Abstract
Recent industrialization has increased human exposure to bio-available aluminum (Al). If more Al enters the brain than leaves, Al concentration will rise in the brain leading to neurodegenerative disorders. The aim of the present study was to determine Al concentration, neurodegeneration, and nicotinic acetylcholine receptor (nAChR) gene expression in the cortex and amygdala after oral ingestion of Al salt. The effect of Al on cortex- and amygdala-dependent learning and memory functions was also assessed. Mice were given AlCl3 (250 mg/kg) in drinking water for 42 days. nAChR gene expression was determined in the cortex and amygdala. The mice were subjected to behavior tests (fear conditioning, fear extinction, and open field), to assess memory deficits. The acquisition of fear memory in the fear conditioning test remained unaffected due to the Al administration. However, fear extinction (which is a new learning) was severely impaired. The behavioral analysis in the open field test showed greater anxiety and less adaptability to the new environment in Al-treated animals. High Al concentration and severe neurodegeneration in the cortex were observed following Al treatment while a slight, non-significant elevation in Al concentration was observed in the amygdala of Al-treated animals. The analysis of nAChR gene expression via RT-PCR showed a significant reduction in expression of α7, α4, and β2 nAChR genes in the cortex of Al-treated animals, while in the amygdala, the level of the α4 nAChR gene remained unaltered. Oral Al ingestion causes neuropathological changes and suppresses expression of nAChR genes that lead to deficits in learning and higher anxiety in Al-treated animals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neurotoxicity is caused by certain chemicals, called neurotoxins, that cause degeneration of neuronal cells [1]. Certain trace elements and few metals, e.g., aluminum (Al), mercury, copper, lead, arsenic, and manganese, can also act as neurotoxins when exposed to high concentrations [2]. Al is abundantly present in the earth’s crust [3], but no physiological role is known for this metal in the human body [4]. Al is released into the environment by soil erosion and anthropogenic activities [5]. Human exposure to Al may occur via oral ingestion and drinking water as Al salts are added to a variety of commercially prepared foods and beverages [6]. Although food is an important contributor for Al exposure to humans, Al present in drinking water is more bio-available [5], due to the reason that Al in drinking water is in uncomplexed form and can be absorbed from the gastrointestinal tract. On the other hand, Al present in food is complexed with other elements and is present in the form of phytates and polyphenols that greatly reduce its absorption [7]. Al salts are added for clarification of drinking water and are used as coagulant to reduce turbidity, microorganisms, and organic matter from drinking water [5]. Though this treatment is useful, it may cause an increase in Al concentration at the final point of consumption [5]. Moreover, high residual concentrations may cause Al to deposit in the distribution system which on disturbance may cause an increase in Al concentration in tap water [8]. Many epidemiological studies, toxicological studies on laboratory animals, and information on biochemical change in the brain after high Al exposure suggest that Al is a powerful neurotoxicant [4, 9]. Dietary intake of Al may exacerbate the underlying events associated with neurodegeneration [10] and may lead to the development of neurodegenerative disorders like amyotrophic lateral sclerosis, Alzheimer’s disease, Guam Parkinson’s dementia, etc. [11].
Al is known to target many voltage-gated and ligand-gated ion channels [11] and is well known for its toxic effects on the cholinergic system [12, 13]. The cholinergic system, which comprises muscarinic and nicotinic acetylcholine receptors (nAChRs), plays an important role in cognition, learning, and memory [14]. Although the effect of Al on muscarinic acetylcholine receptor expression is extensively studied [15–17], its effect on nAChRs, specifically in the cortex and amygdala, is less well known. The neuronal nAChRs play a major role in many physiological functions including learning, memory, and attention [11]. α7 and α4β2 are the most abundant types of nAChRs in the central nervous system [18] and are abundantly expressed in the brain [19]. Keeping in view the importance of nAChRs in cognition and memory processes, and the unexplored effects of Al on these receptors in the cortex and amygdala, the present study was designed to determine the effects of Al on learning, memory, and nAChR gene expression in the cortex and amygdala. Moreover, neurodegeneration, caused by Al exposure, was also investigated.
Materials and Methods
Chemicals
Aluminum chloride hexahydrate AlCl3·6H2O (AL0770), paraformaldehyde (PA0095100), and Cresyl Violet stain (229630250) were purchased from Scharlau, Spain. AlCl3·6H2O had ≤0.005% total impurities of heavy metals such as Pb. The product was kept in an air-tight container in a well-ventilated area. Taq polymerase, 10 mM deoxynucleotide (dNTP), and RT enzyme were obtained from Fermentas® and TRI Reagent from Invitrogen®. Chemical solutions were made fresh every day.
Animals
All experiments complied with the rulings of the Institute of Laboratory Animal Research, Division on Earth and Life Sciences, National Institute of Health, USA (Guide for the Care and Use of Laboratory Animals). The research protocol was approved by the Internal Review Board (IRB), Atta-ur-Rahman School of Applied Biosciences, National University of Sciences and Technology. Male BALB/c mice (3–4 months of age, weight ranging from 30 to 45 g) were purchased from the National Institutes of Health (NIH), Islamabad. Mice were housed in the animal house of ASAB, NUST, under controlled environmental conditions. The temperature was maintained at a constant temperature of 25 ± 2 °C, and a natural light and dark cycle (14 h light and 10 h dark) was followed. Animals were given water and standard diet ad libitum consisting of 30% crude protein, 9% crude fat, 4% crude fiber, and 10% moisture.
