Abstract
Within many populations, some individuals may be more apt to move, and these individuals can substantially impact population dynamics. Invasive Silver Carp (Hypophthalmichthys molitrix) have spread throughout much of the Mississippi River Basin, and their presence has resulted in multiple negative ecosystem effects. Silver Carp are known to move hundreds of km, which has likely contributed to their rapid spread. Our study examined movement patterns and environmental cues for movement in Silver Carp based on acoustic telemetry of tagged fish that ranged widely (i.e., mobile) and those that did not range far from the site of their original capture and tagging (i.e., sedentary) in the Wabash River, USA. Sedentary and mobile designations were made based on observed extremes of mean annual ranges, and these designations were consistent within seasons and among years. Both movement groups displayed seasonal variation in movements, with mobile Silver Carp consistently moving greater distances within each season and sedentary Silver Carp exhibiting lower variability in distances moved than mobile individuals. Discharge (change in discharge) and temperature were significant predictors of mobile and sedentary individuals’ movements. Additional environmental variables (i.e., cumulative growing degree day, day of year, and change in temperature) also related to movement likelihood of sedentary individuals, whereas total length was the only additional variable that influenced movement likelihood of mobile individuals. Total length was significantly related to movement distance for both groups of Silver Carp, but the relationship was negative for sedentary fish and positive for mobile fish. Results point to differences in behavior that may require targeted management strategies to achieve agency goals to interrupt mobile individual movements that can result in range expansion. Such strategies may also limit introductions and invasions by other aquatic invasive species that exhibit similar behaviors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The presence of mobile and sedentary individuals within a population has been documented in a plethora of organisms, both terrestrial and aquatic (Petty and Grossman 2004; Crook 2004; Gu et al. 2006; Stuart and Jones 2006). Even in humans, the act of moving vast distances may be more common for specific individuals, such as individuals carrying genes associated with “wanderlust” (e.g., Chen et al. 1999). The prevalence of mobile and sedentary individuals within a population influences population and community dynamics, including population genetics and species range. Mobile and sedentary tendencies of organisms have been described as variation in personality (Funk 1955; Rasmussen and Belk 2012), a response to some environmental factor (Roy et al. 2012; Mossop et al. 2017), or as an interaction between intrinsic behavior and environmental variability (Rehage et al. 2016). The prevalence of mobile behavior types within a population is moderated by natural selection (Lowe and McPeek 2014); thus, it can be expected to change through time or vary among populations based on changing community structure or environmental conditions (Hobbs et al. 2017) in addition to varying among species (Funk 1955; Dewey 1981).
Movement capacity (i.e., the ability to move frequently and over large distances) is often considered a common invasive species trait (Moyle 1986; Kolar and Lodge 2001; Rehage et al. 2016). Moving individuals incur significant risks (i.e., predation, uncertain resources; Rehage et al. 2016). However, risks can be offset by invasions of new habitats that create additional subpopulations that contribute to a more resilient metapopulation. For example, a larger network of interconnected subpopulations can overcome genetic constraints associated with small populations by providing more reliable access to mates and sharing of alleles among subpopulations (Berthouly-Salazar et al. 2013). Moreover, this distributed metapopulation structure ensures that large numbers of individuals can coexist without depleting local resources while increasing the likelihood of recolonization after one or more subpopulations are eliminated by some catastrophic event. These movement benefits can therefore be factors when determining the likelihood that an introduced species becomes established, spreads, and exerts negative ecological impacts to the extent that it becomes invasive. It is plausible that mobile individuals could disproportionately influence gene flow and population dynamics relative to more stationary individuals (Trakhtenbrot et al. 2005). In addition, mobile individuals may even be more fit than sedentary congeners (Bonte et al. 2014; Bonte and Dahriel 2016) or have higher survival rates (White and Wagner 2021), thereby further increasing likelihood of invasion success and range expansion. Understanding the movements of mobile individuals in populations characterized by individuals exhibiting varying movement tendencies may provide insight to help limit the spread and negative ecological effects of invasive species.
Invasive Silver Carp (Hypophthalmichthys molitrix) in the Wabash River (USA), exhibit individual variation in movement tendencies, with individual ranges from 0 km up to > 400 km (Prechtel et al. 2017). Their movement capacity allows them to rapidly move over hundreds of km in a few days (Coulter et al. 2016a) and has been noted as one of the potential reasons why Silver Carp have become so invasive in many lotic and lentic systems (Kolar et al. 2007). Movement patterns of Silver Carp populations have been well studied, with most responding to temperature and hydrographic cues (DeGrandchamp et al. 2008; Coulter et al. 2016a, 2017) for movement. When examined at a population level, increasing discharge or water levels may positively influence movement rate (DeGrandchamp et al. 2008; Coulter et al. 2016a) and movement distance (Coulter et al. 2016a) but negatively influence likelihood of movement (Coulter et al. 2016a). Large changes in water levels over 24 h have been observed to positively influence the likelihood of Silver Carp moving (Coulter et al. 2016a). Temperature, in combination with changes in discharge, is also known to trigger spawning in Silver Carp (Abdusamadov 1987; Kolar et al. 2007; Kocovsky et al. 2012) and has been attributed to movements for staging (movement to near a spawning site; Coulter et al. 2016a) or spawning (Coulter et al. 2016b). While these studies have developed an understanding of how population-level movement responds to environmental conditions, there remains a need to identify the drivers of intra-population movements, specifically individual movements on the extremes of the movement continuum. As previously discussed, individuals moving the most may have the potential to disperse into new areas and link subpopulations.
