etiopathogenesis of neurodegenerative disorders [10, 11]. However, the exact mechanism by which Al causes these pathological changes is still unknown.
Synaptic physiology and plasticity are crucial for learning, memory, and higher cognitive functions [12]. Synaptic plasticity is regulated by various key components at presynaptic{synaptophysin (Syp), synaptosomal-associated protein (SNAP) 25 and neurexin (Nrxn)} [13,14,15] and post synaptic junction, where scaffolding molecules {post synaptic density (PSD) 95, Shank, and Homer} [16] and signaling molecules {calcium/calmodulin dependent protein kinase IV (CamkIV), neuregulin (Nrg) and kalirin (Kalrn)} [17,18,19,20] play crucial role in synaptic functions. Al modifies neurotrophins in synapse and promotes synaptic degeneration through adhesion molecules [21, 22]. This way Al can alter the complex link between the pre- and postsynaptic membranes and disrupt the cellular communication between synapses, necessary for synaptic signaling and strength. Keeping in view the abovementioned scenario, how Al exposure in time-dependent manner modify this complex process, still needs to be elaborated. Moreover, how the after-effects of Al exposure affect synapses was the prime question of this study and was investigated in recovery groups.
Neuronal membranes have a delicate composition of lipids and proteins making them crucial for normal cellular functioning, especially synaptic transmission in the brain [23]. Poor quality of these cell membrane lipids and proteins can trigger the formation of amyloid plaques, tangles, and other stressful conditions caused by metal neurotoxicity, which hampers normal synaptic transmission [24,25,26,27]. Little is known how Al can modify the balance between the functional groups in membranous macromolecules. Therefore, in this study, we aimed to look for such changes using appropriate method of Fourier transform infrared spectroscopy (FTIR) at the end of Al exposure and compared results with the recovery groups.
Chronic exposure to high doses of Al has been thoroughly investigated [21, 28]. However, no study has comprehensively reported the comparison of the same dose at different time points on synaptic markers. We aimed to understand and compare the adverse outcomes of Al exposure in a time-dependent manner and post-exposure recovery. Al was given through drinking water by keeping the total exposure same for two periods (30 and 45 days) and neurotoxicity was investigated through lipid and protein profile of membrane using FTIR spectroscopy, mRNA expression of synaptic genes and cellular histopathological changes. At the end of both exposure periods, a recovery period of 20 days was given to rats by providing distilled water and allowing them to self-recover. The same parameters were investigated and compared with the respective Al exposed groups.
Materials and Methods
Reagents and Chemicals
Aluminum chloride hexahydrate AlCl3·6H2O (AL0770) was purchased from Scharlau, Spain. AlCl3·6H2O had ≤ 0.005% impurities of heavy metals, such as Pb, and was stored in a ventilated area in an air-tight container. Potassium bromide (KBr) of FTIR grade, sodium hydroxide (NaOH), Congo red stain, silver nitrate (AgNO3), ammonium hydroxide, formaldehyde, citric acid, and sodium thiosulphate were purchased from Merck Germany. Deoxynucleotide (dNTP) 10 mM, 10 mM oligodT and RT enzyme and Maxima SYBR Green/ROX qPCR Master Mix (2 ×) were obtained from Thermo Scientific and TRI Reagent from Invitrogen®.
Experimental Design and Animal Grouping
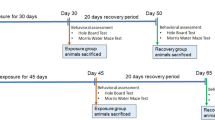
Experiments were performed according to Institute of Laboratory Animal Research, Division on Earth and Life Sciences, National Institute of Health, USA (Guide for the Care and Use of Laboratory Animals) rulings. Experimental protocol was permitted by the Internal Review Board (IRB) of Atta-ur-Rahman School of Applied Biosciences (ASAB), NUST (IRB No. 135). Male Wistar rats (total 40) aging 3–4 months, weighing 150–160 g were obtained from Laboratory Animal House, ASAB, and kept under specific environmental conditions of 25 ± 2 °C temperature, and natural light and dark cycle (14 h light and 10 h dark). Animals were provided water and standard diet ad libitum containing crude protein (30%), crude fat (9%), crude fiber (4%), and moisture (10%) [29]. The rats were classified into five groups, eight rats each group. The number of rats for mRNA expression analysis [30, 31], FTIR [30, 32] and histopathological experiments [33, 34] were carefully selected based on previously reported literature:
-
(a)
control group received distilled water.
-
(b)
30DAl group received 195 mg/kg AlCl3 through drinking water for 30 days.
-
(c)
30DR group received 195 mg/kg AlCl3 through drinking water for 30 days. After Al exposure for 30 days, rats were given distilled water for 20 days for self-recovery.
-
(d)
45DAl group received 130 mg/kg AlCl3 through drinking water for 45 days.
-
(e)
45DR group received 130 mg/kg AlCl3 through drinking water for 45 days. After Al exposure for 45 days, rats were given distilled water for 20 days for self-recovery.
The primary objective of this study was to evaluate the time-dependent effects of Al exposure. Therefore, Al was given in time-dependent manner by keeping the total exposure of 5850 mg/kg at two time points categorized as short- and long-term exposure for 30 and 45 days, respectively. When the same dose of Al is given at different time points, it is presumed that it may have differential effects upon exposure. Humans are exposed to Al due to its omnipresence in daily life, that makes it difficult to set safe intake levels of Al. The limits set by the WHO are routinely exceeded by humans residing in different regions and circumstances [35]. Therefore, mentioned dose was selected to study Al neurotoxicity at the high end of human exposure.
After the completion of the trial, the animals were sacrificed (Fig. 1) through cervical dislocation, and the brain was rapidly isolated to collect the cortex and hippocampus through a scalpel in dry ice. Both the cortex and hippocampi from each cerebral hemisphere (two per brain sample) were collected and kept at − 80 °C till further use. From one hemisphere, the cortex and hippocampus were used for RNA extraction, while the other was used for FTIR analysis.
Body Weight and Formula of Brain Coefficients of Rats
The body weights of the animals were monitored twice a week and then averaged out. Brain, cortex, and hippocampus weights and respective coefficients (%) were measured according to formula described earlier [36]. Whole brains were collected after dissection and weighed. Whole cortex and whole hippocampus were extracted immediately from both hemispheres and weighed collectively after carefully removing all extra parts such as fat [37].
FTIR Spectroscopic Measurements of Membrane Lipids and Proteins Functional Group Changes
FTIR spectroscopy was performed as previously described method [32] for identification of functional groups and chemical structures [38] of biomolecules based on spectral differences. The tissue samples were lyophilized overnight to remove water and then grounded to get fine powder. The tissue powder was mixed with KBr at a ratio 1:100 to collect pellets by subjecting pressure of 1100 kg/cm2 through a hydraulic press (Perkin Elmer, Germany) [39]. The background energy from blank KBr pellet was also measured to give correction to the whole data. An attenuated total reflection (ATR) FTIR spectrometer by Bruker Alpha was used to record the absorption in the spectral region of 4000–500 cm−1 at a spectral resolution of 4 cm−1. The spectra were recorded at room temperature under similar conditions. Each sample was scanned thrice with different pellets and then averaged out. Each spectrum was baseline corrected and normalized to the absorbance of amide I band.
FTIR measurements were then followed by statistical method of Hierarchal cluster analysis (HCA), as described earlier [32]. The HCA is a clustering method that showed the organization of the tested groups in a hierarchy. The HCA results were presented in a dendrogram, which is a plot indicating the groups and their relationships in the form of a tree. There are two main approaches that are usually followed for grouping in HCA: agglomerative or divisive [40]. We followed the agglomerative method measured through suitable metric of Euclidean distance.
RNA Extraction, Quantification, and Reverse Transcription
RNA was extracted from cortical and hippocampal tissues through Tri-reagent, as mentioned in manufacturer’s protocol [41]. mRNA quality was checked both qualitatively and quantitatively through agarose gel (1%) electrophoresis and spectrophotometry at 260 nm (Thermo Scientific Nanodrop), respectively and purity was estimated according to a 260/280 nm ratio of > 1.9. An equal amount of mRNA (3 µg) was reverse transcribed (RT) to cDNA through oligodT primer and MuLV RT.
qRT-PCR for Gene Expression Analysis
The experiment was performed according to method described earlier [42]. Synthesized cDNA from each group was taken as template for real-time PCR (qRT-PCR) amplification through AB QuantGene using Maxima SYBER green. Each reaction was performed with a master mix of 20 μl volume containing 1 µl cDNA, 4 µl 2 × Maxima SYBR green, 1 µl forward, and 1 µl reverse primers of the gene of interest (Table 6) diluted with H2O. The steps of PCR procedures followed were initial denaturation for 10 min at 95 °C, followed by 40 cycles for 15 s at 95 °C and 1 min at the annealing temperature, which varied for every gene (Table 6), followed by a final dissociation step of 45 s at 72 °C, proceeded by melt curve analysis for validation of specificity of the amplified products. The expression level was measured by 2−ΔΔCT method, as explained previously by Livak and Schmittgen [43]. The CT values were then normalized to the CT value of beta actin (Actb) gene in each sample. Actb was used as housekeeping gene, as it was considered as stable gene in cortex and hippocampus [44,45,46].
All primers were designed through NCBI primer BLAST according to rat mRNA sequences obtained from NCBI, except for Syp, PSD95, CamkIV, and Actb, which were selected from literature [42, 47]. Each primer was then tested for nonspecific products and correct amplicon size by 1% ethidium bromide (EtBr) agarose gel. Primer efficiency was also checked by cDNA serial dilutions.
