Abstract
Functional traits define an organism in terms of how the organism interacts with its environment and determine the influence the organism has on dynamic ecosystem processes, such as biogeochemical nutrient cycling. Freshwater mussels (Bivalvia: Unionidae) form hotspots of biogeochemical activity in benthic environments by alleviating nutrient limitation at the sediment–water interface; however, little is known about the influence of mussel functional traits on sediment biogeochemical cycles, particularly nitrogen (N)-removal via denitrification and anammox. Our aim was to model the influence of two mussel functional traits: ammonium (NH4+) excretion and organic matter (OM) biodeposition, on N-removal in stream sediments. We quantified mussel excretion and biodeposition and incubated mussels in microcosms containing river sediment using flow-through methods. We measured nutrient and gas fluxes to quantify real-time ambient N-removal (denitrification + anammox), and conducted isotope pairing techniques to determine the maximum N-removal potential for denitrification and anammox pathways. NH4+ excretion was shown to be a significant predictor of ambient N-removal, whereas OM biodeposition significantly increased the maximum N-removal potential in the sediment. Our study is the first of its kind to link mussel functional traits specifically to N-removal and contributes to the growing knowledge of the role these highly imperiled organisms play by directly and indirectly influencing ecosystem-scale processes in lotic systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biogeochemical cycles are dependent on interactions between organisms and the abiotic environment. The magnitude and direction of an organism’s influence on such dynamic ecosystem processes as biogeochemical nutrient cycling are governed by functional traits (de Bello et al. 2010; Flynn et al. 2011; Lavorel and Garnier 2002; Schmitz et al. 2015), which are characteristics of an organism, expressed as phenotypes, that represent how the organism interacts with its environment (Díaz and Cabido 2001; Violle et al. 2007). Quantifying an organism’s functional traits and assessing the traits’ effects on ecosystem processes defines the functional role the organism plays in its ecosystem (Cardinale et al. 2011; Rusek 1998; Thrush et al. 2006; Wallace and Webster 1996). As such, there is a growing interest in describing functional diversity of biological communities (i.e., functional trait richness; Tilman 2001) rather than focusing on biodiversity (i.e., species richness) alone in studies of ecosystem resiliency (Loreau et al. 2001; Petchey and Gaston 2006; Tilman et al. 1997; Walker 1992).
Lotic ecosystems (i.e., streams and rivers) are hotspots of biogeochemical activity due to nutrient loading in the riparian zone (McClain et al. 2003; Vidon et al. 2010), hyporheic exchange of solutes to geochemically and microbially-active sites (Boulton et al. 1998; Lautz and Fanelli 2008) and biotic and abiotic controls of nutrient retention time (Ensign and Doyle 2005; Grimm et al. 2005) and spiraling lengths (Ensign and Doyle 2006; Newbold et al. 1981). Within the biotic realm of a stream, the coexistence of diverse groups of aquatic animals that process organic matter (OM) in various ways have differing effects on the recycling/repacking dynamics of carbon (C), nitrogen (N) and phosphorus (P) (Covich et al. 1999; Grimm 1988; Wallace and Webster 1996). When density and diversity of aquatic animals are high, functional traits in the community can significantly influence biogeochemical cycling on the ecosystem-scale (Cardinale et al. 2002; Covich et al. 2004; Vaughn 2010). Understanding the functional traits present in ecological communities, their role in provisioning ecosystem function and the persistence of their role over time, we can assess the importance of aquatic fauna on overall ecosystem resiliency (Atkinson et al. 2018). Lotic systems have been modified and degraded globally (Blann et al. 2009; Bunn and Arthington 2002; Carpenter et al. 1998; Poff et al. 2002), and many species have been lost (Ricciardi and Rasmussen 1999; Richter et al. 1997; Strayer and Dudgeon 2010). Many aquatic organisms provision ecosystem functions that directly or indirectly benefit humans (i.e., ecosystem services; Wilson and Carpenter 1999), and when species are lost, the functional traits they possess are lost as well (Estes et al. 2011; Hooper et al. 2012; Vaughn 2010). If we are to understand the cost of species loss, it is imperative to quantify the functional role of aquatic fauna and the spatial/temporal significance of their role at the ecosystem-scale.
Functional traits of aquatic fauna influence biogeochemical nutrient cycling directly and indirectly (Atkinson et al. 2017; Vanni 2002). For example, N and P are recycled through ingestion followed by excretion (Vanni and McIntyre 2016) which is a source of bioavailable nutrients for benthic primary producers (Atkinson et al. 2017). N and P can also be repackaged by an animal through secondary production, representing a net sink of nutrients when either assimilated into long-term biomass such as bone or shell (Strayer and Malcom 2007; Vanni et al. 2013) or when biomass is removed from aquatic systems through predation of aquatic animals by terrestrial predators and the emergence of aquatic insects (Gende et al. 2002; Nakano and Murakami 2001; Paetzold et al. 2005). Additionally, biodeposition (i.e., egestion) by aquatic animals, while considered a source of C to the system, also acts as a relatively long-term sink of N and P (Halvorson et al. 2017). It is recognized that aquatic fauna also indirectly influence biogeochemical nutrient cycling (Atkinson et al. 2017; Vanni 2002). For example, predators not only directly control prey populations, but can also indirectly influence the feeding behavior and excretion and/or tissue stoichiometry of prey through the “ecology of fear”, thus altering local source/sink dynamics (Dalton and Flecker 2014). Aquatic fauna indirectly influence biogeochemical cycles by physically (Mermillod-Blondin 2011; Mermillod-Blondin and Rosenberg 2006) and chemically (Matisoff and Wang 1998) altering their environment. This is particularly conspicuous in the benthic N-cycle, where biogeochemical transformations are dominated by redox gradients in the vertical sediment profile (Berner 1981). Multiple groups of aquatic fauna coexist in the benthic zone, and those that burrow vertically into the sediment profile (e.g., bivalves, chironomid larvae, tubificid worms) have been shown to significantly influence redox reactions (Anschutz et al. 2012; Benelli et al. 2017; Hoellein et al. 2017; Hölker et al. 2015; Turek and Hoellein 2015).
One group of benthic fauna known to have direct and indirect effects on ecosystem function is freshwater mussels (Bivalvia: Unionidae). Freshwater mussels (hereafter mussels) are a species-rich group of burrowing, filter-feeding bivalves that represent a link between the pelagic and benthic compartments of a stream as they filter material from the water column and concentrate it in the sediment through production, excretion and biodeposition (Bogan 2007; Vaughn et al. 2008). By fostering benthic-pelagic coupling, mussels directly concentrate C, N and P in the benthos. Highly reactive forms of N and P contained in mussel excreta can alleviate nutrient limitation and stimulate biofilm growth (Atkinson et al. 2013, 2014). This leads to further indirect effects on biogeochemical cycling by increasing primary production in the benthos and abundance of biofilm-grazing chironomid larvae (Allen et al. 2012; Spooner et al. 2012), which has implications for food web complexity and overall species- and functional-richness (Atkinson et al. 2014). Mussels are ideal model organisms with which to assess the indirect influence of functional traits on biogeochemical nutrient cycling, as they are long-lived (6–100 years), relatively sessile organisms that thrive in multi-species aggregations and can dominate the benthic biomass of rivers (Vaughn 2017; Vaughn and Hakenkamp 2001). Mussel aggregations are patchy throughout a watershed, and patches are often dense and speciose, resulting in mussel beds being classified as hotspots for such ecosystem functions as benthic-pelagic coupling (Atkinson and Vaughn 2015; Spooner et al. 2012; Vaughn et al. 2015), alleviation of nutrient limitation (Atkinson et al. 2013; Vaughn et al. 2007) and stimulation of local primary production (Atkinson et al. 2013; Spooner and Vaughn 2006). These effects are largely influenced by two mussel functional traits: excretion and biodeposition (Vaughn 2017; Vaughn and Hakenkamp 2001).