In Vivo AlCl3 Administration
Animals were divided into two groups designated as the control group (received distilled water) and the Al-treated group (received AlCl3 250 mg/kg/day in drinking water for 42 days). The dose of 250 mg/kg used in our study means that for every kilogram (kg) weight of the animal, the amount of Al should be 250 mg. For a mouse of 40 g weight, the amount of Al should be 10 mg to be ingested by the mouse. Before the start of this study, we calculated water intake for animals and it was found that a mouse of 40 g weight normally drinks 10 ml of water daily. Therefore, in order to administer the required dose (250 mg/kg per day), 10 mg of Al salt was dissolved in 10 ml of water and this water was given to mice.
Behavior Tests
Behavioral tests were performed from 10 a.m. to 5 p.m. on the 42nd day of Al treatment. Animals were transferred to the testing room 30 min prior to beginning of behavior test. The behavior test was recorded with a video camera in the absence of the experimenter and analyzed later on.
Fear Conditioning
The testing procedure was the same as described previously [20] with some modifications. An animal enclosure chamber (17 cm × 17 cm × 25 cm) was used for fear conditioning. The inside of the box was cleaned with 70% ethanol before and after the experiment to prevent bias based on olfactory cues.
The testing procedure started with habituation of the subject animal in the empty chamber for 5 min to avoid any freezing response due to anxiety of the new space during the experimental procedure. The test session consisted of five tones (80 db) for 30 sec, called as conditioned stimulus (CS), each paired with a 1-s 0.5-mA foot shock (US: given at the end of tone/CS) with an inter-tone interval (ITI) of 2 min. The response to the CS was measured as “freezing.” Freezing is a commonly used index of fear and is defined as the complete absence of any body movements except for those associated with respiration [21].
Freezing time was measured during CS delivery by ANY-maze software. The freezing response was expressed as percentage freezing for each CS trial according to the following formula.
Fear Extinction
The testing procedure was the same as described earlier [20] with some modifications. The test was performed in a context, entirely different from that used in fear conditioning. The walls and the floors of the chamber were replaced so that the animal could only associate and recall memory formed with the CS. In order to avoid bias in behavior based on the new environment, the subject was given a habituation time of 7 min in this new context. The test phase consisted of 20 CS trials 80 dB each of 30 sec, and the ITI was also kept for 30 sec. No US was given to the animal during fear extinction. The freezing time was recorded by ANY-maze, and freezing time was plotted as percent freezing. Percent freezing was calculated from the same formula as described for fear conditioning. Upon experiment completion, the box was cleaned with 70% ethanol to avoid any olfactory cues for the next mouse.
Open Field Test
The test is performed to check anxiety, locomotor activity, and exploratory behavior. The testing procedure was the same as described previously [22] with slight modifications. The mouse was placed in the center of a square opaque iron alloy box with dimensions of 40 × 40 × 40 cm. The box was divided into central and peripheral areas. The demarcation for the peripheral area was up to 2 cm away from the walls of the box. The box was placed in a homogenously illuminated area. Each mouse was placed in the box for 30 min. The whole test was recorded with a video camera and was later assessed for the following parameters.
-
1.
Time spent in central and peripheral regions during the whole test duration
-
2.
Number of rearings as a measure of exploratory behavior. Rearing was defined as the posture when the animal was standing in vertical position on its hind limbs.
-
3.
Time spent in relaxed and anxious grooming
The latter two parameters measured in the initial 5 min of the test were separately compared to these activities in the last 5 min of the 30-min test duration to determine how aluminum administration affects the adaptation to a new environment. After completion of the test, the box was cleaned properly with 70% ethanol.
Determination of Al Concentration in Brain
Al concentration was determined in the cortex and amygdala via inductively coupled plasma atomic emission spectrometry (ICP-AES) as described by [23] for blood with some modifications. All propylene glassware was rinsed with distilled water and was then soaked in 10% (v/v) nitric acid for 48 h and was then thoroughly washed with de-ionized water. Accurately 1 g of wet tissue was weighed and was digested by a conventional wet digestion method by adding 3 ml of a freshly prepared HNO3-H2O2 mixture (2:1 v/v). The samples were then digested at 70 °C for 2 h, after which the samples were treated with 2 ml of HNO3 and a few drops of H2O2, and heating was continued at 80 °C until a clear digested mixture was obtained. The excess acid was evaporated to obtain a semi-dry mass to which de-ionized water was added up to 3 ml, after which the concentration of aluminum was measured using ICP-AES at an analytical wavelength of 396.15 λ.
Histological Examination
Mice were anesthetized using ketamine (300 μl/50 g) i.p. After the animal was completely anesthetized and pedal reflex was abolished, the mouse brain was fixed via transcardial perfusion, with 4% paraformaldehyde, according to the method previously described [24]. The brain tissue was fixed in the same fixative for 24–48 h at 4 °C. Then, the brain tissue was processed for paraffin embedding and cut into 3-μm coronal sections.