Histopathological Examination
Congo Red Stain for Detection of Amyloid Beta Deposition
Congo red staining was performed as described earlier [33] for the detection of amyloid aggregates. The rat brain was fixed by transcardial perfusion with 4% paraformaldehyde according to a previously described method [41]. Coronal sections of 5 µm thickness were deparaffinized with xylene, and rehydrated in various concentrations of alcohol, washed with isopropanol (70%), and then with distilled H2O. Congo red stain 49.5 mL (0.5%) and 0.5 mL NaOH (1%) were poured on deparaffinized sections and retained for 20 min. The sections were then washed with distilled H2O and alcohol for 2 min and counterstained with hematoxylin for 30 s. After counterstaining sections were washed with 70% isopropanol (6–10 dips) and final washing was done with distilled H2O. The slides were mounted on coverslips after air-drying for 1 h.
Silver Staining for Detection of Amyloid Plaques Deposition
Silver staining was performed by modified method of Bielshowsky [48] to analyze the black colored amyloid plaque deposits. After deparaffinization of tissues in xylene and rehydration in various alcohol concentrations, slides were placed for 15 min in 10% AgNO3 solution until the sections became light brown. After washing with H2O thrice, concentrated ammonium hydroxide was mixed with AgNO3 till the precipitate became clear. The slides were incubated for 30 min at 40 °C and then kept in developer solution (20 ml of 40% formaldehyde, 0.5 g citric acid, 20 µl conc. nitric acid, and 100 ml distilled H2O) for 3 min. The slides were then placed in 1% ammonium hydroxide for 1 min to stop the silver reaction. After washing with H2O thrice, slides were placed for 5 min in 5% sodium thiosulphate. The sections were dehydrated and mounted on coverslips.
Quantitative Morphometric Analysis
Congo red and silver-stained sections from all groups were visualized through Optika microscope B-150 (Italy). Images were captured using the Optika Vision Lite 2.1 image analysis software. From each animal three slides were prepared and analyzed by calculating total number of cells from layer 2 and 3 of cortex and from dentate gyrus (DG), CA1, CA2 and CA3 regions of hippocampus at 40× resolution. Then number of Congo red and silver positive cells were calculated, and percentage was plotted.
Statistical Analysis
Data were represented as the mean ± standard error of mean (SEM) and n = number of animals used in the experiment. “Graph Pad Prism” software V8.0 was used for analyzing the statistical differences through one-way ANOVA followed by Bonferroni’s test for multiple comparisons for gene expression data and histopathological analysis. Two-way ANOVA was used for analyzing the body weight of rats to analyze the difference between initial and final body weights, and comparisons were made in all tested groups. Results with p < 0.05 was considered statistically significant. For FTIR data, Origin 2022 was used to calculate the band center and full width at half maximum (FWHM), and to perform HCA to study the similarity and differences in all tested rat brain tissues.
Results
Effect of Al Exposure and Post-Exposure Recovery on Body Weight
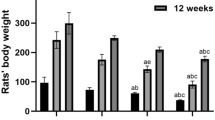
Initial and final body weights were plotted to examine the effect of Al exposure and post-exposure recovery on overall body weights of rats. There was no difference between control group, 30 days of Al exposed group and its respective recovery group (Fig. 2a). However, slight decrease in final body weight (257.67 ± 0.26 g, p < 0.01) of rats treated with Al for 45 days was observed in comparison to final body weight (266.00 ± 0.78 g) of animals of the control group, followed by increase in weight in recovery group (263.25 ± 0.30 g, p < 0.05; Fig. 2b). The brain, cortex and hippocampus weights and their coefficients were measured and compared (Table 1).
FTIR Spectroscopic Measurement of Al Exposure and Post-Exposure Recovery
The average of normalized absorbance of FTIR spectra in range of 4000–500 cm −1, in all groups were recorded (Figs. 3a, 4a). The spectral differences were observed in the functional groups of the molecules as lipids, proteins, and carbohydrates among Al exposure and recovery groups. FTIR spectra of Al exposure after 45 days showed wavenumber shift towards higher wavenumbers in lipid and protein bands compared with control group and 30 days of Al exposure group in both cortex and hippocampus (Tables 2, 4). The decrease in wavenumber shift was only observed in recovery period of 30 days of Al exposure that was parallel to control group suggesting an alleviation in Al induced damage. The band center and FWHM were estimated for cortex and hippocampus through beginning and end of FTIR spectra (Fig. 5a, b). The spectral features of observed bands i.e., center, and FWHM were measured for cortex (Tables 2, 3) and hippocampus (Tables 4, 5).
a Normalized absorbance of FTIR spectra of hippocampus of rat brain, b HCA of hippocampus extracted from all tested groups; 30DAl Al exposure for 30 days, 30DR 20 days recovery period after 30 days of Al exposure, 45DAl Al exposure for 45 days, 45DR 20 days recovery period after 45 days of Al exposure
The differences between IR spectra among groups were further evaluated statistically by HCA. The classification performed through cluster analysis is justified by the fact that there are two groups of Al exposure and their respective recovery groups in addition to control group. In this way, it was possible to substantiate whether the groups are distinct or similar. Normalized absorbance values of FTIR spectra were used for HCA (Figs. 3b, 4b). HCA recognizes every group; and have placed control and recovery group after 30 days of Al exposure in one branch, while Al exposure group in separate group. Moreover, Al exposure after 45 days and its respective recovery group were placed in separate branch confirming the difference in rate of recovery from control group.
Effect of Al Exposure and Post-Exposure Recovery on Synaptic Vesicle Genes
Syp
In the cortex, expression of Syp was not affected after 30 days of Al exposure (0.62 ± 0.15, p > 0.05) whereas expression was downregulated after 45 days of Al exposure (0.31 ± 0.14, p < 0.01) when compared with the control group (1.00 ± 0.01). In recovery group of 45 days Al exposure (0.39 ± 0.06, p > 0.99; Fig. 6a), no difference was observed when compared to its Al exposed group suggesting no improvement in recovery.
Relative mRNA expression levels of synaptic vesicle genes Syp and SNAP25 in cortex (a, b) and hippocampus (c, d) of Al exposed and recovery groups. *p < 0.05, **p < 0.01, ***p < 0.001, refer to significant difference compared with the control group while #p < 0.05, #p < 0.01, ###p < 0.001 refer to significant difference between Al exposed and recovery groups; n number of animals, 30DAl Al exposure for 30 days, 30DR 20 days recovery period after 30 days of Al exposure, 45DAl Al exposure for 45 days, 45DR 20 days recovery period after 45 days of Al exposure
In the hippocampus, levels of Syp were downregulated after 30 days (0.46 ± 0.14, p < 0.05) and 45 days of Al exposure (0.02 ± 0.03, p < 0.001) relative to control group (1.00 ± 0.01). Upon completion of 20 days of recovery after 30 days exposure group, a trend of upregulation was observed but not significant (0.70 ± 0.08, p = 0.76) when compared with its Al exposed group. Syp expression was upregulated (0.47 ± 0.09, p = 0.04; Fig. 6c) in 45 days exposed recovery group when compared with its Al exposed group, and its gene expression recovery was better than 30 days exposed recovery group.
SNAP25
The expression of SNAP25 in the cortex was downregulated after 30 days (0.49 ± 0.09, p < 0.01) and 45 days of Al exposure (0.09 ± 0.02, p < 0.001) in relation to the control group (1.00 ± 0.01). The recovery after 30 days of Al exposure (0.71 ± 0.09, p = 0.43) showed no difference compared to its Al exposed group. Recovery after 45 days of Al exposure showed that SNAP25 expression was significantly upregulated (0.44 ± 0.05, p = 0.05; Fig. 6b) as compared to the Al exposed group.
When studied in hippocampus, SNAP25 expression was downregulated after 30 days (0.30 ± 0.12, p < 0.01) and 45 days (0.09 ± 0.03, p < 0.001) of exposure when compared with the control group (1.00 ± 0.01). No effect was observed in terms of recovery after both exposure groups (p > 0.99; Fig. 6d).
Effect of Al Exposure and Post-Exposure Recovery on Adhesion Molecule Genes
Nrxn1
The analysis of Nrxn1 in the cortex showed no difference in 30 days of Al exposure group (0.62 ± 0.12, p > 0.05) meanwhile expression was downregulated (0.34 ± 0.13, p < 0.01; Fig. 7a) after 45 days of Al exposure when compared with the control group (1.00 ± 0.04). No effect was observed in terms of recovery in after 30 (p = 0.25) and 45 days (p > 0.99) of exposure.
Relative mRNA expression levels of adhesion molecule genes Nrxn1 and Nrxn2 in cortex (a, b) and hippocampus (c, d) of Al exposed and recovery groups. *p < 0.05, **p < 0.01, ***p < 0.001, refer to significant difference compared with the control group while #p < 0.05, #p < 0.01, ###p < 0.001 refer to significant difference between Al exposed and recovery groups; n number of animals, 30DAl Al exposure for 30 days, 30DR 20 days recovery period after 30 days of Al exposure, 45DAl Al exposure for 45 days, 45DR 20 days recovery period after 45 days of Al exposure
When studied in hippocampus, the mRNA expression was downregulated after 30 days (0.49 ± 0.12, p < 0.05) and 45 days of exposure (0.19 ± 0.11, p < 0.001; Fig. 7c) in comparison to the control group (1.00 ± 0.01). Upregulation in expression was observed in recovery period after 45 days of exposure (0.77 ± 0.06, p = 0.006) when compared with the respective Al exposed groups, whereas no difference was observed in recovery after 30 days of exposure (0.77 ± 0.07, p = 0.43).
