Abstract
Naturally fragmented landscapes are adequate systems for evaluating patterns and mechanisms that determine species distribution without confounding effects of anthropogenic fragmentation and habitat loss. We aimed to evaluate an ant metacommunity’s spatiotemporal patterns in montane forest islands amid a grassland-dominated matrix. We assessed these patterns by deconstructing the ant metacommunity into forest-dependent and habitat generalist species. We sampled twice a year (summer and winter) over 2 years (2014 and 2015), using soil and arboreal pitfall traps, in fourteen forest islands (varying in size, shape, and connectivity) in the Espinhaço Range Biosphere Reserve, Brazil. We evaluated the relationship between ant species richness, composition (β-diversity), and predictor variables of forest island structure (canopy cover and understory density) and landscape structure (forest amount, number of forest islands, and shape). We sampled 99 ant species, 66.7% of which were classified as forest-dependent and 33.3% as habitat generalist species. We found that ant β-diversity was higher in space than in time, and that species composition variation in time (temporal β-diversity) differed between ant species groups. Both ant groups responded differently to forest island and landscape structure characteristics. Landscape structure seems to act as a spatial filter and the forest islands’ local characteristics as an environmental filter, which jointly determine the local and regional diversity. We demonstrate the importance that forest archipelagos pose to ant metacommunity’s structure and dynamics in montane tropical regions. Mountaintop conservation and management strategies must consider the forest island archipelago to maintain the biodiversity and the functioning of these systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Unraveling the drivers of local species diversity is urgent and essential. Species detected on a regional scale might be absent in specific localities of such region for several reasons (Kraft et al. 2015). For instance, they could be unable to colonize a given locality due to dispersal limitations (Padial et al. 2014) or because of environmental filtering, when the abiotic conditions inhibit their persistence (Gibb et al. 2015). Even if they are able to colonize a locality, their persistence may be hindered by biotic filtering (Ellwood et al. 2016), when predators, lack of relevant interactions, and better competitors may exclude them. These factors’ interactions determine which of the regions’ species constitute local communities (Gray et al. 2018), with their importance varying in different spatial and temporal scales (Kaspari et al. 2003; da Silva and Hernández 2015). Thus, environmental, spatial, and temporal processes are simultaneously responsible for the local assembly mechanisms and community dynamics (Leibold et al. 2004; Mouquet et al. 2005), although they can have different roles in community assembly depending on the species groups and ecological systems (Kaspari et al. 2003; Graham and Fine 2008; da Silva and Hernández 2014). The effects of these processes across spatiotemporal scales have properly been underlined using the metacommunity approach (da Silva et al. 2021). A metacommunity is characterized by the connection of local communities via dispersal of multiple potentially interacting species (Leibold et al. 2004). Therefore, these multiple perspectives of processes on community patterns can shed light into the main mechanisms driving species distributions across an increasingly patchy and heterogeneous world (Mouquet et al. 2005; da Silva and Hernández 2015).
Naturally fragmented landscapes are adequate systems for evaluating species distribution patterns (Cuissi et al. 2015; Rossetti et al. 2017). However, the majority of these landscapes are oceanic archipelagos. Therefore, the theories describing island diversity were formulated with marine archipelagos as a model. A major example is the theory of island biogeography (TBI) that predicts a positive species-area relationship and a negative species-isolation relationship (MacArthur and Wilson 1967), which is now part of the theoretical framework of the metacommunity approach (Leibold et al. 2004). Since TBI’s elaboration, these predictions have also been applied to anthropogenically fragmented landscapes, where isolated patches are formed due to habitat loss (Leal et al. 2012; Rossetti et al. 2017). Various organisms in terrestrial archipelagos have shown similar patterns to those predicted by TBI (Adams et al. 2017; Palmeirim et al. 2018), but some do not follow these predictions (Haila 2002) due to the differences between the effects of habitat loss and fragmentation per se (Fahrig 2017). This leaves an unanswered and fundamental question of island biogeography: “How the patterns and processes that generate biodiversity behave when comparing marine and terrestrial archipelagos systems?” (Patiño et al. 2017). In order to fully answer this question, it is imperative to gather more information on biodiversity responses in naturally formed terrestrial archipelagos, where the landscape matrix may be permeable for only a few species, generating a metacommunity structure (Leibold et al. 2004). Besides, naturally fragmented landscapes are excellent systems for evaluating patterns and mechanisms that determine the distribution of species in environments without confounding effects of human-driven fragmentation and habitat loss (Fahrig 2017; Haddad et al. 2017).
Although crucial for the maintenance of species diversity, small and isolated patches are often neglected in conservations practices (Wintle et al. 2019). One example of those systems is the forest archipelagos immersed in grassland-dominated ecosystems on tropical mountaintops (Streher et al. 2017). In Brazil, it is common to find such systems across the mountaintops of the Espinhaço Range Biosphere Reserve (hereafter treated as forest islands) (Coelho et al. 2018b). The Espinhaço mountain range extends over 1,200 km from southeast to northeast Brazil and harbors high biodiversity and endemism (Silveira et al. 2016; Fernandes et al. 2020). The forest islands are surrounded by a campo rupestre matrix (montane grasslands), which creates a complex vegetational mosaic in the landscape, giving rise to a typical forest archipelago (Coelho et al. 2016; Itescu 2018). In this scenario, the composition and structure of the landscape (i.e., the habitat types and their configuration) can influence the colonization and establishment of species differently (i.e., a metacommunity dynamic) since different habitats provide distinct resources and conditions (Fahrig et al. 2011). Furthermore, temperature and rainfall vary throughout the year, with a marked influence on forest conditions. There is an average difference of almost 5°C and 140 mm/month between the summer and winter seasons in this forest archipelago (da Silva et al. 2019). Despite its relevance to ecology and conservation, the mountaintop forest islands have increasingly suffered from anthropogenic disturbances, such as illegal fires, grazing, and selective logging (Coelho et al. 2018b). The distribution of these forest islands is also expected to be affected by ongoing climate change, which is predicted to be more intense in mountainous areas (Mayor et al. 2017; Rumpf et al. 2018). Therefore, there is an urgency in defining clear strategies to conserve these mountaintops ecosystems (Noroozi et al. 2018) by establishing priorities and guidelines that can support informed decision-making policy towards sustainable use of the campo rupestre and its forest islands (Coelho et al. 2018b; Fernandes et al. 2020; Neves et al. 2021a).
In this tropical forest archipelago system, vagile organisms like wasps, bees, dung beetles, and fruit-feeding butterflies can benefit from a heterogeneous landscape by seeking different resources and conditions in the various landscape habitats. For instance, wasps and bees use the floral resources of the matrix while nesting in the forest islands (Perillo et al. 2020). Dung beetles and fruit-feeding butterflies also seem to use the favorable conditions of the forest islands during winter, taking shelter from the less favorable conditions of the matrix (Pereira et al. 2017; da Silva et al. 2019). On the other hand, for less vagile organisms, such as ants, the habitat diversity and the landscape configuration influence the sources of colonizing propagules. Once present in a locality, ants have a greater dependence on local resources and conditions, with little or no use of the other landscape features (Spiesman and Cumming 2008; Andersen 2019). Therefore, one might expect different responses between vagile and sessile organisms inhabiting this forest archipelago (Neves et al. 2021a).
