Abstract
Understanding how communities respond to anthropogenic disturbance is considered a stepping-stone to support decision-making by environmental managers and restoration ecology. In endangered biodiverse regions, such as the Neotropics, ecological indicators are particularly useful, by enlighten possible diversity trends and functional shifts. Our objectives were to deepen our understanding on the soil ant assemblages/habitat relationships in the Brazilian Amazon, particularly relevant for a data-deficient and fast changing region, and evaluate if regional ant assemblages, collected within a gradient of disturbance, could fulfill the surrogate role. A sampling effort of 630 pitfall traps and litter collection were used for monitoring ant communities within the gradient of habitats considered. Sequential reductions in ant richness, abundance and simplification of assemblage compositions were detected along the disturbance gradient comprising pristine forests, secondary forests, agriculture and pastures. Additionally, indicator species identified could be used to assess the disturbance level but also to guide restoration efforts in this region. Finally, even though late secondary forests displayed diverse assemblages, old-growth/pristine forests support distinct communities, highlighting its critical contribution for the conservation of ants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Brazilian Amazon is considered a biodiversity hotspot, as it is one of the largest and most important tropical forests in the world, providing a range of ecosystem services across local, regional, and global scales (Garrett et al. 2017; Cajaiba et al. 2018a). However, the demand for food, energy, and raw materials (Laurance et al. 2014; Dou et al. 2018) has driven increased logging and conversion of forests to agriculture and pastures, causing massive landscape changes in recent decades (Gibbs et al. 2016). Global concerns regarding environmental and ecological issues aroused, such as loss of biodiversity, increased greenhouse gas emissions, climate change, and the supply of water, closely linked with the trees’ metabolism (Hoff et al. 2018; Roitman et al. 2017; Sales et al. 2020), threatening wellbeing and socio-economy (Pecl et al. 2017). Despite the increasing research highlighting the significance of this regions’ biodiversity and ecosystem services provided (e.g. Ometto et al. 2011; Campbell et al. 2018), the quantification of the impacts of ongoing landscape changes is far from being understood (Dohm et al. 2011; Ometto et al. 2011; Spiller et al. 2018).
Invertebrates, namely arthropods, are particularly suited to quantify anthropogenic effects on ecosystems, since their population dynamics and mobility allow them to quickly respond to changing environmental conditions (Gerlach et al. 2013; Diame et al. 2015; Buchori et al. 2018). Ants are predominant arthropods in most ecosystems, performing significant ecological functions within diverse trophic levels (as predators, herbivores, and scavengers) (Wilson and Hölldobler 2005; Baccaro et al. 2012; Pfeiffer et al. 2013). Also, their contribution to the physicochemical properties of the soil, nutrient cycling, water drainage, soil aeration, seed dispersion and pollination have been underlined by several authors (Bruna et al. 2011; Belém et al. 2020). Moreover, diversity and abundance evidences its functional dominance in most terrestrial ecosystems (Bharti et al. 2016). For instance, in the Brazilian Amazon, the biomass of ants can reach four times the combined biomass of all vertebrates (Wilson and Hölldoble 2005). Soil dwelling ant species are easily sampled using standard methodologies (e.g. pitfall traps) (Delabie et al. 2009) and considered highly responsive to environmental change, usually by an assemblage offset (Baccaro et al. 2013; Buchori et al. 2018). In fact, works in other regions depict responses to disturbance by ant communities, for instance significant loss of ant diversity in regrowth forests when compared with mature forests (Silva et al. 2007), time-lags in the recovery of assemblages after ecosystem restoration (Dunn 2004) and an increase of dominant invasive species suppressing natives in forest fragments (Achury et al. 2021).
In the Brazilian Amazon, some publications have shown the interest on using ants as ecological indicators of landscape changes. Anyway, there is a gap in data and evidence concerning ant communities’ sensitivity to ongoing land use and management changes in the Amazon region, fundamental to support their use as proxies of specific disturbances and ecosystems’ recovery status (e.g. Solar et al. 2016; Santos et al. 2021; Souza and Fernandes 2021). Our objectives were to deepen our understanding on the soil ant assemblages/habitat relationships in the Brazilian Amazon, particularly relevant for a data-deficient and fast changing region, and evaluate if regional ant assemblages, collected within a gradient of disturbance, could fulfill this surrogate role. The following hypotheses were tested: (i) disturbed habitats harbor less diverse communities than more pristine ones, (ii) disturbance is perceived by increased turnover and nestdness patterns, (iii) habitats’ disturbance/restoration is detectable using indicators species, i.e. associated with a single land use.
Materials and methods
Study area and habitats selection
The study was conducted in the Brazilian Amazon, municipality of Uruará, state of Pará, Brazil (Fig. S1, Supplementary Material). Old-growth/primary rainforest stands-out as the dominant land use, while deforested areas mark the landscape near the main roads (e.g. Transamazonica) and in the south-central region of the municipality (Cajaiba et al. 2018b). Deforested areas are linked with urban development, extensive livestock production, cocoa farming, and small landowners mixed crops (short-rotational cycle agriculture) (Da Silva et al. 2018). The climate is characterized as hot-humid (Köppen classification), with an average annual temperature and precipitation of 26 °C and 2000 mm, respectively (Peel et al. 2007).
Dominant land uses (for here on referred as “habitats”) present in the region were chosen and classified as (from remote sensing, interviews with local residents, and on-site visits) (Da Silva et al. 2018): (i) Primary Forest (PF), little or no disturbance for at least 80 years; (ii) Secondary Forest (SF), 15 years of recovery from anthropogenic disturbance; (iii) Incipient forest (IF), 5 years of recovery from anthropogenic disturbance; (iv) Agriculture (AG), cocoa plantations (Theobroma cacao), and (v) Pastures (PA), extensive livestock pastures.
Ant sampling
Sampling was carried out in 2015, during the rainy season (February/March), the intermediate season (June) and the dry season (September/October), to encompass possible effects of varying environmental variables on ant communities. Individuals were capture using pitfall traps with 26 cm diameter and 13 cm deep, containing approximately 350 ml of solution (water, coarse salt for preserving dead animals, and neutral detergent to break the surface tension of the water) and covered by a suspended roof to prevent rainwater from entering. Two independent sites per habitat were sampled, located with a minimum distance of 24 km from each other. Seven random points in each study site, with a minimum distance of 150 m from each other and a minimum distance of 150 m from the edge were selected (Lacasella et al. 2015). Three traps were installed by point, 10 m apart—two baited (sardines and bananas) and one non-baited. Traps were installed for 48 h, with the protocol being repeated for all habitats, sites and periods of collection, totaling a sampling effort of 630 traps (see Table S1, Supplementary Material, for details).