Movement (used hereafter as a broad term that spans behaviors associated with dispersal, migration, and routine/daily movements) and behavior can influence the success and impacts of invasive species through all stages of invasion (Juette et al. 2014); yet, data needed to assess these factors are lacking for many invasive species (e.g., Chapple et al. 2012). Our study sought to identify differences in the movement patterns and environmental cues related to movements of Silver Carp displaying the extremes in range, classified as sedentary or mobile based on mean annual ranges, using telemetry data collected from acoustically tagged fish in the Wabash River. We sought understanding of drivers for movement (i.e., Driscoll et al. 2014) in both extreme groups (i.e., mobile and sedentary) of Silver Carp. We also wanted to determine whether behavior type is fixed at an individual level vs. occurring within a random subset of the population that varies through time (Rehage et al. 2016). We tested the hypotheses that mobile individuals are consistently mobile and that movements of the two groups differ seasonally, given that seasonal differences in movement have previously been observed in the Wabash River Silver Carp population (i.e., Coulter et al. 2016a). Additionally, we tested the hypothesis that mobile and sedentary individuals’ movements are triggered by different environment cues.
Methods
Study organism
Silver Carp are large (up to 140 cm total length), planktivorous, cyprinids native to southeast Asia that are primarily distributed in eastern China (Kolar et al. 2007). Populations of the species exist in 80 + countries (Kolar et al. 2007), including established invasive populations at risk for further spread across the globe (e.g., United States, South Africa (Crookes et al. 2020), Hungary (Molnár et al. 2021), China (Xie and Chen 2001), Japan (Matsuzawa and Senou 2008)). In some locations, Silver Carp comprise > 80% of the fish biomass (Coulter et al. 2018). Silver Carp can negatively influence invaded ecosystems, and high densities of these fish have been associated with declines in native planktivorous fishes (Irons et al. 2007; Pendleton et al. 2017; Shields et al. 2021) and changes in zooplankton community composition (Sass et al. 2014). Silver Carp have exhibited long distance movements in both their native (see Kolar et al. 2007) and invasive ranges, with some individuals moving 100 s of km (e.g., DeGrandchamp et al. 2008; Coulter et al. 2016a). This substantial movement capacity can pose a greater risk for range expansion.
Study site
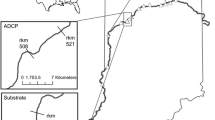
The Wabash River flows from headwaters in Eagle Marsh (Fort Wayne, IN, USA) to the west and south before joining the Ohio River (Shawneetown, IL, USA; Fig. 1). The Wabash River contains a single mainstem dam after which it is free flowing for 661 river km (rkm). Silver Carp were first detected in the Wabash River in 1995 (Kolar et al. 2007), and they now reproduce (Coulter et al. 2013, 2016b) and move freely throughout the free flowing 661 rkm (Coulter et al. 2016a).
Acoustic telemetry and movement calculation
From 2011 to 2013, 300 Silver Carp were implanted with acoustic transmitters (Vemco V16-4H, 120 s mean ping interval, Vemco, Bedford, Nova Scotia, Canada) in the middle and upper portions of the Wabash River (rkm 499–600) as part of a previous study (See Coulter et al. 2016a for full details including surgical procedures). Surgical procedures for implanting of acoustic transmitters into the coelomic cavity were approved by the Purdue University Animal Care and Use Committee (Protocol # 09-040). From 2011 to 2017, tagged fish were monitored with a series of 16 passive stationary receivers (VR2Ws, Vemco, Bedford, Nova Scotia, Canada) placed on the river bottom from rkm 653 downstream to rkm 122. Locations of active stationary receivers varied from year to year (typically 9–15 annually) due to receiver loss resulting in some variation in the possible maximum range over which an individual could move and be detected (Table 1). Stationary receivers were tested at initial deployment using a range testing tag (same specs as transmitters in Silver Carp) to verify that receivers deployed at selected locations could detect tags the entire river width. However, stationary receiver ranges in rivers are not uniform (Abeln 2018) and tag detection can vary with environmental conditions (e.g., Huveneers et al. 2015); thus, the exact range of the stationary receivers at a particular time is unknown. However, post hoc analyses of detection histories for individuals included in this study revealed a likelihood of fish skipping a receiver was 1.2% (48,077 movements and 565 missed receiver observations). This metric was calculated by comparing the detection histories of fishes to the order in which stationary receivers were deployed. Only main channel receivers were included in the analysis (two backwater receivers removed). The greatest number of skips occurred in May (143 skips) and the fewest skips occurred in November and January (4 skips each). Active tracking occurred from rkm 627 to rkm 425 and that entire portion of the river was tracked once per week from 2011 to 2013, April through September (Coulter et al. 2016a; Prechtel et al. 2018). Due to this limited timeframe and coverage of the larger study area, active tracking detections were only used in this study to help evaluate possible mortalities and not in the calculation of range/movement.
Silver Carp ranges were calculated for each individual within each year (annual range) as maximum displacement (i.e., difference in rkm between most upstream and most downstream locations within a year; similar to Clapp et al. 1990). Ranges were not calculated for fish that never moved during the study as we were unable to determine if these were fish that were not moving or possible mortalities. Ranges were also not calculated for fish with ≤ 1 year of data as ranges estimated from 1 year of data were significantly smaller than range estimates from ≥ 2 years of data (Prechtel et al. 2017). No detections that occurred within 48 h of transmitter implantation were used to reduce the impact of behavior related changes due to handling and surgery on results. Detection histories for individual fish were condensed to one detection per day per receiver, with the exception of fish that moved past multiple receivers in a day in which case the first detection at each receiver was retained (Online Resource 1: Fig. S1; Online Resource 2). For example, a fish that moved from receiver A to B then back to receiver A in a single day would have 3 detections for that day.