Nrxn2
Expression of Nrxn2 in the cortex was downregulated after both, 30 days (0.42 ± 0.19, p < 0.05) and 45 days of exposure (0.29 ± 0.10, p < 0.05) relative to the control group (1.00 ± 0.01). The trend in downregulation was also observed in recovery after 30 days (0.86 ± 0.06, p = 0.21) and 45 days (0.36 ± 0.12, p > 0.99; Fig. 7b) of exposure when compared with Al exposed groups.
The mRNA expression analysis of Nrxn2 in hippocampus showed that expression was downregulated after both 30 days (0.34 ± 0.13, p < 0.01) and 45 days of Al exposure (0.14 ± 0.05, p < 0.001) as compared to the control group (1.00 ± 0.05). Recovery group in 30 (0.57 ± 0.10, p > 0.99) and 45 days (0.47 ± 0.08, p = 0.25; Fig. 7d) of Al exposure showed no difference when compared to the respective Al exposed groups suggesting no improvement in recovery.
Effect of Al Exposure and Post-Exposure Recovery on Post-synaptic Scaffolding Molecule Genes
PSD95
When studied in the cortex, no effect was observed (0.66 ± 0.06, p > 0.05) on expression of PSD95 in 30 days of Al exposure group however, after 45 days of exposure expression was downregulated (0.39 ± 0.13, p < 0.01) when compared to the control group (1.00 ± 0.02). No effect was observed in terms of recovery after both exposure groups (p > 0.99; Fig. 8a).
Relative mRNA expression levels of scaffolding molecule genes PSD95, Shank1 and 2 and Homer1 in cortex (a–d) and hippocampus (e–h) of Al exposed and recovery groups. *p < 0.05, **p < 0.01, ***p < 0.001, refer to significant difference compared with control group while #p < 0.05, #p < 0.01, ###p < 0.001 refer to significant difference between Al exposed and recovery groups; n number of animals, 30DAl Al exposure for 30 days, 30DR 20 days recovery period after 30 days of Al exposure, 45DAl Al exposure for 45 days, 45DR 20 days recovery period after 45 days of Al exposure
On the contrary, in hippocampus, expression was significantly downregulated in 30 days (0.41 ± 0.06, p < 0.01) and 45 days of Al exposure (0.02 ± 0.10, p < 0.001). In terms of recovery, expression was upregulated in recovery of 45 days Al exposure (0.49 ± 0.10, p = 0.01) while no significant change in expression was observed in recovery (0.56 ± 0.10, p > 0.99; Fig. 8e) of 30 days Al exposure group when compared with the respective Al exposed group.
Shank1
In the cortex, expression of shank1 was downregulated in both in 30 days (0.44 ± 0.08, p < 0.05) and 45 days of Al exposure groups (0.29 ± 0.07, p < 0.01) as compared to the control group (1.00 ± 0.01). No effect was observed in recovery period of both 30 days (0.73 ± 0.12, p = 0.39) and 45 days (0.54 ± 0.11, p = 0.70; Fig. 8b) of Al exposed groups compared with the respective exposure groups.
In hippocampus, expression was downregulated in 30 days (0.41 ± 0.09, p < 0.01) and 45 days (0.05 ± 0.02, p < 0.001) of Al exposure groups in comparison to the control group (1.00 ± 0.05). The expression was significantly upregulated (0.88 ± 0.06, p = 0.01; Fig. 8f) in recovery group of 30 days Al exposure as compared to its Al exposed group. Meanwhile, no recovery was observed in recovery of 45 days Al exposure (0.36 ± 0.11, p = 0.15) suggesting less recovery than 30 days of recovery period.
Shank2
When analyzed in the cortex no effect was observed in expression of shank2 in 30 days of Al exposure (0.74 ± 0.08, p > 0.05), whereas expression was downregulated (0.34 ± 0.14, p < 0.01; Fig. 8c) after 45 days of Al exposure when compared to the control group (1.00 ± 0.02). In terms of recovery no difference was observed in recovery of 45 days Al exposure (0.74 ± 0.15, p = 0.18) when compared with the respective exposure group.
In hippocampus, expression was downregulated after 30 days (0.56 ± 0.14, p < 0.05) and 45 days (0.24 ± 0.09, p < 0.001) of Al exposure when compared to the control group (1.00 ± 0.02). No upregulation (0.83 ± 0.06, p = 0.48) in expression was observed in recovery period of 30 days Al exposure compared with the respective exposure group, whereas expression was upregulated (0.79 ± 0.04, p = 0.009; Fig. 8g) in recovery after 45 days of Al exposure.
Homer1
In the cortex, the expression of Homer1 was downregulated (0.37 ± 0.12, p < 0.05) in 30 days and 45 days of Al exposure (0.10 ± 0.05, p < 0.001) when compared to control group (1.00 ± 0.05). Expression was upregulated in recovery of 30 days of Al exposure (0.92 ± 0.16, p = 0.03) suggesting an improved recovery as compared to its Al exposed group, however there was no recovery (0.33 ± 0.08, p > 0.99; Fig. 8d) after 45 days of Al exposure.
When analyzed in the hippocampus, downregulation in expression was observed in 30 days (0.67 ± 0.04, p < 0.05) and 45 days (0.36 ± 0.09, p < 0.001) of Al exposure groups when compared to control group (1.00 ± 0.05). No significant upregulation in expression was observed (0.81 ± 0.04, p > 0.99; Fig. 8h) in recovery group of 30 days Al exposure when compared with its exposure group, whereas expression was upregulated (0.68 ± 0.05, p = 0.03) in recovery group of 45 days Al exposure.
Effect of Al Exposure and Post-Exposure Recovery on Signaling Molecule Genes
CamkIV
In the cortex expression of CamkIV was downregulated after 30 days (0.66 ± 0.07, p < 0.05) and 45 days (0.53 ± 0.06, p < 0.01) of Al exposure groups when compared to the control group (1.00 ± 0.05). In terms of recovery, expression was not upregulated (0.84 ± 0.06, p = 0.05) in recovery group of 30 days Al exposure while this was upregulated (0.81 ± 0.05, p = 0.04; Fig. 9a) in recovery after 45 days of Al exposure when compared with the respective exposure groups.
Relative mRNA expression levels of signaling molecule genes CamkIV, Nrg1 and 2 and Kalrn in cortex (a–d) and hippocampus (e–h) of Al exposed and recovery groups. *p < 0.05, **p < 0.01, ***p < 0.001, refer to significant difference compared with the control group while #p < 0.05, #p < 0.01, ###p < 0.001 refer to significant difference between Al exposed and recovery groups; n number of animals, 30DAl Al exposure for 30 days, 30DR 20 days recovery period after 30 days of Al exposure, 45DAl Al exposure for 45 days, 45DR 20 days recovery period after 45 days of Al exposure
In the hippocampus, expression of CamkIV was downregulated after 30 days (0.32 ± 0.09, p < 0.01) and 45 days (0.03 ± 0.02, p < 0.001; Fig. 9e) of Al exposure in comparison to the control group (1.00 ± 0.05). No change in expression was observed in both recovery groups (p > 0.99) compared with the respective exposure groups suggesting no improvement in gene expression.
Nrg1
When studied in the cortex, Nrg1 expression was downregulated after 30 days (0.49 ± 0.07, p < 0.05) and 45 days (0.02 ± 0.04, p < 0.001) of Al exposure in relation to the control group (1.00 ± 0.04). Expression was not upregulated (0.82 ± 0.13, p = 0.26) in recovery period after 30 and 45 days (0.43 ± 0.12, p = 0.09; Fig. 9b) of Al exposure when compared with Al exposed groups.
In the hippocampus, there was no difference in expression after 30 days of Al exposure (0.60 ± 0.09, p > 0.05) but the expression was significantly downregulated in 45 days of Al exposure group (0.03 ± 0.02, p < 0.001) in comparison to the control group (1.00 ± 0.02). No effect was observed in terms of upregulation of expression in recovery groups of both Al exposed groups (p > 0.99; Fig. 9f).
Nrg2
The expression analysis of Nrg2 in the cortex was downregulated after 30 days (0.45 ± 0.21, p < 0.05) and 45 days (0.02 ± 0.04, p < 0.001) of Al exposure when compared to the control group (1.00 ± 0.04). In the recovery group after 30 days Al exposure no difference was observed (0.83 ± 0.07, p = 0.34) when compared with respective Al exposure group. Expression was not upregulated (0.36 ± 0.08, p = 0.51; Fig. 9c) in recovery group of 45 days Al exposure.
When analyzed in the hippocampus, expression of Nrg2 was significantly downregulated in both 30 days (0.46 ± 0.06, p < 0.01) and 45 days of Al exposure groups (0.01 ± 0.03, p < 0.001) when compared to control group (1.00 ± 0.03). The expression was not upregulated (0.77 ± 0.10, p = 0.36) in recovery after 30 and 45 days (0.39 ± 0.15, p = 0.12; Fig. 9g) of Al exposure when compared with respective Al exposed groups.