A less understood indirect ecosystem function provisioned by mussels is their influence on the benthic N-cycle, specifically the removal of dissolved inorganic nitrogen (DIN) via biogeochemical pathways (hereafter N-removal) in stream sediments. Research has shown that native freshwater mussels stimulate significant levels of denitrification (hereafter DNF) in stream sediments (Benelli et al. 2017; Hoellein et al. 2017; Trentman et al. 2018), but the specific mechanisms are poorly understood. Our research aimed to examine the link between mussel functional traits (excretion, biodeposition) and N-removal via the biogeochemical pathways of DNF and anaerobic ammonium oxidation (hereafter anammox). DNF and anammox refer to microbial metabolisms in which DIN is converted to di-nitrogen gas (N2), which diffuses through the water column and is released into the atmosphere (Knowles 1982). In DNF, two molecules of nitrate (NO3−) are converted to N2 through a series of intermediate N-species. In anammox, one molecule of nitrite (NO2−, derived from NO3−) combines with one molecule of ammonium (NH4+) through a series of intermediate N-species to form N2. As a result, DNF and anammox represent the permanent removal of N from an aquatic system. We hypothesized that mussels indirectly stimulate N-removal in stream sediments by physically and chemically altering the benthic environment, and a mussel’s influence on N-removal is contingent on an individual’s excretion and biodeposition rates. We predicted mussel functional traits serve to foster the redox conditions necessary to stimulate biogeochemical N-removal, specifically by excreting NH4+ and depositing OM at or near the oxic/anoxic boundary in the sediment. We quantified rates of NH4+ excretion and OM biodeposition of three common mussel species in a lowland river in the southeastern U.S. and modelled the influence of the two functional traits on ambient N2 flux across the sediment–water interface and maximum DNF and anammox potentials present in the sediment. We predicted mussel NH4+ excretion would increase N-removal by introducing a highly reactive form of N to anoxic sites in the sediment, thus chemically altering the benthic environment. Additionally, we predicted OM biodeposition would increase N-removal by altering the benthic environment either chemically by providing an energy substrate for the DNF pathway in the form of labile C, or physically by creating anoxic microenvironments through the decomposition of OM or the cohesive nature of egesta-associated mucus.
Methods and materials
Experimental design
We collected mussels, sediment and water from the Sipsey River, a fifth-order tributary of the Tombigbee River in the Mobile Basin of Alabama. The main stem of the Sipsey River flows relatively undisturbed through extensive floodplain wetlands for 180 km, and is characterized as harboring remarkably intact and diverse biological communities (Atkinson et al. 2019; McGregor and O’Neil 1992), including 37 of 41 historically-occurring unionid mussel species (Haag and Warren 2010). The undisturbed nature of the river, low nutrient concentrations, and high native mussel populations throughout make the Sipsey River a good model system for studying ecosystem functions provisioned by mussels. We conducted three incubations trials on consecutive weeks in August 2017. Each incubation trial took approximately 5 days (Table 1). We used three common species from the Sipsey River that vary in phylogeny, life history and shell morphology (Williams et al. 2008): Cyclonaias asperata (tribe Quadrulini, short-term brooder, rough shell containing pustules), Fusconaia cerina (tribe Pleurobemini, short-term brooder, satiny-textured shell) and Lampsilis ornata (tribe Lampsilini, long-term brooder, smooth shell). We designed the experiment as a balanced incomplete block design, where each incubation trial consisted of two species treatments and a sediment-only control, all replicated three times for a total of nine experimental units per trial (Table 1). The design allowed us to examine all species combinations with a control, minimizing the risk of pseudoreplication. A total of six replicate individuals per species treatment were used in the experiment. To reduce trial bias, we took care to ensure the time between different stages of an incubation (Table 1) were the same between the three trials.
Field collection and functional trait quantification
We collected sediment from the Sipsey River 48-h before beginning a trial and sieved the sediment into buckets to remove mussels and large sediment particles (≥ 11-mm). We then returned to the lab where we homogenized the sediment and filled ten cylindrical microcosms (9-cm × 30-cm) to a depth of 20-cm. Once filled, the microcosms were fully submerged in oxygenated site water set to site-water temperature (trial 1: 27.3 °C, trial 2: 24.3 °C, trial 3: 26.1 °C). The microcosms sat submerged in the dark for 24-h prior to transport and another 24-h once transported to the incubation chamber (Table 1). Allowing the microcosms to sit submerged in the dark for 48-h aided in reestablishing a redox gradient in the vertical sediment profile (Catallo 1999).
We collected mussels 24-h prior to beginning an incubation trial. We collected mussels from the same reach as the sediment in areas 0.5 to 0.7-m deep, 30-m upstream of an established long-term mussel survey site (site 6 in Haag and Warren 2010). We measured the shell lengths (mm) for each mussel and estimated soft tissue dry mass based on previously established length-mass regressions for each species (C.L. Atkinson, unpublished data). Mussels within a species were relatively the same size (shell length mean ± 1 SE: F. cerina = 52.4 ± 1.40 mm, C. asperata = 51.2 ± 1.79 mm, L. ornata = 80.7 ± 2.74 mm) and were small enough to fit comfortably in the microcosm environment. After the mussels were collected and measured, we characterized mussel excretion and biodeposition with in-field experiments as described in Atkinson et al. (2013). Briefly, we filled nine 1-L chambers with filtered site water (GF/F filter, 0.7 μm pore size, EMD Millipore, Burlington, MA). We gently scrubbed each mussel (n = 3 species−1) to remove algae and sediment attached to the shell and placed each individual in a chamber. We collected and cleaned empty mussel shells to use in three control chambers to account for nutrients and solids in the chamber produced by shell-associated algae and microbes. we sealed the chambers and partially submerged them in site water to maintain temperature. After 1-h, we carefully removed the mussels and control shells. Following the experiment, we wrapped the mussels in damp towels and placed them in a cooler for transport. We immediately placed the chambers on ice and brought them to the lab where we filtered the contents through a GF/F filter (0.7 μm pore size). The filters and water were promptly frozen until analysis for biodeposition and excretion rates, respectively.
We dried filters to a constant weight at 50 °C, weighed them on an analytical balance (± 0.0001 g), combusted them at 500 °C for 2-h and weighed them again to determine ash-free dry mass (AFDM). We subtracted AFDM from the mass of the dried filter to estimate the rate of OM biodeposition (mg OM h−1) for each mussel. We analyzed the filtered chamber water for NH4+ concentration using a Lachat QuickChem flow injection analyzer (Hach Company, Loveland, Colorado, USA). We estimated individual mussel NH4+ excretion rate (µmol NH4+ h−1) by subtracting the average NH4+ concentration in the control chambers (representing background NH4+) from the NH4+ concentration in each mussel chamber.
Chamber incubations
Immediately following the in-field excretion/biodeposition experiment, we sealed the microcosms air-tight with a rubber stopper and transported the mussels, microcosms and site water from the University of Alabama (UA) to Dauphin Island Sea Lab (DISL) where the incubation experiments took place. Upon arrival to DISL, we unpacked the microcosms in a temperature-controlled incubation chamber, set the chamber to site-water temperature, and placed the microcosms in holding tanks of aerated site water to acclimate. Once the microcosms were warmed to site-water temperature, we placed one mussel in the center of each microcosm and allowed the mussels to acclimate to the experimental conditions for 12-h prior to beginning the incubation trail (Table 1).
We utilized continuous flow-through incubations (Miller-Way and Twilley 1996) to assess the influence of native mussels on ambient gas and nutrient flux in benthic sediments (Fig. 1). Each microcosm contained 10-cm of overlying water and 20-cm of sediment. We fit caps to each microcosm while submerged in site water so to insure the microcosms were free of any air. A cap contained a magnetic stir-bar and two valves to which inflow and outflow tubing were connected. While still submerged, we placed a microcosm in a rack which was used to keep the cap securely tightened throughout the incubation trial, and inflow and outflow tubing were tightened onto the valves (Fig. 1). Once assembled, we removed the microcosms from the holding tanks and placed them on shelves in the environmental chamber. The inflow for all nine microcosms was supplied from one 50-L reservoir of aerated, unfiltered site water. Each inflow tubing was connected to a peristatic pump, and the flow for all trials was set at 1.5-mL min−1. As per the design of the experiment, unfiltered site water was constantly flowing into the microcosm, and outflow water flowed into a reservoir. An additional length of tubing was installed to flow directly from the inflow reservoir to the waste reservoir and was used to assess the concentrations of the analytes in the inflow reservoir. Finally, we fit a stir-bar motor into the rack atop each microcosm (Fig. 1). The motor turned the magnetic stir-bar inside the microcosm to simulate flow. We began the 24-h incubation trial once all the motors were installed, the stir-bars were turning, and the lights within the environmental chamber were turned off.
Continuous flow-through incubation setup modified from Miller-Way and Twilley (1996). Black lines represent the direction of the flow through the apparatus (~ 1.5 mL min−1). The microcosms were 9 cm in diameter and 30 cm tall (20 cm of sediment, 10 cm of overlying water)
At the end of the trial, we collected water samples from the inflow tubing and from the outflow for each microcosm. We collected triplicate water samples by overflowing 3X times the volume of 12-mL exetainers to measure dissolved oxygen (DO) concentration, which we used to estimate total respiration (O2 influx) within a microcosm. We analyzed samples for DO immediately in the incubation chamber while still dark with a Unisense OX-500 O2 microsensor calibrated according to the manufacturer (Unisense A/S, Aarhus, Denmark). Following DO sample collection, we collected samples for N2 flux by overflowing 3X times the volume of 12-mL exetainers, which we then treated with 250-µL ZnCl2 (50% w/v) to stop all microbial activity. We also collected water samples that we filtered through a GF/F filter (0.7 μm pore size) and stored at − 20 °C until analysis for NH4+, NO2− and NO3− fluxes. We analyzed ambient N2 fluxes with the N2:Ar method on a membrane inlet mass spectrometer (MIMS, Kana et al. 1994) equipped with a copper column heated to 600 °C to remove oxygen (Eyre et al. 2002). We analyzed water samples for nutrient fluxes on a Lachat QuickChem flow injection analyzer (Hach Company, Loveland, Colorado, USA). We calculated fluxes of O2, N2, NH4+, NO2−, and NO3− for each microcosm by subtracting the concentration of an analyte in the inflow from the corresponding concentrations from each microcosm’s outflow. Concentrations (µmol L−1) were normalized by flow rate (~ 0.1 L h−1) and sediment surface area (0.007 m2) to report areal flux rates (µmol m−2 h−1).