From the 232 Silver Carp that were detected post-tagging, 11 individuals were removed due to no observed movements and 20 individuals were removed due to < 1 year of data. Using the remaining 201 Silver Carp, we established sedentary and mobile groups using the extremes of mean annual range values. Groupings included fish with the smallest 20% of mean annual ranges and largest 20% of study ranges, respectively, resulting in 40 individuals per movement group (Fig. 2). These groupings were created as a balance between classifying fishes that displayed the most extreme differences in range and still retaining a sufficient sample size within each category for statistical analyses. More extreme classifications were initially attempted (extreme 10% of mean annual ranges) but resulted in insufficient sample size for analyses. Larger cut-offs (25% and 35%) were also analyzed, and results are included in Online Resource 1. On average, mean annual ranges were calculated from 4.5 years of data (± 1.5 sd). To verify that this grouping resulted in use of different river lengths, we calculated both total (sum of all detected movements) and net distance (sum of all detected movements with upstream movements being positive and downstream movements being negative) moved over the study (2011–2017) for each individual. Net and total distances moved over the study were then compared using an analysis of variance (ANOVA). Mobile individuals (StudyTotal[mean ± sd] 886 rkm ± 471; StudyNet[mean ± sd] − 58 rkm ± 102) moved consistently greater distances over the study than sedentary individuals (StudyTotal[mean ± sd] 49 rkm ± 37; StudyNet[mean ± sd] − 2 rkm ± 7) (Total: F = 125.52, df = 1,78, p < 0.0001; Net: F = 12.33, df = 1,78, p = 0.0007). There was no significant difference in total length at tagging between fish classified as mobile (mean = 707.6 mm ± 55.9 SD) and sedentary (mean = 694.3 mm ± 67.4 SD; t-test: T = − 0.96, p = 0.17; Online Resource 1: Table S2) and total length was not correlated with mean annual range size (correlation: R = 0.16, p = 0.17). In addition to the annual ranges of sedentary and mobile individuals, we calculated several additional response metrics, including mean rkm of annual range, minimum rkm of annual range, maximum rkm of annual range, and total and net movement distances (distance between consecutive detections, net movement factors in directionality; Coulter et al. 2016a). Inclusion of these additional metrics provides a more complete examination of the different ways fish may move (e.g., apparent migrations vs. unidirectional movement).
Mean annual range size (river km [rkm] ± standard deviation [sd]) of Silver Carp (n = 201) in the Wabash River, IN (USA) estimated from acoustic telemetry data. Sedentary and mobile individuals were chosen from the far right and far left sides of the x-axis with cut-offs indicated by vertical dashed lines. Fish with a mean annual range size = 0 or ≤ 1 year of data were excluded. On average, mean annual ranges were calculated from 4.5 years of data (± 1.5 sd)
Analyses
Since annual variation in environmental conditions (e.g., water levels and temperature) could potentially influence differences in range and movement, we first examined whether annual range sizes or rkm used (mean, max, min) varied between sedentary and mobile groups within each study year. To do this, we used linear mixed-effects models (‘lmer’; Bates et al. 2015) with movement group and year as fixed effects and individual fish ID as a random effect. Next, we examined whether the seasonal movement patterns of sedentary and mobile fish were similar or if a total or net movement in a particular season contributed to differences in range between the two groups. For these comparisons, we used a linear mixed-effects model with movement group and season as fixed effects and individual fish ID and year as random effects. Lastly, we examined the effects of biotic (total length) and abiotic (temperature, change in temperature over 24 h (ΔTemp), discharge, change in discharge over 24 h (ΔDis), day of year [DOY], and cumulative growing degree day [CGDD, base 10 °C]) on the total and net movements of mobile and sedentary fish as well as whether an individual would move (be detected on a different stationary receiver) or not (detected on the same stationary receiver) between detections. Temperature (mean daily air temperature, °C) was obtained from a weather station near the center of the study site (NOAA station GHCND: USC00129430) and used to calculate CGDD. Water temperature data were not available for the duration of the study; however, water temperatures in the Wabash River were previously shown to be highly correlated with air temperatures from this weather station (Coulter et al. 2016b). We calculated growing degree day (GDD) as [(maximum daily temperature − minimum daily temperature)/2] − 10 °C base temperature (Chezik et al. 2014; Coulter et al. 2016b). If GDD was negative, a zero was substituted. Cumulative growing degree day is the sum of all previous GDD within a given year. We obtained discharge information from the U.S. Geological Survey stream gaging station (Gage 03335500) at Lafayette, IN (USA), which is located near the center of the study site. Discharge data were not available from this gaging station from 3/31/2012 through 7/17/12 and 5/17/2014 through 5/25/2014. We therefore used discharge information from the next gaging station downstream (Covington, IN [USA], gage 03336000) to estimate Lafayette discharge using a regression created from dates when concurrent gage measurements were available at both gages (Lafayette discharge [m] = 28.21[Covington discharge]2 + 44.34[Covington discharge] + 18.40; R2 = 0.996). Movements (pairs of consecutive detections) were assigned environmental data using either the mean value over the movement (temperature, discharge), change in values over the movement (ΔDis, ΔTemp), or the value at the start of the movement (DOY, GDD, CGDD, total length). We measured total length (mm) of each fish during transmitter implantation and adjusted these values for years since tagging by adding estimated growth to measured total length at tagging. We estimated growth using the growth equation from Stuck et al. (2015) for Silver Carp from the Wabash River.
For each group and response variable (i.e., move/no move, total distance, net distance), we used generalized linear mixed-effects models (‘glmer’; Bates et al. 2015) with abiotic and biotic variables listed above as fixed effects and individual fish ID as a random effect. To examine if fish would move or not, we used a binomial family model with the same fixed and random effects as the linear models examining movement distance. We evaluated environmental predictor variables for correlations prior to inclusion in models and no variables were significantly correlated (Pearson correlations, all < r = 0.45). We assessed models of every possible combination of biotic and abiotic predictors using Akaike’s Information Criterion with small sample size correction (AICC) using the ‘dredge’ command (Package MuMIn, Barton 2018) and we averaged models with delta AICC (ΔAICC) < 2 using conditional model averaging. We conducted all statistical analyses in R (v. 3.5.2, R Core Team 2018) with an α = 0.05.