Due to their dependence on microclimate and local resources (Andersen et al. 2002) and considering their extreme diversity and distribution (Levy 1995; Dunn et al. 2007; Parr et al. 2017), ants have been used as good model organisms for understanding the effects of habitat loss and fragmentation (Vasconcelos et al. 2006; Cuissi et al. 2015; Ahuatzin et al. 2019). Moreover, ants are sensitive to environmental changes, especially changes in vegetation structure (Andersen 2019; Corro et al. 2019) and forest cover (Ahuatzin et al. 2019). Therefore, forest islands should harbor ant species that exhibit narrow environmental tolerances, constraining them to a forested environment (forest-dependent species) and species with broader environmental tolerances, which thrive in a higher diversity of habitats (habitat generalist species). In order to find the most important processes for both species’ groups (forest-dependent and habitat generalists), we can deconstruct the metacommunity according to these tolerances (Pandit et al. 2009). Doing so, we avoid the interference of one group’s ecological responses on another’s. For instance, the habitat generalist species mask the ecological response of the forest-dependent dung beetle species to both patch and landscape features (da Silva et al. 2019). Nevertheless, we still do not know if this is the case for sessile organisms in the forest archipelago.
Here, we aimed to evaluate the spatial and temporal patterns of ant metacommunity’s structure and dynamics according to their habitat tolerance (forest-dependent and habitat generalist species) over the local (forest island) and landscape (forest archipelago) features of a montane forest archipelago. In order to do so, we have tested the following predictions: (i) the variation in species composition (β-diversity) is higher spatially than temporally due to the low mobility and longevity of ants for both groups (forest-dependent and habitat generalist species); (ii) the forest islands’ vegetation structure and landscape structure determine the ant richness and temporal species composition variation (temporal β-diversity), since larger, denser, and less isolated forest islands are richer and more temporally stable; and (iii) the effect of forest island and landscape structure differs for each species group, since forest-dependent species are more sensitive to variations in local vegetation structure (such as canopy cover and understory density) and landscape structure (such as habitat amount and isolation) than habitat generalist species.
Material and methods
Study area
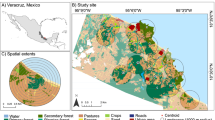
This study was carried out in the forest islands from a naturally formed montane forest archipelago at the Serra do Cipó (see Coelho et al. 2018b) located in the southern portion of the Espinhaço Range Biosphere Reserve, Brazil (19°14′19″S, 43°31′35″W; Fig. 1). The Espinhaço is an important mountain range in terms of biodiversity and water resources in Brazil (Fernandes 2016). It extends over 1200 km from southern Minas Gerais to the center of Bahia States, and it is amidst three great Brazilian biomes, the Caatinga (semi-arid) to its north, the Cerrado (Brazilian savanna) to its west, and the Atlantic Forest to its east; these last two are considered hotspots of biodiversity conservation (Myers et al. 2000).
Associated with these mountaintops occurs the campo rupestre, an ancient, climatically buffered, and diverse ecosystem that is considered the hottest Brazilian biodiversity hotspot and hosts various endangered and endemic species (Fernandes et al. 2020; Hopper et al. 2021). This ecosystem has a high edaphic and vegetational heterogeneity (Le Stradic et al. 2015; Ferrari et al. 2016) and enables the occurrence of relictual forest islands associated with specific edaphic-climatic characteristics, such as erosion valleys that lack boulders (Coelho et al. 2016). In addition, the occurrence of forest islands in the Espinhaço mountain range starts at 1200 m a.s.l. and is also frequently associated with headwaters (Coelho et al. 2018b). These forest islands’ floristic resembles semi-deciduous Atlantic Forests (Coelho et al. 2018a), and the most common plant families are Myrtaceae, Lauraceae, Melastomataceae, and Fabaceae.
The regional climate is Cwb (dry-winter subtropical highland climate) according to Köppen’s classification (Alvares et al. 2013). In the summer, the temperature was 19.22°C and humidity 88.62% (mean values of December, January, and February 2014 and 2015), while in the winter, they were 14.68°C and 92.58%, respectively (June, July, and August 2014 and 2015 mean values). In the Espinhaço range’s high elevations, the relative humidity is always high due to the nebular condensation of humid air (Coelho et al. 2016), but the temperature varies seasonally, with a mean difference of 4.54°C between seasons (data from a weather station installed at 1400 m a.s.l., near our study site: Onset HOBO® U30 data logger; Long-Term Ecological Research Program Project – PELD Campos Rupestres da Serra do Cipó; see Silveira et al. (2019)).
Sampling design
We selected 14 forest islands, varying in dimension, shape, and distances from each other and from a continuous forest (Table S1) to access ant diversity. All of them occur in the same elevation belt (ca. 1300 m a.s.l.). In the nuclear region of each forest island, we installed a permanent plot of 1000 m2 (20 × 50 m). In each plot, we installed five soil pitfall traps (9 cm deep × 15 cm diameter) (Bestelmeyer et al. 2000) and five identical arboreal pitfall traps (Ribas et al. 2003), which were left in the field for 48 h per sampling campaign. The soil traps were installed at the vertices and at the center of each plot (20 × 50 m), while arboreal traps were installed 1.5 m above the ground in the nearest tree from each soil trap. Pitfall trapping is the best method to capture ants given our aim and the conditions of our study area (Montgomery et al. 2021). We sampled these plots four times: two times in the summer season (February) and two times in the winter season (August) in 2014 and 2015.
The sampled material was taken to the Laboratório de Ecologia de Insetos (Insect Ecology Lab) at the Universidade Federal de Minas Gerais (LEI-UFMG) for processing and identification. We followed Baccaro et al. (2015) for genera identification and used LEI-UFMG Ant Collection to confirm species identification. The LEI-UFMG is an important collection of campo rupestre’s ant species that has been continuously reviewed by various ant specialists. We used a qualitative approach to separate the species into groups according to their habitat occurrence. Based on published information (Costa et al. 2015; Castro et al. 2020; Nunes et al. 2020; Neves et al. 2021a), our own field experience, and by comparing the ant species sampled in forest islands with the pre-existing ant species collection from the LEI-UFMG, we classified each species as “forest-dependent species” (species only found in forest islands) and “habitat generalist species” (species found in both forest islands and campo rupestre in local studies). A similar approach was used to classify ant species of Cerrado (Vasconcelos et al. 2018).
Forest island and forest archipelago structure
We measured forest island size and distances (from each other and from the continuous forest) using the latest satellite images of Google Earth Pro v.7.3.2 software. Each forest island was manually delimited, and its area was measured in km2. The distances from each forest island to its neighbors and from the continuous forest were measured in a straight line in km. To calculate the canopy cover and understory structure, we took four canopy hemispheric photos and 16 understory photos within each forest island in each sampling event. The canopy photos were taken from 1.5 m above ground using a fish eye’s lens attached to a Canon T5i camera (Nassar et al. 2008). These images were processed using the Gap Light Analyzer software (Frazer et al. 1999), which calculates the number of white pixels (i.e., open spaces) in each image. In order to determine the understory density, we used a method recommended by Nobis and Hunziker (2005), which consists of analyzing digital images of the local shrub and herbaceous vegetation. Understory images were taken using a 1 m2 white sheet as background, positioning the camera 3 m far from it and 1 m above the ground. Therefore, we measured the following variables as local predictive variables: (i) forest island size, (ii) distance to the continuous forest, (iii) distance to the nearest forest island, (iv) canopy cover, and (v) understory density (see also Pereira et al. 2017; Perillo et al. 2020).