Litter sampling, considering litter the layer of leaves and debris that can be easily removed from the more compact soil (Ivanov and Keiper 2009; Da Silva et al. 2018), was applied to complemented pitfalls: 10 random areas of 1 m2 (1 × 1 m—total of 300 areas, see Table S2 Supplementary Material), with a minimum distance of 100 m between them, were selected by habitat, site and sampling period. Scrapping gently with a metal spatula the litter and the topsoil was essential so that most ants in the samples were captured (Nakamura et al. 2007; Cajaiba et al. 2017b). Each soil and litter sample were placed in a tightly closed tissue bag and kept in an insulated box, screened in the laboratory for ants within the leaves, branches, and soil.
Ants were preserved in 70% ethanol solution and identified until the species level (whenever possible), according to reference bibliography (Bolton 1994; Palácio and Fernández 2003; Baccaro et al. 2015): in cases considered dubious or with incomplete referenced information, individuals where classified as morphospecies (Swart et al. 2018). Even though not relevant for taxonomic studies, morphospecies have an added value for conservation, environmental impact assessment and for comparing regional patterns diversity in biodiverse regions (e.g., Derraik et al. 2002). Collected specimens were deposited at the Laboratory of Ecology and Conservation, Federal Institute of Education, Science and Technology of Maranhão in Brazil.
Environmental variables monitoring
Fifteen environmental variables with potential influence on ant communities and ecosystem functioning were monitored within the sampled habitats (Cajaiba et al. 2017a): temperature (T), humidity (H), precipitation (P), circumference at breast height (CBH), circumference at ankle height (CAH), canopy cover (CC), richness of plants (RP), abundance of plants (AP), tree density (TD), richness of shrubs (RS), abundance of shrubs (AS), percentage of exposed soil (PES), percentage of green (vegetation) cover (GC), percentages of leaf litter cover (LLC), height of leaf litter (HLL). Information concerning the methods associated with each variable and the correlation between environmental variables and habitats are depicted in Tables S3, S4 (Supplementary materials).
Statistical analysis
Species rarefaction curves were plotted using the presence-absence data matrices using EstimateS 9.1.0 software (Colwell 2013). In order to standardize the comparisons between habitats and to estimate sampling completeness, curves and the Chao2 non-parametric estimator of total species richness were calculated (Colwell et al. 2004). In order to discriminate richness and abundance between ecosystems, Generalized Linear Models (GLzMs) with a Poisson distribution for richness and gaussian for abundance, were fitted. The response variables were the richness and abundance and the explanatory variables were the different habitats. Subsequently, pairwise contrast analyses using the Tukey test were performed to detect possible differences among habitats (Crawley 2010). In order to homogenize the variances and normalize the residues the abundance was transformed by log(x + 1).
The taxonomic composition of ant communities across habitats was compared using Permutational Multivariate Analysis of Variance (PERMANOVA). To inspect particular differences, two axes Non-Metric Multidimensional Scaling (NMDS) was applied, considering the low degree of stress (0.21). To reduce possible biases in the NMDS, least correlated environmental variables were selected (Spearman's rho < 0.75) (Graham 2003): temperature (T), humidity (H), canopy cover (CC), percentage of exposed soil (PES), LLC percentages of leaf litter cover (LLC), tree density (TD), richness of shrubs (RS) and adjusted to the corresponding NMDS axes. Bray–Curtis similarity index was used within PERMANOVA and NMDS, with 999 permutations (Clarke and Warwick 2001).
We used Beta diversity analysis to verify the dissimilarity between habitats (Baselga 2010). Pairwise dissimilarity index (βsør), ranging from 0 (associations of identical species) to 1 (associations of different species), was partitioned in two summing components, turnover (βsim) and nestedness (βnes) (Baselga 2010). The use of this approach enables testing (i) differences in the total dissimilarity values (βsor), but also (ii) the relative contribution of species replacement (βsim) and species erosion (βnes).
Single-value indicator analysis (IndVal) (Dufrêne and Legendre 1997) with 9999 permutations was used to detect possible indicator species, by combining the specificity (relative abundance patterns) of a species in a given habitat with its fidelity within that habitat (patterns of incidence). Species with high specificity and high fidelity within a habitat are considered to have the highest indicator value (IndVal > 25%, Dufrêne and Legendre 1997).
Envfit function (Oksanen et al. 2007) of the vegan package (Oksanen et al. 2013), Beta.pair function of the betapart package (Baselga et al. 2017) and indicspecies 1.7.5 package (De Cáceres and Jansen 2015) within program R 3.2.4 (Core Team 2016) were used in our statistical analysis.
Results
General results and diversity comparisons
Species-accumulation curves per habitat approximate asymptotes, indicating a satisfactory sampling effort (Fig. 1). These data suggest that the number of ant species sampled is close to the total number of species in each type of habitat.
Ant rarefaction species-accumulation curves for the studied habitats. Central lines represent occurrence-based rarefaction curves and their 95% confidence intervals. PF—little or no disturbance for at least 80 years, (ii) SF—15 years of recovery from anthropogenic disturbance, (iii) IF—5 years of recovery from anthropogenic disturbance, (iv) AG—cocoa plantations (T. cacao), and (v) PA—extensive livestock pastures
A total of 9727 individuals from 131 species, 31 genera and 8 subfamilies were captured (Supplementary Material, Table S4). Myrmicinae (66 species and 13 genera) and Ponerinae (25 species and 7 genera) were the diverse subfamilies. On the other hand, the genus Pheidole (15 species and 2,872 individuals), Camponotus (12 species and 1016 individuals), Solenopsis (10 species and 1128 individuals) and Pachycondyla (6 species and 1038 individuals) attained the highest abundances. Conversely, 48 species (36.64%) included less than ten individuals, and 37 species (28.25%) less than five individuals (Supplementary Material, Table S4). PF were associated with outstanding richness and abundance (105 species and 3643 individuals), followed by SF (82 species and 2018 individuals), contrasting with PA (61 species, 1308 individuals) (Table S4, supplementary material).