Results
Annual variation
Fish used in this study were detected for a mean of > 4 years (mobile: 4.70 years ± 0.25 SE; sedentary: 4.73 years ± 0.20 SE) for an average of 30–40 day per year (mobile: 31.79 day/year ± 7.47 SE; sedentary: 39.90 day/year ± 14.43 SE). Ranges used by sedentary fish were significantly smaller than mobile fish (F1,6 = 122.54, p < 0.0001), with pairwise comparisons showing differences between the groups in every year (Fig. 3). Years 2015–2017 had significantly smaller ranges overall than earlier years except for 2012 (F1,6 = 6.483, p < 0.0001). However, mobile fish ranges were driving this trend, and sedentary ranges showed no significant difference from year to year. Mean rkm was not different between sedentary and mobile groups (F1,6 = 0.979, p = 0.326; Fig. 4); however, mobile fish used significantly higher and lower max and min rkm than sedentary fish (max rkm: F1,6 = 14.06, p = 0.0004; min rkm: F1,6 = 46.09, p < 0.0001; Fig. 4). Similarly to range values, differences among years (min rkm: F1,6 = 8.35, p < 0.0001; max rkm: F1,6 = 13.132, p < 0.0001) were driven by mobile fish, and sedentary fish showed no significant annual variation in these values. Pairwise comparisons revealed that mobile fish max rkm was significantly higher in 2011–2015 compared to 2017. Additionally, mobile fish min rkm was lower in 2012–2014, 2016 and 2017 compared to 2011 and 2015.
Annual ranges in river km (rkm) (± standard deviation [sd]) of mobile and sedentary Silver Carp in the Wabash River, IN (USA) from 2011 to 2017. Mobile Silver Carp exhibited significantly larger range sizes than sedentary Silver Carp in all study years (p < 0.05). Numbers of Silver Carp in each movement group varied annually and are provided in Table 1
Mean a, b maximum, and c minimum river km (rkm) (± standard deviation bars [sd]) used by acoustically tagged mobile and sedentary Silver Carp in the Wabash River, IN (USA). Mean rkm was not different between movement groups. Maximum and minimum rkm were both different between movement groups which were higher and lower in the mobile group. Differences among years within the mobile group are indicated by letters. The sedentary group was never different among years
Seasonal variation
Total distances moved were significantly greater in mobile fish then sedentary fish (F1,3 = 131.25, p < 0.0001), but movements were not different among seasons (F = 1.12, df = 1,3, p = 0.340; Fig. 5). However, net distances showed both a significant difference between groups (F1,3 = 35.49, p < 0.0001) and among seasons (F1,3 = 29.91, p < 0.0001; Fig. 6). Pairwise comparison showed net movement was lower in the fall and winter compared to other seasons.
Mean net distances (river km [rkm]) (± standard deviation bars [sd]) moved by acoustically tagged Silver Carp in the Wabash River, IN (USA). Positive values indicate net upstream movements and negative values indicate net downstream movements. When year and individual fish were accounted for, net distances moved were different between movement groups and among seasons (fall and winter < spring and summer). Net distances moved were not different among seasons for sedentary fish and were different among seasons for mobile fish (indicated by *)
Abiotic and biotic drivers of movements
Discharge, ΔDis, and temperature were significantly related to whether an individual would move or remain stationary for both groups (Table 2; Supplemental File 1: Table S3—move/no move average model coefficients). Average model coefficients for discharge and temperature were 41 and 3.2 times higher, respectively, for the mobile group than for the sedentary group. Additionally, total length was a significant predictor for the mobile group to move or remain stationary, and all other variables (CGDD, ΔTemp, DOY) except total length were significant predictors in the averaged model for the sedentary group. Movement distance was related to total length for both groups, although the direction of the relationship was different (sedentary—negative, mobile—positive; Table 3; Supplemental File 1: Table S4—averaged model coefficients). Discharge, ΔDis, and CGDD were significantly related to distance moved in sedentary Silver Carp. Only DOY, in addition to length, was related to movement distance for mobile individuals.
Net distance, a metric of movement distance and direction combined, did not have fish total length as a significant predictor for sedentary or mobile groups. Change in discharge was the only significant predictor in the averaged model for the sedentary group (Table 4; Supplemental File 1: Table S5—averaged model coefficients). An averaged model could not be generated for the mobile Silver Carp as only one model had a ΔAIC < 2 (Online Resource 1). Temperature and CGDD were both significant predictors in this model for the mobile group.
Discussion
Sedentary fish exhibited consistently less variability in their movements than mobile fish, and their movements were not as strongly associated with environmental variables when the same environmental predictors were significant (i.e., smaller coefficients; e.g. temperature), suggesting that sedentary fish may be less reactive than mobile fish to the examined environmental predictors. However, a greater variety of environmental variables were related to sedentary fish movements than mobile fish, especially in determining whether an individual would move or remain stationary. Alternatively, mobile individuals’ movements were generally related to variables with a temporal component (DOY or CGDD) and/or total length. In addition to reacting more to environmental cues, mobile fish may have also been sensitive to factors not included in this study (e.g., densities, Rasmussen and Belk 2012), resulting in higher movement frequencies. Many of the observed significant relationships with movement distance were different, or different in magnitude, between the two movement groups, indicating that resource managers may benefit from targeting a specific movement group for management.
