Abstract
Many organisms must move among habitats to fulfill life history requirements. Fish movements have been widely studied and tend to be either fine-scale (i.e., routine) and governed by factors such as food availability and cover, or broad-scale and associated with spawning migrations. However, movements of invasive fishes in non-native ecosystems are comparatively poorly understood despite the often critical importance of fish movement and dispersal for invasion success. We examined invasive Silver Carp (Hypophthalmichthys molitrix) movements using acoustic telemetry to monitor the timing, distance, and direction of fish movements and assessed movements in relation to seasonal, annual, environmental, and individual factors in the Wabash River (Indiana, USA), a largely unregulated Midwestern river. Silver Carp exhibited highly variable movements that could be rapid and large in magnitude; however, tagged individuals remained stationary most of the time. Despite high variability, several trends emerged, indicating the importance of backwater habitats, avoidance of small tributaries, and tendencies of tagged fish to exhibit directed spring and fall broad-scale movements. Summer movements were smaller in magnitude, characterized by lower movement rates, and evenly split between upstream and downstream directions, although tagged Silver Carp moved more frequently during summer months. Our results indicate that specific seasons (i.e., spring and early fall) and locations (i.e., backwaters) are likely targets for Silver Carp control in the Wabash River and should also be useful targets for early detection and control in other largely unconstrained rivers over a broad geographic range (e.g., Great Lakes tributaries and upper Mississippi River mainstem and tributaries).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Invasive species’ movements are often critical for successful establishment and spread in novel environments (Kolar and Lodge 2001; Lindström et al. 2013; Perkins et al. 2013) and can also reflect species’ habitat preferences (Bain et al. 1988; Sharma and Jackson 2007; Belica and Rahel 2008; Rowe et al. 2009). Moreover, non-native ecosystems can provide novel cues for invasive species that may elicit unique behaviors compared to those within a species’ native range, thus yielding differences in habitat preferences and even life history attributes (Kolar et al. 2007; Coulter et al. 2013; Liu et al. 2015). Therefore, examining invasive species’ movements can yield valuable information on the biology and ecological impacts of invasive species as well as inform management and control efforts. Despite the potential utility of movement data for informing management efforts, relatively little is known about non-native fish movements in invaded ecosystems.
Movements allow individual fishes to utilize a variety of habitats and resources (Diana 1995) and are generally divided into two categories: broad-scale migrations and fine-scale, routine movements. Broad-scale fish migrations generally reflect seasonal movements, including those related to spawning (Taylor et al. 1996; Koel and Peterka 2003), and are often initiated by environmental cues such as temperature (e.g., Achord et al. 2007; Keefer et al. 2009; Dahl et al. 2004). In contrast, routine movements are usually associated with daily or short-term activities and influenced by food availability (Hill and Grossman 1993), foraging (Clough and Ladle 1997), predation (Gilliam and Fraser 1987; Fraser et al. 2006; Belica and Rahel 2008), and competition (Swan and Palmer 2000; Gilliam and Fraser 2001; Kahler et al. 2001; Fraser et al. 2006). Variation in all types of movements can be based on individual factors including body size (Gowan and Fausch 1996; Skalski and Gilliam 2000; Roberts and Angermeier 2007), morphology (Hanson et al. 2007), sex (Hanson et al. 2008), and maturity (Hutchings and Gerber 2002; Croft et al. 2003; Albanese et al. 2004; Petty and Grossman 2007). Therefore, temporal, ecosystem, and individual factors may all impact the movements and dispersal of invasive fish.
Silver Carp (Hypophthalmichthys molitrix) have spread rapidly throughout many non-native freshwater ecosystems and exhibit broad-scale spawning migrations apparently triggered by changes in water level and water temperature (Abdusamadov 1987; Peters et al. 2006; Kolar et al. 2007; DeGrandchamp et al. 2008; Calkins et al. 2012). Silver Carp habitat varies from the native range, where mature fish primarily occupy lentic habitats, to invaded ranges, where fishes are often found in lotic systems (Kolar et al. 2007); although, backwater lentic-like habitat may also be used in invaded rivers (DeGrandchamp et al. 2008). Recent observations suggest that Silver Carp behavior in North America differs from the native range (e.g., Coulter et al. 2013; Deters et al. 2013), and previous conclusions regarding the behavioral ecology in native ranges of Silver Carp may thus be inaccurate in invaded environments. Models have been developed to predict the invasion of Silver Carp for management purposes, although data from closely related species and/or information collected from native ecosystems have been primarily used to build the models (e.g., Cooke and Hill 2010). Understanding behavioral plasticity in Silver Carp movements across invaded ecosystems would not only contribute to more robust models to predict future invasions and spread, it would also provide additional evidence to support the idea that plasticity is a valuable shared trait among invasive species (e.g., Davidson et al. 2011).