To classify the landscape structure of the forest archipelago, we used images from a high-resolution multispectral satellite (RapidEye ~5m resolution) and classified these images using the R package randomForest v4.6-12. Then, we selected 10 landscape metrics from each one of the 14 forest islands, using data from buffers with a radius of 250 m considering the sampled plot center as the centroid areas (Table S2) (see also da Silva et al. 2019; Perillo et al. 2020).
Statistical analysis
We performed all the analyses using data of forest-dependent and habitat generalist species separately in the R software (R Core Team 2020). We calculated the sampling coverage as a measure of sample completeness for each forest island using the R package iNEXT (Chao and Jost 2012; Hsieh et al. 2016) using two times the reference sample size for the extrapolation curves. We calculated the accumulated richness of all sampling seasons for each forest island (n = 14) and the richness of each sampling (four events in each forest island; n = 56). Furthermore, we calculated the relative frequency of occurrence for each species as a specific indicator of the spatiotemporal distribution using the percentage of sampling unities where they are present.
To determine the variation of the ant species composition between forest islands (spatially) and between seasons (temporally), we calculated the β-diversity for forest-dependent and habitat generalist species based on the Sorensen dissimilarity coefficient using the R package betapart (Baselga and Orme 2012). We chose the Sorensen dissimilarity coefficient because it gives more importance to shared species in a sample (Baselga 2012) and indicates the proportion of shared species between sites (Anderson et al. 2011). In our case, as the study area does not occupy a vast region (less than 25 km2), the occurrence of spatiotemporally shared species is expected. The spatial β-diversity represents the composition dissimilarity among the 14 forest islands, while the temporal β-diversity represents the dissimilarity in each forest island through time (the four sampling periods). We decomposed the total β-diversity (Sorensen dissimilarity) into species replacement (or turnover) and nestedness-resultant components for forest-dependent and habitat generalist species to verify which is the main process driving spatiotemporal patterns (Baselga 2010; Baselga 2012). Both components were calculated accounting for multiple sites (spatial) or sampling periods (temporal) and accounting for pairwise dissimilarities, using the function beta.multi of the R package betapart (Baselga and Orme 2012). This function calculates the overall spatial and temporal β-diversity and its turnover and nestedness-resultant components.
Finally, to verify the effects of the vegetation structure and landscape structure on species richness (pooled values for all samplings) and temporal variation of species composition (temporal β-diversity) of forest island ants, we first tested the correlation between the forest island structure variables (local scale) and between forest archipelago structure variables (landscape scale). Those variables with ≥ 50% correlation (or p-value < 0.05) were removed from the analyses (see correlated variables in Figure S1). After that, we used the remaining six non-correlated metrics (local variables: (i) canopy cover, (ii) distance to a continuous forest; landscape variables: (iii) percentage of the forest island in the landscape, (iv) number of forest islands in the landscape, (v) mean distance to forest island neighbors, (vi) forest island’s total edge) as explanatory variables in generalized linear models (GLMs). We used species richness and temporal β-diversity of forest-dependent and habitat generalist species as response variables. We also simplified them by removing the non-significant variables one by one considering the p-value. Hence, we reached the most adequate model, in which we performed a residual analysis to verify its error distribution (Crawley 2013). The most adequate distributions were Poisson for richness models and Binomial for β-diversity models, adjusting both for overdispersion (with their quasi families).
Results
We sampled 99 ant species distributed in 40 genera and nine subfamilies across all forest islands (Table S3). Among the sampled species, 18 species were singletons (18%), 15 doubletons (15%), and seven tripletons (7%), totaling 40 rare species (40%). Only eight species (8.1%) occurred in all forest islands. From the total number of species, 65.7% were classified as forest-dependent (N = 65) and 34.3% as habitat generalist species (N = 34). The most frequent species were Pachycondyla striata Smith (100% frequency in the forest islands), followed by Gnamptogenys striatula Mayr (89%) and Pheidole jelskii Mayr (82%), all classified as habitat generalists. The most frequent forest-dependent species were Camponotus lespesii Forel (76%) and two morphospecies of Pheidole Westwood: Pheidole sp.13 (57%) and Pheidole sp.2 (55%) (Table S3). We obtained between 78.3 and 94.6% of sample completeness (average ± standard deviation = 88.8 ± 5.1%; Table S3).
Variation of the ant species composition (β-diversity)
We found that the spatial variation (spatial β-diversity) in ant species composition was greater than the temporal variation (temporal β-diversity) regardless of the species group evaluated (1.8 and 2.1 times higher for forest-dependent and habitat generalist species, respectively) (Fig. 2). Species replacement contributed more to spatial β-diversity than nestedness for both forest-dependent and habitat generalist species (95.0% and 92.3%, respectively). For temporal β-diversity, we found an increase of the nestedness-resultant component for both forest-dependent and habitat generalist species (41.6% and 50.5%, respectively). In all cases, the spatiotemporal β-diversity of habitat generalist species was lower when compared to β-diversity of forest-dependent species (Fig. 2).
Partitioning of β-diversity (Sorensen dissimilarity) of forest-dependent (FD) and habitat generalist (HG) ant’s species into its components of replacement (βrep; darker bars) and nestedness (βnes; lighter bars) spatially (among forest islands) and temporally (among seasons per forest island) in forest islands of Serra do Cipó, southern portion of the Espinhaço Range Biosphere Reserve, Minas Gerais, Brazil
Effect of environmental variables
We found that forest-dependent species richness was not affected by any of the local or landscape variables, while canopy cover and average distance to neighbor forest islands influenced habitat generalist species richness (Table 1, Fig. 3). Surprisingly, we found more habitat generalist species in forest islands with denser canopies and also in forest islands that are closer to their neighbors (Fig. 3a, b). In addition, we found that an increase in the amount of forest or in the number of forest islands in the landscape negatively affected the temporal composition variation of forest-dependent species (Table 1, Fig. 3c, d). Moreover, the number of islands in the landscape affected in the same way the habitat generalist species (Fig. 3d), thus determining an increase of temporal stability for both groups.
Relationship between species richness (upper panels) and temporal β-diversity (Sorensen dissimilarity; lower panels) of forest-dependent and habitat generalist ant species with canopy cover (local variable), mean distance to neighbor forest islands at 250 m buffer, habitat amount represented by forest island at 250 m buffer, and the number of forest islands at 250 m buffer (landscape variables)
Discussion
We evaluated the effects of local and landscape attributes on spatial and temporal patterns of an ant metacommunity inhabiting the montane, naturally formed forest archipelago. According to our predictions, we highlight as main findings that (i) the species composition variation across forest islands (spatial β-diversity) is higher than the species composition variation across sampling periods (temporal β-diversity); (ii) the forest island vegetation structure and the landscape structure influence the ant richness of habitat generalist species only; (iii) only the landscape structure determines the temporal β-diversity for either group; and (iv) both forest island and landscape structure metrics affected ant species’ groups differently.