Kalrn
The expression of Kalrn in the cortex was downregulated in both 30 days (0.31 ± 0.04, p < 0.05) and 45 days of Al exposure (0.22 ± 0.12, p < 0.01) when compared to control group (1.00 ± 0.05). In recovery group after 30 days (0.69 ± 0.18, p = 0.37; Fig. 9d) and 45 days (0.42 ± 0.11, p > 0.99) of Al exposure no difference was observed when compared with respective Al exposure groups.
On the contrary, in hippocampus no prominent change in expression was observed in both 30 days (0.65 ± 0.07 p > 0.05) and 45 days of Al exposure groups (0.79 ± 0.08, p > 0.05; Fig. 9h) in comparison to control group (1.00 ± 0.05). No effect on recovery groups were also observed (p > 0.99).
Effect of Al Exposure and Post-Exposure Recovery on Brain Histopathology
Cortex
In the cortical layer 2 and 3, percentage of Congo red positive cells significantly increased after both 30 days (42.20 ± 1.20, p < 0.05) and 45 days of Al exposure (57.30 ± 2.19, p < 0.001) as compared to the control group (26.5 ± 0.76). However, in 30 days exposure recovery group no notable increase in Congo red positive cells (38.10 ± 2.37, p > 0.05) were observed when compared to the control group suggesting an improvement in recovery. Meanwhile in recovery period given after 45 days of Al exposure, there was a significant increase in Congo red positive cells (50.90 ± 5.22, p < 0.01; Fig. 10b), relative to control group showing no improvement in recovery.
a The figure showing representative slides of cortical layers 2 and 3 at 40× magnification. White arrows show normal cells while yellow arrows represent red colored plaques in cortical layers 2 and 3. b Histogram shows the percent positive of Congo red cells in cortex. *p < 0.05, **p < 0.01, ***p < 0.001 refer to significant difference compared with control while #p < 0.05, #p < 0.01, ###p < 0.001 refer to significant difference between Al exposed and recovery groups; n number of animals, 30DAl Al exposure for 30 days, 30DR 20 days recovery period after 30 days of Al exposure, 45DAl Al exposure for 45 days, 45DR 20 days recovery period after 45 days of Al exposure
Al exposure after both 30 days (60.40 ± 3.78, p < 0.05) and 45 days (81.31 ± 2.66, p < 0.001) have resulted in significant increase in silver deposits when compared to the control group (37.21 ± 1.59). The percentage of silver deposits was significantly decreased in recovery period given after 30 days of Al exposure (43.91 ± 5.10, p > 0.05) whereas number was slightly decreased in recovery group of 45 days of Al exposure (63.81 ± 7.01, p < 0.05; Fig. 11b).
a The figure showing representative slides of cortical layers 2 and 3 at 40× magnification. White arrows show normal cells while yellow arrows represent black colored deposits in cortical layers 2 and 3. b Histogram shows the percent positive of silver deposits in cortex. *p < 0.05, **p < 0.01, ***p < 0.001 refer to significant difference compared with the control group while #p < 0.05, #p < 0.01, ###p < 0.001 refer to significant difference between Al exposed and recovery groups; n number of animals, 30DAl Al exposure for 30 days, 30DR 20 days recovery period after 30 days of Al exposure, 45DAl Al exposure for 45 days, 45DR 20 days recovery period after 45 days of Al exposure
Hippocampus
Congo red staining in both 30 and 45 days of Al exposure resulted in significant increase in Congo red positive cells in DG (57.20 ± 3.01, p < 0.01 and 82.41 ± 0.30, p < 0.001, respectively), CA1 (58.71 ± 4.37, p < 0.01 and 85.70 ± 4.96, p < 0.001, respectively), CA2 (47.91 ± 4.11, p < 0.01 and 87.30 ± 5.26, p < 0.001, respectively) and CA3 (72.10 ± 1.89, p < 0.01 and 82.71 ± 4.37, p < 0.001, respectively) when compared to the percentage of cells in the control group in DG (37.10 ± 2.92; Fig. 12b), CA1 (31.91 ± 2.34; Fig. 12c), CA2 (24.50 ± 3.78; Fig. 12d) and CA3 (42.81 ± 5.21; Fig. 12e). In terms of recovery, for 30 days exposure group, percentage of Congo red cells were significantly decreased that showed an improved recovery in DG, CA1, CA2 and CA3 (43.81 ± 3.36; 37.90 ± 3.01; 35.30 ± 1.83 and 45.42 ± 5.73, p > 0.05, respectively). In recovery after 45 days exposure group percentage of cells were only improved in CA1 (45.62 ± 4.46, p > 0.05) while the percentage of Congo red cells were increased in DG, CA2 and CA3 (56.20 ± 1.58, p < 0.01, 60.12 ± 0.97, p < 0.001 and 67.32 ± 2.41, p < 0.05, respectively).
a The image shows representative slides of hippocampus (Congo red staining) sections at 40× magnification. White arrows show normal cells while yellow arrows represent red colored plaques in hippocampus regions. Histogram shows the percent positive of Congo red cells in b DG, c CA1, d CA2 and e CA3. *p < 0.05, **p < 0.01, ***p < 0.001 refer to significant difference compared with the control group while #p < 0.05, #p < 0.01, ###p < 0.001 refer to significant difference between Al exposed and recovery groups; n number of animals, 30DAl Al exposure for 30 days, 30DR 20 days recovery period after 30 days of Al exposure, 45DAl Al exposure for 45 days, 45DR 20 days recovery period after 45 days of Al exposure
Silver staining in both 30 and 45 days of Al exposure resulted in significant increase in silver deposits in DG (64.31 ± 2.33, p < 0.05 and 77.00 ± 2.08, p < 0.001, respectively), CA1 (58.80 ± 1.95, p < 0.01 and 80.71 ± 2.89, p < 0.001, respectively), CA2 (81.23 ± 2.30 and 86.84 ± 3.45, p < 0.001, respectively) and CA3 (65.45 ± 3.08, p < 0.01 and 81.00 ± 2.01, p < 0.001, respectively) as compared to silver deposits in control group in DG (50.32 ± 2.91; Fig. 13b), CA1 (35.12 ± 2.51; Fig. 13c), CA2 (41.90 ± 3.97; Fig. 13d) and CA3 (44.12 ± 3.67; Fig. 13e). In recovery group after 30 days of exposure, percentage of silver deposits decreased in DG, CA2 and CA3 (53.31 ± 3.38, 41.10 ± 0.34 and 55.51 ± 0.356, p > 0.05, respectively), except for CA1 where no recovery was observed (52.32 ± 1.31, p < 0.05). In recovery group after 45 days of Al exposure, improvement was observed only in DG (59.71 ± 2.60, p > 0.05) while percentage of silver deposits remained high in CA1, CA2 and CA3 (64.43 ± 4.47, 65.71 ± 4.83 and 77.72 ± 3.14, p < 0.001, respectively).
a The image shows representative slides of hippocampus (silver staining) sections at 40× magnification. White arrows show normal cells while yellow arrows represent black colored deposits in hippocampus regions. Histogram shows the percent positive of silver deposits in b DG, c CA1, d CA2 and e CA3. *p < 0.05, **p < 0.01, ***p < 0.001 refer to significant difference compared with control while #p < 0.05, #p < 0.01, ###p < 0.001 refer to significant difference between Al exposed and recovery groups; n number of animals, 30DAl Al exposure for 30 days, 30DR 20 days recovery period after 30 days of Al exposure, 45DAl Al exposure for 45 days, 45DR 20 days recovery period after 45 days of Al exposure
Discussion
Studies on Al neurotoxicity are almost a century old [49]. Humans are exposed to Al through different routes, and it is absorbed through gastrointestinal tract and excreted in urine [50]. During normal functioning of kidneys, Al elimination occurs from body almost completely with very little accumulation, specifically in the brain. However, when accumulated, Al is responsible for cognitive deficits [51]. Animal model studies have also suggested that Al is responsible for hampering synaptic plasticity [31]. Synaptic membranes hold many receptors and channels; therefore, any alteration in lipid and protein content of synaptic membranes due to Al exposure can affect neurotransmitter release, thus altering synapse function [24, 27].
This study was designed to investigate the toxic effects of Al on synaptic elements in terms of critical gene expression studies and lipid and protein content of cortical and hippocampal tissues through FTIR. High doses of Al were selected to ensure the Al accumulation in brain with aim to understand the next objective of our study which is post-exposure recovery effect after Al exposure. The administered doses of Al to rats per day in our study does not exceed from doses taken by individuals consuming antacids and buffered aspirin chronically that contribute to thousands of mg/kg of Al [50]. Furthermore, a longitudinal study from China revealed the high amount of Al intake from daily food items [52]. For example, pancakes provide 180 mg Al per serving [53]. Another study suggested that 5% of the American population ingested more than 95 mg/kg/day through food items [54]. Previous studies also reported that administration of 260 mg/kg Al for 5 weeks refers to maximum estimated human intake [2, 55]. Additionally, the LD50 for AlCl3 given through oral route is 200–1000 mg/kg in mice and rats [56]. According to these studies, as well as previous studies on Al neurotoxicity by our research group [28, 29], the above-mentioned doses were selected.