We examined possible predictors of movement using model selection procedures. The study objective was to examine Silver Carp movement patterns to detect trends in direction, rate, and magnitude and to relate movements to individual (e.g., sex), seasonal (e.g., DOY, month, year), and environmental (e.g., water level) characteristics. We hypothesized that spawning behavior would yield broad-scale upstream movements in the spring associated with changes in water levels and temperature known to initiate spawning. Additionally, we hypothesized that post-spawning movements would be smaller in magnitude and less directed (i.e., not predominantly upstream or downstream). As a large river with limited backwater habitat available and a single mainstem dam, the Wabash River (Indiana, USA) is unique compared to other rivers in which Silver Carp movements have been studied (e.g., DeGrandchamp et al. 2008; Calkins et al. 2012) and is more similar to rivers susceptible to future invasion (e.g., tributaries of the Laurentian Great Lakes and upper Mississippi River).
Methods
Study area
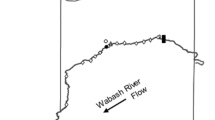
The Wabash River, Indiana, USA, has only one mainstem dam located upstream of the study area, with ≈660 km of free-flowing river below the dam. The watershed is 85,326 km2 with ≈66 % agricultural land use. Upper reaches of the river are higher velocity with exposed bedrock substrate while lower portions are dominated by sandy substrates (Gammon 1998). This study focused on the upper and middle portions of the Wabash River (Fig. 1). There are few backwater habitats in this portion of the river, although two are present near the middle of the study area. Backwater 1 is connected to the main channel except during drought conditions (i.e., summer 2012), and Backwater 2 is connected to the main channel ≈50 % of the year. The Tippecanoe River is the largest tributary in the upper and middle reaches of the Wabash River (5050 km2 watershed; Hoggatt 1975), while the Little, Eel, and Salamonie Rivers are smaller tributaries in the study area (740, 1440, and 2110 km2 respectively; Hoggatt 1975). The Little River has been identified as a potential hydrological connection between the Mississippi River Basin and the Great Lakes by which Silver Carp may invade the Great Lakes (USACE 2010).
Locations of Vemco VR2W stationary receivers within the Wabash River, Indiana, USA, indicated by circles. Area included in the enlarged image of the Wabash River is indicated by the black square. Area of river covered by active tracking is included in the shaded square. Scale bar is for enlarged area of Wabash River
Surgery
Silver Carp to be implanted with acoustic transmitters were captured from river kilometer (RKm) 499 to RKm 600 of the Wabash River, from 2011 to 2013, using boat electrofishing (Model SR16H; Smith-Root Inc., Vancouver, WA, USA). The electrofishing boat was powered by a generator with a pulsator running at either 3–4 A of direct current at 30 pulses s−1 and 20–50 % range of pulse width or 7–8 A of direct current at 120 pulses s−1. In accordance with the manufacturer’s recommendations, only fish of sufficient size such that tag weight was <2 % of individual’s body mass were retained from electrofishing samples to be implanted with transmitters (Winter 1996).
Fish to be implanted with telemetry tags were sedated using electroanesthesia and measured for total length (mm) and mass (g). Sex was determined, if possible, through the expression of eggs or milt or visualization of the gonads within the surgical field (classification: male, female, unknown). Fish to be tagged were prepared for an incision on the left side of the body by removing scales from a 5 cm × 3 cm patch on the ventral portion of the body between the anal and pelvic fins. The cleared area was treated with betadine to minimize infection risk and a ≈5 cm incision was made into the coelomic cavity of the fishes with care given not to damage internal organs. A Vemco (Bedford, Nova Scotia, Canada) V16-4H 69 Hz (28 g, 63 × 16 mm) individually coded acoustic transmitter was inserted into the coelomic cavity, and the incision was closed with three sutures (PDS II violet monofilament absorbable sutures model Z970, Ethicon, Inc., Sommerville, NJ, USA). All handling was completed in ≈2 min, and surgical instruments were sterilized in 90 % ethanol between fish. Tagged individuals were returned to the river within 2 km of the site of capture once equilibrium was recovered. Fishes tagged in 2011 were visually identified as Silver Carp, Bighead Carp (H. nobilis), or hybrid bigheaded carp based on morphology. Fin clips were taken from the left pectoral fin of fishes tagged in 2012 and 2013 and stored at −80 °C for later genetic identification using polymerase chain reaction (PCR) as described below. Only telemetry data from fish visually (2011) or genetically (2012–2013) identified as Silver Carp were utilized for this study.