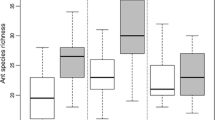
Statistical significant differences were detected for habitats’ richness and abundance (GLzM, F = 14.49, p < 0.001 and F = 49.13, p < 0.001, respectively). PF attained significantly higher richness and abundance than all other habitats (Fig. 2A). SF presented also significantly higher richness and abundance when compared IF, AG and PA (Fig. 2B). On the other hand, IF, AG and PA depicted equivalent richness and abundance, significantly lower than PF and SF (Fig. 2A, B) (see supplementary material, Table S5, for details of the associated differences and Tukey post-hoc values).
Box-plots depicting Ants’ Richness (A) and Abundance (B) for the habitats studied. The values followed by the same letters are not statistical significantly different according to the Tukey test. PF—little or no disturbance for at least 80 years, (ii) SF—15 years of recovery from anthropogenic disturbance, (iii) IF—5 years of recovery from anthropogenic disturbance, (iv) AG—cocoa plantations (T. cacao), and (v) PA—extensive livestock pastures
Taxonomic composition
Of the 131 species collected, 45 (34.35%) were shared among all habitats, while 36 (27.50%) were exclusive of specific habitats. The largest number of exclusive species were captured in PF (17), followed by SF (7 species). Complementary, the largest species’ sharing occurred between PF and SF (13 species) (Table S6, Supplementary Material). In fact, ant assemblages showed significant differences in composition (PERMANOVA, F = 13.43, p < 0.0001), reinforced by the dissimilarity between all pairwise comparisons (Table 1). Additionally, pristine assemblages were also apart from the other habitats, even though with a small overlap with late secondary forest (Non-metric Multidimensional Scaling, NMDS; Fig. 3). NMDS also depicts a general overlap between disturbed habitats (IF, AG and PA) and a trend in the assemblages composition associated with the gradient of disturbance under study (Fig. 3). The distinct assemblages of PF were linked with canopy cover (CC), tree density, litter cover on soil (LTC), moisture (H), and shrub richness (NSS), while PA, AG and partially IF were correlated with higher temperatures (T) and percentage of exposed soil (PES) (Fig. 3).
Non-metric Multidimensional Scaling (NMDS) depicting the composition of ant assemblages according to the habitats: PF—little or no disturbance for at least 80 years, (ii) SF—15 years of recovery from anthropogenic disturbance, (iii) IF—5 years of recovery from anthropogenic disturbance, (iv) AG—cocoa plantations (T. cacao), and (v) PA—extensive livestock pastures. Bray–Curtis similarity algorithm, stress 0.21 was used. The detailed description of each environmental variables Table S3, Supplementary material
Beta diversity dissimilarity index (βsor) highest values (βsor) were found when comparing PF with PA (βsor = 0.87), PF with IF (βsor = 0.77), and SF with AG (βsor = 0.78) (Fig. 4); even the lower dissimilarities, such as between PF and SF (βsor = 0.52) and PF and IF (βsor = 0.58) show significant variation in species composition between habitats (Fig. 4). The isolation of assemblages was accentuated by the predominant Turnover (βsim) component: e.g. when comparing PF with PA (βsim: 0.66), SF with AG (βsim: 0.59) (Fig. 4). Nestdedness (βnes), depicting close assemblages’ erosion, was particularly significant when comparing IF with PA (βnes: 0.30) and AG with PA (βnes: 0.29) and only marginal for SR vs IF (βnes = 0.17) and PF vs SF (βnes: 0.19). Detailed comparisons are depicted in Table S7, Supplementary material.
Comparison of dissimilarity values for βsor (overall dissimilarity), βnes (dissimilarity resulting from nestedness), and βsim (turnover) for the ants assemblages associated with the different habitats. PF—little or no disturbance for at least 80 years, (ii) SF—15 years of recovery from anthropogenic disturbance, (iii) IF—5 years of recovery from anthropogenic disturbance, (iv) AG—cocoa plantations (T. cacao), and (v) PA—extensive livestock pastures
Indicator species—Indval
From the species collected, 16 were considered particularly relevant for indicating habitats, five species associated with PF, two with SF, two with PA and one with AG and none with IF (Table 2). Additionally, four indicated less disturbed areas (PF and SF simultaneously) and two highly disturbed areas (PA and AG simultaneously) (Table 2).
Discussion
Ant assemblages’ characteristics and habitats disturbance
Anthropogenic change in the forest landscapes created a toll on ants’ diversity, as demonstrated by the decline in richness and abundance between the less disturbed (primary and secondary forest) and all other habitats. This biodiversity trend is in agreement with the habitat heterogeneity hypothesis (Tews et al. 2004), but also with studies demonstrating increasing diversity with habitat complexity (Staab et al. 2014; Rabello et al. 2015). Both link habitat heterogeneity and complexity with diverse diets and nest location possibilities, thus allowing the coexistence of a large set of species and populations (Klimes et al. 2012; Ahuatzin et al. 2019). Additionally, highly disturbed habitats, particularly those with reduced canopy coverage, litter, and high solar incidence, constrain microclimates and create more extreme conditions (i.e., soil moisture, temperature, and solar radiation) that apparently curb ant communities (Reynolds et al. 2018).
The previous factors are also considered decisive for the shifts in composition of assemblages, by modulating potential niches (Read and Andersen 2000). Trees’ age and diversity is considered one of the most relevant factors for providing nesting locations (Frizzo and Vasconcelos 2013), food resources (Arnan et al. 2007) and complex microclimates (Carvalho et al. 2020). Indirectly, trees’ shading in the forest soil is also inversely related with the cover of shrubs that dominate the first stages of forest regeneration and with the arthropod communities that are closer to the communities of highly modified habitats (Cajaiba et al. 2018a; Da Silva et al. 2018). Erosion in richness and abundance has been ascribed to cattle trampling in pasture areas, that contributes to soil compaction, reduced pathways through the litter layer, as well as less hiding places and hunting grounds for ants (Chen et al. 2011; Cajaiba et al. 2017b). Additionally, change in soil characteristics associated with pasture management creates complementary (and partially unknown) challenges to many soil dwelling ant communities (Spellmeier et al. 2019), which become dominated by opportunistic generalists, as well as alien invasive eurytherms (Lassau and Hochuli 2004).