Most of the β-diversity of the ant metacommunity, both spatial and temporal, is explained by species replacement. Species replacement has been described as the primary process driving spatial differences in species compositions for most organisms across many spatial scales and ecosystems (Soininen et al. 2018). The mechanisms behind this phenomenon may be related to the low dispersal capacity and the high dependence on forest habitats of the ants sampled (65.7% of forest-dependent species), which would be directly related to landscape structure, especially distance to neighbor forest islands, determining their dispersal dynamics (Soininen et al. 2007). The contribution of the nestedness-resultant component was a little higher than the replacement component only for temporal β-diversity of habitat generalist species. This implies more losses than replacement of species in the ant assemblages found when the environmental conditions are harsher and the resources scarcer (winter season). This result contrasts with those found by Nunes et al. (2020) and Neves et al. (2021b), who have reported a high temporal replacement of ant species in the campo rupestre and in a tropical dry forest, respectively. Both systems are seasonal, so there is a high temporal variation in resource availability, which implies more replacement than loss (or nested loss) of ant species over time. Habitat generalist species are also those that contribute more to nestedness when compared with habitat specialists in the Espinhaço Range, showing a spatial dynamic related to the mass effects’ metacommunity process (Neves et al. 2021a). Ants are sessile organisms, so we are aware that either a variation in species activity or a sampling artifact can contribute to the values of species replacement found. Accumulating spatiotemporal replicated data via long-term studies will help unravel these issues, in addition to contributing to the advancement of metacommunity research (Record et al. 2021).
Although the species-area relationship is well documented for ants, especially in areas with anthropogenic fragmentation (Schoereder et al. 2004; Vasconcelos and Bruna 2012; Ohyama et al. 2021), species richness of some ants’ groups may not be associated with or dependent on forest fragment area (Lozano-Zambrano et al. 2009), as we also found here. For instance, Lozano-Zambrano et al. (2009) found distinct relationships between the richness of some ant species’ groups related to habitat requirements and fragment area of a tropical dry forest in Colombia. Therefore, the ant metacommunity inhabiting the forest archipelago studied, which is formed by a high diversity of species sampled by both soil and arboreal pitfall traps, may harbor species with different niche requirements that are not affected by forest island area per se in terms of species richness. Similarly, no relationship between species richness of bees and wasps (Perillo et al. 2020), fruit-feeding butterflies (Pereira et al. 2017), dung beetles (da Silva et al. 2019), and forest island area in the same montane forest archipelago has been found. This implies that for ants, the deconstruction of the metacommunity into intra-groups within the forest-dependent species group according to habitat requirements can be an appropriate approach to investigate such relationships. In addition, habitat quality can be more important than habitat amount to determine the species habitat use in naturally fragmented landscapes (Regolin et al. 2021).
As we have shown, forest island features and landscape structure metrics affected ant species’ groups differently. Surprisingly, the species richness of forest-dependent species was not affected by any forest island and landscape variables. Besides, the canopy cover positively affected the species richness of habitat generalist species, while the mean distance to neighbor forest islands negatively affected this group. Canopy cover varies seasonally and spatially among the forest islands sampled (Pereira et al. 2017; da Silva et al. 2019). However, the seasonal variation in canopy cover was insufficient to affect forest-dependent ant species in terms of species richness and compositional changes. Our results for habitat generalist species also contrast with those found by Ahuatzin et al. (2019). On the other hand, for dung beetles, da Silva et al. (2019) found a positive relationship between habitat generalist species’ abundance and richness and canopy cover only during the cold season. These authors suggested that these species may use the forest islands as refuges during times of harsher climatic conditions and scarcer resource availability in the campo rupestre open matrix. Although we do not address this pattern in this study, the accumulated richness of habitat generalist species over the cold and hot seasons sampled may suggest an even larger flow of or use by habitat generalist species inhabiting canopy-denser forest islands and consequently more climatically buffered habitats. Another possible explanation is that the preference of habitat generalist species by forest island conditions and resources can be a general pattern. Ant species richness is positively influenced by higher forest structural complexity in tropical forests, especially when soil to canopy strata is considered (Andersen 1986; Vasconcelos and Vilhena 2006; Neves et al. 2013). Habitat generalist species richness also decreased with increasing distance between neighbor forest islands. This implies a direct relationship between forest islands’ aggregation and habitat generalist species richness, which can generate a high spatial flow that decreases the possibility of habitat generalist species entry when forest islands are more isolated. This result also suggests a forest islands’ spatial distribution effect on the species richness of these ants: the closest the forest islands are on average, the higher the overall species richness is. In terms of metacommunity patterns, we can assume this result as a dispersal-driven effect, which implies a higher probability of a locality being influenced by closer sites (Leibold et al. 2004; Soininen et al. 2007), with important effects on the temporal changes or stability of the ant metacommunity.
The amount of forest island in the landscape, however, was important in determining the species composition temporal changes (β-diversity) in the assembly of forest-dependent ant species. In our study, the amount of forest island in the landscape was positively correlated with other similar variables such as forest island size, largest patch index, total core area, and area-weighted mean core area index distribution (see Figure S1; see Table S2 for variable description), but also positively correlated with forest islands’ understory density (Pearson’s rho = 0.67). Forests with low tree density (or understory density) can favor the occurrence of specialized opportunistic ant species, which can result in high species richness (Ahuatzin et al. 2019) but can also result in decreases in resource availability used by more sensitive forest-dependent species. Seasonal changes in resource availability and climatic conditions are known to influence ant foraging activity (Calazans et al. 2020) and consequently the diversity and composition of assemblages through time (Nunes et al. 2020). Therefore, forest-dependent ant species are more temporally stable in less isolated, bigger forest islands with a denser understory, which can indicate high resource availability (Philpott and Foster 2005; Klimes et al. 2012; Nunes et al. 2020).
The negative relationship between temporal β-diversity and the number of forest islands in the 250 m buffer was the only common response of both ant species’ groups. This result can be explained by the fact that more islands in the neighborhood mean less isolation, providing higher stability in local communities over time (lower temporal β-diversity) due to dispersal dynamics, since isolation inhibits ants’ dispersion (Tonhasca Jr et al. 2003; Leibold et al. 2004; Fahrig 2013). Changes in patterns of temporal β-diversity due to some kind of isolation (e.g., distance to closest forest island, distance to continuous forest) have been documented for bees and wasps (Perillo et al. 2020) and for both forest-dependent and habitat generalist dung-beetles (da Silva et al. 2019) in the same mountainous system. Habitat generalist species, especially considering vagile organisms such as butterflies and dung beetles, also benefit from a more forested landscape since the forest patches are more stable micro-climatically and buffer the severe climate in this mountaintop during the winter season (Pereira et al. 2017; da Silva et al. 2019). We now add new information on the effects of isolation on temporal patterns of ants, a less vagile group of organisms.