Genetics
Genetic material from fin clips was used to distinguish Silver Carp from Bighead Carp and hybrid bigheaded carp as the latter can appear morphologically similar to Silver Carp (Lamer et al. 2010). Extracted DNA from fin clips was obtained using QIAmp mini DNA kits (Qiagen Inc., Valencia, CA, USA). Samples were run in duplicate with negative controls using nuclease free water with three previously developed primers (Mia et al. 2005). Each reaction totaled 20 µl with 5 µM each of forward and reverse primers, 50 ng µl−1 DNA template, SensiMix (Bioline USA, Inc., Tauton, MA, USA), and nuclease-free water with a total of 50 ng of genomic DNA. Conditions for PCR were as follows: 94 °C for 2.5 min, 50 °C for 45 s, and 72 °C for 1 min for 45 amplification cycles with an extension period of 72 °C for 10 min. Results were visualized in a 2 % agarose gel run for 75 min at 3.8 V cm−1 and interpreted by basepair length and banding pattern as described in Mia et al. (2005). Comparison of visual and genetically identified individuals (2012 and 2013 fish) indicated that there was a 9 % chance that fish visually identified as Silver Carp were actually hybrids.
Telemetry
Detections of tagged Silver Carp were collected using both stationary receivers and active tracking. Active tracking allows for fine-scale, routine movements to be detected, while stationary receivers provide continuous monitoring that is useful for detecting broad-scale movements of individual fishes. Omnidirectional stationary receivers (Vemco VR2W) were deployed using custom rebar stands on the river bottom in tributaries (Eel River, Little River, Salamonie River, Tippecanoe River) and at multiple locations in the Wabash River, including the two backwaters (Fig. 1), covering ≈400 river kilometers (RKm). This was judged to be sufficient to cover the potential range of tagged Silver Carp based on maximum movements of ≈430 km in the Illinois River (DeGrandchamp et al. 2008).
The number and location of stationary receivers active at any given time varied due to occasional losses of the VR2Ws. Stationary receivers were tested to evaluate detection efficiency using a Vemco-supplied range testing tag at initial deployment and were found to detect the tag across the entire channel width. However, changes in water level and turbidity throughout the study may have impacted the range at which telemetered fish were detected, thus allowing the potential for undetected passage of tagged fishes in the vicinity of stationary receivers. Stationary receivers from RKm 565 upstream were deployed on bedrock, the RKm 545 stationary receiver was deployed over cobble, and the remaining Wabash River stationary receivers were over sandy substrate. Stationary receivers in tributaries were placed 1–2 km upstream of river confluences to avoid detections of fish in the Wabash River main channel. Backwater stationary receivers were not placed directly inside the entrances to minimize detections from the main channel. Overall, stationary receivers were placed near parks or boat ramps to allow easy maintenance and downloading. Stationary receivers were also placed in areas that tended to be deeper than the surrounding river to prevent exposure of the equipment during low-flow periods.
Active tracking was performed by deploying hydrophones from a boat or canoe traveling downstream at <8 km h−1 covering ≈100 RKm. An omnidirectional hydrophone (Vemco VH110) connected to a manual receiver (Vemco VR100) was used to locate tagged Silver Carp. Once a detection of ≥75 dB was achieved on the manual receiver, the position of the tagged fish was recorded using a handheld GPS (GPSMap 60, Garmin Ltd., Olathe, KS, USA; ±10 m). Additional detections (n = 3) of tagged Silver Carp in 2012 and 2013 were collected by the R.E. Columbo lab at Eastern Illinois University (Charleston, IL, USA) in the lowest reaches of the Wabash River (≈RKm 200-0).