Seasonal and annual variations in movements were apparent for mobile, but not sedentary fish, suggesting that mobile fish pose the greatest risk of moving into uninvaded habitats at specific times and related to life history events. Although ascertaining the purpose of a movement (e.g., spawning vs. dispersal) is beyond the scope of this study, data from the mobile fish do indicate that spawning movement may be a potential influence on movements of these fishes (e.g., high movement in spring/summer). Additionally, positive net distances moved by the most mobile fish indicate that there could be potential disperers within this group. Additionally, mobile fish tended to move more frequently under conditions that coincided with an “open river” (i.e., dams open to allow free-flowing water) in many regulated rivers and flooding allowing fishes to move more freely among river sections. Open dams and high-water levels would allow mobile fish to invade locations that are otherwise inaccessible at a time during which they are moving the greatest distances. The use of temporary barriers (e.g., seasonal electrical barriers) could constrain movements during this critical time and limit both dispersal and spawning activity. This strategy could be used to prevent and/or control invasion without requiring the full-time use of expensive.
Movements (upstream rkm used and range) in mobile fish generally declined over the course of the study, although the cause of this is unclear. Little of this annual variation appears to correspond to river conditions. For example, 2012 was a severe drought year in Midwestern North America, yet the movements of mobile individuals were not reduced in this year. Instead, the decline in movement through time may be more likely related to study design issues such as reduced stationary receiver coverage in later years.
The presence and dispersal behavior of mobile individuals in an invasive population is particularly important, as these individuals contribute to invasion success (Cote et al. 2010; Juette et al. 2014). Mobile Silver Carp were consistently mobile throughout our study (i.e., they consistently exhibited movements typical of those at the mobile extreme of the movement continuum). However, expression of a particular behavioral type (i.e., mobile vs. sedentary) may still vary in relation to factors we did not examine. For example, dispersal behaviors may decrease with increasing genetic interrelatedness (Cote et al. 2010), which could occur in cases where invasive species establish with limited founder populations. Our study examined the movement of individuals from the middle of an invasive population, and future work should examine the prevalence of mobile individuals at invasion fronts to better understand the role they play in invasion success. Mobile individuals at the invasion front may be more apt to move and able to colonize new habitats once sedentary individuals are recruited to that habitat (Rehage et al. 2016). Mobile individuals, especially at the invasion front, could therefore lead to more rapid establishment and expansion of invasive species ranges (Thomas et al. 2001; Phillips et al. 2006; Bénichou et al. 2012).
The presence of sedentary individuals in a population may also promote the invasiveness of introduced species, yet many studies focus solely on the importance of mobile individuals within a population (e.g., Fobert et al. 2019). For example, sedentary individuals can contribute to establishment and resilience of metapopulations that serve to sustain introduced species’ populations. In this situation, sedentary fish occupy favorable habitat patches with sufficient resources, while mobile fish invade new habitats, potentially creating new subpopulations. If new, favorable habitats are not encountered by mobile fish and they die, subpopulations of sedentary fish persist to maintain the metapopulation. Although previously dispersing individuals may have failed to establish, the continued pressure by new mobile recruits sustained by sedentary individuals provides continued opportunities for successful spread. Without sedentary individuals in a population, highly mobile introduced species metapopulations may not be able to maintain viable subpopulations that can backstop failed dispersal. Therefore, it is important to improve our understanding of both sedentary and mobile individuals and their prevalence in invasive populations.
Sedentary and mobile individuals remained consistent in their movement type throughout this study. However, all fish in this study were likely mature (> 45 cm total length, Coulter 2015), and adult movements may not reflect movement behaviors throughout a fish’s entire life history (Morrissey and Ferguson 2011). Mobile or sedentary fish may have different advantages or disadvantages at different ontogenetic stages. For example, juvenile Mottled Sculpin (Cottus bairdii) that were more mobile grew faster than sedentary juveniles, but the opposite was true for adults (Petty and Grossman 2004). Previous studies have also documented periods of mobile and sedentary movements within the same individual (Alldredge et al. 2011). However, even when examining seasonal movements, mobile and sedentary fish in our study were consistently different from each other despite seasonal variation between movement types. Future work should evaluate the consistency of behavioral type at finer time scales and across varying ontogenetic stages.
In our study, movement distances of both sedentary and mobile fish were significantly related to total length, but the relationships were in contrasting directions (sedentary—negative; mobile—positive). Within a species, larger fish are physically more capable of moving longer distances; therefore, the trend of increasing movement with increasing total length we observed in mobile Silver Carp may be partially explained by swimming capability. In a different invaded river (Illinois River, USA), current management actions to reduce Silver Carp numbers use size selective capture gears (e.g., gill nets) and have reduced Silver Carp total lengths (Coulter et al. 2018). In this case, managers may be reducing the risk of spread posed by mobile individuals by selectively removing the largest fish in the population. Sedentary and mobile behavioral types are often also linked with other personality traits such as aggressiveness (mobile individuals) and sociability (sedentary individuals; Rasmussen and Belk 2012; Rehage et al. 2016). In the case of Silver Carp, perhaps sedentary individuals are also less aggressive, resulting in displacement of small sedentary individuals from preferred habitat and moving more than larger sedentary fish. Differences in other personality traits between sedentary and mobile individuals could contribute to the negative relationship of movement distance to total length. In the future, determining if any other traits are linked with dispersal could reveal additional factors that positively influence invasion success.
Dispersal syndrome refers to cases in which multiple traits, such as higher fecundity and survival, are related to dispersal tendencies (Clobert et al. 2009; Stevens et al. 2014). These traits may all interact to positively influence invasion success (Juette et al. 2014; Rehage et al. 2016). Additionally, aggressiveness is also often associated with dispersal and presence at an invasion front (e.g., Groen et al. 2012). Such aggressive individuals may be better able to obtain and defend resources, thus enhancing the impacts of the invasive species. Examinations of prevalence of specific genes or gene combinations are necessary to further compare sedentary and mobile individuals (Rasmussen and Belk 2017) and evaluate how traits linked with mobility may influence invasion success. Bigheaded carps (Hypophthalmichthys spp.) specifically have an additional complicating factor because individuals that appear to be Silver Carp may be hybrids of Bighead Carp (Hypophthalmichthys nobilis) and Silver Carp (Lamer et al. 2015). Hybrids may disperse differently, and hybrid groups could have greater or reduced prevalence of potentially risky mobile individuals (Coulter et al. 2020).