Even if pristine forest ant assemblages look as partial isolated from the other habitats, secondary forests results—more than 15 years of recovery were not sufficient to bring ant assemblages closer to the PF communities—depict the halfway of recovery between degraded and pristine habitats (Ottonetti et al. 2006; Ribas et al. 2012a). Composition similarities found here support this idea, namely the gradient in beta diversity within the gradient of habitats, in line with chronosequential studies concerning ants and ecosystem restoration (Laste et al. 2019). The significant turnover found, circa 80% of the total beta diversity, indicates that each habitat has a (partially) distinct subset of species (Lyra-Jorge et al. 2010; Cajaiba et al. 2017b). Age from disturbance could be part of the explanation the of increasing dissimilarity (Schmidt et al. 2013). Perennial crops, such as cocoa, sustain higher diversities and complex assemblages than pastures and even incipient secondary forests, and presented partial similarities with PFs (Groc et al. 2017). Apparently, cocoa plantations extensive management, high canopy cover and particularly thick litter (Groc et al. 2017) contribute to an increase in the resources and stability in environmental conditions, that enable the conservation of ant diversity (and in general soil arthropods) in neotropical regions (Delabie et al. 2007; Schroth et al. 2011).
With did not monitored other types of crops, namely annual intensive which results would probably include additional shifts and biodiversity loss. Limitations of nesting locations for ants, caused by the lack of litter, decaying trunks, or live trees (Klimes et al. 2012) is one of the most important factors hindering ant diversity (Pacheco et al. 2013). Also, agricultural intensification, specifically cultivation operations, pesticides, fertilizer inputs, permanent arable land, and simplification of habitat structure are considered the main factors creating colds pots in the Neotropics (Ekroos et al. 2010; Cajaiba et al. 2019).
Neotropical ants as ecological indicators of anthropogenic disturbances
Ants are dominant organisms in most terrestrial regions and, therefore, have a long and successful history as indicators of ecosystem integrity (Chen et al. 2011; Ribas et al. 2012b; Bharti et al. 2016). The interpretation of its environmental change surrogate potential is achieved when species-habitat associations are explained and considered significant (Chen et al. 2011). Anyway, in diverse regions such as the Neotropics, this interpretation could be hindered considering that species level identification is not always possible, due to lack of available taxonomists, unknown (new) species and costs associated (Cajaiba et al 2018a). In such regions, the morphospecies concept might be used to create the first assessment of the relation between diversity and habitats, while reducing the time and expenses associated with species identification (Groc et al. 2010; Maveety et al. 2011). In fact, surrogate taxonomic approaches have been developed to address the short-term need of providing scientific advice to resource managers and policy makers (Hackman et al. 2017; Cajaiba et al. 2018b). Also, several authors highlight that differences observed at the species level usually can be seen at higher taxonomic levels, namely genus (Andersen et al. 2002; Derraik et al. 2002; Schnell et al. 2003).
In our study, we identified 16 species/morphospecies indicating habitats within diverse levels of disturbance. Most indicator species associated with pristine habitats were found almost exclusively there, whereas indicators from disturbed habitats, were mostly generalist present within all habitats but specially abundant in the former, e.g. Pheidole species/morphospecies (see Table S4 supplementary material and Table 2) (Delabie et al. 2009). In this way, the dominance of the generalist species/guilds further suggests that the replacement of pristine forests by anthropogenic habitats is accompanied by a replacement of the biomass of specialized forest ants species by the generalist species (Philpott et al. 2010) that are able to cope and take advantage of novel conditions created (Groc et al. 2017). Apparently, reduction in biomass/diversity seem to ultimate “indicators” of disturbance in the Neotropics.
Final remarks
Comprehensive biodiversity surveys are generally unfeasible, due to limitations in time, financial resources and taxonomists, justifying the application of surrogates in ecological diagnostics (Chen et al. 2011; Andersson et al. 2014). Several works demonstrate that certain organisms’ behaviors, inter and intra specific relationships, services and response to environmental change might be used for estimating the conservation status of ecosystems and landscapes (Kemerich et al. 2014; Lima et al. 2015). Noss (1990), Dale and Beyeler (2001), Lawton and Gaston (2001), and Scott et al. (2006) among many other scientists highlighted the features of indicator/surrogate organisms: relevant for ecosystem homeostasis (not only keystone species), wide geographical range, low-cost straightforward sampling and sensitive to environmental change. Noteworthy has been the pertinence of indicators for supporting decision-making of ecosystem restoration and sustainable management practices (e.g. Semprucci et al. 2015; Cajaiba et al. 2017a; Belém et al. 2020).
Our results support the hypotheses that ants are particularly reactive to anthropogenic disturbance in the Brazilian Amazon, changing their assemblages’ richness, abundance and composition. Also, several species were considered indicators of pristine and or disturbed habitats. Thus, we conclude that ants might be useful for assisting in decision-making regarding environmental conservation and habitats restoration in this region. Furthermore, as ants are intimately related to almost all other taxa and secondary forests were not able are to substitute PFs diversity, significant patches of pristine vegetation should be left in landscape to ensure biodiversity conservation (Kalamandeen et al. 2018). Interestingly, cocoa plantations’ ant assemblages high diversity, suggests as a potential rescue habitat for ants. Future studies should focus on farm characteristics, such as plantation size and age, to verify how these productive areas contribute to the maintenance of biodiversity in this region. We would like to emphasize the need to conduct more research with ants in other areas of the Amazon, including large scale and long-term continuous sampling of data and environmental variables, logistically unfeasible in our study. Also, it will be fundamental to achieve more works in secondary forests, within diverse stages of recovery, to confirm (or not) the results obtained by our humble study (Cajaiba et al. 2019).