Movement trends
To examine movement patterns, movement rate and distance were determined as follows. Silver Carp detections were imported into ArcMap 10.1 (ESRI, Redlands, CA, USA) to measure movement distances and direction. Movement distances and rates represent minimum estimates of movements because individuals may move outside of the study area or in between the ranges of the stationary receivers. Distances were measured between consecutive detections for each detected fish and were considered to constitute a movement if >50 m. 50 m was used as a cutoff for movement based on the error during tracking (GPS locations). Only one detection per location, per fish, per day was used when examining movements to minimize autocorrelation, and no detections within 48 h of tagging were included included. Movements were not carried over between years as limited data were available for winter months. Movement rate was calculated as the distance moved per day (km day−1). For comparison among months and years, mean movement rate and distance were calculated for the time period of interest (e.g., month or year). Separate movements (not averaged but month or year) were used in the movement distance, rate, and probability models.
To assess trends in movement distance and rate, movements were compared using unbalanced analysis of variance (ANOVA), including only months in which enough data were available (March–October). ANOVAs contained month and year as factors and also included the interaction of month and year, and a random effect of individual Silver Carp to account for possible lack of independence of movements coming from the same individual. Post hoc pairwise comparisons were done using Tukey’s test on significant variables.
Growing degree day (GDD) and cumulative growing degree day (CGDD) with a base of 10 °C were calculated based on temperature data from a weather station near RKm 498. No water temperature data were available, so GDD was used as an indicator of daily water temperature. Water temperatures measured on select dates in 2012 and 2013 and were significantly correlated with GDD (r = 0.655, p < 0.0001, n = 33). CGDD is used in risk assessments (e.g., Kocovsky et al. 2012) and can indicate when Silver Carp spawning may occur, which is known to relate to movement. Water level information was obtained from a US Geological Survey (USGS) river gage (#03335500; Fig. 2) near RKm 498. Current gage height was the gage level at 12:00 p.m. on a given day and change in gage height over 24 h was the change in water level from 12:00 p.m. the day before to 12:00 p.m. on the current day. Positive values indicated rising water levels and negative values indicated declining water levels. Similarly, the change in gage height over 48 h was the change in water level from 12:00 p.m. 2 days before to 12:00 p.m. on the current day. Models were parameterized using values for the day the movement was completed.
Model selection
To determine ecological variables impacting movement distance and rate, all possible subsets of linear mixed-effects models (lme in Package ‘nlme’; http://cran.r-project.org/web/packages/nlme/index.html) were evaluated using Akaike’s Information Criterion with small sample size correction (AICc). The full models for movement distance and rate included gage height, change in gage height over 24 h, change in gage height over 48 h, GDD, CGDD, day of year (DOY), month, year, and sex to reveal potential predictors for movements. Month, year, and sex were categorical while all other variables were continuous and calculated as previously described. Pearson’s correlations were used to determine if variables were uncorrelated and change in gage height over 48 h was removed as it was strongly correlated with change in gage height over 24 h (r = 0.9, p < 0.0001). Only data from March to October were used in models due to low sample size in other months. All models contained a random effect of individual fishes, as movements made by the same fish could not be assumed to be independent. A logistic mixed-effects model, the same model selection procedures, and the same full model parameters were also used to examine predictors of Silver Carp movement probability: move (1) or remain stationary (0). Reported models, and those which were included in model averaging, were models with ΔAIC < 2.00. Significant coefficients in the averaged model were examined. All analyses were done in R (v. 2.14.1).
Results
Movement trends
Total lengths of tagged Silver Carp ranged from 374 to 940 mm, with a mean total length of 699.3 mm ± 4.26SE. Overall, individual Silver Carp averaged 1194 ± 586SE detections fish−1 (Table 1). Movement data from 184 tagged fishes indicated movements >50 m and were used for analysis. Fishes not included for analysis were individuals with either a single detection, those detected at only one stationary receiver, or individuals with all movements <50 m. Individuals suspected of being deceased were also eliminated from analyses. Detections indicated no movement 92.3 % of the time, with the greatest percentages of fish moving >50 m occurring in May–July (Fig. 3a). Fishes exhibiting movements >50 m moved an average distance of 12.4 ± 1.0SE km at an average rate of 4.4 ± 0.38SE km day−1 (n = 184), although movement distance and rate varied over time (Fig. 3b). The greatest distances and most rapid movement rates occurred in September and October, with a secondary peak in March. The largest movement detected was 409 km (August 2012), and the most rapid movement rate was just over 95 km in a single day (September 2013). Both movement distance and rate declined through the summer, with the lowest values occurring in July and August. Silver Carp movements were predominantly upstream directed in March and April and predominantly downstream directed in September and October, while movements were evenly split between upstream and downstream from May to August (Fig. 3c).