Cresyl Violet Staining
Paraffin sections were stained with cresyl violet for neuronal Nissl bodies and to determine neurodegeneration. Sections were de-waxed by giving two washes in xylene each for 5 min and then rehydrated with descending concentrations of isopropanol for 5 min each. Then, the sections were stained with Cresyl Violet for 4 min. After rinsing with distilled water, the sections were de-stained with 70% acid alcohol (2 ml glacial acetic acid in 200 ml of 70% ethanol) for 2 min. Slides were mounted with Canada balsam and were observed under a light microscope.
Quantitative Analysis of Neurodegeneration
Quantitative analysis of the cell number was carried out in layers 1, 2–4, 5, and 6 of the motor cortex, sensory motor cortex (hind limb and forelimb regions), and sensory motor cortex (dysgranular zone and barrel field area). The analysis was performed in an area of 10,000 μm2 from three randomly selected sites in each layer of each region. Later, the average of values from all three sites were taken and plotted.
Gene Expression Studies
On the 43rd day of treatment, animals were sacrificed and the cortex and amygdala were harvested from the brain for gene expression studies. Gene expression studies were carried out according to the method as described earlier [25]. Briefly, RNA was extracted from the cortex and amygdala using TRIzol with standard TRI reagent protocol. The integrity of RNA samples was checked qualitatively (on 2% agarose gel) and quantitatively (with a spectrophotometer). Complementary DNA (cDNA) (40 μl) was made by RT-PCR using 1 μg of extracted RNA from each sample. The cDNA was then processed for PCR, and the reaction mixture recipe was as follows: 10 μM primer, 25 mM MgCl2, 10 mM dNTP, and 0.625 units of 25 μl Taq polymerase (Thermo Scientific). Primers specific for the genes (sequence mentioned in Table 1) were used for PCR with initial denaturation of 95 °C for 5 min, followed by denaturation at 94 °C for 30 s, annealing at respective primer temperatures (Table 1) for 30 s, and extension at 72 °C for 30 s. The reaction was terminated with a final extension at 72 °C for 10 min. The reaction was repeated for 35 cycles. Actin was used as the housekeeping gene and to normalize respective genes. PCR products were run on 2% agarose gel, visualized by ethidium bromide (adding 10 μl of 10 mg/ml solution for every 100 ml of gel) staining. Each PCR product band was quantified for densitometry using NIH software “Image J.”
Statistical Analysis:
Data is expressed as mean ± standard error of mean (SEM; n = number of animals). Results were analyzed statistically using “GraphPad Prism” V5.0 for windows (GraphPad software, San Diego, CA). A two-tailed t test was applied to analyze the significance of the results. Results were taken significant only if the “p” value was less than 0.05.
Results
Effect of Aluminum on Fear Memory
The amygdala-dependent delay fear conditioning test results showed that Al administration has no effect on fear memory acquisition as control and Al-treated animals showed similar learning of fear memory (similar freezing response in both groups) across five cue foot shock pairing trials (Fig. 1a).
Effect of Al on fear memory. a Graph showing percent freezing response in control and Al-treated animals across five cue foot shock pairing trials of amygdala-dependent delay fear conditioning test. b Graph showing percent freezing response in control and Al-treated animals across 20 tone (CS) trials of fear extinction test. c The graph showing average freezing response in control and Al-treated animals during the last five tones (CS) of fear extinction. **P < 0.01; n = sample size
During fear extinction learning, the control group showed a progressive decline in the freezing response (Fig. 1b) and reduced freezing was observed during the last five CS trials (3.52 ± 1.38 s; Fig. 1c). While Al-treated animals showed impaired fear extinction learning demonstrated by high freezing response (27.39 ± 6.26 s, p < 0.01, Fig. 1c) even during the last five CS trials of fear extinction.
Open Field Test
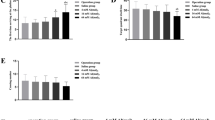
High anxiety level was observed in Al-treated animals in the open field test, as these animals preferred to stay in the periphery of the test box (22.19 ± 0.5 s) as compared to the control group (16.71 ± 0.4 s, p < 0.001) and the control group spent significantly greater time in the center (8.03 ± 0.41 s, p < 0.001) as compared to the Al-treated group (2.95 ± 0.57 s; Fig. 2a). Greater exploratory activity in the new environment was observed in control animals as revealed by higher rearing number (45 ± 3.06), as compared to Al-treated animals (22.57 ± 2.68, p < 0.001) during the initial 5 min of the 30-min test duration. Whereas control animals showed greater adaptation to the new environment as depicted by decreased rearings (7.5 ± 1.47) compared to Al-treated animals (21.28 ± 1.94, p > 0.05; Fig. 2b) during the last 5 min of test duration. During the initial 5 min of test, animals did not show relaxed grooming. Relaxed grooming increased in the control group (41.93 ± 7.21 s) as compared to the Al-treated group (4.38 ± 1.07 s, p < 0.001) in the last 5 min of the test (Fig. 2c), revealing a greater adaptation to the novel environment. The Al-treated group showed high anxious grooming (45.28 ± 5.18 s) during the initial 5 min of the test as compared to the control group (11.79 ± 1.89 s, p < 0.001). During the last 5 min, the control group showed almost negligible anxious grooming (2.8 ± 0.31 s), while the Al-treated animals were unable to adapt to the new environment and showed high anxious grooming during the last 5 min (21.54 ± 2.42 s, p < 0.001; Fig. 2d).