References
Achury R, Holway DA, Suarez AV (2021) Pervasive and persistent effects of ant invasion and fragmentation on native ant assemblages. Ecology 102:e03257. https://doi.org/10.1002/ecy.3257
Ahuatzin D, Corro E, Jaimes A, González J, Feitosa R, Ribeiro M, Acosta J, Coates R, Dáttilo W (2019) Forest cover drives leaf litter ant diversity in primary rainforest remnants within human-modified tropical landscapes. Biodivers Conserv 28:1091–1107. https://doi.org/10.1007/s10531-019-01712-z
Andersen A, Hoffmann B, Müller W, Griffiths A (2002) Using ants as bioindicators in land management: simplifying assessment of ant community responses. J Appl Ecol 39:8–17. https://doi.org/10.1046/j.1365-2664.2002.00704.x
Andersson K, Bergman K-O, Andersson F, Hedenström E, Jansson N, Burman J, Winde I, Larsson MC, Milberg P (2014) High-accuracy sampling of saproxylic diversity indicators at regional scales with pheromones: the case of Elater ferrugineus (Coleoptera, Elateridae). Biol Conserv 171:156–166. https://doi.org/10.1016/j.biocon.2014.01.007
Arnan X, Rodrigo A, Retana J (2007) Uncoupling the effects of shade and food resources of vegetation on Mediterranean ants: an experimental approach at the community level. Ecography 30:161–172. https://doi.org/10.1111/j.2006.0906-7590.04796.x
Baccaro FB, Souza JLP, Franklin E, Landeiro VL, Magnusson WE (2012) Limited effects of dominant ants on assemblage species richness in three Amazon forests. Ecol Entomol 37:1–12. https://doi.org/10.1111/j.1365-2311.2011.01326.x
Baccaro FB, Rocha IF, Aguila BEG, Schietti J, Emilio T, Pinto JLPV, Lima AP, Magnusson WE (2013) Changes in ground-dwelling ant functional diversity are correlated with water-table level in an amazonian terra firme forest. Biotropica 45:755–763. https://doi.org/10.1111/btp.12055
Baccaro FB, Feitosa RM, Fernández F, Fernandes IO, Izzo TJ, Souza JLP, Solar R (2015) Guia Para os Gêneros de Formigas do Brasil. Editora INPA, Manaus, p 388
Baselga A (2010) Partitioning the turnover and nestedness components of beta diversity. Global Ecol Biogeogr 19:134–143. https://doi.org/10.1111/j.1466-8238.2009.00490.x
Baselga A, Orme D, Villeger S, De Bortoli J, Leprieur F (2017) Package ‘betapart’: Partitioning Beta Diversity into Turnover and Nestedness Components. R package version 1.4–1. https://CRAN.R-project.org/package=betapart. Accessed 01 March 2021
Belém SO, Guia BP, Campbell AJ, Maués MM, Viana JH (2020) Effects of ants (Hymenoptera: Formicidae) on flying insect visitor behaviour and fruit production in açaí palm (Euterpe oleracea Martius). Austral Entomol 59:612–618. https://doi.org/10.1111/aen.12458
Bharti H, Bharti M, Pfeiffer M (2016) Ants as bioindicators of ecosystem health in Shivalik Mountains of Himalayas: assessment of species diversity and invasive species. Asian Myrmecol 8:1–15. https://doi.org/10.20362/am.008023
Bolton B (1994) Identification guide to the ant genera of the world. Harvard University Press, London, p 222
Bruna EM, Izzo TJ, Inouye BD, Uriarte M, Vasconcelos HL (2011) Asymmetric dispersal and colonization success of amazonian plant-ants Queens. PLoS ONE 6:e22937. https://doi.org/10.1371/journal.pone.0022937
Buchori D, Rizali A, Rahayu GA, Mansur I (2018) Insect diversity in post-mining areas: Investigating their potential role as bioindicator of reclamation success. Biodiversitas 19:1696–1702. https://doi.org/10.13057/biodiv/d190515
Cajaiba RL, Périco E, Caron E, Dalzochio MS, Da Silva WB, Santos M (2017a) Are disturbance gradients in neotropical ecosystems detected using rove beetles? A case study in the Brazilian Amazon. For Ecol Manag 405:319–327. https://doi.org/10.1016/j.foreco.2017.09.058
Cajaiba RL, Perico E, Dalzochio MS, Silva WB, Bastos R, Cabral JA, Santos MG (2017b) Does the composition of Scarabaeidae (Coleoptera) communities reflect the extent of land use changes in the Brazilian Amazon? Ecol Indic 74:285–294. https://doi.org/10.1016/j.ecolind.2016.11.018
Cajaiba RL, Périco E, Da Silva WB, Leote P, Santos M (2018a) Are small dung beetles (Aphodiinae) useful for monitoring neotropical forests’ ecological status? Lessons from a preliminary case study in the Brazilian Amazon. For Ecol Manag 429:115–123. https://doi.org/10.1016/j.foreco.2018.07.005
Cajaiba RL, Périco E, Da Silva WB, Vieira TB, Dalzochio MS, Bastos R, Cabral JA, Santos M (2018b) How informative is the response of ground beetles’ (Coleoptera: Carabidae) assemblages to anthropogenic land use changes? Insights for ecological status assessments from a case study in the Neotropics. Sci Total Environ 636:1219–1227. https://doi.org/10.1016/j.scitotenv.2018.04.392
Cajaiba RL, Périco E, Da Silva WB, Caron E, Buss BC, Dalzochio M, Santos M (2019) Are primary forests irreplaceable for sustaining Neotropical landscapes’ biodiversity and functioning? Contributions for restoration using ecological indicators. Land Degrad Dev 31(508–517):2019. https://doi.org/10.1002/ldr.3467
Campbell AJ, Carvalheiro LG, Maués MM, Jaffé R, Giannini TC, Freitas MAB, Coelho BWT, Menezes C (2018) Anthropogenic disturbance of tropical forests threatens pollination services to açaí palm in the Amazon river delta. J Appl Ecol 55:1–12. https://doi.org/10.1111/1365-2664.13086
Carvalho R, Andersen A, Anjos D, Pacheco R, Chagas L, Vasconcelos H (2020) Understanding what bioindicators are actually indicating: linking disturbance responses to ecological traits of dung beetles and ants. Ecol Indic 108:105764. https://doi.org/10.1016/j.ecolind.2019.105764
Chen Y, Li O, Chen Y, Lu Z, Zhou X (2011) Ant diversity and bio-indicators in land management of lac insect agroecosystem in Southwestern China. Biodivers Conserv 20:3017–3038. https://doi.org/10.1007/s10531-011-0097-x