Summary of Silver Carp movements by month. a Proportion of consecutive detections indicating no movement. Of those individuals that did move. b Mean (1/2SD) minimum movement rate and distances varied by month. c Directionality of Silver Carp movements upstream and downstream of a previous detection. Movements from January, February, November, and December were not included as small sample sizes prevented accurate comparisons (n = 1, 2, 8, and 1 respectively)
Silver Carp mean movement distances were statistically similar among years (F2,1550 = 27.34, p < 0.0001). There was no significant effect of month or the interaction between month and year on movement distance (Fig. 4a, b). Movement rates were different among years (F2,1550 = 44.45, p < 0.0001), with the highest movement rates observed in 2013. Movement rates showed a significant interaction between month and year, indicating that the effect of month was inconsistent among years (F10,1533 = 16.17, p < 0.0001; Fig. 4c). Movement rates were higher from May to August 2013 compared to the same months in 2011 and 2012. Within a given year, movement rate was always greater in October compared to all other months except September. For all 3 years, movement rates were greater in September than May–August of the same year. Movement rate in March 2012 was greater than May–August of 2012. Silver Carp exhibited greater frequencies of smaller movements from May to August, while other months included greater frequencies of broad-scale movements (Fig. 5). The trend of greater numbers of smaller movements in the summer and more broad-scale movements in the spring and fall was consistent across years.
Minimum movement distances and rates from 2011 to 2013 across months included in two-factor blocked ANOVA (month and year) with an individual Silver Carp random effect. a Movement distance and rate (1/2 SD) was greater in 2013 than in 2011 and 2012. Mean monthly b movement distance (1/2 SD) and c movement rate (1/2 SD) of Silver Carp from 2011 to 2013. The highest movement rates for most months were in 2013 (>2011 in June and August; >2012 in May–July). September had significantly higher movements rates (2011–2013) as did October (2011) than summer months (May–August). In 2012, March also had higher movement rates than summer months (May–August)
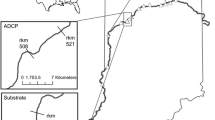
Numbers of Silver Carp at specific stationary receivers varied over relatively short periods of time (Fig. 6). Number of detections fish−1 was consistently high in Backwaters 1 (3987 ± 667SE, 179 individuals) and 2 (7556 ± 1458SE, 74 individuals), as well as RKm 497 (3042 ± 1204 SE, 28 individuals; Table 2). RKm 545, 521, 510 exhibited moderate numbers of detections fish−1 (<121 detections fish−1). Downstream stationary receivers at RKm 479, 344, and 265 recorded <46 detections fish−1. All other upstream stationary receivers (RKm 600, 627, 653) in the Wabash River main channel showed <11 detections fish−1 (19, 11, and 5 individuals). The stationary receiver at RKm 565 had very high mean detections fish−1 (>20,000). No Silver Carp were detected in the Little, Salamonie, or Eel Rivers, but tagged fishes were detected in the remaining Wabash River tributary monitored in this study, the Tippecanoe River (79 ± 14SE detections fish−1, 54 individuals).
Number of tagged Silver Carp located and hydrograph at different locations on the Wabash River, Indiana, USA, in 2013. Days of year (DOY) included were selected to cover pre-spawn and spawning time periods. Shaded area in (a) indicates time period enlarged in (b–d). Locations of the stationary receivers (VR2Ws) for which data are displayed are: a, b Backwater 1 located near river kilometer (RKm) 499; c RKm510; and d RKm521. Dashed line is gage height and solid line is number of tagged fish
Model selection
Movement distance had three best models with ΔAIC < 2 (Table 3) all of which contained gage height, DOY, month, and year. Movement rate had two best models (Table 4), which both included gage height, GDD, CGDD, DOY, month, and year. Model averaging of best models for movement distance and rate both exhibited significant positive effects of river gage height and GDD (Table 5). The movement distance averaged model also contained significant positive effects of DOY, month (August, September, October), and year (2012, 2013). Of the months with significant effects, October had the greatest positive effect on movement distances. 2013 had a greater effect than 2012 in the averaged model. The averaged movement distance model also contained a significant negative effect of DOY.