Management programs for invasive species often fail to account for individual variation in behavior (Juette et al. 2014). Removal programs that target specific behavioral types could result in a shift in behavioral types within the population, ultimately leading to alterations in population dynamics and ecosystem effects (Juette et al. 2014). A better understanding of individual heterogeneity will allow individual variation in movement and habitat use to be incorporated into models (Tyler and Rose 1994). Ideally these investigations will be coupled with genetic analyses to determine how observed movements match with dispersal (Morrissey and Ferguson 2011).
Data availability
Movement data analyzed during the current study are available in Supplementary File 2. Environmental data is publicly available, and sources are mentioned in the text.
References
Abdusamadov AS (1987) Biology of white amur (Ctenopharyngodon idella), silver carp (Hypophthalmichthys molitrix), and bighead (Aristichthys nobilis), acclimatized in the Terek Region of the Caspian Basin. J Ichthyol 26:41–49
Abeln J (2018) Environmental drivers of habitat use by bigheaded carps to inform harvest in the Starved Rock Pool of the Illinois River. M.S. thesis, Southern Illinois University—Carbondale
Alldredge P, Gutierrez M, Duvernell D, Schaefer J, Brunkow P, Matamoros W (2011) Variability in movement dynamics of topminnow (Fundulus notatus and F. olivaceus) populations. Ecol Freshw Fish 20:513–521. https://doi.org/10.1111/j.1600-0633.2011.00499.x
Barton K (2018) MuMIn: multi-model inference. R package version 1.42.1. https://CRAN.R-project.org/package=MuMIn
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Bénichou O, Calvez V, Meunier N, Voituriez R (2012) Front acceleration by dynamic selection in Fisher population waves. Phys Rev E 86:041908. https://doi.org/10.1103/PhysRevE.86.041908
Berthouly-Salazar C, Hui C, Blackburn TM, Gaboriaud C, van Rensberg BJ, van Vuuren BJ, Le Rous JJ (2013) Long-distance dispersal maximizes evolutionary potential during rapid geographic range expansion. Mol Ecol 22:5793–5804. https://doi.org/10.1111/mec.12538
Bonte D, Dahriel M (2016) Dispersal: a central and independent trait in life history. Oikos 126:472–497. https://doi.org/10.1111/oik.03801
Bonte D, De Roissart A, Wybouw N, Van Leeuwen T (2014) Fitness maximization by dispersal: evidence from an invasion experiment. Ecology 95:3104–3111. https://doi.org/10.1890/13-2269.1
Chapple DG, Simmonds SM, Wong BBM (2012) Can behavioral and personality traits influence the success of unintentional species introductions? Trends Ecol Evol 27:57–64. https://doi.org/10.1016/j.tree.2011.09.010
Chen C, Burton M, Greenberger E, Dmitrieva J (1999) Population migration and the variation of Dopamine D4 receptor (DRD4) allele frequencies around the globe. Evol Hum Behav 20:309–324. https://doi.org/10.1016/S1090-5138(99)00015-X
Chezik KA, Lester NP, Venturelli PA (2014) Fish growth and degree-days I: selecting a base temperature for a within population study. Can J Fish Aquat Sci 71:47–55
Clapp DF, Clark RD Jr, Diana JS (1990) Range, activity, and habitat of large, free-ranging brown trout in a Michigan stream. Trans Am Fish Soc 119:1022–1034. https://doi.org/10.1577/1548-8659(1990)119%3c1022:RAAHOL%3e2.3.CO;2
Clobert J, Le Galliard J, Cote J, Meylan S, Massot M (2009) Informed dispersal, heterogeneity in animal dispersal syndromes and the dynamics of spatially structured populations. Ecol Lett 12:197–209. https://doi.org/10.1111/j.1461-0248.2008.01267.x
Cote J, Fogarty S, Weinersmith K, Brodin T, Sih A (2010) Personality traits and dispersal tendency in the invasive mosquitofish (Gambusia affinis). Proc R Soc B 277:1571–1579. https://doi.org/10.1098/rspb.2009.2128
Coulter AA, Keller D, Amberg JJ, Bailey EJ, Goforth RR (2013) Phenotypic plasticiaty in the spawning traits of bigheaded carp (Hypophthalmichthys spp.) in novel ecosystems. Freshw Biol 58:1029–1037. https://doi.org/10.1111/fwb.12106
Coulter AA, Bailey EJ, Keller D, Goforth RR (2016a) Invasive Silver Carp movement patterns in the predominantly free-flowing Wabash River (Indiana, USA). Biol Invasions 18:471–485. https://doi.org/10.1007/s10530-015-1020-2
Coulter AA, Keller D, Bailey EJ, Goforth RR (2016b) Predictors of bigheaded carp drifting egg density and spawning activity in an invaded, free-flowing river. J Gt Lakes Res 42:83–89. https://doi.org/10.1016/j.jglr.2015.10.009
Coulter AA, Schultz D, Tristano E, Brey MK, Garvey JE (2017) Restoration versus invasive species: bigheaded carps’ use of a rehabilitated backwater. River Res Appl 33:662–669. https://doi.org/10.1002/rra.3122
Coulter DP, MacNamara R, Glover DC, Garvey JE (2018) Possible unintended effects of management at an invasion front: reduced prevalence corresponds with high condition of invasive bigheaded carps. Biol Conserv 221:118–126. https://doi.org/10.1016/j.biocon.2018.02.020
Coulter AA, Brey MK, Lamer JT, Whitledge GW, Garvey JE (2020) Early generation hybrids may drive range expansion of two invasive fishes. Freshw Biol 65:716–730. https://doi.org/10.1111/fwb.13461
Coulter AA (2015) Biology and ecology of bigheaded carps in an invaded ecosystem. Ph.D. thesis, Purdue University