In this study, we assessed the body weights of animals to analyze the progress of model development for Al neurotoxicity. Body and brain weights are one of the key indexes that initially indicate the toxicity influence on animal's health. In our study, Al exposure for 45 days resulted in reduced body weight compared to Al exposure for 30 days, where no change was observed in relation to the control group. This decrease in body weight is consistent with previous research [57, 58]. The decrease in body weight was recovered after 20 days, whereas in previous studies, no recovery was made in terms of body weight [59] which could be due to the recovery period of one week after exposure to 200 mg/kg of Al for 21 days. Furthermore, the cortex and hippocampus tissues were weighed, and the respective coefficients were measured. Brain coefficients provide important information in toxicological studies in which target organs are tested for toxic chemicals [60] and it is expressed by dividing the weight of each animal by the weight of corresponding organ of an animal [61]. The decrease in hippocampal weight and coefficient was observed after 45 days of Al exposure. These findings were according to previous studies [31], which further confirmed the establishment of an animal model by indicating the improvement in hippocampal weight and coefficient after recovery group. However, no effect was observed on the cortex weight. Moreover, no animal died during the trial.
FTIR spectroscopy was recorded for both Al exposure and post-exposure recovery group that provides information about biomolecules, i.e., proteins, lipids and carbohydrates and in-depth analysis about protein secondary structure, membrane ordering, fluidity, and information as hydrocarbon chain length can also be obtained [32]. In the cortex, decrease in wavenumber of amide A band was observed only in 45DAl group that represent the N–H stretching in proteins. This may be because of Al induced free radicals that can damage the N–H bond in proteins [62]. This was restored in its respective recovery group, while FWHM was significantly altered in both Al exposed groups, which was fully recovered in 30DR group and slightly restored in 45DR group. However, amide A band was not noticed in hippocampal spectra.
Band assignment 2955, 2920 and 2850 cm−1 represents the lipid profile. In cortex no change was noticed in asymmetric stretching of CH2, however prominent shift was observed in spectral features of both 30DAl and 45DAl group with no recovery in 45DR group in hippocampus, which indicates the increase in membrane fluidity and disoriented acyl chains [26, 63]. Membrane fluidity is vital for regulating axonal information, receptors, and ion channels [64] and any alterations in fluidity affect signaling pathways. Previous studies have suggested that free radicals can increase the membrane fluidity by disrupting acyl chain [65, 66]. Studies have also reported that Al causes increase in fluidity [67, 68]. Reports have also shown that amyloid beta peptide 1–40 increases neuronal membrane fluidity in brain tissues [69, 70]. On the contrary, decrease in fluidity in brain was taken as positive factor due to decreased cholesterol levels [71]. These alterations in fluidity are dependent on many factors and cholesterol level is an important factor, but it should be taken with caution as alteration in cholesterol have also varying implications, as studies have reported that reduced cholesterol levels causes decrease in fluidity, accompanied by reduction in amyloid beta clustering and causes amyloid induced neurotoxicity [72]. However, our studies are limited only to FTIR analysis of lipid and protein profile, in future it can be validated by separately collecting the membranes.
In the cortex, spectra of asymmetric stretching of CH3 were not noticed. However, in the hippocampus for both 30DAl and 45DAl groups, spectral characters of asymmetric stretching of CH3 were significantly changed with recovery in 30DR, showing an increase in unsaturated lipids in hippocampal membrane, resulting in degradation of fatty acid to shorter chains because of Al induced oxidative stress [65]. Brain is susceptible to oxidative stress due to unsaturated lipid content specially in axonal layer of hippocampus than the cortical region, and low levels of antioxidant enzymes. Previous studies from our lab have confirmed that prolonged exposure to Al results in irreversible oxidative stress [73]. Spectral data from symmetric stretching of CH2 in cortex have shifted significantly in 45DAl group with recovery in 45DR group while in hippocampus shift was prominent in both Al exposure groups with no recovery in 45DR group that indicates structural loss of acyl chain which happens during trans-to-gauche transition [74]. Increase in wavenumber shift means higher disordering in membrane and unsaturation in gauche rotomers forms [69].
In the cortex, spectra of amide, I and II was changed in both Al exposed groups with no recovery in 45DR group that suggests change in secondary structure of proteins. In the hippocampus, amide I and II was changed only in 45DAl group, with no recovery in 45DR group. Furthermore, amide III spectra were only shifted in 45DAl group with no restoration of protein structure in 45DR group. It is an established fact that aggregation and misfolding of proteins in body can cause pathologies like, Alzheimer’s, Parkinson's disease, and Diabetes type II [75]. The protein misfolding is associated with changes in secondary structure of protein. In the cortex, spectral data of symmetric stretch of carbohydrates was modified in both 30DAl and 45DAl groups with only recovery in 30DR group, while in hippocampus, shift was seen in 45DAl group which was restored in 45DR group. The alteration in carbohydrate vibrations may indicate the modifications in sugar backbone of molecules [76]. The results of our study coincided with previous studies where Al-induced model of Alzheimer’s disease altered the lipid profile of cortex [67]. Similarly in a separate study, Al induced toxicity resulted in modification of hippocampal membrane lipids that was alleviated through CoQ [32]. Moreover, recent studies on rat brain tissues, through Al-lactate resulted in alterations in both lipid and protein profile of membrane. In the same study, recovery period of 2 weeks were given after Al exposure, but no improvement was observed which may be because of higher Al doses [33].
The exact mechanism by which Al hampers synaptic transmission is not well understood, as there is lack of enough evidence about Al toxicity studies at molecular expression level of critical genes which were studied here. Synaptic changes are accompanied by neurotransmitter’s release and exocytosis of synaptic vesicles which alters the signaling cascade in post synaptic membrane [77]. Results of mRNA expression of genes studied here showed that order of genes affected the most during Al exposure in the cortex were SANP25, Homer1, Nrg1/2, Shank1, CamkIV, Kalrn and Nrxn2, and in the hippocampus were SNAP25, PSD95, Shank1, CamkIV, Syp, Nrxn1, Shank2, Homer1 and Nrg2. Similarly, in recovery groups, the order of improved recovery in the cortex were SNAP25, CamkIV and Homer1 while in the hippocampus were Nrxn1, PSD95, Shank1, Syp, Shank2 and Homer1.
Earlier studies have also reported decreased expression of Syp in hippocampus, after induction of amyloid beta and iron [47, 78, 79]. Moreover, cadmium, lead and manganese exposure have also resulted in downregulation of Nrxn in zebra fish [80]. Although fluoride exposure does not change SNAP25 levels in the rat hippocampus [81], protein expression studies on lead exposure in the hippocampus have resulted in decreased expression of SNAP25 [82]. The irreversibility in damage studied here may be because of possibility of Al binding to ionotropic nicotinic receptor that modulates transmitter release at presynaptic end, making its clearance difficult [83]. Previous studies also showed that mRNA expression of PSD95 and CamkIV were reduced in both cortex and hippocampus after amyloid induction [47] that were upregulated after curcuminoid treatment. Lead exposure decreases Homer1 expression in rat offspring’s in hippocampal cells [84]. Another study suggested that protein expression levels of Kalrn were downregulated in hippocampus of developing rat brain after treatment with Sevofurane anesthesia, whereas no effect was noted in adult rat brain, which is relevant to our studies, however we have only measured the mRNA expression level and studies were not validated at the protein level in this study [85]. Similarly, exposure to carbofuran also resulted in significant downregulation of mRNA expression of Nrg in female rats [86]. Increased levels of copper does not modify the mRNA expression levels of member of shank family genes in mice [87], whereas in vitro studies have resulted in alterations of biometal profile of ASD by decreasing the expression of shank gene [88]. Our literature search has shown that no studies have been performed in post-exposure recovery of these genes on Al exposure.
The gene expression results can be correlated with proteins expression; however, caution is needed in this respect as expression analysis depends on various factors. Earlier studies have suggested that gene expression can be correlated with respective protein expression. Study results on data set of 2044 proteins showed positive correlation of 0.66 between mRNA expression and protein [89], which indicates that mRNA expression changes can be correlated with proteins [90] in our study.
The main role of the presynapse is to ensure neurotransmitter release, which is maintained by Ca2+ influx by voltage dependent channels. This triggers the neurotransmitter fusion with synaptic vesicles for their release [91]. Binding of neurotransmitters causes ion channels in the postsynaptic membrane to open or close and changes the membrane potential of the postsynaptic membrane molecules, thus mediating information transfer across synapses [92]. Al interaction with cholinergic system has been well reported, that disrupts the Ca2+ regulation by reducing the nicotinic acetylcholine gene expression [29]. It also downregulates the expression of PKC by inhibiting expression of NMDAR through CamkII [93]. Therefore, any modifications induced by Al may alter Ca2+ release or its binding at target site, which may disrupt the release of neurotransmitters through presynaptic vesicles in the synapse (Fig. 14). In the FTIR studies, no difference was noted in the wavenumber shift of the deoxyribose band (990 cm−1), which indicates that change in phosphate may be due to alterations in lipids [30]. This suggests that changes in synaptic vesicles induced by Al may be due to alterations in lipid content and altered genes expression, as synaptic vesicle lipids are crucial for recruiting components of the exo- and endocytic machinery [94]. Furthermore, alterations in membrane fluidity lead to change in membrane-bound enzymes, ion channels, and receptors [95]. The decrease in mRNA expression of Nrg1/2 may be due to changes in membrane fluidity, which may further disrupt the function of associated receptors and ion channels. In many cases, lipid peroxidation may serve as an initial step in events that cause oxidative modification of DNA and proteins [30, 96]. This contributes to excitotoxicity and neuronal injury, by decreasing the activity of Ca2+ pump [95], which may have altered the expression of CamkIV and other studied genes.