In addition to significant effects of gage height and GDD, averaging of the best movement rate models also had significant positive effects for DOY and year. All months except for September and October had significant effects in the averaged model. All significant month effects were negative except for March. However, averaged models were only able to explain a small portion of the variability in the observed data (distance: R2 = 0.23; rate: R2 = 0.28).
The two best models to predict whether Silver Carp will move or remain stationary (i.e., movement probability) both contained gage height, 24 h change in gage height, CGDD, sex, DOY, month, and year (Table 6). The averaged model contained a significant negative effect of gage height, while change in gage height over 24 h had a significant positive effect. CGDD and DOY both had significant negative effects in the model. Female and unknown sex Silver Carp also had significant negative effects in the averaged model. Years had significant negative effects in the averaged model. 2013 had the greatest negative effect and 2012 had the least negative coefficient. All months except for April had significant effects in the averaged model. October had the greatest positive coefficient, while May had the smallest positive value. March was the only month with a negative effect.
Discussion
Silver Carp in the Wabash River exhibited multiple trends in movement direction, distance, and rate that were largely consistent with study expectations. For example, Silver Carp made broad-scale upstream movements in the spring 1–2 months prior to the onset of spawning, presumably to stage until environmental conditions became optimal for spawning. Silver Carp also exhibited expected downstream movements in fall, likely for overwintering. Similar directed movements have been observed in other fishes (Manion 1977; Lucas and Batley 1996; Ovidio and Philippart 2002) and likely reflect seasonal changes in habitat requirements (e.g., temperature, Meyers et al. 1992). Movements from May to August were less directed (50 % upstream/downstream) and smaller in magnitude, as expected. Many fish species have been found to move with greater frequency during the summer (Matheney and Rabeni 1995; Lucas and Batley 1996; Albanese et al. 2004), and summer movements of Silver Carp also occurred more often (i.e., there were fewer stationary fish during the summer months). Summer movements probably represented routine movements associated with foraging and searching for suitable habitat with seasonally declining water levels. Frequent summer movements may also have been associated with fish moving from staging areas to engage in protracted spawning events known to occur throughout the summer (Coulter et al. 2013).
Silver Carp exhibited year-to-year variation in movements in the Wabash River, with greater movement distances and rates in 2013. Larger fish are often associated with deeper habitats (Harvey and Stewart 1991), and so higher water levels would be expected to allow Silver Carp to move more easily over greater distances, while lower water levels should negatively affect movement distances and rates. 2012 was a severe drought year with considerably lower water levels throughout much of the year compared to 2013, and this appeared to have a negative effect on both Silver Carp movement distance and rate. Interestingly, 2011 also exhibited a negative effect on movement rate and distance despite generally similar water levels compared to 2013. This may have been due, in part, to the lower number of tagged fish at large during this first year of the study. Additionally, changes in climatic conditions may also have influenced annual variability in fish movements. In this case, the juxtaposition of a severe drought year (2012) and a comparably much wetter year that included one of the highest gage heights on record for USGS gaging station #03335500 (2013) may have triggered greater movement activity in the latter year. However, additional telemetry data over a longer time period that include additional comparable climate events are needed to test this hypothesis.
Water level has previously been shown to play a role in invasive Silver Carp movements (Abdusamadov 1987; Peters et al. 2006; DeGrandchamp et al. 2008; Calkins et al. 2012), and this was also the case in the present study. Movement distance and rate were positively related with water level, and numbers of tagged fishes at specific stationary receivers changed in conjunction with water level changes. The importance of water level is further illustrated by the increased movement distances and rates observed in the spring and fall, when water levels are typically higher. Rising and falling water levels are common triggers of upstream and downstream movements in stream fishes (Manion 1977; Reynolds 1983; Lucas and Batley 1996), and fish movements, including routine movements, are also known to be greatly influenced by flow (Taylor and Cooke 2012). Relationships of movements with water levels support the hypothesis that annual variation in movements may also be influenced by inter-annual, climatically driven water level differences as previously indicated.