N-removal potential
After water samples were collected for nutrient and gas fluxes, we uncapped the microcosms and gently removed the mussels. The mussels remained in an aerated holding tank of site water until being reintroduced to the Sipsey River. We discarded the bottom 10-cm of sediment from each microcosm, and retained top 10-cm (the sediment in which the mussel was active) to be analyzed for N-removal potential in sediment slurries with the isotope pairing techniques (IPT, Thamdrup and Dalsgaard 2002).
To prepare the slurries for the IPT measurements, we wet-sieved (2-mm mesh) the top 10-cm of sediment from a microcosm. We homogenized the < 2-mm sediment and subsampled it into six 12-mL exetainers. Each exetainer received approximately 2-g of wet sediment. We then filled each exetainer with anoxic NO3−-free artificial river water (ARW; Supplemental Material). We formulated the ARW to contain the major ions of Sipsey River water at baseflow concentrations, with the exception of NO3− (Atkinson et al. 2019; McGregor and O’Neil 1992), and we synthesized the ARW using methods described by Smith et al. (2002). We filled an exetainer with anoxic ARW, then capped the exetainer air-tight and placed the samples on a shaker table overnight to remove any residual NO3− and O2. The following day, we added isotopically-labelled 15NO3− to each exetainer to a final concentration of [15NO3−] = 50-µM0. We then immediately added a 250-μL solution of ZnCl2 (50% w/v) to three of the six replicate exetainers, ceasing all microbial activity and representing the concentration of N2 isotopes at time zero (T0). We sealed the remaining exetainers and they shook anaerobically for an additional 6-h. After 6-h, we spiked the remaining three replicates with 250-μL of ZnCl2, ceasing all microbial activity and representing the concentration of N2 isotopes at time final (T6). We recapped the exetainers and measured the concentrations of 29N2 and 30N2 within 24-h on the MIMS. Following analysis on the MIMS, we dried the slurry sediment to constant weight at 50 °C and weighed each sample on an analytical balance (± 0.0001 g) to determine dry mass. We normalized concentrations of 29N2-N and 30N2-N (µmol L−1) by slurry incubation time (6 h) and sediment dry mass (kg) to report maximum N-removal potential as an hourly rate per kg of sediment (µmol N2-N kg−1 h−1). Anammox rates were low, and some nearly equivalent to the baseline fluctuation (noise) of the MIMS signal. Anammox potential values that had a MIMS signal < 2 × the value of noise were considered to be “0”.
Statistical analyses
To compare size (soft tissue DM) and rates of NH4+ excretion and OM biodeposition between the three species used in our experiment, we utilized 1-way analysis of variance (ANOVA). We also used 1-way ANOVA to determine if the fluxes of NH4+, NO3−, N2-N, and O2, as well as DNF and anammox potentials, differed amongst our species treatments and if species treatments differed from the sediment-only controls. Because we used a balanced incomplete block design, we incorporated the variation due to the random effect of incubation trial (block effect) using the R package multcomp version 1.4–8. We conducted a post hoc analysis following a significant ANOVA (p < 0.05) with Tukey’s Honestly Significant Difference (HSD) multiple comparisons (α = 0.05) and also took into account the random effect of incubation trial by running the posthoc analyses in the multcomp package using the ghlt() function.
We conducted simple linear regression (SLR) to examine the relationship between our functional traits of interest and N-removal parameters. Prior to running SLRs, we conducted an analysis of covariance (ANCOVA) to examine species-specific differences. Species was the explanatory variable in the ANCOVA model, and soft tissue DM was the covariate, as it was biologically relevant that larger individuals excreted more NH4+ and deposited more OM. From the 24-h chamber incubations, we examined the relationship between ambient N-removal and the functional traits of respiration, NH4+ excretion and OM biodeposition. This was done to assess the real-time influence of functional traits on ambient N-removal over a relatively short period of time. For the maximum N-removal potential from the IPT slurry experiments, we only examined the relationship DNF and anammox potential had with OM biodeposition. We excluded respiration and NH4+ excretion from comparison with N-removal potentials due to the fact that the IPT method involved a high degree of sediment perturbation, and we assumed any biogeochemical effect of respiration and NH4+ excretion in the microcosms would be negligible following perturbation and preparation of the slurries. Contrarily, the OM produced from mussel biodeposition became a permanent component of the sediment, thus potentially influencing sediment biogeochemistry during the IPT slurry experiments.
To test the influence of mussel functional traits (NH4+ excretion, OM biodeposition) on ambient N-removal, we first removed the effect of respiration, which by drawing down the oxygen in the water column could influence the redox distribution in the sediments, from the analyses. First, we estimated mussel respiration by subtracting the mean O2 influx of the three sediment-only control microcosms (respiration due to heterotrophic microbes and meiofauna) in an incubation trial from each mussel treatment microcosm in that same trial. The same was done for N2-N efflux of mussel microcosms by subtracting the mean N2-N efflux of the controls to correct for DNF occurring outside of the presence of a mussel. Second, we fit the control-corrected respiration and DNF estimates into a SLR with N2-N efflux as the response variable and mussel respiration as the explanatory variable. Then, to remove the effect of respiration from the analysis, the residuals from the above SLR analysis were used as a response variable to examine the role of specific mussel functional traits on N-removal. To test for outliers among the residuals, we conducted a Bonferroni-adjusted outlier test using the R package car version 2.1–3. If outliers were detected (Bonferroni p < 0.05), we removed them from subsequent analyses. Finally, after correcting for outliers, the residual values were treated as the response variable in SLRs to test for the effects of NH4+ excretion and OM biodeposition on ambient N2-N efflux. All analyses were conducted using R version 3.3.1 (R Core Team 2016).
Results
Mussel parameters and functional traits
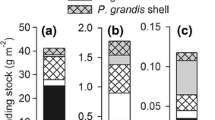
L. ornata individuals were significantly heavier (F2,13 = 19.28, p < 0.001, Table 2, Fig. 2a) than F. cerina and C. asperata. There was a significant difference in NH4+ excretion rates among species (F2,19 = 4.47, p = 0.033, Table 2, Fig. 2b) with L. ornata having the highest NH4+ excretion rate. Although F. cerina contained less soft tissue dry mass than C. asperata on average, the mean excretion rate of F. cerina was higher than that of C. asperata. Additionally, L. ornata deposited significantly more OM as feces and pseudofeces than F. cerina and C. asperata (F2,19 = 4.12, p = 0.043, Table 2, Fig. 2c). Unlike NH4+ excretion, OM biodeposition followed the trend of heaviest to lightest species with L. ornata depositing the most, followed by C. asperata and F. cerina, respectively.
Boxplots highlighting differences in a soft tissue dry mass, b NH4+ excretion and c OM biodeposition among the mussel species used in the incubation trials. Letters over a boxplot represents a significantly different mean, determined via Tukey’s HSD posthoc analysis following a 1-way ANOVA (α = 0.05) taking into account the random effect of incubation trial (block effect)
Ambient gas and nutrient flux
The 24-h chamber incubation allowed us to measure small-scale ambient gas (N2, O2) and nutrient (NH4+, NO3−) flux across the sediment–water interface, which were calculated as µmol m−2 h−1 (Table 3). Before taking water samples at the 24-h mark, we closely examined each microcosm for the presence of bubbles, which would introduce bias into such fine-scale gas flux measurements. Two microcosms, both controls (one from trial 1, one from trial 3), contained microbubbles and were not sampled for ambient fluxes. Our results showed a significant treatment effect of NH4+ flux (F3,19 = 3.42, p = 0.038, Table 3), but a Tukey’s HSD posthoc analysis indicated no individual differences between treatments, and no treatment was significantly different from the control (Fig. 3a, light grey bars). The control treatments showed a slight average influx of NH4+ into the sediment. L. ornata treatments showed a larger average influx of NH4+ into the sediment, while F. cerina and C. asperata showed an average efflux (Fig. 3). In terms of NO3– flux, there was a significant treatment effect (F3,19 = 4.03, p = 0.022, Table 3), but our posthoc test indicated none of the mussel treatments were significantly different from the control (Fig. 3a, dark grey bars). Dissimilar to NH4+ flux, the control treatments showed an average efflux of NO3− (Fig. 3a). NO3− flux among mussel treatments showed a similar trend to that of NH4+ flux in that L. ornata treatments showed an average influx of NO3−, while F. cerina and C. asperata showed an average efflux. There was a significant treatment effect on the efflux of N2 gas (F3,19 = 74.73, p ≪ 0.001, Table 3) with all mussel treatments showing significantly higher rates of N2 efflux than the controls, and L. ornata showing significantly higher rates than F. cerina and C. asperata (Fig. 3b, dark grey bars). A similar pattern was observed for O2 consumption, with there being a significant treatment effect (F3,19 = 252.3, p ≪ 0.001, Table 3) and all mussel treatments showing significantly higher respiration rates than the controls, as well as L. ornata showing significantly higher rates than F. cerina and C. asperata (Fig. 3b, light grey bars).