Clarke KR, Warwick RM (2001) Change in marine communities: an approach to statistical analysis and interpretation, 2nd edn. PRIMER-E Ltd, Plymouth Marine Laboratory, Plymouth
Colwell RK, Mao CX, Chang J (2004) Interpolating, extrapolating, and comparing incidence-based species accumulation curves. Ecology 85:2717–2727. https://doi.org/10.1890/03-0557
Colwell RK (2013) EstimateS: statistical estimation of species richness and shared species from samples. Version 9. User’s Guide. http://purl.oclc.org/estimates. Accessed 05 February 2021
Crawley MJ (2010) The R Book. Wiley, Chichester
Da Silva WB, Périco E, Dalzochio MS, Santos M, Cajaiba RL (2018) Are litterfall and litter decomposition processes indicators of forest regeneration in the neotropics? Insights from a case study in the Brazilian Amazon. For Ecol Manag 429:189–197. https://doi.org/10.1016/j.foreco.2018.07.020
Dale VH, Beyeler SC (2001) Challenges in the development and use of ecological indicators. Ecol Indic 1:3–10. https://doi.org/10.1016/S1470-160X(01)00003-6
De Cáceres M, Jansen F (2015) Package ‘indicspecies’. http://CRAN.R-project.org/package=indicspecies. Accessed 01 March 2021
Delabie JHC, Jahyny B, Nascimento IC, Mariano CSF, Lacau S, Campiolo S, Philpott SM, Leponce M (2007) Contribution of cocoa plantations to the conservation of native ants (Insecta: hymenoptera: Formicidae) with a special emphasis on the Atlantic Forest fauna of southern Bahia, Brazil. Biodivers Conserv 16:2359–2384. https://doi.org/10.1007/s10531-007-9190-6
Delabie JHC, Céréghino R, Groc S, Dejean A, Gibernau M, Corbara B, Dejean A (2009) Ants as biological indicators of Wayana Amerindian land use in French Guiana. C R Biol 332:673–684. https://doi.org/10.1016/j.crvi.2009.01.006
Derraik J, Closs G, Dickinson K, Sirvid P, Barratt B, Patrick B (2002) Arthropod morphospecies versus taxonomic species: a case study with Araneae, Coleoptera, and Lepidoptera. Conserv Biol 16:1015–1023. https://doi.org/10.1046/j.1523-1739.2002.00358.x
Diame L, Blatrix R, Grechi I, Rey J-Y, Sane CAB, Vayssieres J-F, Bom H, Diarra K (2015) Relations between the design and management of Senegalese orchards and ant diversity and community composition. Agric Ecosyst Environ 212:94–105. https://doi.org/10.1016/j.agee.2015.07.004
Dohm C, Leal IR, Tabarelli M, Meyer ST, Wirth R (2011) Leaf-cutting ants proliferate in the Amazon: an expected response to forest edge? J Tropic Ecol 27:645–649. https://doi.org/10.1017/S0266467411000447
Dou Y, Silva RFB, Yang H, Liu J (2018) Spillover effect offsets the conservation effort in the Amazon. J Geogr Sci 28:1716–1732. https://doi.org/10.1007/s11442-018-1539-0
Dufrêne M, Legendre P (1997) Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecol Monogr 67:345–366. https://doi.org/10.1890/0012-9615(1997)067[0345:SAAIST]2.0.CO;2~
Dunn RR (2004) Recovery of faunal communities during tropical forest regeneration. Conserv Biol 18:302–309. https://doi.org/10.1111/j.1523-1739.2004.00151.x
Ekroos J, Heliola J, Kuussaari M (2010) Homogenization of lepidopteran communities in intensively cultivated agricultural landscapes. J Appl Ecol 47:459–467. https://doi.org/10.1111/j.1365-2664.2009.01767.x
Frizzo TL, Vasconcelos HL (2013) The potential role of scattered trees for ant conservation in an agriculturally dominated neotropical landscape. Biotropica 45:644–651. https://doi.org/10.1111/btp.12045
Garrett RD, Gardner TA, Fonseca T, Marchand S, Barlow J, Blas DE, Ferreira J, Lees AC, Parry L (2017) Explaining the persistence of low income and environmentally degrading land uses in the Brazilian Amazon. Ecol Soc 22:e27. https://doi.org/10.5751/ES-09364-220327
Gerlach J, Samways M, Pryke J (2013) Terrestrial invertebrates as bioindicators: an overview of available taxonomic groups. J Insect Conserv 17:831–850. https://doi.org/10.1007/s10841-013-9565-9
Gibbs HK, Munger J, Roe JL, Barreto P, Pereira R, Christie M, Amaral T, Walker NF (2016) Did ranchers and slaughterhouses respond to zero-deforestation agreements in the Brazilian Amazon? Conserv Lett 9:32–42. https://doi.org/10.1111/conl.12175
Graham MH (2003) Confronting multicollinearity in ecological multiple regression. Ecol Lett 84:2809–2815. https://doi.org/10.1890/02-3114
Groc S, Delabie J, Longino J, Orivel J, Majer J, Vasconcelos H, Dejean A (2010) A new method based on taxonomic sufficiency to simplify studies on Neotropical ant assemblages. Biol Conserv 143:2832–2839. https://doi.org/10.1016/j.biocon.2010.07.034
Groc S, Delabie J, Fernandez F, Petitclerc F, Corbara B, Leponce M, Céréghino R, Dejean A (2017) Litter-dwelling ants as bioindicators to gauge the sustainability of small arboreal monocultures embedded in the Amazonian rainforest. Ecol Indic 82:43–49. https://doi.org/10.1016/j.ecolind.2017.06.026
Hackman KO, Gong P, Venevsky S (2017) A rapid assessment of landscape biodiversity using diversity profiles of arthropod morphospecies. Lands Ecol 32:209–223. https://doi.org/10.1007/s10980-016-0440-4
Hoff R, Rajão R, Leroy P (2018) Clashing interpretations of REDD+ “results” in the Amazon Fund. Clim Change 150:433–444. https://doi.org/10.1007/s10584-018-2288-x
Ivanov K, Keiper J (2009) Effectiveness and biases of winkler litter extraction and pitfall trapping for collecting ground-dwelling ants in northern temperate forests. Environ Entomol 38:1724–1736. https://doi.org/10.1603/022.038.0626
Kalamandeen M, Gloor E, Mitchard E et al (2018) Pervasive rise of small-scale deforestation in Amazonia. Sci Rep 8:1–10. https://doi.org/10.1038/s41598-018-19358-2
Kemerich PDC, Ritter LG, Borba WF (2014) Indicadores de sustentabilidade ambiental: métodos e aplicações. Rev Monog Ambient 13:3723–3736. https://doi.org/10.5902/2236130814411