Effect of Al on anxiety behavior in the open field test. a Graph showing time spent (s) by control and Al-treated animals in the center and periphery of the open field test box. b Graph showing comparison of number of rearings in control and Al-treated animals during the initial and last 5 min of the open field test. c The graph showing time spent (s) by control and Al-treated animals in relaxed grooming during the initial and last 5 min of the test. d The graph showing time spent (s) by control and Al-treated animals in anxious grooming during the initial and last 5 min of the test. **P < 0.01, ***P < 0.001; n = sample size
Aluminum Concentration in Brain
The evaluation of Al concentration in the cortex and amygdala showed that the Al-treated group had significantly high concentration of Al in the cortex (462.57 ± 121.08 μg/g) as compared to the control animals (98.85 ± 6.71 μg/g, p < 0.05). Whereas Al did not accumulate much in the amygdala, as a slight, non-significant increase in the amygdala of the Al-treated group (870.83 ± 251.90 μg/g) was observed as compared to the control group (488 ± 73.23 μg/g, p > 0.05; Fig. 3). The basal levels of Al in the amygdala were higher than in the cortex of control animals.
Histological Examination
The Cresyl Violet staining in the motor cortex of Al-treated animals revealed that there was a significant reduction in cell number in layers 2–4 (28.5 ± 1.91), layer 5 (26.5 ± 0.99), and layer 6 (29.66 ± 1.53) as compared to layers 2–4 (34.8 ± 0.76, p < 0.01), layer 5 (32.86 ± 1.13, p < 0.01), and layer 6 (35.13 ± 1.09, p < 0.05) of control animals. The cell number in layer 1 of the motor cortex in Al-treated animals (9.77 ± 0.26) remained unaltered as compared to control animals (11.13 ± 0.79, p > 0.05; Fig. 4a). No difference in cell number in any of the layer in the somatosensory cortex (hind limb and forelimb regions) was observed between control and Al-treated animals (Fig. 4b). In the dysgranular zone and barrel field area of the somatosensory cortex, there was a significant reduction in layers 2–4 (24.83 ± 1.3) and layer 5 (20 ± 1.25) of Al-treated animals as compared to layers 2–4 (32.73 ± 1, p < 0.001) and layer 5 (24.73 ± 1.56, p < 0.05) of control animals. While the cell number in layer 1 (5.52 ± 0.61) and layer 6 (27.72 ± 0.89) of the somatosensory cortex (dysgranular zone, barrel field area) in Al-treated animals remained unaffected as compared to layer 1 (7 ± 0.9, p > 0.05) and layer 6 (30.33 ± 1.96, p > 0.05; Fig. 4c) of control animals. Representative slides from various brain areas are shown (Fig. 5).
The graph showing the cell number in different areas of the cortex in control and Al-treated mice groups. a Graph showing cell count in different layers of the motor cortex in control and Al-treated animals. b Graph showing cell count in different layers of the hind limb and fore limb sensory motor cortex in control and Al-treated animals. c Graph showing cell count in different layers of the dysgranular zone and barrel field area sensory motor cortex in control and Al-treated animals. Reduced cell number represents neurodegeneration after Al treatment. *P < 0.05, **P < 0.01, ***P < 0.001; n = sample size
The graph shows representative slides of cortex histology (Nissl staining) sections at ×40 magnification. a Motor cortex of control animals. b Motor cortex of Al-treated animals. c Hind limb and fore limb sensory motor cortex of control animals. d Hind limb and fore limb sensory motor cortex of Al-treated animals. e Dysgranular zone and barrel field area sensory motor cortex of control animals. f Dysgranular zone and barrel field area sensory motor cortex of Al-treated animals
Effect of Al on Nicotinic Acetylcholine Receptor Gene Expression
There was a significant reduction in the gene expression of α7 (0.81 ± 0.08), α4 (0.19 ± 0.02), and β2 (0.91 ± 0.14) nAChRs in the cortex of the Al-treated group as compared to the α7 (2.35 ± 0.61, p < 0.05), α4 (0.88 ± 0.0.15, p < 0.05), and β2 (2.06 ± 0.52, p < 0.05; Fig. 6a) nAChRs in the cortex of control animals. In the case of amygdala, only the expression of α7 (0.57 ± 0.16) and β2 (1.06 ± 0.12) nAChRs was reduced in Al-treated animals as compared to the α7 (1.74 ± 0.18, p < 0.001) and β2 (1.6 ± 0.12, p < 0.05) nAChRs of the control animals, while the level of the α4 nAChR remained unaltered after Al treatment (Fig. 6b).
Discussion
Al is abundantly present in the earth’s crust [26]. Al is used in many industries, e.g., in the automotive electric and construction industries. Moreover, it is also used in the production of metal alloys and food packaging and cooking utensils [8]. Due to the industrial revolution, the distribution and availability of Al have greatly increased to biological systems [26]. There is considerable evidence that Al causes neurotoxicity and plays a role in the pathogenesis and etiology of Alzheimer’s disease [9].
In the present study, the focus was to determine Al neurotoxicity at high end of human exposure. Certain individuals who consume antacids or buffered aspirin, chronically, are exposed to very high amounts of Al [1]. It has been reported previously that 260 mg/kg Al administered for 5 weeks corresponds to the estimated maximal human intake [27–29]. Therefore, a high dose of Al was selected in this study and Al toxicity was induced by administration of 250 mg/kg AlCl3 for a period of 6 weeks in drinking water.