Mean a ambient nutrient (NH4+, NO3−) flux and b ambient gas (N2-N, O2) flux measured at the end of the 24-h chamber incubation trials. Error bars represent ± 1 SE. Letters over a bar represents a significantly different mean, determined via Tukey’s HSD posthoc analysis following a 1-way ANOVA (α = 0.05) taking into account the random effect of incubation trial (block effect)
N-removal potentials
Using the IPT method, we quantified N-removal potentials via two biogeochemical pathways, DNF and anammox (Table 3). The two control microcosms that were not sampled for ambient nutrient and gas flux due to the presence of microbubbles were included in IPT experiments, as we assumed microbubbles would not significantly influence N-removal potentials in the sediment. Results showed a significant treatment effect on N-removal potential via the DNF pathway (F3,23 = 5.63, p = 0.005, Table 3). L. ornata treatments were shown to foster significantly higher average DNF potentials than the controls and the F. cerina and C. asperata treatments, but F. cerina and C. asperata treatments were not significantly different from the controls (Fig. 4, dark grey bars). L. ornata treatments showed the highest average anammox potentials and were greater than the control, while mean anammox potentials for F. cerina and C. asperata were not significantly different from the control treatments (Fig. 4, light grey bars). There was not a significant treatment effect for anammox potentials (F3,23 = 2.33, p > 0.1, Table 3).
Mean N-removal potentials (DNF, anammox) measured during the IPT slurry experiments. Error bars represent ± 1 SE. Letters over a bar represents a significantly different mean, determined via Tukey’s HSD posthoc analysis following a 1-way ANOVA (α = 0.05) taking into account the random effect of incubation trial (block effect)
Modelling influence of functional traits on N-removal
Results from ANCOVA showed soft tissue DM to be a significant indicator of an individual mussel’s influence on ambient N2 flux (F1,14 = 10.35, p = 0.006), DNF potential (F1,14 = 11.46, p = 0.004) and anammox potential (F1,14 = 4.96, p = 0.043). When corrected for soft tissue DM, there were no species-specific differences in ambient N2 flux (F2,14 = 1.66, p > 0.2), DNF potential (F1,14 = 1.62, p > 0.2) or anammox potential (F1,14 = 0.569, p > 0.5). Thus, all SLRs were conducted using all mussels in a single data set without taking into effect species-specific relationships.
Ambient N2 efflux during the 24-h chamber incubations was significantly correlated to mussel respiration (R2 = 0.756, p ≪ 0.001, Fig. 5a), NH4+ excretion (R2 = 0.320, p = 0.008, Fig. 5b) and OM biodeposition (R2 = 0.592, p < 0.001, Fig. 5c). One data point from the N-removal vs. respiration SLR (L. ornata datum; residual variation = − 391.88) was determined to be a significant outlier (Bonferroni p < 0.05) and was removed from SLRs examining the influence of functional traits on the residual variation from the N-removal vs. respiration relationship (Fig. 6a; note n = 5 for L. ornata). After accounting for respiration in the incubations (~ 24.4% residual variation remaining in data), NH4+ excretion was significantly correlated to the remaining variation in the data (R2 = 0.263, p = 0.020, Fig. 6b), suggesting NH4+ excretion is a significant predictor of ambient N-removal in our experimental setup. Additionally, results showed a moderate positive relationship between OM biodeposition and the remaining variation in the data (R2 = 0.187, p = 0.053, Fig. 6c), suggesting a moderate influence of OM biodeposition on ambient N-removal.
Scatterplots highlighting relationships between ambient N2-N efflux and a mussel respiration, b NH4+ excretion and c OM biodeposition. Species are represented by the following symbols: F. cerina (open circle), C. asperata (open square), L. ornata (open triangle). Results of SLRs are reported as the coefficient of determination (R2) and p-values. Bolded p-values represent a significant relationship between the explanatory and response variable. Significant regression equations are: (a) y ≈ 0.735*x – 154, (b) y ≈ 133*x + 711, (c) y ≈ 200*x + 638
a Distribution among species of the residual variation present in the ambient N2 flux versus respiration SLR. These data were used as the response variable for SLRs testing the influence of b NH4+ excretion and c OM biodeposition on ambient N2 flux once the effect (caveat) of respiration had been removed from the data. Species are represented by the following symbols: F. cerina (open circle), C. asperata (open square), L. ornata (open triangle). Results of SLRs are reported as the coefficient of determination (R2) and p value. Bolded p-values represent a significant relationship between the explanatory and response variable. The dashed line in the OM biodeposition plot represents a moderate (0.05 < p < 0.1) linear relationship. Significant regression equations are: (b) y ≈ 46.0*x = 73.1
The maximum N-removal potential in the sediment was significantly correlated to mussel OM biodeposition (Fig. 7). This result was observed for both N-removal pathways, with the rate of OM biodeposition positively influencing DNF (R2 = 0.386, p = 0.005, Fig. 7a) and anammox (R2 = 0.229, p = 0.030, Fig. 7b) potential.
Scatterplots highlighting relationships between a DNF and c anammox potentials and OM biodeposition. Species are represented by the following symbols: F. cerina (open circle), C. asperata (open square), L. ornata (open triangle). Results of SLRs are reported as the coefficient of determination (R2) and p-values. Bolded p-values represent a significant relationship between the explanatory and response variable. Significant regression equations are: (a) y ≈ 0.329*x + 0.071, (b) y ≈ 0.055*x + 0.048
Discussion
Our results provide evidence that, within our experimental system, the presence of mussels in riverine sediment stimulated permanent N-removal, and the influence of mussels on N-removal is biomass-associated rather than species-specific. Additionally, our results describe an added functional role of mussel physiological traits in the benthos, as the products of excretion and biodeposition were shown to chemically and physically alter sediment and foster N-removal via the biogeochemical pathways of DNF and anammox. These functional traits have been shown to have significant effects on ecosystem functions such as nutrient limitation (Atkinson et al. 2013; Vaughn et al. 2007), primary production (Atkinson et al. 2013; Spooner and Vaughn 2006) and benthic biodiversity (Spooner et al. 2012; Vaughn et al. 2008). Here, our results show that these functional traits also have a significant effect on the ecosystem function of biogeochemical N-removal. We showed that excreta-derived NH4+ served as a bioavailable N source needed for microbially-mediated efflux of N2 across the sediment–water interface, and OM biodeposition played a significant role in the maximum N-removal potential present in the sediment. Previous studies have shown native freshwater mussels to stimulate N-removal (Benelli et al. 2017; Hoellein et al. 2017; Trentman et al. 2018), but our study is the first to link this stimulation to specific mussel functional traits.
The role of NH4 + excretion in N-removal
We hypothesized NH4+ excretion would chemically alter the benthic environment by introducing a highly reactive form of N to anoxic sites, thus stimulating N-removal. We found that, even when accounting for 76% of the variation in the data by removing the effect of respiration, NH4+ excretion remained a significant influence on ambient N-removal (DNF + anammox), explaining roughly 26% of the remaining variation in the data. This represents strong evidence that excreta-derived N can be a prominent reactant in N-removal pathways in freshwater sediments containing mussels. Because the incubations ran under dark conditions, we assumed the absence of NH4+ uptake by autotrophic microbes and microphytobenthos. Thus, we assumed the fate of excreta-derived NH4+ was via uptake by heterotrophic microbes, including those that facilitate such biogeochemical pathways as nitrification and anammox. If excreta-derived N underwent the DNF pathway, it would first need to be nitrified before being denitrified. Though we did not directly measure nitrification rates in the microcosms, we would have expected to see higher rates of NO3− efflux in the mussel treatments relative to the controls if nitrification was occurring in the system without DNF occurring as well. Though containing large, NH4+ excreting infauna, the three mussel species treatments did not show significantly different NH4+ nor NO3− fluxes relative to the controls. This suggests a fate of excreta-derived NH4+ to be utilization by nitrifying microbes and subsequent DNF, indicating the presence of coupled nitrification–denitrification (hereafter coupled-DNF).