Klimes P, Idigel C, Rimandai M, Fayle TM, Janda M, Weiblen GD, Novotny V (2012) Why are there more arboreal ant species in primary than in secondary tropical forests? J Anim Ecol 81:1103–1112. https://doi.org/10.1111/j.1365-2656.2012.02002.x
Lacasella F, Gratton C, Felici S, Isaia M, Zapparoli M, Marta S, Sbordoni V (2015) Asymmetrical responses of forest and ‘“beyond edge”’ arthropod communities across a forest–grassland ecotone. Biodivers Conserv 24:447–465. https://doi.org/10.1007/s10531-014-0825-0
Lassau SA, Hochuli DF (2004) Effects of habitat complexity on ant assemblage. Ecography 27:157–164. https://doi.org/10.1111/j.0906-7590.2004.03675.x
Laste K, Durigan G, Andersen A (2019) Biodiversity responses to land-use and restoration in a global biodiversity hotspot: ant communities in Brazilian Cerrado. Austral Ecol 44:313–326. https://doi.org/10.1111/aec.12676
Laurance WF, Sayer J, Cassman KG (2014) Agricultural expansion and its impacts on tropical nature. Trends Ecol Evol 29:107–116. https://doi.org/10.1016/j.tree.2013.12.001
Lawton JH, Gaston KJ (2001) Indicator species. Encycl Biodiv 3:437–450. https://doi.org/10.1016/B0-12-226865-2/00156-5
Lima PAF, Bachêco BS, Sousa SR, Gatto A, Aquino FG, Albuquerque LB (2015) Indicadores ecológicos: ferramentas para monitoramento do processo de restauração ecológica. Embrapa cerrado, Planaltina, p 44
Lyra-Jorge MC, Ribeiro MC, Ciocheti G, Tambosi LR, Pivello VR (2010) Influence of multi-scale landscape structure on the occurrence of carnivorous mammals in a human-modified savanna. Brazil Eur J Wildl Res 56:359–368. https://doi.org/10.1007/s10344-009-0324-x
Maveety SA, Browne RA, Erwin TL (2011) Carabidae diversity along an altitudinal gradient in a Peruvian cloud forest (Coleoptera). Zookeys 147:651–666. https://doi.org/10.3897/zookeys.147.2047
Nakamura A, Catterall CP, House AP, Kitching R, Burwell C (2007) The use of ants and other soil and litter arthropods as bio-indicators of the impacts of rainforest clearing and subsequent land use. Journal Insect Conserv 11:177–186. https://doi.org/10.1007/s10841-006-9034-9
Noss RF (1990) Indicators for monitoring biodiversity: a hierarchical approach. Conserv Biol 4:355–364. https://doi.org/10.1111/j.1523-1739.1990.tb00309.x
Oksanen J, Kindt R, Legendre P, O’Hara B, Stevens MHH, Oksanen MJ, Suggests MAS (2007) The Vegan Package. Community Ecology Package. http://cran.rproject.org, http://vegan.r-forge. Accessed 25 January 2021
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara RB, Simpson GL, Solymos R (2013) Vegan: Community Ecology Package. R package. Available at http://cran.r-project.org/web/packages/vegan/index.html. Accessed 15 March 2021
Ometto JP, Aguiar APD, Martinelli LA (2011) Amazon deforestation in Brazil: effects, drivers and challenges. Carbon Management 2:575–585. https://doi.org/10.4155/CMT.11.48
Ottonetti L, Tucci L, Santini G (2006) Recolonization patterns of ants in a rehabilitated lignite mine in central Italy: potential for the use of Mediterranean ants as indicators of restoration processes. Restor Ecol 14:60–66. https://doi.org/10.1111/j.1526-100X.2006.00105.x
Pacheco P, Vasconcelos HL, Groc S, Camacho GP, Frizzo TL (2013) The importance of remnants of natural vegetation for maintaining ant diversity in Brazilian agricultural landscapes. Biodivers Conserv 22:983–997. https://doi.org/10.1007/s10531-013-0463-y
Palácio EE, Fernández F (2003) Claves para las subfamilias y géneros. In: Fernández F (ed) Introducción a las hormigas de la región Neotropical. Instituto de Investigación de Recursos Biológicos Alexander Von Humboldt, Bogotá, pp 233–260
Pecl GT, Araujo MB, Bell J, Blanchard J, Bonebrake TC, Chen I, Clark TD, Colwell RK, Danielsen F, Evengard B, Robinson S (2017) Biodiversity redistribution under climate change: impacts on ecosystems and human well-being. Science 355:1–9. https://doi.org/10.1126/science.aai9214
Peel MC, Finlayson BL, McMahon TA (2007) Updated world map of the Koppen-Geiger climate classification. Hydrol Earth Syst Sc 11:1633–1644. https://doi.org/10.5194/hess-11-1633-2007
Pfeiffer M, Mezger D, Dyckmans J (2013) Trophic ecology of tropical leaf litter ants (Hymenoptera: Formicidae)—a stable isotope study in four types of Bornean rain forest. Myrmecol News 19:31–41
Philpott SM, Perfecto I, Armbrecht I, Parr C (2010) Ant diversity and function in disturbed and changing habitats. In: Lach L, Parr C, Abbott K (eds) Ant ecology. Oxford University Press, New York, pp 137–156
R Core Team (2016) R: A language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria. https://www.R-project.org/
Rabello A, Queiroz A, Lasmar C, Cuissi R, Canedo-Júnior E, Schmidt F, Ribas C (2015) When is the best period to sample ants in tropical areas impacted by mining and in rehabilitation process? Insect Soc 62:227–236. https://doi.org/10.1007/s00040-015-0398-2
Read J, Andersen A (2000) The value of ants as early warning bioindicators: responses to pulsed cattle grazing at an Australian arid zone locality. J Arid Environ 45:231–251. https://doi.org/10.1006/jare.2000.0634
Reynolds C, Fletcher RJ, Carneiro CM et al (2018) Inconsistent effects of landscape heterogeneity and land-use on animal diversity in an agricultural mosaic: a multi-scale and multi-taxon investigation. Lands Ecol 33:241–255. https://doi.org/10.1007/s10980-017-0595-7
Ribas C, Schmidt F, Solar R, Campos R, Valentim C, Schoereder J (2012a) Ants as indicators of the success of rehabilitation efforts in deposits of gold mining tailings. Restor Ecol 20:712–720. https://doi.org/10.1111/j.1526-100X.2011.00831.x