Schematic representation of Al exposure on synaptic genes that may alter Ca2+ signaling thus disrupting the release of neurotransmitters at postsynaptic membrane. This may lead to neuronal damage and loss of synaptic strength. Downward arrows indicate the downregulation of mRNA expression levels of tested genes. The figure was drawn using the Biorender software (https://www.biorender.com/)
To understand Al induced toxicity and post-exposure recovery effect on cellular morphology of cortex and hippocampal tissues, Congo red and silver stain confers best information. Congo red staining is used to study in vitro formation of amyloid fibrils by forming lipophilic derivatives that penetrates the blood brain barrier [97]. Similarly, silver staining is used for identification and detection of pathological deposits in brain as amyloid beta and neurofibrillary tangles [98]. In our study, increased number of Congo red positive cells in cortical layers in 45DAl group with no recovery in its relative group suggested an irreversible damage. Meanwhile in the hippocampal subfields DG, CA1, CA2 and CA3 Congo red deposited in both 30DAl and 45DAl groups with only recovery in 30DR group. Results of study are in accordance with the earlier studies where deposition occurred in DG of mouse hippocampus after Al exposure [99]. Similarly, Congo red cells are formed in Al-induced Alzheimer’s disease model in cerebellum of rat brain [100]. In terms of recovery, studies conducted by Verma and colleagues that showed no difference between Al exposed and recovery group [33]. Results from silver staining showed black colored deposition in cells in both cortical layers and hippocampal regions in both 30DAl and 45DAl groups with recovery only in 30DR groups, while only DG showed recovery in 45DR group. Reports of earlier research have also suggested that Al exposure can lead to shrinkage of pyknotic Purkinje cells with formation of dark irregular amyloid plaques in cerebellum [100], hippocampus and cortex [33] of rat brain.
Findings of our studies indicate that, although Al is given while keeping the total exposure same for two different time points, it has differential effect on rat brain tissues. This implies that exposure to Al has not just dose-dependent but also has time-dependent effect. Consequently, it also provides answer to our next question that why similar recovery period has different effect. The possible reason for this could be the fact that when Al was given in time-dependent manner has different effect, then clearance or removal of metal would also be dependent on time, therefore, short-term exposure lead to better recovery compared to the prolonged exposure. This could be a major application of our study in terms of studying the toxic effects of Al metal on the human population.
Limitations of our study are that we have used only one time point of recovery. To better understand the phenomenon of recovery, more time points are needed in future studies. The accumulation and elimination of Al can be studied in a better way when levels of Al are measured at multiple time points, not only in brain tissues but also in plasma, which will provide detailed insight into the accumulation pattern as well as the removal of Al from the body. Moreover, along with the mRNA expression, proteins level and their functional studies can further elucidate the exact mechanisms underlying Al-induced neurotoxicity.
Conclusion
In conclusion, Al exposure for prolonged period resulted in irreversible damage to lipid and protein component of membrane, mRNA expression levels of synaptic genes and accumulation of red and black colored deposits in cortical and hippocampal tissues. However, recovery after 30 days of Al resulted in improvement in lipid and protein profile of brain tissues and inhibition of plaques aggregation occurred due to Al exposure. Self-recovery of 20 days in Al exposed rats for 45 days did not cause any notable improvement that suggested period of 20 days is too short for self-recovery after prolonged exposure even when using the same dose which was being used for the 30 days. It could be due to the reason that in the rat brain Al has half-life of more than 100 days and Al elimination from brain is slow process when given at lower doses. It appears that when given high dose for shorter duration of 30 days, there might be some saturation of the system, that did not cause Al accumulation, or somehow the elimination may be faster, this should be further studied with Al detection and measuring its kinetics. In conclusion, further studies need to be done with post-exposure recovery at varying time-points to better understand the phenomenon of self-recovery after Al exposure.
Data availability
The data will be made available upon reasonable request.
Abbreviations
- Al:
-
Aluminum
- FTIR:
-
Fourier transform infrared spectroscopy
- HCA:
-
Hierarchical cluster analysis
- PSD:
-
Post synaptic density
- Syp:
-
Synaptophysin
- SNAP25:
-
Synaptosomal-associated protein 25
- Nrxn1, 2:
-
Neurexin 1, 2
- CamkIV:
-
Calcium/calmodulin-dependent protein kinase IV
- Kalrn:
-
Kalirin
- Nrg1, 2:
-
Neuregulin 1, 2
- Actb:
-
Beta actin
- ATR:
-
Attenuated total reflection
- qRT-PCR:
-
Real-time PCR
- FWHM:
-
Full width at half maximum
- DG:
-
Dentate gyrus
References
Linert W, Kozlowski H (2012) Metal ions in neurological systems. Springer, New York
Golub MS, Donald JM, Gershwin ME, Keen CL (1989) Effects of aluminum ingestion on spontaneous motor activity of mice. Neurotoxicol Teratol 11:231–235
Walton JR (2007) A longitudinal study of rats chronically exposed to aluminum at human dietary levels. Neurosci Lett 412:29–33
World Health Organization (2003) Aluminium in drinking-water: background document for development of WHO Guidelines for drinking-water quality. World Health Organization, Geneva
Yokel RA (2000) The toxicology of aluminum in the brain: a review. Neurotoxicology 21:813–828
World Health Organisation (2004) Guidelines for drinking-water quality. World Health Organization, Geneva
Ferreira PC, Piai KdA, Takayanagui AMM, Segura-Muñoz SI (2008) Aluminum as a risk factor for Alzheimer’s disease. Rev Lat Am Enfermagem 16:151–157
Roskams AJ, Connor JR (1990) Aluminum access to the brain: a role for transferrin and its receptor. Proc Natl Acad Sci 87:9024–9027
Nagasawa K, Ito S, Kakuda T, Nagai K, Tamai I, Tsuji A, Fujimoto S (2005) Transport mechanism for aluminum citrate at the blood–brain barrier: kinetic evidence implies involvement of system Xc− in immortalized rat brain endothelial cells. Toxicol Lett 155:289–296
Flaten TP (2001) Aluminium as a risk factor in Alzheimer’s disease, with emphasis on drinking water. Brain Res Bull 55:187–196
Yasui M, Kihira T, Ota K (1992) Calcium, magnesium and aluminum concentrations in Parkinson’s disease. Neurotoxicology 13:593–600
Stampanoni Bassi M, Iezzi E, Gilio L, Centonze D, Buttari F (2019) Synaptic plasticity shapes brain connectivity: implications for network topology. Int J Mol Sci 20:6193
Jahn R, Südhof TC (1999) Membrane fusion and exocytosis. Annu Rev Biochem 68:863–911
Furuya TK, Silva PNO, Payão SLM, Bertolucci PHF, Rasmussen LT, De Labio RW, Braga ILS, Chen ES, Turecki G, Mechawar N (2012) Analysis of SNAP25 mRNA expression and promoter DNA methylation in brain areas of Alzheimer’s Disease patients. Neuroscience 220:41–46
Krueger DD, Tuffy LP, Papadopoulos T, Brose N (2012) The role of neurexins and neuroligins in the formation, maturation, and function of vertebrate synapses. Curr Opin Neurobiol 22:412–422
Tomasetti C, Iasevoli F, Buonaguro EF, De Berardis D, Fornaro M, Fiengo ALC, Martinotti G, Orsolini L, Valchera A, Di Giannantonio M (2017) Treating the synapse in major psychiatric disorders: the role of postsynaptic density network in dopamine-glutamate interplay and psychopharmacologic drugs molecular actions. Int J Mol Sci 18:135
Remmers C, Sweet RA, Penzes P (2014) Abnormal kalirin signaling in neuropsychiatric disorders. Brain Res Bull 103:29–38
Hook SS, Means AR (2001) Ca (2+)/CaM-dependent kinases: from activation to function. Annu Rev Pharmacol Toxicol 41:471
Naz H, Islam A, Ahmad F, Hassan MI (2016) Calcium/calmodulin-dependent protein kinase IV: a multifunctional enzyme and potential therapeutic target. Prog Biophys Mol Biol 121:54–65
Mei L, Xiong W-C (2008) Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat Rev Neurosci 9:437–452
Liu W, Liu J, Gao J, Duan X, Zhang L (2022) Effects of subchronic aluminum exposure on learning, memory, and neurotrophic factors in Rats. Neurotox Res:1–15