In this study, we showed the importance of a naturally formed forest island archipelago for the maintenance of the mountaintop’s ant diversity. Without these relictual forest islands, a large proportion of the ant fauna would not be able to establish and persist. In addition to being suitable as a refuge during adverse winter conditions for habitat generalist species (da Silva et al. 2019), most ants found in these forest islands are directly dependent on their resources and conditions. The responses of the ant metacommunity seem to be determined jointly by the landscape structure of the forest island archipelago (i.e., isolation) and local features (e.g., forest island area, canopy cover). The landscape structure influences the dispersal capacity of ants between forest islands, and local forest structure influences their establishment capacity, which in conjunction determine the local (α) and regional diversity (γ). In summary, the landscape structure acts as a spatial filter and the forest island structure as an environmental filter. We also advocate the importance of deconstructing communities into habitat specialists and habitat generalists (da Silva et al. 2019; Neves et al. 2021a), or other habitat-related requirements (Lozano-Zambrano et al. 2009). Besides, habitat specialists are expected to be more sensitive to forest habitat loss and land-use changes — the current main causes of entomofauna loss (Sánchez-Bayo and Wyckhuys 2019). Therefore, management plans of the mountaintop areas must also consider the forest island archipelago in order to maintain the biodiversity and functioning of these montane systems.
References
Adams BJ, Schnitzer SA, Yanoviak SP (2017) Trees as islands: canopy ant species richness increases with the size of liana-free trees in a Neotropical forest. Ecography 40:1067–1075. https://doi.org/10.1111/ecog.02608
Ahuatzin DA, Corro EJ, Jaimes AA, Valenzuela González JE, Feitosa RM, Ribeiro MC, Acosta JCL, Coates R, Dáttilo W (2019) Forest cover drives leaf litter ant diversity in primary rainforest remnants within human-modified tropical landscapes. Biodivers Conserv 28:1091–1107. https://doi.org/10.1007/s10531-019-01712-z
Alvares CA, Stape JL, Sentelhas PC, Gonçalves JLM, Sparovek G (2013) Köppen's climate classification map for Brazil. Meteorol Z 22:711–728. https://doi.org/10.1127/0941-2948/2013/0507
Andersen AN (1986) Diversity, seasonality and community organization of ants at adjacent heath and woodland sites in Southeastern Australia. Aust J Zool 34:53–64. https://doi.org/10.1071/ZO9860053
Andersen AN (2019) Responses of ant communities to disturbance: five principles for understanding the disturbance dynamics of a globally dominant faunal group. J Anim Ecol 88:350–362. https://doi.org/10.1111/1365-2656.12907
Andersen AN, Hoffmann BD, Müller WJ, Griffiths AD (2002) Using ants as bioindicators in land management: simplifying assessment of ant community responses. J Appl Ecol 39:8–17. https://doi.org/10.1046/j.1365-2664.2002.00704.x
Anderson MJ, Crist TO, Chase JM et al (2011) Navigating the multiple meanings of beta diversity: a roadmap for the practicing ecologist. Ecol Lett 14:19–28. https://doi.org/10.1111/j.1461-0248.2010.01552.x
Baccaro FB, Feitosa RM, Fernández F, Fernandes IO, Izzo TJ, de Souza JLP, Solar RRC (2015) Guia para os gêneros de formigas do Brasil. Editora INPA, Manaus
Baselga A (2010) Partitioning the turnover and nestedness components of beta diversity. Glob Ecol Biogeogr 19:134–143. https://doi.org/10.1111/j.1466-8238.2009.00490.x
Baselga A (2012) The relationship between species replacement, dissimilarity derived from nestedness, and nestedness. Glob Ecol Biogeogr 21:1223–1232. https://doi.org/10.1111/j.1466-8238.2011.00756.x
Baselga A, Orme CDL (2012) betapart: an R package for the study of beta diversity. Methods Ecol Evol 3:808–812. https://doi.org/10.1111/j.2041-210X.2012.00224.x
Bestelmeyer BT, Agosti D, Alonso LE, Brandão CRF, Brown WL, Delabie JHC, Silvestre R (2000) Field techniques for the study of ground-dwelling ants: an overview, description, and evaluation. In: Agosti D, Majer JD, Alonso LE, Schultz TR (eds) Ants: Standard methods for measuring and monitoring biodiversity. Smithsonian Institution Press, Washington, pp 122–144
Calazans EG, Costa FV, Cristiano MP, Cardoso DC (2020) Daily dynamics of an ant community in a mountaintop ecosystem. Environ Entomol 49:383–390. https://doi.org/10.1093/ee/nvaa011
Castro FS, da Silva PG, Solar R, Fernandes GW, Neves FS (2020) Environmental drivers of taxonomic and functional diversity of ant communities in a tropical mountain. Insect Conserv Diver 13:393–403. https://doi.org/10.1111/icad.12415
Chao A, Jost L (2012) Coverage-based rarefaction and extrapolation: standardizing samples by completeness rather than size. Ecology 93:2533–2547. https://doi.org/10.1890/11-1952.1
Coelho MS, Fernandes GW, Pacheco P, Diniz V, Meireles A, Santos RM (2016) Archipelago of montane forests surrounded by rupestrian grasslands: new insights and perspectives. In: Fernandes GW (ed) Ecology and conservation of mountaintop grasslands in Brazil. Springer, New York, pp 129–156
Coelho MS, Carlos PP, Pinto VD, Meireles A, Negreiros D, Morellato LPC, Fernandes GW (2018a) Connection between tree functional traits and environmental parameters in an archipelago of montane forests surrounded by rupestrian grasslands. Flora 238:51–59. https://doi.org/10.1016/j.flora.2017.04.003
Coelho MS, Neves FS, Perillo LN, Morellato LPC, Fernandes GW (2018b) Forest archipelagos: a natural model of metacommunity under the threat of fire. Flora 238:244–249. https://doi.org/10.1016/j.flora.2017.03.013
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Corro EJ, Ahuatzin DA, Jaimes AA, Favila ME, Ribeiro MC, López-Acosta JC, Dáttilo W (2019) Forest cover and landscape heterogeneity shape ant–plant co-occurrence networks in human-dominated tropical rainforests. Landsc Ecol 34:93–104. https://doi.org/10.1007/s10980-018-0747-4
Costa FV, Mello R, Lana TC, Neves FS (2015) Ant fauna in megadiverse mountains: a checklist for the rocky grasslands. Sociobiology 62:228–245. https://doi.org/10.13102/sociobiology.v62i2.228-245
Crawley MJ (2013) The R Book. Wiley, Chichester