The overall fit of the averaged models for movement distance and rate was relatively poor (R2 < 0.3), suggesting that other variables such as food availability (Calkins et al. 2012) or water velocity (Kolar et al. 2007; DeGrandchamp et al. 2008) may strongly influence Silver Carp movements. Additionally, variation may occur at the individual level because some individuals may be more sedentary while others are more mobile (Bradford and Taylor 1997; Fraser et al. 2001; Morrissey and Ferguson 2011). This strategy would be advantageous for insuring that at least some individuals remain in suitable habitat (i.e., sedentary fish) while others disperse in search of additional viable habitats that have not yet been colonized (i.e., mobile or transient fish). Indeed, mobile individuals in Silver Carp populations have no doubt contributed strongly to the species’ spread, while sedentary individuals have maintained local populations throughout the introduced range in the Mississippi River basin. Regardless, environmental cues are often important for initiating life history events and associated movements (Bennett and Burau 2014; Crook et al. 2014; Winkler et al. 2014; Pagon et al. 2013; Schmaljohann et al. 2012), and this study highlights the influence of water levels on the movements of a large, invasive riverine fish.
Averaged models for Silver Carp movement distance and rate both included a significant positive effect of temperature (GDD). Water temperature is known to influence the onset of Silver Carp spawning (Abdusamadov 1987; Kocovsky et al. 2012), and it was therefore expected to influence movements of tagged fishes in this study. Additionally, temperature is a critical variable that strongly influences the metabolism and behaviors of poikilotherms, including fishes. Thus, movement for thermoregulatory purposes is common in fish (Baird and Krueger 2003; Armstrong et al. 2013; Westhoff et al. 2014), and increasing temperatures can increase movements (Beamish 1970). Movement distance and rate were positively influenced by GDD. However, CGDD exhibited a significant negative effect on Silver Carp movement rate and probability. While a minimum CGDD is required for Silver Carp spawning to occur, the high CGDD occurring later in the year when some of the most rapid movements occurred may have resulted in the observed negative effect.
Females and unknown sex Silver Carp both had significant negative effects on movement probability. Silver Carp of unknown sex included some individuals that were immature at the time of tagging. Immature fishes may move less because they do not engage in broad-scale spawning movements (Shaw et al. 2013). The lower movement probability observed in females may relate to physiological processes (e.g., conserved energy for egg development) or sex-biased dispersal. Differences in dispersal distances between sexes have been observed in other fish species (Hutchings and Gerber 2002), including other invasive fishes (Marentette et al. 2011), and may function to minimize inbreeding (Pusey 1987; Perrin and Mazalov 2000) and thus promote invasion success.
Stationary receivers in both backwaters had high numbers of detections, with over 70 tagged Silver Carp present on some occasions. Previous studies of Silver Carp in invaded rivers indicate a preference for backwater and lower velocity habitats (DeGrandchamp et al. 2008; Calkins et al. 2012). Backwater habitats therefore represent likely targets for control efforts. The use of lower velocity habitats may function to conserve energy and provide access to resources that may not be as abundant in the river mainstem (e.g., zooplankton communities; Burdis and Hoxmeier 2011). Resources and environmental conditions may also differ among rivers and tributaries, and most Wabash River tributaries had no detections of Silver Carp. The Tippecanoe River, the largest tributary in the study area, did show some use by several tagged individuals, with one individual moving >10 km upstream. However, the limited use of the Tippecanoe River by tagged fishes and absence of detections in smaller tributaries suggest that small streams are resistant to Silver Carp invasion, especially for larger adults, possibly resulting from lower water levels, increased velocity, or lower primary productivity. Recent work has also suggested that some smaller tributaries to the Missouri River may be resistant to invasion by Silver Carp (Hayer et al. 2014), and further investigations are necessary to determine what factors (e.g., biotic resistance, habitat limitations) may inhibit expansion into small tributaries. It should also be noted that, while tagged Silver Carp were in the Wabash River near the mouth of the Little River, no tagged individuals traveled up the Little River, a possible corridor for invasion of the Great Lakes basin (USACE 2010).
As in many telemetry studies, the placement and spacing of stationary receivers may have influenced some of observed trends. Stationary receivers allow an estimation of minimum movement distances and rates, and individuals must move as far as the next stationary receiver to be categorized as moving or may leave the study area. Active tracking allowed for the detection of smaller movements, but detections from active tracking represent a relatively small portion of the collected data. Movements observed in telemetry studies may also be influenced by tag effects (e.g., Frank et al. 2009); however, the large number of movements, the repetition of spring and fall trends across years, and the exclusion of any detections collected within 48 h of tagging likely minimized the influence of tagging observed patterns.