In coupled-DNF, nitrification and DNF occur as a seemingly single pathway converting NH4+ to N2 through NO2−/NO3− intermediates on small spatial and/or temporal scales (Seitzinger et al. 2006). Bivalves in both freshwater and marine systems have been shown to stimulate coupled-DNF. In regards to unionid mussels, Benelli et al. (2017) observed an increase specifically in coupled-DNF in sediment containing mussels relative to sediment only using IPT slurry methods. Additionally, Hoellein et al. (2017) and Turek and Hoellein (2015), using chamber incubation methods similar to our study, attributed the influence of unionid mussels and the invasive Asian clam (Corbicula fluminea), respectively, on DNF to be a result of their burrowing behavior. Burrowing behavior increased O2 penetration into the sediment and deposited excreta and egesta products deeper in the vertical sediment profile, representing benthic alterations that the authors suggested resulted in conditions favoring coupled-DNF (Hoellein et al. 2017; Turek and Hoellein 2015). Effects are similar in marine systems, with coupled-DNF stimulation being attributed to such burrowing marine bivalves as Austrovenus stutchburyi (Jones et al. 2011) and Macoma baltica (Bonaglia et al. 2014), the intertidal ribbed mussel Guekensia demissa (Bilkovic et al. 2017), and the reef-building eastern oyster (Crassostrea virginica, Newell et al. 2002, 2005).
Although we recognize DNF is not the only fate possible for NO3− within our system, multiple pieces of evidence suggest the presence of coupled-DNF, namely (1) the absence of autotrophy reducing N uptake by microbes not involved in N-removal pathways, (2) the fact that neither NH4+ nor NO3− flux in mussel treatments differed from the sediment-only control, but N2 flux did, and (3) the significant positive relationship between mussel NH4+ excretion and N2 efflux. Additionally, coupled-DNF is optimized in environments with low water column NO3− concentrations and high sediment oxygen (Seitzinger et al. 2006), which are conditions reflective of the low background NO3− concentrations of the Sipsey site water (Atkinson et al. 2019; McGregor and O’Neil 1992) and relatively large sediment particle size (≤ 11-mm) used in our experiment. Future studies examining the influence of mussel excretion on N-removal could benefit from feeding the mussels 15N-enriched algae for a period of time prior to incubation, as was done in a study by Atkinson et al. (2014) that traced the fate of mussel excreta in riverine food webs. Using this method in conjunction with continuous flow-through incubations, excreta-derived 15NH4+ could be traced to reveal the contribution of mussel excretion to specific N-removal pathways (DNF, coupled-DNF, anammox) under ambient conditions. Also, NO3− assimilation can contribute to a large portion of N-retention in marine and freshwater systems when such systems are N-limited (Hou et al. 2012; Veuger et al. 2007) and, due to our experimental design, we were not able elucidate the proportion of NO3− that was removed from the system by either assimilatory or dissimilatory processes. As such, using an isotopic tracer such as 15NH4+-enriched algae would also aid in assessing the contribution of NO3− assimilation by heterotrophic microorganisms in our system.
Our results concerning the fate of excreta-derived N contrast with those found in a similar microcosm study by Hoellein et al. (2017) in which the authors observed significant increases in NH4+ efflux in mussel treatments relative to sediment-only controls under ambient conditions. The authors’ study was conducted in an urban stream in the midwestern U.S. with much higher background DIN concentrations than that of the Sipsey River. With the low background DIN in our study system (Atkinson et al. 2019; McGregor and O’Neil 1992), highly reactive N from mussel excreta was readily taken-up by microbially-active sediment, even in the absence of autotrophy. The stark differences among our results support the claim that mussel aggregations act as biogeochemical hotspots for alleviating N limitation when DIN is low (Atkinson and Vaughn 2015), and add to that assertion by showing excreta-derived N not only stimulates the activity of primary producers (Atkinson et al. 2013; Spooner and Vaughn 2006), but denitrifying microbes as well.
The role of OM biodeposition in N-removal
The addition of mussel biodeposits to the sediments in the slurries increased the maximum N-removal potential via both DNF and anammox pathways. We predicted mussel biodeposits could stimulate N-removal by both chemically and physically altering the benthic environment. OM biodeposition introduces an energy substrate (electron donor) for the DNF pathway in the form of labile C (Grenz et al. 1990; Mirto et al. 2000). Due to the method we used to collect mussel biodeposit, we were not able to differentiate between feces and pseudofeces. Feces is the egesta product of mussels, while pseudofeces is a conglomerate of rejected material captured while filter-feeding (Nichols et al. 2005). Mussel feces is richer in C, N and P than pseudofeces (Hoellein et al. 2017), and therefore we expected most of the labile OM we measured in the biodeposits were a product of feces. For OM to influence the DNF pathway, feces would need to be deposited in or near, or transported to, the oxic/anoxic redox boundary where denitrifying microbes can utilize the labile C. In the slurries where the biodeposits were well-mixed with sediment in an anoxic environment and with abundant NO3−, there was a significant positive relationship between mussel OM biodeposition rate and the maximum DNF potential in the sediment. This result suggested OM derived from mussel feces chemically alters the benthic environment by providing the energy substrate necessary to stimulate N-removal via the DNF pathway. Similar results in the marine literature support our result, as C. virginica biodeposits have been shown to be a source of labile C able to stimulate DNF within oyster reefs at multiple locations in the eastern U.S. (Hoellein et al. 2015; Newell et al. 2002). Authors have also found the role of marine bivalve biodeposits in stimulating DNF to be less than predicted due to small sediment particle size, short O2 penetration depths and high NO3− concentrations in the water column, the effects of which resulted in biodeposits not having a large effect on subsurface N dynamics (Higgins et al. 2013; Hoellein et al. 2015; Kellogg et al. 2013). Thus, in gravel-dominated sediment with low background NO3− concentrations (the conditions present in our microcosms), OM biodeposition could have a greater influence on subsurface N dynamics, stimulating N-removal via DNF.
We observed a significant positive relationship between mussel OM biodeposition rate and maximum anammox potential in the sediment slurries. As anammox does not require C as an energy substrate as does DNF, this result suggests there could be additional mechanisms by which OM biodeposition could stimulate N-removal, potentially by creating anoxic microenvironments in the sediment via decomposition that activate those microbial metabolisms involved in DNF and anammox pathways. Studies of high density marine mussel farms have shown the decomposition of biodeposits to significantly decrease DO in sediment (Carlsson et al. 2010; Giles and Pilditch 2006; Nizzoli et al. 2005). The population densities of the mussel farms studies were similar to those currently found in natural mussel aggregations in the Sipsey River. Though OM decomposition will occur at different rates in marine and freshwater systems, the evidence of bivalve biodeposits decreasing sediment DO is likely, as decomposition is an oxygen-consuming process. Similar results were observed with reef-building oysters as well. Newell et al. (2002) examined the effect of simulated C. virginica biodeposits from high-density reefs and observed a significant decrease of local DO concentrations in the presences of massive amounts of feces and pseudofeces. High quantities of biodeposit-derived OM decomposition in natural mussel aggregations could satisfy two of the three requirements for N-removal in sediments by both making the environment more anoxic and providing labile C. Although we were not able to elucidate the mechanism by which OM biodeposition stimulates N-removal, our results show the added material to be an important component of overall N-removal potential in freshwater sediments.
OM biodeposition had a moderate influence on ambient N2 efflux during the 24-h chamber incubation. In Carlsson et al. (2010), there was not a measurable amount of biodeposit decomposition until > 88-h post-deposition. The weak influence of mussel OM biodeposition on ambient DNF in our chamber incubations could be explained by the fact that the 24-h incubation time did not allow sufficient time for mussel biodeposits to chemically and physically alter the benthic environment. Also, unlike the extreme IPT microenvironment, there may have not been enough available N nor enough anoxic sites in the sediment to foster the necessary colonization of DNF and anammox microbes on mussel biodeposits. Our results suggest that OM biodeposits act as a more long-term influence on sediment biogeochemical processes, whereas highly reactive excreta-derived N stimulates N-removal on much shorter time scales. OM biodeposition has been suggested as playing a role in mussels’ influence on N-removal (Hoellein et al. 2017; Vaughn 2017), with authors calling for empirical tests to reveal the specific functional role of mussel biodeposits. The results of our SLRs support these claims by suggesting a direct positive relationship between OM biodeposition rate and N-removal potential in sediment. Future studies could benefit from isolating mussel biodeposits using such a method as the “biodeposition tray method” (Hoellein et al. 2015) and measuring N-removal potentials on the biodeposits or biodeposit-sediment mixtures.