Behavior is the net output of motor, sensory, and cognitive functions of the nervous system. Therefore, behavioral alterations are potentially a sensitive indicator of xenobiotic-induced neurotoxicity [30]. In order to determine how Al affected the cortex- and amygdala-dependent behavioral functions, behavior tests, such as open field test (cortex-dependent) and fear memory tests (amygdala-dependent), were performed. In order to study the neuronal substrates of learning and memory, classical fear conditioning is a powerful tool [31]. It is identified that the amygdala is critically involved in the acquisition and storage of fear memory [32]. Although we observed a reduction in the gene expression of α4 and α7 nAChRs in the amygdala, our results revealed that Al administration had no effect on fear conditioning. Previously, it is reported that in the dorsal hippocampus, trace fear conditioning, a hippocampus-dependent behavior, is regulated by cholinergic transmission, but delay fear conditioning, an amygdala-dependent fear conditioning paradigm used in our experiments, is not dependent on cholinergic transmission [33]. Moreover, another study on the hippocampus reported that nicotine administration enhances trace fear conditioning but has no effect on delay fear conditioning [34]. In view of this previous literature, it is stated that the deficit in nAChR gene expression in the amygdala does not influence delay fear conditioning as both groups show similar learning during this testing paradigm, but this needs to be further investigated. Our results show that fear extinction is greatly impaired in Al-treated animals as these animals are unable to develop new memories related to fear extinction. As the cholinergic system is known to play a very important role in fear extinction [35], therefore, the lack of fear extinction observed in our experiments might be due to the nAChR gene expression deficit in the amygdala reported in our data.
In the open field test grooming activity, exploration and anxiety were measured. Grooming is evolutionarily an important behavior in all animals, but in addition to being a normal behavior, this is also displayed by animals in anxiety because grooming helps in stress reduction [36]. In our study, the grooming behavior, in the open field was evaluated according to the criteria defined by Smolinsky et al. [37], which states that grooming in mouse follows cephalocaudal direction and any grooming activity that does not follow this pattern is due to anxiety [37]. Based on this criterion, the relaxed and anxious grooming was scored in our experiments. Our results indicate that the Al-treated animals showed more anxious grooming during the first and last 5 min of the open field test as compared to the control animals. This high anxious grooming, even during the last 5 min of the test period, manifests high anxiety and lesser adaptability to the novel environment as a result of Al administration. Moreover, elevated level of relaxed grooming observed in control animals during the last 5 min of the test duration also manifest that control animals are better able to adapt to a new environment as compared to Al-treated animals. Higher anxiety in Al-treated animals was further validated by the observation that the Al-treated animals spent more time in the periphery of the test box. Our results are in accordance to the previously reported high anxiety in the open field after Al treatment [38]. Intriguingly, although a higher level of anxiety was observed in Al-treated animals, this high anxiety does not seem to influence exploratory activity in Al-treated animals, as the Al-treated animals maintained high rearing during the entire test duration. An increase in rearing is in accordance to previously reported results after Al exposure [39]. Rearing is a measure of the exploratory behavior in rodents [40], but with increasing familiarity of the environment, the animals show a reduction in rearing as was observed in control animals in our experiment. High rearings observed in our experiments are contradictory to those observed by Platt et al., who reported no change in rearing after Al administration [41]. This difference might be due to the lack of Al accumulation in the cortex in experiments of Platt et al. [41]. In the histological examination, we observed neurodegeneration in layer 4 of the barrel field cortex. The specific neuronal arrangement in the form of barrels in the barrel cortex is associated with whiskers [42], which plays an important role during exploratory activity [43]. Therefore, in spite of the damage caused by Al in the barrel field cortex, the preservation of high exploration is quite intriguing and needs further investigations. This preservation of rearing in Al-treated animals might be due to decreased expression of α4 nAChR in the cortex after Al treatment. As α4 nAChRs are required to activate some inhibitory neural circuits, those which inhibit some behavioral patterns, absence of these receptors can therefore result in the elevation of some behavior topographies [44] and rearing might be one of them. Moreover, the possibility that locomotor hypo-activity may be associated with higher anxiety could be ruled out from our histological examination results of the sensory motor cortex of the hind limb and forelimb regions. There was no significant difference between the cell number in control and Al-treated animals in all these cortical areas that are associated with locomotion.
After Al ingestion, the entry of Al in the brain through the blood-brain barrier had been established a long time ago [45]. Our results also support this notion as we observed a high Al concentration in the cortex of Al-treated animals. Previously, high Al content has been reported in the whole brain after oral ingestion of Al [46]. Similar high accumulation of Al in the cortex of rats was observed that was orally administered with Al [47], but our results are in contradiction to those observed by Doming et al. [48]. The difference in our results from that of Doming et al. might be due to the reason that Doming et al. had studied Al accumulation in the brain of aged rats (8- and 16-month-old rats) while the mice used in our experiments were 3 months old. In old-age rats, there might be a decline in the retention of Al [49] as it is reported previously that high Al accumulation occurs in the brain of 3-month-old rats while 8- and 16-month-old rats do not show much Al accumulation in the brain [50]. Although the difference in Al accumulation in the amygdala of control and Al-treated animals was non-significant, the amygdala showed higher Al accumulation as compared to the cortex. This might be due to the reason that Al exposure results in glutamate overproduction [51]; in turn, Al binds to glutamic acid and forms a stable glutamic acid salt which gets deposited in the brain [52]. Since, amygdala is rich in glutamatergic neurons [52], therefore, Al is likely to accumulate more in the amygdala. Moreover, Al is known to cause depression in animals, as revealed by our open field results, and depression leads to an increase in the number of neurovascular cells in the amygdala resulting in impaired blood-brain barrier functioning [53]. Therefore, it is postulated that in addition to increased accumulation of Al and impaired clearance from the amygdala, it can result in high concentration in the amygdala.