Crook DA (2004) Is the home range concept compatible with the movements of two species of lowland river fish? J Anim Ecol 73:353–366
Crookes S, Heer T, Castañeda RA, Mandrak NE, Heath DD, Weyl OLE, MacIsaac HJ, Foxcroft LC (2020) Monitoring the silver carp invasion in Africa: a case study using environmental DNA (eDNA) in dangerous watersheds. NeoBiota 56:31–47. https://doi.org/10.3897/neobiota.56.47475
DeGrandchamp KL, Garvey JE, Colombo RE (2008) Movement and habitat selection by invasive Asian carps in a large river. Trans Am Fish Soc 137:45–56. https://doi.org/10.1577/T06-116.1
Dewey MR (1981) Seasonal abundance, movement and diversity of fishes in an Ozark stream. J Ark Acad Sci 35:33–39
Driscoll DA, Banks SC, Barton PS, Ikin K, Lentini P, Lindenmayer DB, Smith AL, Berry LE, Burns EL, Edworthy A, Evans MJ, Gibson R, Heinsohn R, Howland B, Kay G, Munro N, Scheele BC, Stirnemann I, Stojanovic D, Sweaney N, Villaseñor NR, Westgate MJ (2014) The trajectory of dispersal research in conservation biology. Systematic review. PLoS ONE 9:e95053. https://doi.org/10.1371/journal.pone.0095053
Fobert EK, Treml EA, Swearer SE (2019) Dispersal and population connectivity are phenotype dependent in a marine metapopulation. Proc R Soc B 286:20191104. https://doi.org/10.1098/rspb.2019.1104
Funk JL (1955) Movement of stream fishes in Missouri. Trans Am Fish Soc 85:39–57
Groen M, Sopinka NM, Marentette JR, Reddon AR, Brownscombe JW, Fox MG, Marsh-Rollo SE, Balshine S (2012) Is there a role for aggression in round goby invasion fronts? Behaviour 149:685–703
Gu H, Hughes J, Dorn S (2006) Trade-off between mobility and fitness in Cydia pomonella L. (Lepidoptera: Tortricadae). Ecol Entomol 31:68–74. https://doi.org/10.1111/j.0307-6946.2006.00761.x
Hobbs RJ, Valentine LE, Standish RJ, Jackson ST (2017) Movers and stayers: novel assemblages in changing environments. Trends Ecol Evol 33:116–128. https://doi.org/10.1016/j.tree.2017.11.001
Huveneers C, Simpfendorder CA, Kim S, Semmens JM, Hobday AJ, Pederson H, Stieglitz T, Vallee R, Webber D, Heupel MR, Peddemors V, Harcourt RG (2015) The influence of environmental parameters on the performance of detection range of acoustic receivers. Methods Ecol Evol 7:825–835. https://doi.org/10.1111/2041-210X.12520
Irons KS, Sass GG, McClelland MA, Stafford JD (2007) Reduced condition factor of two native fish species coincident with the invasion of non-native Asian carps in the Illinois River, U.S.A. Is this evidence for competition and reduced fitness? J Fish Biol 71:258–273. https://doi.org/10.1111/j.1095-8649.2007.01670.x
Juette T, Cucherousset J, Cote J (2014) Animal personality and the ecological impacts of freshwater non-native species. Curr Zool 60:417–427. https://doi.org/10.1093/czoolo/60.3.417
Kocovsky PM, Chapman DC, McKenna JE (2012) Thermal and hydrologic suitability for Lake Erie and its major tributaries for spawning of Asian carps. J Gt Lakes Res 38:159–166
Kolar CS, Lodge DM (2001) Progress in invasion biology: predicting invaders. Trends Ecol Evol 16:199–204. https://doi.org/10.1016/S0169-5347(01)02101-2
Kolar CS, Chapman DC, Courtenay WR, Housel CM, Williams JD, Jennings DP (2007) Bigheaded carps: a biological synopsis and environmental risk assessment, vol 33. American Fisheries Society Special Publication, Bethesda
Lamer JT, Ruebush BC, Arbieva ZH, McClelland MA, Epifanio JM, Sass GG (2015) Diagnostics SNPs reveal widespread introgressive hybridization between introduced bighead and silver carp in the Mississippi River Basin. Mol Ecol 24:3931–3943. https://doi.org/10.1111/mec.13285
Lowe WH, McPeek MA (2014) Is dispersal neutral? Trends Ecol Evol 29:444–450. https://doi.org/10.1016/j.tree.2014.05.009
Matsuzawa Y, Senou H (2008) Alien fishes of Japan. Bun-ichi Co. Ltd, Tokyo, p 160
Molnár T, Lehoczky I, Meleg EE, Boros G, Specziár A, Mozsár A, Vitál Z, Józsa V, Allele W, Urbányi B, Fatle FAA, Kovács B (2021) Comparison of the genetic structure of invasive bigheaded carp (Hypophthalmichthys spp) populations in Central-European lacustrine and riverine habitats. Animals 11:2018. https://doi.org/10.3390/ani11072018
Morrissey MB, Ferguson MM (2011) Individuals variation in movement through the life cycle of a stream-dwelling salmonid fish. Mol Ecol 20:253–248. https://doi.org/10.1111/j.1365-294X.2010.04921.x
Mossop KD, Moran NP, Chapple DG, Wong BBM (2017) Connectivity and habitat type shape divergent dispersal behavior in a desert-dwelling fish. Landsc Ecol 32:1065–1078. https://doi.org/10.1007/s10980-017-0509-8
Moyle PB (1986) Fish Introduction in to North America: patterns and ecological impact. In: Mooney HA, Drake JA (eds) Ecological and biological invasion of North America and Hawaii. Springer, New York, pp 27–43
Pendleton RM, Schwinghamer C, Solomon LE, Casper AF (2017) Competition among river planktivores: are native planktivores still fewer and skinnier in response to the Silver Carp invasion? Environ Biol Fish 100:1213–1222. https://doi.org/10.1007/s10641-017-0637-7
Petty JT, Grossman GD (2004) Restricted movement by mottled sculpin (pisces: cottidae) in a southern Appalachian stream. Freshw Biol 49:631–645