Cuissi RG, Lasmar CJ, Moretti TS, Schmidt FA, Fernandes WD, Falleiros AB, Schoereder JH, Ribas CR (2015) Ant community in natural fragments of the Brazilian wetland: species–area relation and isolation. J Insect Conserv 19:531–537. https://doi.org/10.1007/s10841-015-9774-5
da Silva PG, Hernández MIM (2014) Local and regional effects on community structure of dung beetles in a mainland-island scenario. PLoS One 9:e111883. https://doi.org/10.1371/journal.pone.0111883
da Silva PG, Hernández MIM (2015) Scale-dependence of processes structuring dung beetle metacommunities using functional diversity and community deconstruction approaches. PLoS One 10:e0123030. https://doi.org/10.1371/journal.pone.0123030
da Silva PG, Nunes CA, Ferreira LF, Braga RF, Beiroz W, Perillo LN, Solar RRC, de Siqueira Neves F (2019) Patch and landscape effects on forest-dependent dung beetles are masked by matrix-tolerant dung beetles in a mountaintop rainforest archipelago. Sci Total Environ 651:1321–1331. https://doi.org/10.1016/j.scitotenv.2018.09.195
da Silva PG, Cañedo-Argüelles M, Bogoni JA, Heino J (2021) Editorial: Spatio-temporal dynamics of metacommunities - implications for conservation and management. Front Ecol Evol 9:670212. https://doi.org/10.3389/fevo.2021.670212
Dunn RR, Sanders NJ, Fitzpatrick MC et al (2007) Global ant (Hymenoptera: Formicidae) biodiversity and biogeography - a new database and its possibilities. Myrmecol News 10:77–83
Ellwood MDF, Blüthgen N, Fayle TM, Foster WA, Menzel F (2016) Competition can lead to unexpected patterns in tropical ant communities. Acta Oecol 75:24–34. https://doi.org/10.1016/j.actao.2016.06.001
Fahrig L (2013) Rethinking patch size and isolation effects: the habitat amount hypothesis. J Biogeogr 40:1649–1663. https://doi.org/10.1111/jbi.12130
Fahrig L (2017) Ecological responses to habitat fragmentation per se. Annu Rev Ecol Evol Syst 48:1–23. https://doi.org/10.1146/annurev-ecolsys-110316-022612
Fahrig L, Baudry J, Brotons L, Burel FG, Crist TO, Fuller RJ, Sirami C, Siriwardena GM, Martin JL (2011) Functional landscape heterogeneity and animal biodiversity in agricultural landscapes. Ecol Lett 14:101–112. https://doi.org/10.1111/j.1461-0248.2010.01559.x
Fernandes GW (2016) Ecology and conservation of mountaintop grasslands in Brazil. Springer, Cham
Fernandes GW, Arantes-Garcia L, Barbosa M, Barbosa NPU, Batista EKL, Beiroz W, Resende FM, Abrahão A, Almada ED, Alves E, Alves NJ, Angrisano P, Arista M, Arroyo J, Arruda AJ, Bahia TO, Braga L, Brito L, Callisto M, Caminha-Paiva D, Carvalho M, Conceição AA, Costa LN, Cruz A, Cunha-Blum J, Dagevos J, Dias BFS, Pinto VD, Dirzo R, Domingos DQ, Echternacht L, Fernandes S, Figueira JEC, Fiorini CF, Giulietti AM, Gomes A, Gomes VM, Gontijo B, Goulart F, Guerra TJ, Junqueira PA, Lima-Santos D, Marques J, Meira-Neto J, Miola DTB, Morellato LPC, Negreiros D, Neire E, Neves AC, Neves FS, Novais S, Oki Y, Oliveira E, Oliveira RS, Pivari MO, Junior EP, Ranieri BD, Ribas RP, Scariot A, Schaefer CE, Sena L, da Silva PG, Siqueira PR, Soares NC, Soares-Filho B, Solar R, Tabarelli M, Vasconcellos R, Vilela E, Silveira FAO (2020) Biodiversity and ecosystem services in the Campo Rupestre: a road map for the sustainability of the hottest Brazilian biodiversity hotspot. Perspect Ecol Conserv 18:213–222. https://doi.org/10.1016/j.pecon.2020.10.004
Ferrari LT, Schaefer CEGR, Fernandes RBA, Mendonça BAF, Gjorup DF, Corrêa GR, Senra EO (2016) Thermic and hydric dynamics of ironstone (Canga) and quartzite rupestrian grasslands in the quadrilátero ferrífero: the ecological importance of water. In: F G (ed) Ecology and Conservation of Mountaintop Grasslands in Brazil. Springer, Cham, pp 71–86
Frazer G, Canham C, Lertzman K (1999) Gap Light Analyzer (GLA): Imaging software to extract canopy structure and gap light transmission indices from true-colour fisheye photographs. Users Manual and Program Documentation. http://rem-main.rem.sfu.ca/downloads/Forestry/GLAV2UsersManual.pdf. Accessed 15 May 2017
Gibb H, Stoklosa J, Warton DI, Brown AM, Andrew NR, Cunningham SA (2015) Does morphology predict trophic position and habitat use of ant species and assemblages? Oecologia 177:519–531. https://doi.org/10.1007/s00442-014-3101-9
Graham CH, Fine PVA (2008) Phylogenetic beta diversity: linking ecological and evolutionary processes across space in time. Ecol Lett 11:1265–1277. https://doi.org/10.1111/j.1461-0248.2008.01256.x
Gray REJ, Ewers RM, Boyle MJW, Chung AYC, Gill RJ (2018) Effect of tropical forest disturbance on the competitive interactions within a diverse ant community. Sci Rep 8:5131. https://doi.org/10.1038/s41598-018-23272-y
Haddad NM, Gonzalez A, Brudvig LA, Burt MA, Levey DJ, Damschen EI (2017) Experimental evidence does not support the habitat amount hypothesis. Ecography 40:48–55. https://doi.org/10.1111/ecog.02535
Haila Y (2002) A conceptual genealogy of fragmentation research: from island biogeography to landscape ecology. Ecol Appl 12:321–334. https://doi.org/10.2307/3060944
Hopper SD, Lambers H, Silveira FAO, Fiedler PL (2021) OCBIL theory examined: reassessing evolution, ecology and conservation in the world’s ancient, climatically buffered and infertile landscapes. Biol J Linn Soc 133:266–296. https://doi.org/10.1093/biolinnean/blaa213
Hsieh TC, Ma KH, Chao A, McInerny G (2016) iNEXT: an R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol Evol 7:1451–1456. https://doi.org/10.1111/2041-210x.12613
Itescu Y (2018) Are island-like systems biologically similar to islands? A review of the evidence. Ecography 42:1298–1314. https://doi.org/10.1111/ecog.03951
Kaspari M, Yuan M, Alonso L (2003) Spatial grain and the causes of regional diversity gradients in ants. Am Nat 161:459–477. https://doi.org/10.1086/367906
Klimes P, Idigel C, Rimandai M, Fayle TM, Janda M, Weiblen GD, Novotny V (2012) Why are there more arboreal ant species in primary than in secondary tropical forests? J Anim Ecol 81:1103–1112. https://doi.org/10.1111/j.1365-2656.2012.02002.x
Kraft NJB, Adler PB, Godoy O, James EC, Fuller S, Levine JM (2015) Community assembly, coexistence and the environmental filtering metaphor. Funct Ecol 29:592–599. https://doi.org/10.1111/1365-2435.12345
Le Stradic S, Buisson E, Fernandes GW (2015) Vegetation composition and structure of some Neotropical mountain grasslands in Brazil. J Mt Sci 12:864–877. https://doi.org/10.1007/s11629-013-2866-3