The histological examination in the cortex showed that there was a severe neurodegeneration in the motor cortex which is in agreement with the previously observed motor neuron degeneration by Al administration [54]. However, the results obtained in our study are contradictory to those obtained by Platt et al. who have reported no histological changes in cortical tissue following Al administration [41]. Platt et al. have not observed histological effects of Al on specific cortex areas; moreover, in their study, Platt et al. had administered Al via intracerebroventricular injection while in our study Al was administered via drinking water which is more close to the natural route of Al intoxication in humans. Moreover, Platt et al. had observed Al accumulation in the brain areas which were in immediate vicinity of the injection site which might be the reason that they were unable to observe degenerative changes caused by Al in the cortex.
The exact mechanism by which Al causes neurodegeneration is not known, but it is well documented that Al interacts with the cholinergic system, causing suppression of cholinergic receptor expression and disruption of calcium regulation [55]. Effects of Al on nAChR gene expression in the cortex and amygdala were not investigated earlier than this. Among several different possible combinations of the nAChRs, the α7 and α4β2 receptor subtype combinations are most abundant in the mammalian and rodent brain [56]. Therefore, these two receptor combinations were the focus of interest in this study. The nicotinic receptors are cation-permeable channels, and especially, the flow of Ca+2 ions is particularly important. The Ca+2-regulated acetylcholine release is of vital importance in the process of cognition and memory [57]. Our results demonstrate that oral Al administration results in reduced expression of nAChR genes in both cortex and amygdala. It can be concluded that the reduced gene expression will lead to reduced expression of the nAChRs in neurons. Similar observations were made by Gulya et al., who reported a reduced nicotine binding in Al-treated animals [12]. The reduced expression of nAChRs may result in reduced excitation of these receptors which may affect not only the postsynaptic depolarization but also the presynaptic Ca+2-dependent intracellular signaling cascades and neurotransmitter release [10]. As a result, all these factors will affect cognition and memory in Al-treated animals which may lead to neurobehavioral changes, similar to those observed in patients with AD [30].
Conclusion
Our results demonstrate that oral exposure to high Al content causes high Al accumulation in the brain which leads to neurodegeneration, reduction of nAChR gene expression, and deficits in learning and memory. To the best of our knowledge, this is the first study that reports the effect of Al on cortex- and amygdala-dependent functions like grooming and fear memory. Moreover, the effect of Al on the nAChR gene in the cortex and amygdala has also not been reported earlier. In view of the results obtained in our study, it is suggested that human exposure to Al should be limited, which, in addition to avoiding head trauma, will be the only change in lifestyle that may reduce the risk of developing neurodegenerative disorders like Alzheimer’s disease.
References
Abd-Elhady RM, Elsheikh AM, Khalifa AE (2013) Anti-amnestic properties of Ginkgo biloba extract on impaired memory function induced by aluminum in rats. Int J Dev Neurosci 31(7):598–607
Hashmi AN, Yaqinuddin A, Ahmed T (2015) Pharmacological effects of Ibuprofen on learning and memory, muscarinic receptors gene expression and APP isoforms level in pre-frontal cortex of AlCl3-induced toxicity mouse model. Int J Neurosci 125(4):277–287
Jalbani N, Kazi TG, Jamali MK, Arain BM, Afridi HI, Baloch A (2007) Evaluation of aluminum contents in different bakery foods by electrothermal atomic absorption spectrometer. J Food Compos Anal 20(3):226–231
McLachlan D, Kruck TP, Lukiw WJ, Krishnan SS (1991) Would decreased aluminum ingestion reduce the incidence of Alzheimer’s disease? CMAJ: Canadian Medical Association Journal 145(7):793
Ferreira PC, Piai KA, Takayanagui AMM, Segura-Muñoz SI (2008) Aluminum as a risk factor for Alzheimer’s disease. Rev Lat Am Enfermagem 16(1):151–157
Walton J (2007) A longitudinal study of rats chronically exposed to aluminum at human dietary levels. Neurosci Lett 412(1):29–33
Yokel RA, Hicks CL, Florence RL (2008) Aluminum bioavailability from basic sodium aluminum phosphate, an approved food additive emulsifying agent, incorporated in cheese. Food Chem Toxicol 46(6):2261–2266
Organization WH (2004) Guidelines for drinking-water quality: recommendations, vol 1. World Health Organization