Ribas CR, Campos RBF, Schmidt FA, Solar RRC (2012b) Ants as indicators in Brazil: a review with suggestions to improve the use of ants in environmental monitoring programs. Psyche. https://doi.org/10.1155/2012/636749
Roitman I, Vieira LCG, Jacobson TKB, Bustamante MMC, Marcondes NJS, Cury KS, Delabie J, Fernandez F, Petitclerc F, Corbara B, Leponce M, Céréghino R, Dejean A (2017) Litter-dwelling ants as bioindicators to gauge the sustainability of small arboreal monocultures embedded in the Amazonian rainforest. Ecol Indic 82:43–49. https://doi.org/10.1016/j.ecolind.2017.06.026
Sales L, Ribeiro BR, Chapman CA, Loyola R (2020) Multiple dimensions of climate change on the distribution of Amazon primates. Perspect Ecol Conserv 18:83–90. https://doi.org/10.1016/j.pecon.2020.03.001
Santos JC, Anjos DV, Almeida WR, Aguiar JJ, Delabie J (2021) Ground-dwelling ant diversity in Amazonian secondary forests and neighboring pastures. Trop Ecol 62:279–287. https://doi.org/10.1007/s42965-021-00146-5
Schmidt F, Ribas C, Schoereder H (2013) How predictable is the response of ant assemblages to natural forest recovery? Implications for their use as bioindicators. Ecol Indic 24:158–166. https://doi.org/10.1016/j.ecolind.2012.05.031
Schnell MR, Pik AJ, Dangerfield JM (2003) Ant community succession within eucalypt plantations on used pasture and implications for taxonomic sufficiency in monitoring. Austral Ecol 28:553–555. https://doi.org/10.1046/j.1442-9993.2003.01312.x
Schroth G, Faria D, Araujo M, Bede L, Van Bael SA, Cassano CR, Oliveira LC, Delabie JHC (2011) Conservation in tropical landscape mosaics: the case of the cacao landscape of southern Bahia, Brazil. Biodiver Conserv 20:1635–1654. https://doi.org/10.1007/s10531-011-0052-x
Scott AG, Oxford GS, Selden PA (2006) Epigeic spiders as ecological indicators of conservation value for peat bogs. Biol Conserv 127:420–428. https://doi.org/10.1016/j.biocon.2005.09.001
Semprucci F, Losi V, Moreno M (2015) A review of Italian research on free-living marine nematodes and the future perspectives on their use as ecological indicators (EcoInds). Mediterr Mar Sci 16:352–365. https://doi.org/10.12681/mms.1072
Silva RR, Feitosa RM, Eberhardt F (2007) Reduced ant diversity along a habitat regeneration gradient in the southern Brazilian Atlantic forest. Forest Ecol Manag 240:61–69. https://doi.org/10.1016/j.foreco.2006.12.002
Solar RC, Barlow J, Andersen A, Schoereder JH, Berenguer E, Ferreira J, Gardner TA (2016) Biodiversity consequences of land-use change and forest disturbance in the Amazon: a multi-scale assessment using ant communities. Biol Conserv 197:98–107. https://doi.org/10.1016/j.biocon.2016.03.005
Souza JLP, Fernandes IO (2021) Do spatial and temporal scales affect the efficiency of surrogates in ant monitoring on the hydroelectric power-plant area in Brazilian Amazon? Ecol Indic 121:107158. https://doi.org/10.1016/j.ecolind.2020.107158
Spellmeier J, Périco E, Haetinger C, Freitas EM, Morás APB (2019) Effect of grazing on the plant community of a Southern Brazilian swamp. Floresta e Ambient 26:e20180339. https://doi.org/10.1590/2179-8087.033918
Spiller MS, Spiller C, Garlet J (2018) Arthropod bioindicators of environmental quality. Rev Agro@mbiente 12:41–57. https://doi.org/10.18227/1982-8470ragro.v12i1.4516
Staab M, Schuldt A, Assmann T, Bruelheide H, Klein A (2014) Ant community structure during forest succession in a subtropical forest in South-East China. Acta Oecol 61:32–40. https://doi.org/10.1016/j.actao.2014.10.003
Swart RC, Pryke JS, Roets F (2018) Arthropod assemblages deep in natural forests show different responses to surrounding land use. Biodivers Conserv 27:583–606. https://doi.org/10.1007/s10531-017-1451-4
Tews J, Brose U, Grimm V, Tielbörger K, Wichmann MC, Schwager M, Jeltsch F (2004) Animal species diversity driven by habitat heterogeneity/diversity: the importance of keystone structures. J Biogeogr 31:79–92. https://doi.org/10.1046/j.0305-0270.2003.00994.x
Wilson EO, Hölldobler B (2005) The rise of the ants: a phylogenetic and ecological explanation. PNAS 102:7411–7414. https://doi.org/10.1073/pnas.0502264102
Acknowledgements
This research was supported by European Investment Funds by FEDER/COMPETE/POCI—Operational Competitiveness and Internationalization Programme, under Project POCI-01-0145-FEDER-006958, National Funds by FCT—Portuguese Foundation for Science and Technology, under the project UIDB/04033/2020 and INTERACT, Integrative Research in Environment, Agro-Chains and Technology, Project Norte 2020 45/2015/02. WBS thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazil through a doctorate fellowship (Finance Code 001). EP thanks Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil for the research grant (Research Productivity no. 307303/2019-5).
Author information
Authors and Affiliations
Contributions
All the authors contributed to the conception and design of the study. The material was prepared and the data were collected by WBS and RLC. The data were analyzed and discussed by WBS, RLC, MS and EP. The first draft of the manuscript was written by WBS and RLC and all the authors commented on the previous versions of the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no confict of interest.
Additional information
Communicated by P. Ponel.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to the Topical Collection: Forest and plantation biodiversity.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
da Silva, W.B., Cajaiba, R.L., Santos, M. et al. Effectiveness of ant communities to detect anthropogenic disturbance in Neotropical forest landscapes: a case study in the Brazilian Amazon. Biodivers Conserv 31, 211–226 (2022). https://doi.org/10.1007/s10531-021-02329-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-021-02329-x