Leal IR, Filgueiras BKC, Gomes JP, Iannuzzi L, Andersen AN (2012) Effects of habitat fragmentation on ant richness and functional composition in Brazilian Atlantic forest. Biodivers Conserv 21:1687–1701. https://doi.org/10.1007/s10531-012-0271-9
Leibold MA, Holyoak M, Mouquet N, Amarasekare P, Chase JM, Hoopes MF, Holt RD, Shurin JB, Law R, Tilman D, Loreau M, Gonzalez A (2004) The metacommunity concept: a framework for multi-scale community ecology. Ecol Lett 7:601–613. https://doi.org/10.1111/j.1461-0248.2004.00608.x
Levy R (1995) Identification Guide To the Ant Genera of the World. Syst Entomol 20:372–372. https://doi.org/10.1111/j.1365-3113.1995.tb00102.x
Lozano-Zambrano FH, Ulloa-Chacón P, Armbrecht I (2009) Hormigas: relaciones especies-área en fragmentos de bosque seco tropical. Neotrop Entomol 38:44–54. https://doi.org/10.1590/S1519-566X2009000100004
MacArthur RH, Wilson EO (1967) The theory of island biogeography. Princeton University Press, Princeton
Mayor JR, Sanders NJ, Classen AT, Bardgett RD, Clément JC, Fajardo A, Lavorel S, Sundqvist MK, Bahn M, Chisholm C, Cieraad E, Gedalof Z’, Grigulis K, Kudo G, Oberski DL, Wardle DA (2017) Elevation alters ecosystem properties across temperate treelines globally. Nature 542:91–95. https://doi.org/10.1038/nature21027
Montgomery GA, Belitz MW, Guralnick RP, Tingley MW (2021) Standards and best practices for monitoring and benchmarking insects. Front Ecol Evol 8:579193. https://doi.org/10.3389/fevo.2020.579193
Mouquet N, Carr MC, Cottenie K (2005) The world is patchy and heterogeneous! Trade-off and source-sink dynamics in competitive metacommunities. In: Holyoak M, Leibold MA, Holt RD (eds) Metacommunities: spatial dynamics and ecological communities. The University of Chicago Press, Chicago, pp 237–262
Myers N, Mittermeier RA, Mittermeier CG, Fonseca GAB, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403:853–845. https://doi.org/10.1038/35002501
Nassar JM, Rodríguez JP, Sánchez-Azofeifa A, Garvin T, Quesada M (2008) Manual of methods: human, ecological and biophysical dimensions of tropical dry forests. Gráficas Lauki, Caracas
Neves FS, Queiroz-Dantas KS, da Rocha WD, Delabie JHC (2013) Ants of three adjacent habitats of a transition region between the Cerrado and Caatinga biomes: the effects of heterogeneity and variation in canopy cover. Neotrop Entomol 42:258–268. https://doi.org/10.1007/s13744-013-0123-7
Neves FS, da Silva PG, Solar R et al (2021a) Habitat generalists drive nestedness in a tropical mountaintop insect metacommunity. Biol J Linn Soc 133:577–586. https://doi.org/10.1093/biolinnean/blaa059
Neves FS, Antoniazzi R, Camarota F, Pacelhe FT, Powell S (2021b) Spatiotemporal dynamics of the ant community in a dry forest differ by vertical strata but not by successional stages. Biotropica 53:372–383. https://doi.org/10.1111/btp.12918
Nobis M, Hunziker U (2005) Automatic thresholding for hemispherical canopy-photographs based on edge detection. Agric For Meteorol 128:243–250. https://doi.org/10.1016/j.agrformet.2004.10.002
Noroozi J, Talebi A, Doostmohammadi M, Rumpf SB, Linder HP, Schneeweiss GM (2018) Hotspots within a global biodiversity hotspot - areas of endemism are associated with high mountain ranges. Sci Rep 8:10345. https://doi.org/10.1038/s41598-018-28504-9
Nunes CA, Castro FS, Brant HSC, Powell S, Solar R, Fernandes GW, Neves FS (2020) High temporal beta diversity in an ant metacommunity, with increasing temporal functional replacement along the elevational gradient. Front Ecol Evol 8:571439. https://doi.org/10.3389/fevo.2020.571439
Ohyama L, Holt RD, Matthews TJ, Lucky A (2021) The species–area relationship in ant ecology. J Biogeogr. https://doi.org/10.1111/jbi.14149
Padial AA, Ceschin F, Declerck SA et al (2014) Dispersal ability determines the role of environmental, spatial and temporal drivers of metacommunity structure. PLoS One 9:e111227. https://doi.org/10.1371/journal.pone.0111227
Palmeirim AF, Benchimol M, Vieira MV, Peres CA (2018) Small mammal responses to Amazonian forest islands are modulated by their forest dependence. Oecologia 187:191–204. https://doi.org/10.1007/s00442-018-4114-6
Pandit SN, Kolasa J, Cottenie K (2009) Contrasts between habitat generalists and specialists: an empirical extension to the basic metacommunity framework. Ecology 90:2253–2262. https://doi.org/10.1890/08-0851.1
Parr CL, Dunn RR, Sanders NJ, Weiser MD, Photakis M, Bishop TR, Fitzpatrick MC, Arnan X, Baccaro F, Brandão CRF, Chick L, Donoso DA, Fayle TM, Gómez C, Grossman B, Munyai TC, Pacheco R, Retana J, Robinson A, Sagata K, Silva RR, Tista M, Vasconcelos H, Yates M, Gibb H (2017) GlobalAnts: a new database on the geography of ant traits (Hymenoptera: Formicidae). Insect Conserv Diver 10:5–20. https://doi.org/10.1111/icad.12211
Patiño J, Whittaker RJ, Borges PAV, Fernández-Palacios JM, Ah-Peng C, Araújo MB, Ávila SP, Cardoso P, Cornuault J, de Boer EJ, de Nascimento L, Gil A, González-Castro A, Gruner DS, Heleno R, Hortal J, Illera JC, Kaiser-Bunbury CN, Matthews TJ, Papadopoulou A, Pettorelli N, Price JP, Santos AMC, Steinbauer MJ, Triantis KA, Valente L, Vargas P, Weigelt P, Emerson BC (2017) A roadmap for island biology: 50 fundamental questions after 50 years of The Theory of Island Biogeography. J Biogeogr 44:963–983. https://doi.org/10.1111/jbi.12986
Pereira GCN, Coelho MS, Beirão MDV, Braga RF, Fernandes GW (2017) Diversity of fruit-feeding butterflies in a mountaintop archipelago of rainforest. PLoS One 12:e0180007. https://doi.org/10.1371/journal.pone.0180007
Perillo LN, Barbosa NPU, Solar RRC, Neves FS (2020) Patterns of diversity in a metacommunity of bees and wasps of relictual mountainous forest fragments. J Insect Conserv 24:17–34. https://doi.org/10.1007/s10841-019-00194-2
Philpott SM, Foster PF (2005) Nest-site limitation in coffee agroecosystems: artificial nests maintain diversity of arboreal ants. Ecol Appl 15:1478–1485. https://doi.org/10.1890/04-1496
Record S, Voelker NM, Zarnetske PL, Wisnoski NI, Tonkin JD, Swan C, Marazzi L, Lany N, Lamy T, Compagnoni A, Castorani MCN, Andrade R, Sokol ER (2021) Novel insights to be gained from applying metacommunity theory to long-term, spatially replicated biodiversity data. Front Ecol Evol 8:612794. https://doi.org/10.3389/fevo.2020.612794