Strengths and weaknesses of the microcosm design
A review of the feeding behavior of mussels reported that an adult mussel roughly 61-mm in length can filter water at a rate of ~ 0.5 to 1-L h−1 (Vaughn et al. 2008, and references within). This estimated filtration-rate is roughly equivalent the flow-rate we used in our continuous-flow design. Additionally, it has been shown that riverine mussels will gape (indication of filtering activity) roughly 50% of the time under dark laboratory conditions (Chen 1998). Although we did not assess the quantity or quality of seston in the unfiltered site water contained in the inflow reservoir, a recent study on the Sipsey River reported OM concentrations in the water column to be ~ 2 mg AFDM L−1 during baseflow conditions near the site at which we collected the water used in the incubations (Atkinson et al. 2019). Therefore, we assumed mussels were exposed to adequate OM for feeding within the microcosms. Further, If we assumed our mussels were actively filtering for 50% of the time during the incubation, and the mussel filtration-rate was roughly 50–100% of the flow-rate within the chamber, the excreta-derived N, be it in the form of NH4+ or NO3−, will have a much longer residence time in a microcosm than it would naturally in the Sipsey River, where reach-scale discharge at the field site is roughly 3 × 104 L s−1 during baseflow conditions (C.L. Atkinson, unpublished data). Therefore, excreta-derived N from mussels with fully exposed siphons was excreted directly to the overlying water, but not lost “downstream” as quickly as would occur in the natural stream environment, which increased localized DNF beyond what would be expected in the natural environment.
Another component of natural mussel habitat missing from the microcosm design was interstitial flow, which is important as mussels in the Sipsey River and elsewhere (Amyot and Downing 1991) will often completely bury themselves. We were not able to quantify how far each mussel buried itself into the sediment, but we did note that no mussel was completely buried, but most F. cerina and C. asperata individuals buried to where only their siphons were exposed. Most L. ornata individuals had > 50% of their shell exposed, likely due to their size relative to the sediment surface area keeping them from being able to burrow within the microcosm. We therefore assume NH4+ for all mussels was excreted directly into the overlying water and diffused into the sediment to undergo coupled-DNF. Little is known about the feeding behavior of buried mussels, but we can assume that unless mussels completely cease metabolism when buried, they must continue to filter-feed, excrete, and produce biodeposits. In a stream, the flow of interstitial water is slower than that of the overlying water column and, therefore, excreta-derived NH4+ will be more available to local food webs if deposited below the sediment–water interface, potentially increasing local nitrification and coupled-DNF. Our results support this notion by showing that when excreta-derived NH4+ was concentrated locally in the sediment in the absence of light, it significantly increased ambient DNF. As a result, the continuous flow-through design utilized in our chamber incubation experiments could represent a better proxy of what occurs when highly reactive N from mussel excretion is introduced to slow-moving, microbially-active [heterotrophic] interstitial water, rather than directly into the water column. In natural mussel assemblages, especially those found in lowland systems like the Sipsey River where the benthos is characterized as being predominantly composed of gravel and sand, a large proportion of mussels are completely buried beneath the sediment–water interface (Allen and Vaughn 2009; Schwalb and Pusch 2007). This could indeed be relevant to our study system the Sipsey River. In our study system, during quantitative surveys in the summer and fall of 2016, 81% of L. ornata individuals (n = 222), 52% of F. cerina individuals (n = 726) and 51% of C. asperata individuals (n = 1067) were found completely buried (C.L. Atkinson, unpublished data). Though the issue of interstitial flow cannot be addressed with the continuous-flow through microcosm design, future studies should attempt to optimize the flow-rate through the microcosms to closer mimic mussels’ natural habitat. This would reduce the retention time of N within the system, as well as help minimize the influence of respiration on ambient DNF.
We designed the microcosm study to focus on the effect of individual mussels on N-removal on small spatial and temporal scales. Our experimental design allowed us to observe how small-scale N2 flux was influenced by the excretion and egestion of an individual mussel, but, due to the constraints of the small-scale microcosm design, we suggest any extrapolation of our results to larger community scales (e.g., patch, reach) be done with caution. Additionally, the mussels, sediment and water we used in our experiment were extracted from the Sipsey River during baseflow conditions in the late summer. Therefore, we also suggest temporal extrapolation of the rates measured in our study be done with care.
Conclusion
Freshwater mussels are one of the most imperiled faunal groups in the world (Strayer et al. 2004). Mussel conservation efforts are hindered by the overall lack of understanding of mussel ecology, behavior, and the provisioning of ecosystem functions. Our results suggest the functional traits of NH4+ excretion and OM biodeposition contribute to a mussel’s indirect provisioning of ecosystem function by stimulating biogeochemical N-removal in sediments through chemical and physical alteration of the benthic environment. Our microcosm design also allowed us to observe biogeochemical processes on a small scale, model the influence of individual mussels on biogeochemical processes in freshwater sediment, and add to the growing knowledge of the functional roles of mussels in aquatic ecosystems. With growing recognition of the importance of mussels for aquatic ecosystem health and resiliency (see review in Vaughn 2017 and references within), our research indicates that conservation efforts could benefit from viewing functional traits holistically by not only assessing the direct effects, but also the latent effects that can ripple through the biotic and abiotic realms of an aquatic ecosystem.
References
Allen DC, Vaughn CC (2009) Burrowing behavior of freshwater mussels in experimentally manipulated communities. J N Am Benthol Soc 28:93–100
Allen DC, Vaughn CC, Kelly JF, Cooper JT, Engel MH (2012) Bottom-up biodiversity effects increase resource subsidy flux between ecosystems. Ecology 93:2165–2174
Amyot J-P, Downing JA (1991) Endo- and epibenthic distribution of the unionid mollusc Elliptio complanata. J N Am Benthol Soc 10:280–285
Anschutz P, Ciutat A, Lecroart P, Gérino M, Boudou A (2012) Effects of tubificid worm bioturbation on freshwater sediment biogeochemistry. Aquat Geochem 18:475–497
Atkinson CL, Vaughn CC (2015) Biogeochemical hotspots: temporal and spatial scaling of the impact of freshwater mussels on ecosystem function. Freshw Biol 60:563–574
Atkinson CL, Vaughn CC, Forshay KJ, Cooper JT (2013) Aggregated filter-feeding consumers alter nutrient limitation: consequences for ecosystem and community dynamics. Ecology 94:1359–1369
Atkinson CL, Kelly JF, Vaughn CC (2014) Tracing consumer-derived nitrogen in riverine food webs. Ecosystems 17:485–496
Atkinson CL, Capps KA, Rugenski AT, Vanni MJ (2017) Consumer-driven nutrient dynamics in freshwater ecosystems: from individuals to ecosystems. Biol Rev 92:2003–2023
Atkinson CL, Allen DC, Davis L, Nickerson ZL (2018) Incorporating ecogeomorphic feedbacks to better understand resiliency in streams: a review and directions forward. Geomorphology 305:123–140
Atkinson CL, van Ee BC, Lu Y, Zhong W (2019) Wetland floodplain flux: temporal and spatial availability of organic matter and dissolved nutrients in an unmodified river. Biogeochemistry 142:395–411
Benelli S, Bartoli M, Racchetti E, Moraes PC, Zilius M, Lubiene I, Fano EA (2017) Rare but large bivalves alter benthic respiration and nutrient recycling in riverine sediments. Aquat Ecol 51:1–16