Phillips BL, Brown GP, Webb JK, Shine R (2006) Invasion and the evolution of speed in toads. Nature 439:803. https://doi.org/10.1038/439803a
Prechtel AR, Coulter AA, Etchison L, Jackson PR, Goforth RR (2017) Range estimates and habitat use of invasive Silver Carp (Hypophthalmichthys molitrix): evidence of sedentary and mobile individuals. Hydrobiologia 805:203–218. https://doi.org/10.1007/s10750-017-3296-y
Prechtel AR, Coulter AA, Etchison L, Jackson PR, Goforth RR (2018) Range estimates and habitat use of invasive Silver Carp (Hypophthalmichthys molitrix): evidence of sedentary and mobile individuals. Hydrobiologia 805:203–218. https://doi.org/10.1007/s10750-017-3296-y
R Core Team (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rasmussen JE, Belk MC (2012) Dispersal behavior correlates with personality of a North American fish. Curr Zool 58:260–270. https://doi.org/10.1093/czoolo/58.2.260
Rasmussen JE, Belk MC (2017) Individual movement of stream fishes: linking ecological drivers with evolutionary processes. Rev Fish Sci Aquac 25:70–83. https://doi.org/10.1080/23308249.2016.1232697
Rehage JS, Cote J, Sih A (2016) The role of dispersal behavior and personality in post-establishment spread. In: Weis JS, Sol D (eds) Biological invasions and animal behavior. Cambridge University Press, Cambridge, pp 96–116
Roy LL, Roy AG, Grant JWA, Bergeron NE (2012) Individual variability in the movement behavior of juvenile Atlantic salmon. Can J Fish Aquat Sci 70:339–347. https://doi.org/10.1139/cjfas-2012-0234
Sass GG, Hinz C, Erickson AC, McClelland NN, McClelland MA, Epifanio JM (2014) Invasive bighead and silver carp effects on zooplankton communities in the Illinois River, Illinois, USA. J Gt Lakes Res 40:911–921. https://doi.org/10.1016/j.jglr.2014.08.010
Shields R, Pyron M, Minder M, Etchison L (2021) Long-term trends in CPUE and relative weight of size fish species in the Wabash River, USA, prior to and following silver carp invasion. Hydrobiologia. https://doi.org/10.1007/s10750-021-04652-4
Stevens VM, Whitmee S, Le Galliard J, Clobert J, Böhning-Gaese K, Bonte D, Brändle M, Dehling DM, Hof C, Trochet A, Baguette M (2014) A comparative analysis of dispersal syndromes in terrestrial and semi-terrestrial animals. Ecol Lett 17:1039–1052. https://doi.org/10.1111/ele.12303
Stuart IG, Jones MJ (2006) Movement of common carp, Cyprinus carpio, in a regulated lowland Australian river: implications for management. Fish Manag Ecol 13:213–219. https://doi.org/10.1111/j.1365-2400.2006.00495.x
Stuck JG, Porreca AP, Wahl DH, Colombo RE (2015) Contrasting population demographics of invasive Silver Carp between and impounded and free-flowing river. N Am J Fish Manag 35:114–122. https://doi.org/10.1080/02755947.2014.986343
Thomas CD, Bodsworth EJ, Wilson RJ, Simmons AD, Davies ZG, Musche M, Conradt L (2001) Ecological and evolutionary processes at the expanding range margins. Nature 411:577–581
Trakhtenbrot A, Nathan R, Perry G, Richardson DM (2005) The importance of long-distance dispersal in biodiversity conservation. Divers Distrib 11:173–181. https://doi.org/10.1111/j.1366-9516.2005.00156.x
Tyler JA, Rose KA (1994) Individual variability of spatial heterogeneity in fish population models. Rev Fish Sci Aquac 4:91–123
White SL, Wagner T (2021) Behaviour at short temporal scales drives dispersal dynamics and survival in a metapopulation of brook trout (Salvelinus fontinalis). Freshw Biol 66:278–285. https://doi.org/10.1111/fwb.13637
Xie P, Chen Y (2001) Invasive carp in China’s plateau lakes. Science 294:999–1001
Acknowledgements
We thank D. Keller, T. Stefanavage, and C. Jansen for assisting with this research and several anonymous reviewers for comments which led to the improvement of this manuscript. We also thank the many Goforth lab students and technicians at Purdue University who have worked to collect the data used in this study, including P. Stipe, T. Thoren, J. Beugly, C. Cripe, W. Goldsmith, A. Escobedo, M. Gunn, E. Bailey, C. Rennaker, and A. Lenaerts.
Funding
Funding for this study was provided by the Indiana Department of Natural Resources (EDS E2-1-D639) and the Great Lakes Restoration Initiative awarded to Reuben R. Goforth. Funding from the National Institute of Food and Agriculture also supported R. Goforth and the Goforth lab during this study.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and design of the study, and revision of this manuscript. Additionally, all authors approve of the submitted manuscript. Data collection and initial writing of the manuscript were performed by Alison A. Coulter and Austin R. Prechtel. Alison A. Coulter performed data analyses and created figures/tables.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
Capture, handling, and surgical procedures described in this study was approved by the Purdue University Animal Care and Use Committee under protocol 09-040.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Coulter, A.A., Prechtel, A.R. & Goforth, R.R. Consistency of mobile and sedentary movement extremes exhibited by an invasive fish, Silver Carp Hypophthalmichthys molitrix. Biol Invasions 24, 2581–2596 (2022). https://doi.org/10.1007/s10530-022-02795-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-022-02795-6