Kawahara M, Kato-Negishi M, Hosoda R, Imamura L, Tsuda M, Kuroda Y (2003) Brain-derived neurotrophic factor protects cultured rat hippocampal neurons from aluminum maltolate neurotoxicity. J Inorg Biochem 97:124–131
Martinez-Gardeazabal J, de San Román EG, Moreno-Rodríguez M, Llorente-Ovejero A, Manuel I, Rodríguez-Puertas R (2017) Lipid mapping of the rat brain for models of disease. Biochim Biophys Acta Biomembranes 1859:1548–1557
Kumar V, Gill KDJN (2014) Oxidative stress and mitochondrial dysfunction in aluminium neurotoxicity and its amelioration: a review. Neurotoxicology 41:154–166
Turker S, Severcan M, Ilbay G, Severcan F (2014) Epileptic seizures induce structural and functional alterations on brain tissue membranes. Biochim Biophys Acta Biomembranes 1838:3088–3096
Ahmed GAR, Khalil SKH, Abbas L, Sherif HHA, Abdel-Rahman EA, Saber SH, Hassan M, Hassan MH, Ali SS (2020) ATR-IR and EPR spectroscopy for detecting the alterations in cortical synaptosomes induced by aluminium stress. Spectrochim Acta Part A Mol Biomol Spectrosc 228:117535
Silva VS, Oliveira L, Gonçalves PP (2013) Alteration of aluminium inhibition of synaptosomal (Na+/K+) ATPase by colestipol administration. J Inorg Biochem 128:208–214
Farhat SM, Mahboob A, Iqbal G, Ahmed T (2017) Aluminum-induced cholinergic deficits in different brain parts and its implications on sociability and cognitive functions in mouse. Biol Trace Elem Res 177:115–121
Farhat SM, Mahboob A, Ahmed T (2017) Cortex-and Amygdala-dependent learning and nicotinic acetylcholine receptor gene expression is severely impaired in Mice orally treated with AlCl3. Biol Trace Elem Res 179:91–101
Furber KL, Lacombe RJ, Caine S, Thangaraj MP, Read S, Rosendahl SM, Bazinet RP, Popescu BF, Nazarali AJ (2022) Biochemical alterations in white matter tracts of the aging mouse brain revealed by FTIR spectroscopy imaging. Neurochem Res 47:795–810
Yan D, Jin C, Cao Y, Wang L, Lu X, Yang J, Wu S, Cai Y (2017) Effects of aluminium on long-term memory in rats and on SIRT 1 mediating the transcription of CREB-dependent gene in hippocampus. Basic Clin Pharmacol Toxicol 121:342–352
Saeed A, Qusti SY, Almarwani RH, Jambi EJ, Alshammari EM, Gusty NF, Balgoon MJ (2021) Effects of aluminum chloride and coenzyme Q10 on the molecular structure of lipids and the morphology of the brain hippocampus cells. RSC Adv 11:29925–29933
Verma S, Ranawat P, Nehru B (2020) Studies on the neuromodulatory effects of Ginkgo biloba on alterations in lipid composition and membrane integrity of rat brain following aluminium neurotoxicity. Neurochem Res 45:2143–2160
Qian X, Wang Z-R, Zheng J-J, Ding J-Q, Zhong J-G, Zhang T-Y, Li W, Zhang M (2019) Baicalein improves cognitive deficits and hippocampus impairments in temporal lobe epilepsy rats. Brain Res 1714:111–118
Wong WWK, Chung SWC, Kwong KP, Yin Ho Y, Xiao Y (2010) Dietary exposure to aluminium of the Hong Kong population. Food Addit Contam 27:457–463
Wang N, She Y, Zhu Y, Zhao H, Shao B, Sun H, Hu C, Li Y (2012) Effects of subchronic aluminum exposure on the reproductive function in female rats. Biol Trace Elem Res 145:382–387
Sellers RS, Mortan D, Michael B, Roome N, Johnson JK, Yano BL, Perry R, Schafer K (2007) Society of Toxicologic Pathology position paper: organ weight recommendations for toxicology studies. Toxicol Pathol 35:751–755
Mantsch HH, Chapman D (1996) Infrared spectroscopy of biomolecules. Wiley-Liss, New York
Sivakumar S, Sivasubramanian J, Raja B (2012) Aluminium induced structural, metabolic alterations and protective effects of desferrioxamine in the brain tissue of mice: an FTIR study. Spectrochim Acta Part A Mol Biomol Spectrosc 99:252–258
Granato D, Santos JS, Escher GB, Ferreira BL, Maggio RM (2018) Use of principal component analysis (PCA) and hierarchical cluster analysis (HCA) for multivariate association between bioactive compounds and functional properties in foods: a critical perspective. Trends Food Sci Technol 72:83–90
Mehpara Farhat S, Mahboob A, Ahmed T (2021) Oral exposure to aluminum leads to reduced nicotinic acetylcholine receptor gene expression, severe neurodegeneration and impaired hippocampus dependent learning in mice. Drug Chem Toxicol 44:310–318
Mirza FJ, Amber S, Hassan D, Ahmed T, Zahid S (2021) Rosmarinic acid and ursolic acid alleviate deficits in cognition, synaptic regulation and adult hippocampal neurogenesis in an Aβ1-42-induced mouse model of Alzheimer’s disease. Phytomedicine 83:153490
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408
Orlowski D, Elfving B, Müller HK, Wegener G, Bjarkam CR (2012) Wistar rats subjected to chronic restraint stress display increased hippocampal spine density paralleled by increased expression levels of synaptic scaffolding proteins. Stress 15:514–523
Julian GS, de Oliveira RW, Perry JC, Tufik S, Chagas JR (2014) Validation of housekeeping genes in the brains of rats submitted to chronic intermittent hypoxia, a sleep apnea model. PLoS ONE 9:e109902
Tanic N, Perovic M, Mladenovic A, Ruzdijic S, Kanazir S (2007) Effects of aging, dietary restriction and glucocorticoid treatment on housekeeping gene expression in rat cortex and hippocampus—evaluation by real time RT-PCR. J Mol Neurosci 32:38–46
Ahmed T, Enam SA, Gilani AH (2010) Curcuminoids enhance memory in an amyloid-infused rat model of Alzheimer’s disease. Neuroscience 169:1296–1306
Yamamoto T, Hirano A (1986) A comparative study of modified Bielschowsky, Bodian and thioflavin S stains on Alzheimer’s neurofibrillary tangles. Neuropathol Appl Neurobiol 12:3–9
Tomljenovic L (2011) Aluminum and Alzheimer’s disease: after a century of controversy, is there a plausible link? J Alzheimers Dis 23:567–598
Krewski D, Yokel RA, Nieboer E, Borchelt D, Cohen J, Harry J, Kacew S, Lindsay J, Mahfouz AM, Rondeau V (2007) Human health risk assessment for aluminium, aluminium oxide, and aluminium hydroxide. J Toxicol Environ Health Part B 10:1–269
Zhong W, Wang L, Fang S, Qin D, Zhou J, Yang G, Duan H (2020) Two novel colorimetric fluorescent probes: Hg 2+ and Al 3+ in the visual colorimetric recognition environment. RSC Adv 10:3048–3059
Ma J, Jiang G, Zheng W, Zhang M (2019) A longitudinal assessment of aluminum contents in foodstuffs and aluminum intake of residents in Tianjin metropolis. Food Sci Nutr 7:997–1003
Saiyed SM, Yokel RA (2005) Aluminium content of some foods and food products in the USA, with aluminium food additives. Food Addit Contam 22:234–244
Greger JL (1993) Aluminum metabolism. Annu Rev Nutr 13:43–63
Commissaris RL, Cordon JJ, Sprague S, Keiser J, Mayor GH, Rech RH (1982) Behavioral changes in rats after chronic aluminum and parathyroid hormone administration. Neurobehav Toxicol Teratol 4:403–410
Who A, World Health O (1997) International programme on chemical safety. Environmental Health Criteria, Geneva, p 194
Li X, Hu C, Zhu Y, Sun H, Li Y, Zhang Z (2011) Effects of aluminum exposure on bone mineral density, mineral, and trace elements in rats. Biol Trace Elem Res 143:378–385
Abu-Taweel GM, Al-Mutary MG (2021) Pomegranate juice reverses AlCl3-Induced neurotoxicity and improves learning and memory in female mice. Environ Res 199:111270
Elizabeth MA, Samson P, Itohan ORJMBD (2020) Histomorphological evaluations on the frontal cortex extrapyramidal cell layer following administration of N-Acetyl cysteine in aluminum induced neurodegeneration rat model. Metab Brain Dis 35:829–839
Bailey SA, Zidell RH, Perry RW (2004) Relationships between organ weight and body/brain weight in the rat: what is the best analytical endpoint? Toxicol Pathol 32:448–466
Zhang R, Zhang L, Jiang D, Zheng K, Cui Y, Li M, Wu B, Cheng S (2014) Mouse organ coefficient and abnormal sperm rate analysis with exposure to tap water and source water in Nanjing reach of Yangtze River. Ecotoxicology 23:641–646
Saad-El-Din AA, Mazhar A, Khalil W (2020) Role of Spirulina on gamma-irradiated rats using Fourier transform infrared attenuated total reflectance and Electron spin resonance for brain. J Radiat Res Appl Sci 13:528–541
Casal HL, Mantsch HH (1984) Polymorphic phase behaviour of phospholipid membranes studied by infrared spectroscopy. Biochim Biophys Acta Rev Biomembr 779:381–401
Yehuda S, Rabinovitz S, Carasso RL, Mostofsky DI (2002) The role of polyunsaturated fatty acids in restoring the aging neuronal membrane. Neurobiol Aging 23:843–853
Cakmak G, Miller LM, Zorlu F, Severcan F (2012) Amifostine, a radioprotectant agent, protects rat brain tissue lipids against ionizing radiation induced damage: an FTIR microspectroscopic imaging study. Arch Biochem Biophys 520:67–73