Berner RA (1981) A new geochemical classification of sedimentary environments. J Sediment Res 51:359–365
Bilkovic DM, Mitchell MM, Isdell RE, Schliep M, Smyth AR (2017) Mutualism between ribbed mussels and cordgrass enhances salt marsh nitrogen removal. Ecosphere 8:1–18
Blann KL, Anderson JL, Sands GR, Vondracek B (2009) Effects of agricultural drainage on aquatic ecosystems: a review. Crit Rev Environ Sci Technol 39:909–1001
Bogan AE (2007) Global diversity of freshwater mussels (Mollusca, Bivalvia) in freshwater. In: Balian EV et al (eds) Freshwater animal diversity assessment. Springer, Dordrecht, pp 139–147
Bonaglia S, Nascimento FA, Bartoli M, Klawonn I, Brüchert V (2014) Meiofauna increases bacterial denitrification in marine sediments. Nat Commun 5:5133–5142
Boulton AJ, Findlay S, Marmonier P, Stanley EH, Valett HM (1998) The functional significance of the hyporheic zone in streams and rivers. Annu Rev Ecol Syst 29:59–81
Bunn SE, Arthington AH (2002) Basic principles and ecological consequences of altered flow regimes for aquatic biodiversity. Environ Manage 30:492–507
Cardinale BJ, Palmer MA, Collins SL (2002) Species diversity enhances ecosystem functioning through interspecific facilitation. Nature 415:426
Cardinale BJ, Matulich KL, Hooper DU, Byrnes JE, Duffy E, Gamfeldt L, Balvanera B, O’Connor MI, Gonzalez A (2011) The functional role of producer diversity in ecosystems. Am J Bot 98:572–592
Carlsson MS, Glud RN, Petersen JK (2010) Degradation of mussel (Mytilus edulis) fecal pellets released from hanging long-lines upon sinking and after settling at the sediment. Can J Fish Aquat Sci 67:1376–1387
Carpenter SR, Caraco NF, Correll DL, Howarth RW, Sharpley AN, Smith VH (1998) Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecol Appl 8:559–568
Catallo WJ (1999) Hourly and daily variation of sediment redox potential in tidal wetland sediments. US Geological Survey, Biological Resources Division, Reston
Chen Y (1998) The respiratory physiology and energy metabolism of freshwater mussels and their responses to lack of oxygen. Dissertation, Virginia Polytechnic Institute and State University
Covich AP, Palmer MA, Crowl TA (1999) The role of benthic invertebrate species in freshwater ecosystems: zoobenthic species influence energy flows and nutrient cycling. Bioscience 49:119–127
Covich AP, Austen MC, Bärlocher F, Chauvet E, Cardinale BJ, Biles CL, Inchausti P, Dangles O, Solan M, Gessner MO, Statzner B, Moss B (2004) The role of biodiversity in the functioning of freshwater and marine benthic ecosystems. Bioscience 54:767–775
Dalton CM, Flecker AS (2014) Metabolic stoichiometry and the ecology of fear in Trinidadian guppies: consequences for life histories and stream ecosystems. Oecologia 176:691–701
de Bello FF, Lavorel S, Díaz S, Harrington R, Cornelissen JHC, Bardgett RD, Berg MP, Cipriotti P, Feld CK, Hering D, da Silva PM, Potts SG, Sandin L, Sousa JP, Storkey J, Wardle DA, Harrison PA (2010) Towards an assessment of multiple ecosystem processes and services via functional traits. Biodivers Conserv 19:2873–2893
Díaz S, Cabido M (2001) Vive la difference: plant functional diversity matters to ecosystem processes. Trends Ecol Evol 16:646–655
Ensign SH, Doyle MW (2005) In-channel transient storage and associated nutrient retention: evidence from experimental manipulations. Limnol Oceanogr 50:1740–1751
Ensign SH, Doyle MW (2006) Nutrient spiraling in streams and river networks. Biogeosci, J Geophys Res. https://doi.org/10.1029/2005JG000114
Estes JA, Terborgh J, Brashares JS, Power ME, Berger J, Bond WJ, Carpenter SR, Essington TE, Holt RD, Jackson JBC, Marquis RJ, Oksanen L, Oksanen T, Paine RT, Pikitch EK, Ripple WJ, Sandin SA, Scheffer M, Schoener TW, Shurin JB, Sinclair ARE, Soulé ME, Virtanen R, Wardle DA (2011) Trophic downgrading of planet Earth. Science 333:301–306
Eyre BD, Rysgaard S, Dalsgaard T, Christensen PB (2002) Comparison of isotope pairing and N2: Ar methods for measuring sediment denitrification—assumption, modifications, and implications. Estuaries 25:1077–1087
Flynn DF, Mirotchnick N, Jain M, Palmer MI, Naeem S (2011) Functional and phylogenetic diversity as predictors of biodiversity–ecosystem function relationships. Ecology 92:1573–1581
Gende SM, Edwards RT, Willson MF, Wipfli MS (2002) Pacific salmon in aquatic and terrestrial ecosystems. Bioscience 52:917–928
Giles H, Pilditch CA (2006) Effects of mussel (Perna canaliculus) biodeposit decomposition on benthic respiration and nutrient fluxes. Mar Biol 150:261–271
Grenz C, Hermin M-N, Baudinet D, Daumas R (1990) In situ biochemical and bacterial variation of sediments enriched with mussel biodeposits. Hydrobiologia 207:153–160
Grimm NB (1988) Role of macroinvertebrates in nitrogen dynamics of a desert stream. Ecology 69:1884–1893
Grimm NB, Sheibley RW, Crenshaw CL, Dahm CN, Roach WJ, Zeglin LH (2005) N retention and transformation in urban streams. J N Am Benthol Soc 24:626–642
Haag WR, Warren ML (2010) Diversity, abundance, and size structure of bivalve assemblages in the Sipsey River, Alabama. Aquat Conserv: Mar Freshw Ecosyst 20:655–667
Halvorson HM, Hall DJ, Evans-White MA (2017) Long-term stoichiometry and fates highlight animal egestion as nutrient repackaging, not recycling, in aquatic ecosystems. Funct Ecol 31:1802–1812
Higgins CB, Tobias C, Piehler MF, Smyth AR, Dame RF, Stephenson K, Brown BL (2013) Effect of aquacultured oyster biodeposition on sediment N2 production in Chesapeake Bay. Mar Ecol Prog Ser 473:7–27
Hoellein TJ, Zarnoch CB, Grizzle RE (2015) Eastern oyster (Crassostrea virginica) filtration, biodeposition, and sediment nitrogen cycling at two oyster reefs with contrasting water quality in Great Bay Estuary (New Hampshire, USA). Biogeochemistry 122:113–129
Hoellein TJ, Zarnoch CB, Bruesewitz DA, DeMartini J (2017) Contributions of freshwater mussels (Unionidae) to nutrient cycling in an urban river: filtration, recycling, storage, and removal. Biogeochemistry 135:307–324
Hölker F, Vanni MJ, Kuiper JJ, Meile C, Grossart HP, Stief P, Adrian R, Lorke A, Dellwig O, Brand A, Hupfer M, Mooij WM, Nützmann G, Lewandowski J (2015) Tube-dwelling invertebrates: tiny ecosystem engineers have large effects in lake ecosystems. Ecol Monogr 85:333–351
Hooper DU, Adair EC, Cardinale BJ, Byrnes JEK, Hungate BA, Matulich KL, Gonzalez A, Duffy JE, Gamfeldt L, O’Connor MI (2012) A global synthesis reveals biodiversity loss as a major driver of ecosystem change. Nature 486:105–109
Hou L, Liu M, Carini SA, Gardner WS (2012) Transformation and fate of nitrate near the sediment–water interface of Copano Bay. Cont Shelf Res 35:86–94
Jones HF, Pilditch CA, Bruesewitz DA, Lohrer AM (2011) Sedimentary environment influences the effect of an infaunal suspension feeding bivalve on estuarine ecosystem function. PLoS ONE 6:e27065
Kana TM, Darkangelo C, Hunt MD, Oldham JB, Bennett GE, Cornwell JC (1994) Membrane inlet mass spectrometer for rapid high-precision determination of N2, O2, and Ar in environmental water samples. Anal Chem 66:4166–4170
Kellogg ML, Cornwell JC, Owens MS, Paynter KT (2013) Denitrification and nutrient assimilation on a restored oyster reef. Mar Ecol Prog Ser 480:1–19
Knowles R (1982) Denitrification. Microbiol Rev 46:43–70
Lautz L, Fanelli R (2008) Seasonal biogeochemical hotspots in the streambed around restoration structures. Biogeochemistry 91:85–104
Lavorel S, Garnier É (2002) Predicting changes in community composition and ecosystem functioning from plant traits: revisiting the Holy Grail. Funct Ecol 16:545–556
Loreau M, Naeem S, Inchausti P, Bengtsson J, Grime JP, Hector A, Hooper DU, Huston MA, Raffaelli C, Schmid B, Tilman D, Wardle DA (2001) Biodiversity and ecosystem functioning: current knowledge and future challenges. Science 294:804–808
Matisoff G, Wang X (1998) Solute transport in sediments by freshwater infaunal bioirrigators. Limnol Oceanogr 43:1487–1499
McClain ME, Boyer EW, Dent CL, Gergel SE, Grimm NB, Groffman PM, Hart SC, Harvey JW, Johnston CA, Mayorga E, McDowell WH, Pinay G (2003) Biogeochemical hot spots and hot moments at the interface of terrestrial and aquatic ecosystems. Ecosystems 6:301–312
McGregor SW, O’Neil PE (1992) The biology and water-quality monitoring of the Sipsey River and Lubbub and Bear Creeks, Alabama, 1990-91. Geological Survey of Alabama, Biological Resources Division, vol. 169