Regolin AL, Oliveira-Santos LG, Ribeiro MC, Bailey LL (2021) Habitat quality, not habitat amount, drives mammalian habitat use in the Brazilian Pantanal. Landsc Ecol. https://doi.org/10.1007/s10980-021-01280-0
Ribas CR, Schoereder JH, Pic M, Soares SM (2003) Tree heterogeneity, resource availability, and larger scale processes regulating arboreal ant species richness. Austral Ecol 28:305–314. https://doi.org/10.1046/j.1442-9993.2003.01290.x
Rossetti MR, Tscharntke T, Aguilar R, Batáry P (2017) Responses of insect herbivores and herbivory to habitat fragmentation: a hierarchical meta-analysis. Ecol Lett 20:264–272. https://doi.org/10.1111/ele.12723
Rumpf SB, Hülber K, Klonner G, Moser D, Schütz M, Wessely J, Willner W, Zimmermann NE, Dullinger S (2018) Range dynamics of mountain plants decrease with elevation. Proc Natl Acad Sci USA Biol Sci 115:1848–1853. https://doi.org/10.1073/pnas.1713936115
Sánchez-Bayo F, Wyckhuys KAG (2019) Worldwide decline of the entomofauna: a review of its drivers. Biol Conserv 232:8–27. https://doi.org/10.1016/j.biocon.2019.01.020
Schoereder JH, Sobrinho TG, Ribas CR, Campos RBF (2004) Colonization and extinction of ant communities in a fragmented landscape. Austral Ecol 29:391–398. https://doi.org/10.1111/j.1442-9993.2004.01378.x
Silveira FAO, Negreiros D, Barbosa NPU, Buisson E, Carmo FF, Carstensen DW, Conceição AA, Cornelissen TG, Echternacht L, Fernandes GW, Garcia QS, Guerra TJ, Jacobi CM, Lemos-Filho JP, le Stradic S, Morellato LPC, Neves FS, Oliveira RS, Schaefer CE, Viana PL, Lambers H (2016) Ecology and evolution of plant diversity in the endangered campo rupestre: a neglected conservation priority. Plant Soil 406:129–152. https://doi.org/10.1007/s11104-015-2637-8
Silveira FAO, Barbosa M, Beiroz W, Callisto M, Macedo DR, Morellato LPC, Neves FS, Nunes YRF, Solar RR, Fernandes GW (2019) Tropical mountains as natural laboratories to study global changes: a long-term ecological research project in a megadiverse biodiversity hotspot. Perspect Plant Ecol Evol Syst 38:64–73. https://doi.org/10.1016/j.ppees.2019.04.001
Soininen J, McDonald R, Hillebrand H (2007) The distance decay of similarity in ecological communities. Ecography 30:3–12. https://doi.org/10.1111/j.0906-7590.2007.04817.x
Soininen J, Heino J, Wang J (2018) A meta-analysis of nestedness and turnover components of beta diversity across organisms and ecosystems. Glob Ecol Biogeogr 27:96–109. https://doi.org/10.1111/geb.12660
Spiesman BJ, Cumming GS (2008) Communities in context: the influences of multiscale environmental variation on local ant community structure. Landsc Ecol 23:313–325. https://doi.org/10.1007/s10980-007-9186-3
Streher AS, Sobreiro JFF, Morellato LPC, Silva TSF (2017) Land surface phenology in the tropics: the role of climate and topography in a snow-free mountain. Ecosystems 20:1436–1453. https://doi.org/10.1007/s10021-017-0123-2
Tonhasca A Jr, Albuquerque GS, Blackmer JL (2003) Dispersal of euglossine bees between fragments of the Brazilian Atlantic Forest. J Trop Ecol 19:99–102. https://doi.org/10.1017/S0266467403003122
Vasconcelos HL, Bruna EM (2012) Arthropod responses to the experimental isolation of Amazonian forest fragments. Zoologia 29:515–530. https://doi.org/10.1590/S1984-46702012000600003
Vasconcelos HL, Vilhena JMS (2006) Species turnover and vertical partitioning of ant assemblages in the Brazilian Amazon: a comparison of forests and savannas. Biotropica 38:100–106. https://doi.org/10.1111/j.1744-7429.2006.00113.x
Vasconcelos HL, Vilhena JMS, Magnusson WE, Albernaz ALKM (2006) Long-term effects of forest fragmentation on Amazonian ant communities. J Biogeogr 33:1348–1356. https://doi.org/10.1111/j.1365-2699.2006.01516.x
Vasconcelos HL, Maravalhas JB, Feitosa RM, Pacheco R, Neves KC, Andersen AN (2018) Neotropical savanna ants show a reversed latitudinal gradient of species richness, with climatic drivers reflecting the forest origin of the fauna. J Biogeogr 45:248–258. https://doi.org/10.1111/jbi.13113
Wintle BA, Kujala H, Whitehead A, Cameron A, Veloz S, Kukkala A, Moilanen A, Gordon A, Lentini PE, Cadenhead NCR, Bekessy SA (2019) Global synthesis of conservation studies reveals the importance of small habitat patches for biodiversity. Proc Natl Acad Sci USA Biol Sci 116:909–914. https://doi.org/10.1073/pnas.1813051115
Acknowledgements
We would like to highlight the invaluable help of the following students for assistance with field work: Luiz Eduardo Macedo Reis, Daniela Melo, Luiz Fernando Ferreira, Geanne Pereira, Heron Hilário, Caio Silveira Marques, and Thaís Silva Tavares. We also thank Flávio Camarota, Scott Powel (Cephalotes), and Rodrigo Feitosa for ant identification. PGdS thanks the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for postdoctoral grant (PNPD 88882.316025/2019-01, Code 001). LNP thanks DAAD for the postdoctoral grant (BIOBRAS Project). Thanks also go to Reserva Vellozia, Instituto Chico Mendes para Biodiversidade (ICMBio), Parque Nacional da Serra do Cipó and GSG for logistical support. We also thank the editor and reviewers for their valuable suggestions, and Flávio Camarota for English revision.
Funding
We are thankful to Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil (PELD - 441515/2016-9), for funding the long-term ecological research “PELD Campos Rupestres da Serra do Cipó” and to Fundação de Amparo à Pesquisa do Estado de Minas Gerais, Brazil (CRA - APQ-00311-15) for providing additional funding.
Author information
Authors and Affiliations
Contributions
FdSN formulated the concept and design. HSCB, FSdC, and LNP carried out data collection. HSCB and PGdS analyzed the data and lead the writing. All authors contributed critically to the drafts and gave final approval for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Edited by Heraldo Vasconcelos
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 395 kb)
Rights and permissions
About this article
Cite this article
Brant, H.S.C., da Silva, P.G., de Castro, F.S. et al. Spatiotemporal Patterns of Ant Metacommunity in a Montane Forest Archipelago. Neotrop Entomol 50, 886–898 (2021). https://doi.org/10.1007/s13744-021-00901-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13744-021-00901-2