Avdulov NA, Chochina SV, Igbavboa U, O’Hare EO, Schroeder F, Cleary JP, Wood WG (1997) Amyloid β-peptides increase annular and bulk fluidity and induce lipid peroxidation in brain synaptic plasma membranes. J Neurochem 68:2086–2091
Ahmed GAR, Khalil SKH, El Hotaby W, Abbas L, Farrag ARH, Aal WEA, Sherif HHA, Abdel-Rahman EA, Saber SH, Hassan M (2020) ATR-IR and EPR spectroscopy for following the membrane restoration of isolated cortical synaptosomes in aluminium-induced Alzheimer’s disease–Like rat model. Chem Phys Lipids 231:104931
VlS S, Cordeiro JM, Matos MJ, Oliveira CR, Gonçalves PP (2002) Aluminum accumulation and membrane fluidity alteration in synaptosomes isolated from rat brain cortex following aluminum ingestion: effect of cholesterol. Neurosci Res 44:181–193
Ahmed GAR, El Hotaby W, Abbas L, Sherif HHA, Kamel G, Khalil SKH (2020) Synchrotron Fourier transform infrared microspectroscopy (sFTIRM) analysis of Al-induced Alzheimer’s disease in rat brain cortical tissue. Spectrochim Acta Part A Mol Biomol Spectrosc 239:118421
Mason RP, Jacob RF, Walter MF, Mason PE, Avdulov NA, Chochina SV, Igbavboa U, Wood WG (1999) Distribution and fluidizing action of soluble and aggregated amyloid β-peptide in rat synaptic plasma membranes. J Biol Chem 274:18801–18807
Eckert GP, Wood WG, Müller WE (2001) Effects of aging and β-amyloid on the properties of brain synaptic and mitochondrial membranes. J Neural Transm 108:1051–1064
Fernández-Pérez EJ, Sepúlveda FJ, Peters C, Bascuñán D, Riffo-Lepe NO, González-Sanmiguel J, Sánchez SA, Peoples RW, Vicente B, Aguayo LG (2018) Effect of cholesterol on membrane fluidity and association of Aβ oligomers and subsequent neuronal damage: a double-edged sword. Front Aging Neurosci 10:226
Iqbal G, Ahmed T (2019) Co-exposure of metals and high fat diet causes aging like neuropathological changes in non-aged mice brain. Brain Res Bull 147:148–158
Ripanti F, Di Venere A, Cestelli Guidi M, Romani M, Filabozzi A, Carbonaro M, Piro MC, Sinibaldi F, Nucara A, Mei G (2021) The puzzling problem of cardiolipin membrane-cytochrome c interactions: a combined infrared and fluorescence study. Int J Mol Sci 22:1334
Kawahara M, Kato-Negishi M (2011) Link between aluminum and the pathogenesis of Alzheimer's disease: the integration of the aluminum and amyloid cascade hypotheses. Int J Alzheimer Dis
Akkas SB, Inci S, Zorlu F, Severcan F (2007) Melatonin affects the order, dynamics and hydration of brain membrane lipids. J Mol Struct 834:207–215
Min D, Kim K, Hyeon C, Hoon Cho Y, Shin Y-K, Yoon T-Y (2013) Mechanical unzipping and rezipping of a single SNARE complex reveals hysteresis as a force-generating mechanism. Nat Commun 4:1–10
Reddy PH, Manczak M, Yin X, Grady MC, Mitchell A, Kandimalla R, Kuruva CS (2016) Protective effects of a natural product, curcumin, against amyloid β induced mitochondrial and synaptic toxicities in Alzheimer’s disease. J Investig Med 64:1220–1234
Da Silva VK, de Freitas BS, da Silva DA, Nery LR, Falavigna L, Ferreira RDP, Bogo MR, Hallak JEC, Zuardi AW, Crippa JAS (2014) Cannabidiol normalizes caspase 3, synaptophysin, and mitochondrial fission protein DNM1L expression levels in rats with brain iron overload: implications for neuroprotection. Mol Neurobiol 49:222–233
Tu H, Fan C, Chen X, Liu J, Wang B, Huang Z, Zhang Y, Meng X, Zou F (2017) Effects of cadmium, manganese, and lead on locomotor activity and neurexin 2a expression in zebrafish. Environ Toxicol Chem 36:2147–2154
Han H, Du W, Zhou B, Zhang W, Xu G, Niu R, Sun Z (2014) Effects of chronic fluoride exposure on object recognition memory and mRNA expression of SNARE complex in hippocampus of male mice. Biol Trace Elem Res 158:58–64
Li N, Yu ZL, Wang L, Zheng YT, Jia JX, Wang Q, Zhu MJ, Liu XH, Xia X, Li WJ (2009) Early-life lead exposure affects the activity of TNF-α and expression of SNARE complex in hippocampus of mouse pups. Biol Trace Elem Res 132:227–238
Farhat SM, Ahmed T (2018) Aluminum suppresses effect of nicotine on gamma oscillations (20–40 Hz) in mouse hippocampal slices. CNS Neurolo Disorder Drug Targets 17:404–411
Pang S, Li Y, Chen W, Li Y, Yang M, Zhao L, Shen Q, Cheng N, Wang Y, Lin X (2019) Pb exposure reduces the expression of SNX6 and Homer1 in offspring rats and PC12 cells. Toxicology 416:23–29
Li C, Liu S, Mei Y, Wang Q, Lu X, Li H, Tao F (2022) Differential effects of sevoflurane exposure on long-term fear memory in neonatal and adult rats. Mol Neurobiol 59:2799–2807
Mishra D, Tiwari SK, Agarwal S, Sharma VP, Chaturvedi RK (2012) Prenatal carbofuran exposure inhibits hippocampal neurogenesis and causes learning and memory deficits in offspring. Toxicol Sci 127:84–100
Baecker T, Mangus K, Pfaender S, Chhabra R, Boeckers TM, Grabrucker AM (2014) Loss of COMMD1 and copper overload disrupt zinc homeostasis and influence an autism-associated pathway at glutamatergic synapses. Biometals 27:715–730
Hagmeyer S, Mangus K, Boeckers TM, Grabrucker AM (2015) Effects of trace metal profiles characteristic for autism on synapses in cultured neurons. Neural Plast 2015
Greenbaum D, Colangelo C, Williams K, Gerstein M (2003) Comparing protein abundance and mRNA expression levels on a genomic scale. Genome Biol 4:1–8
Koussounadis A, Langdon SP, Um IH, Harrison DJ, Smith VA (2015) Relationship between differentially expressed mRNA and mRNA-protein correlations in a xenograft model system. Sci Rep 5:1–9
Melland H, Carr EM, Gordon SL (2021) Disorders of synaptic vesicle fusion machinery. J Neurochem 157:130–164
Sheng M, Kim E (2011) The postsynaptic organization of synapses. Cold Spring Harb Perspect Biol 3:a005678
He C, Ji J, Zhao X, Lei Y, Li H, Hao Y, Zhang S, Zhang J, Liu C, Nie J (2021) The role of PKC in regulating NMDARs in aluminum-induced learning and memory impairment in rats. Neurotox Res 39:2042–2055
Binotti B, Jahn R, Pérez-Lara Á (2021) An overview of the synaptic vesicle lipid composition. Arch Biochem Biophys 709:108966
Turker S, Ilbay G, Severcan M, Severcan F (2014) Investigation of compositional, structural, and dynamical changes of pentylenetetrazol-induced seizures on a rat brain by FT-IR spectroscopy. Anal Chem 86:1395–1403
Sasaki T, Mogi SI, Kaneko T, Kojima H, Katoh S, Sano A, Kojima S (2014) Relationship between tissue hydroxyl radical and oxidatively modified macromolecule levels. Geriatr Gerontol Int 14:498–507
Maezawa I, Hong HS, Liu R, Wu CY, Cheng RH, Kung MP, Kung HF, Lam KS, Oddo S, LaFerla FM (2008) Congo red and thioflavin-T analogs detect Aβ oligomers. J Neurochem 104:457–468
Uchihara T (2007) Silver diagnosis in neuropathology: principles, practice and revised interpretation. Acta Neuropathol 113:483–499
Malik N, Amber S, Zahid S (2022) Rosmarinus officinalis and methylphenidate exposure improves cognition and depression and regulates anxiety-like behavior in AlCl3-induced mouse model of Alzheimer’s Disease. Front Pharmacol 13
Mustafa HN (2020) Neuro-amelioration of cinnamaldehyde in aluminum-induced Alzheimer’s disease rat model. J Histotechnol 43:11–20
Acknowledgements
We like to acknowledge Atta-ur-Rahman School of Applied Biosciences (ASAB), National University of Sciences and Technology (NUST), Pakistan, and Higher Education Commission (HEC) of Pakistan for providing support and facilities for this study. First author is recipient of Indigenous 5000 Ph.D. Scholarship program, HEC. HEC is not involved in study design, data interpretation, writing of manuscript, and decision to submit article for publication.
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
H.A.: Methodology, Formal analysis, Investigation, Visualization, Writing - original draft; T.A.: Conceptualization, Methodology, Investigation, Visualization, Resources, Writing - original draft, Writing - review & editing, Supervision. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors have no financial and non-financial interests to disclose.
Ethical Approval
All experimental procedures were approved by institutes Internal Review Board. Approval number: IRB-135.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Asghar, H., Ahmed, T. Comparative Study of Time-Dependent Aluminum Exposure and Post-Exposure Recovery Shows Better Improvement in Synaptic Changes and Neuronal Pathology in Rat Brain After Short-Term Exposure. Neurochem Res 48, 2731–2753 (2023). https://doi.org/10.1007/s11064-023-03936-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-023-03936-6