Mermillod-Blondin F (2011) The functional significance of bioturbation and biodeposition on biogeochemical processes at the water–sediment interface in freshwater and marine ecosystems. J N Am Benthol Soc 30:770–778
Mermillod-Blondin F, Rosenberg R (2006) Ecosystem engineering: the impact of bioturbation on biogeochemical processes in marine and freshwater benthic habitats. Aquat Sci 68:434–442
Miller-Way T, Twilley RR (1996) Theory and operation of continuous flow systems for the study of benthic-pelagic coupling. Mar Ecol Prog Ser 140:257–269
Mirto S, Danovaro R, Mazzola A (2000) Microbial and meiofaunal response to intensive mussel-farm biodeposition in coastal sediments of the western Mediterranean. Mar Pollut Bull 40:244–252
Nakano S, Murakami M (2001) Reciprocal subsidies: dynamic interdependence between terrestrial and aquatic food webs. Proc Natl Acad Sci 98:166–170
Newbold JD, Elwood JW, O’Neill RV, Winkle WV (1981) Measuring nutrient spiralling in streams. Can J Fish Aquat Sci 38:860–863
Newell RI, Cornwell JC, Owens MS (2002) Influence of simulated bivalve biodeposition and microphytobenthos on sediment nitrogen dynamics: a laboratory study. Limnol Oceanogr 47:1367–1379
Newell RI, Fisher T, Holyoke R, Cornwell J (2005) Influence of eastern oysters on nitrogen and phosphorus regeneration in Chesapeake Bay, USA. In: Dame RF, Olenin S (eds) The comparative roles of suspension-feeders in ecosystems. Springer, Dordrecht, pp 93–120
Nichols SJ, Silverman H, Dietz TH, Lynn JW, Garling DL (2005) Pathways of food uptake in native (Unionidae) and introduced (Corbiculidae and Dreissenidae) freshwater bivalves. J Great Lakes Res 31:87–96
Nizzoli D, Welsh DT, Bartoli M, Viaroli P (2005) Impacts of mussel (Mytilus galloprovincialis) farming on oxygen consumption and nutrient recycling in a eutrophic coastal lagoon. Hydrobiologia 550:183–198
Paetzold A, Schubert CJ, Tockner K (2005) Aquatic terrestrial linkages along a braided-river: riparian arthropods feeding on aquatic insects. Ecosystems 8:748–759
Petchey OL, Gaston KJ (2006) Functional diversity: back to basics and looking forward. Ecol Lett 9:741–758
Poff N, Brinson MM, Day J (2002) Aquatic ecosystems and global climate change. Pew Center GlobClim Change, Arlington 44:1–36
R Core Team (2016) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Ricciardi A, Rasmussen JB (1999) Extinction rates of North American freshwater fauna. Conserv Biol 13:1220–1222
Richter BD, Braun DP, Mendelson MA, Master LL (1997) Threats to imperiled freshwater fauna. Conserv Biol 11:1081–1093
Rusek J (1998) Biodiversity of Collembola and their functional role in the ecosystem. Biodivers Conserv 7:1207–1219
Schmitz OJ, Buchkowski RW, Burghardt KT, Donihue CM (2015) Functional traits and trait-mediated interactions: connecting community-level interactions with ecosystem functioning. Adv Ecol Res 52:319–343
Schwalb AN, Pusch MT (2007) Horizontal and vertical movements of unionid mussels in a lowland river. J N Am Benthol Soc 26:261–272
Seitzinger S, Harrison JA, Böhlke JK, Bouwman AF, Lowrance R, Peterson B, Tobias C, Drecht GV (2006) Denitrification across landscapes and waterscapes: a synthesis. Ecol Appl 16:2064–2090
Smith E, Davison W, Hamilton-Taylor J (2002) Methods for preparing synthetic freshwaters. Water Res 36:1286–1296
Spooner DE, Vaughn CC (2006) Context-dependent effects of freshwater mussels on stream benthic communities. Freshw Biol 51:1016–1024
Spooner DE, Vaughn CC, Galbraith HS (2012) Species traits and environmental conditions govern the relationship between biodiversity effects across trophic levels. Oecologia 168:533–548
Strayer DL, Dudgeon D (2010) Freshwater biodiversity conservation: recent progress and future challenges. J N Am Benthol Soc 29:344–358
Strayer DL, Malcom HM (2007) Shell decay rates of native and alien freshwater bivalves and implications for habitat engineering. Freshw Biol 52:1611–1617
Strayer DL, Downing JA, Haag WR, King TL, Layzer JB, Newton TJ, Nichols SJ (2004) Changing perspectives on pearly mussels, North America’s most imperiled animals. Bioscience 54:429–439
Thamdrup B, Dalsgaard T (2002) Production of N2 through anaerobic ammonium oxidation coupled to nitrate reduction in marine sediments. Appl Environ Microbiol 68:1312–1318
Thrush SF, Hewitt JE, Gibbs M, Lundquist C, Norkko A (2006) Functional role of large organisms in intertidal communities: community effects and ecosystem function. Ecosystems 9:1029–1040
Tilman D (2001) Functional diversity. Encyclopedia of Biodiversity 3:109–120
Tilman D, Knops J, Wedin D, Reich P, Ritchie M, Siemann E (1997) The influence of functional diversity and composition on ecosystem processes. Science 277:1300–1302
Trentman MT, Atkinson CL, Brant JD (2018) Native freshwater mussel effects on nitrogen cycling: impacts of nutrient limitation and biomass dependency. Freshwater Science 37:276–286
Turek KA, Hoellein TJ (2015) The invasive Asian clam (Corbicula fluminea) increases sediment denitrification and ammonium flux in 2 streams in the midwestern USA. Freshw Sci 34:472–484
Vanni MJ (2002) Nutrient cycling by animals in freshwater ecosystems. Annu Rev Ecol Syst 33:341–370
Vanni MJ, McIntyre PB (2016) Predicting nutrient excretion of aquatic animals with metabolic ecology and ecological stoichiometry: a global synthesis. Ecology 97:3460–3471
Vanni MJ, Boros G, McIntyre PB (2013) When are fish sources vs. sinks of nutrients in lake ecosystems? Ecology 94:2195–2206
Vaughn CC (2010) Biodiversity losses and ecosystem function in freshwaters: emerging conclusions and research directions. Bioscience 60:25–35
Vaughn CC (2017) Ecosystem services provided by freshwater mussels. Hydrobiologia 810:11–27
Vaughn CC, Hakenkamp CC (2001) The functional role of burrowing bivalves in freshwater ecosystems. Freshw Biol 46:1431–1446
Vaughn CC, Spooner DE, Galbraith HS (2007) Context-dependent species identity effects within a functional group of filter-feeding bivalves. Ecology 88:1654–1662
Vaughn CC, Nichols SJ, Spooner DE (2008) Community and foodweb ecology of freshwater mussels. J N Am Benthol Soc 27:409–423
Vaughn CC, Atkinson CL, Julian JP (2015) Drought-induced changes in flow regimes lead to long-term losses in mussel-provided ecosystem services. Ecol Evol 5:1291–1305
Veuger B, Eyre BD, Maher D, Middelburg JJ (2007) Nitrogen incorporation and retention by bacteria, algae, and fauna in a subtropical, intertidal sediment: an in situ 15 N-labeling study. Limnol Oceanogr 52:1930–1942
Vidon P, Allan C, Burns D, Duval TP, Gurwick N, Inamdar S, Lowrance R, Okay J, Scott D, Sebestyen S (2010) Hot spots and hot moments in riparian zones: potential for improved water quality management. J Am Water Resour Assoc 46:278–298
Violle C, Navas ML, Vile D, Kazakou E, Fortunel C, Hummel I, Garnier E (2007) Let the concept of trait be functional! Oikos 116:882–892
Walker BH (1992) Biodiversity and ecological redundancy. Conserv Biol 6:18–23
Wallace JB, Webster JR (1996) The role of macroinvertebrates in stream ecosystem function. Annu Rev Entomol 41:115–139
Williams JD, Bogan AE, Garner JT (2008) Freshwater mussels of Alabama and the Mobile basin in Georgia, Mississippi, and Tennessee. University of Alabama Press, Tusaloosa
Wilson MA, Carpenter SR (1999) Economic valuation of freshwater ecosystem services in the United States: 1971–1997. Ecol Appl 9:772–783
Acknowledgements
Funding was provided by the University of Alabama, Dauphin Island Sea Lab, the Center for Freshwater Studies at University of Alabama, and through research grants awarded by the Birmingham Audubon Society and the Conchologists of America, Inc. Land access to the Sipsey River was granted by the Weyerhaeuser Company. For assistance in the field and lab, we thank Anne Bell, Alice Kleinhuizen, Corianne Tatariw, Brian van Ee, Anastasia Nickerson, Derek Tollette and Taylor Ledford.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Breck Bowden.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nickerson, Z.L., Mortazavi, B. & Atkinson, C.L. Using functional traits to assess the influence of burrowing bivalves on nitrogen-removal in streams. Biogeochemistry 146, 125–143 (2019). https://doi.org/10.1007/s10533-019-00612-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-019-00612-2