Flaten TP (2001) Aluminium as a risk factor in Alzheimer’s disease, with emphasis on drinking water. Brain Res Bull 55(2):187–196
Stevanović ID, Jovanović MD, Čolić M, Jelenković A, Bokonjić D, Ninković M (2010) Nitric oxide synthase inhibitors protect cholinergic neurons against AlCl 3 excitotoxicity in the rat brain. Brain Res Bull 81(6):641–646
Hu W-P, Li X-M, Chen J-G, Li Z-W (2007) Potentiation of the nicotinic acetylcholine receptor by aluminum in mammalian neurons. Neuroscience 149(1):1–6
Gulya K, Rakonczay Z, Kasa P (1990) Cholinotoxic effects of aluminum in rat brain. J Neurochem 54(3):1020–1026
Maheswari S, Venkatakrishna Murali R, Balaji R (2014) Aluminium induced cholinotoxicity in zebra fish brain—a sequel of oxidative stress. Int J Adv Res 2:322–335
Johnson GV, Jope RS (1986) Aluminum impairs glucose utilization and cholinergic activity in rat brain in vitro. Toxicology 40(1):93–102
Hashmi AN, Yaqinuddin A, Ahmed T (2014) Pharmacological effects of Ibuprofen on learning and memory, muscarinic receptors genes expression and APP isoforms levels in Pre-frontal cortex of AlCl3-induced toxicity mouse model. International Journal of Neuroscience (0):1–37
Grammas P, Caspers M (1991) The effect of aluminum on muscarinic receptors in isolated cerebral microvessels. Res Commun Chem Pathol Pharmacol 72(1):69–79
Harkany T, Lengyel Z, Kasa P, Gulya K (1995) Chronic aluminum treatment results in aluminum deposits and affects Ml muscarinic receptors in rat brain. Neurobiology (Budapest, Hungary) 4(1–2):35–43
Pohanka M (2012) Alpha7 nicotinic acetylcholine receptor is a target in pharmacology and toxicology. Int J Mol Sci 13(2):2219–2238
Orr-Urtreger A, Göldner FM, Saeki M, Lorenzo I, Goldberg L, De Biasi M, Dani JA, Patrick JW, Beaudet AL (1997) Mice deficient in the α7 neuronal nicotinic acetylcholine receptor lack α-bungarotoxin binding sites and hippocampal fast nicotinic currents. J Neurosci 17(23):9165–9171
Lee S, Ahmed T, Kim H, Choi S, Kim DS, Kim SJ, Cho J, Shin HS (2012) Bidirectional modulation of fear extinction by mediodorsal thalamic firing in mice. Nat Neurosci 15(2):308–314
Blanchard RJ, Blanchard DC (1969) Crouching as an index of fear. J Comp Physiol Psychol 67(3):370
Arendash GW, Lewis J, Leighty RE, McGowan E, Cracchiolo JR, Hutton M, Garcia MF (2004) Multi-metric behavioral comparison of APPsw and P301L models for Alzheimer’s disease: linkage of poorer cognitive performance to tau pathology in forebrain. Brain Res 1012(1):29–41
Kazi TG, Afridi HI, Jamali MK, Arain MB, Jalbani N, Syed N (2007) Evaluation of zinc status in whole blood and scalp hair of female cancer patients. Clin Chim Acta 379(1):66–70
Gage GJ, Kipke DR, Shain W (2012) Whole animal perfusion fixation for rodents. Journal of visualized experiments: JoVE (65)
Ahmed T, Enam S, Gilani A (2010) Curcuminoids enhance memory in an amyloid-infused rat model of Alzheimer’s disease. Neuroscience 169(3):1296–1306
Kaizer R, Correa M, Gris L, Da Rosa C, Bohrer D, Morsch V, Schetinger MRC (2008) Effect of long-term exposure to aluminum on the acetylcholinesterase activity in the central nervous system and erythrocytes. Neurochem Res 33(11):2294–2301
Commissaris R, Cordon J, Sprague S, Keiser J, Mayor G, Rech R (1982) Behavioral changes in rats after chronic aluminum and parathyroid hormone administration. Neurobehav Toxicol Teratol 4(3):403
Farhat SM, Mahboob A, Iqbal G, Ahmed T (2016) Aluminum-induced cholinergic deficits in different brain parts and its implications on sociability and cognitive functions in mouse. Biol Trace Elem Res:1–7
Golub MS, Donald JM, Gershwin ME, Keen CL (1989) Effects of aluminum ingestion on spontaneous motor activity of mice. Neurotoxicol Teratol 11(3):231–235
Julka D, Sandhir R, Gill KD (1995) Altered cholinergic metabolism in rat CNS following aluminum exposure: implications on learning performance. J Neurochem 65(5):2157–2164
Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C, Lüthi A (2009) Amygdala inhibitory circuits and the control of fear memory. Neuron 62(6):757–771
Raybuck JD, Lattal KM (2011) Double dissociation of amygdala and hippocampal contributions to trace and delay fear conditioning. PLoS One 6(1):e15982
Pang M-H, Kim N-S, Kim I-H, Kim H, Kim H-T, Choi J-S (2010) Cholinergic transmission in the dorsal hippocampus modulates trace but not delay fear conditioning. Neurobiol Learn Mem 94(2):206–213
Gould TJ, Feiro O, Moore D (2004) Nicotine enhances trace cued fear conditioning but not delay cued fear conditioning in C57BL/6 mice. Behav Brain Res 155(1):167–173
Wilson MA, Fadel JR (2016) Cholinergic regulation of fear learning and extinction. J Neurosci Res
Terry RL (1970) Primate grooming as a tension reduction mechanism. The Journal of psychology 76(1):129–136
Smolinsky AN, Bergner CL, LaPorte JL, Kalueff AV (2009) Analysis of grooming behavior and its utility in studying animal stress, anxiety, and depression. In: Mood and anxiety related phenotypes in mice. Springer, pp 21–36
Sethi P, Jyoti A, Singh R, Hussain E, Sharma D (2008) Aluminium-induced electrophysiological, biochemical and cognitive modifications in the hippocampus of aging rats. Neurotoxicology 29(6):1069–1079
MISAWA T, SHIGETA S (1992) Behavioral effects of repeated aluminum administration in the rat. Tokai J Exp Clin Med 17(5):155–159
Rosana A, Marco Antonio Campana V (2012) High-and low-rearing rats differ in the brain excitability controlled by the allosteric benzodiazepine site in the GABA A receptor. Journal of Behavioral and Brain Science 2012
Platt B, Fiddler G, Riedel G, Henderson Z (2001) Aluminium toxicity in the rat brain: histochemical and immunocytochemical evidence. Brain Res Bull 55(2):257–267
Petersen CC (2007) The functional organization of the barrel cortex. Neuron 56(2):339–355
Grant R, Itskov PM, Towal B, Prescott TJ (2014) Active touch sensing: finger tips, whiskers, and antennae. Front Behav Neurosci 8:50
Ross SA, Wong JY, Clifford JJ, Kinsella A, Massalas JS, Horne MK, Scheffer IE, Kola I, Waddington JL, Berkovic SF (2000) Phenotypic characterization of an α4 neuronal nicotinic acetylcholine receptor subunit knock-out mouse. J Neurosci 20(17):6431–6441
Yokel RA, Allen DD, Ackley DC (1999) The distribution of aluminum into and out of the brain. J Inorg Biochem 76(2):127–132
Linardaki ZI, Orkoula MG, Kokkosis AG, Lamari FN, Margarity M (2012) Investigation of the neuroprotective action of saffron (< i> Crocus sativus</i> L.) in aluminium-exposed adult mice through behavioral and neurobiochemical assessment. Food Chem Toxicol
Sánchez-Iglesias S, Soto-Otero R, Iglesias-Gonzalez J, Barciela-Alonso MC, Bermejo-Barrera P, Méndez-Álvarez E (2007) Analysis of brain regional distribution of aluminium in rats via oral and intraperitoneal administration. J Trace Elem Med Biol 21:31–34
Doming J, Llorens J, Sanchez D, Gomez M, Llobet J, Corbella J (1996) Age-related effects of aluminum ingestion on brain aluminum accumulation and behavior in rats. Life Sci 58(17):1387–1395
Golub MS, Germann SL, Han B, Keen CL (2000) Lifelong feeding of a high aluminum diet to mice. Toxicology 150(1):107–117
Gómez M, Sánchez DJ, Llobet JM, Corbella J, Domingo J (1997) The effect of age on aluminum retention in rats. Toxicology 116(1):1–8
Nayak P, Chatterjee A (2001) Effects of aluminium exposure on brain glutamate and GABA systems: an experimental study in rats. Food Chem Toxicol 39(12):1285–1289
Chen S-M, Fan C-C, Chiue M-S, Chou C, Chen J-H, Hseu R-S (2013) Hemodynamic and neuropathological analysis in rats with aluminum trichloride-induced Alzheimer’s disease. PLoS One 8(12):e82561
Rubinow MJ, Mahajan G, May W, Overholser JC, Jurjus GJ, Dieter L, Herbst N, Steffens DC, Miguel-Hidalgo JJ, Rajkowska G (2016) Basolateral amygdala volume and cell numbers in major depressive disorder: a postmortem stereological study. Brain Struct Funct 221(1):171–184
Shaw CA, Petrik MS (2009) Aluminum hydroxide injections lead to motor deficits and motor neuron degeneration. J Inorg Biochem 103(11):1555–1562
Kaizer RR, Correa MC, Spanevello RM, Morsch VM, Mazzanti CM, Goncalves JF, Schetinger MRC (2005) Acetylcholinesterase activation and enhanced lipid peroxidation after long-term exposure to low levels of aluminum on different mouse brain regions. J Inorg Biochem 99(9):1865–1870. doi:10.1016/j.jinorgbio.2005.06.015
Dani JA (2001) Overview of nicotinic receptors and their roles in the central nervous system. Biol Psychiat 49(3):166–174. doi:10.1016/S0006-3223(00)01011-8
Wang HY, Lee DH, Davis CB, Shank RP (2000) Amyloid peptide Aβ1-42 binds selectively and with picomolar affinity to α7 nicotinic acetylcholine receptors. J Neurochem 75(3):1155–1161
Acknowledgements
We are thankful to the Atta-ur-Rahman School of Applied Biosciences, National University of Sciences and Technology, Islamabad, Pakistan, for providing funding, support, and research facilities to carry out this research project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All applicable international, national, and institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
Rights and permissions
About this article
Cite this article
Farhat, S.M., Mahboob, A. & Ahmed, T. Cortex- and Amygdala-Dependent Learning and Nicotinic Acetylcholine Receptor Gene Expression is Severely Impaired in Mice Orally Treated with AlCl3 . Biol Trace Elem Res 179, 91–101 (2017). https://doi.org/10.1007/s12011-017-0942-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-017-0942-1