Invasive fish movements can provide insight into factors that contribute to invasiveness and how invasion success may vary across non-native ecosystems. For example, Common Carp (Cyprinus carpio) experience frequent hypoxia in native waters (Stecyk and Farrell 2007) and are thus able to move into hypoxic areas of invaded ecosystems that are unexploited by native fishes to spawn competition free. This ultimately contributes to high levels of reproduction and recruitment by Common Carp during hypoxic years (McNeil and Closs 2007; Bajer and Sorensen 2010). In the present study, Silver Carp generally were occasionally highly mobile, especially during spring and fall, allowing them to potentially exert substantial propagule pressure on non-invaded ecosystems via dispersal through connected waters. Such high mobility has likely contributed strongly to the rapid invasion and spread of Silver Carp throughout much of the middle and lower Mississippi River basin. Moreover, Silver Carp were responsive to environmental cues (i.e., water level changes, temperature) provided in the Wabash River, thus allowing them to move appropriately for critical life history events, such as staging, spawning, and overwintering. Control efforts may be targeted to times of high mobility or when fish are stationary and in backwaters, depending on the management technique employed. For example, temporary barriers to movement coinciding with predictable movement behaviors could be implemented to disrupt the ability of Silver Carp to move adequately to fulfill life history stages (e.g., broad scale-migrations to viable spawning and overwintering habitats and routine movements from staging areas to spawning habitats).
Silver Carp exhibit a high degree of plasticity in movements (i.e., variability in movements among years) and have the potential to rapidly disperse into and persist in non-native ecosystems. However, the observed variation does exhibit strong seasonal patterns in movement distance, rate, and directionality that allow for the prediction of dispersal patterns that could be incorporated into dispersal models and control strategies.
References
Abdusamadov AS (1987) Biology of white amur (Ctenopharyngodon idella), silver carp (Hypophthalmichthys molitrix) and bighead (Aristichthys nobilis), acclimatized in the Terek Region of the Caspian Basin. J Ichthyol 26:41–49
Achord S, Zabel RW, Sandford BP (2007) Migration timing, growth, and estimated parr-to-smolt survival rates of wild snake river spring-summer Chinook salmon from the Salmon River Basin, Idaho, to the lower Snake River. Trans Am Fish Soc 136:142–154. doi:10.1577/T05-308.1
Albanese B, Angermeier PL, Dorai-Raj S (2004) Ecological correlates of fish movement in a network of Virginia streams. Can J Fish Aquat Sci 61:857–869. doi:10.1139/F04-096
Armstrong JB, Schindler DE, Ruff CP, Brooks GT, Bentley KE, Torgersen CE (2013) Diel horizontal migration in streams: juvenile fish exploit spatial heterogeneity in thermal and trophic resources. Ecology 94:2066–2075
Bain MB, Finn JT, Booke HE (1988) Streamflow regulation and fish community structure. Ecology 69:382–392
Baird OE, Krueger CC (2003) Behavioral thermoregulation of brook and rainbow trout: comparison of summer habitat use in an Adirondack River, New York. Trans Am Fish Soc 132:1194–1206
Bajer PG, Sorensen PW (2010) Recruitment and abundance of an invasive fish, the common carp, is driven by its propensity to invade and reproduce in basins that experience winter-time hypoxia in interconnected lakes. Biol Invasions 12:1101–1112. doi:10.1007/s10530-009-9528-y
Beamish FWH (1970) Oxygen consumption of largemouth bass Micropterus salmoides, in relation to swimming speed and temperature. Can J Zoolog 48:1221–1228
Belica LAT, Rahel FJ (2008) Movements of creek chub, Semotilus atromaculatus, among habitat patches in a plains stream. Ecol Freshw Fish 17:258–272. doi:10.1111/j.1600-0633.2007.00277.x
Bennett WA, Burau JR (2014) Riders on the storm: selective tidal movements facilitate the spawning migration of threatened delta smelt in the San Francisco estuary. Estuaries Coasts 2014:1–10. doi:10.1007/s12237-014-9877-3
Bradford MJ, Taylor GC (1997) Individual variation in dispersal behaviour of newly emerged chinook salmon (Oncorhynchus tshawytscha) from the Upper Fraser River, British Columbia. Can J Fish Aquat Sci 54:1585–1592
Burdis RM, Hoxmeier RJH (2011) Seasonal zooplankton dynamics in main channel and backwater habitats of the Upper Mississippi River. Hydrobiologia 667:68–87
Calkins HA, Tripp SJ, Garvey JE (2012) Linking silver carp habitat selection to flow and phytoplankton in the Mississippi River. Biol Invasions 14:949–958. doi:10.1007/s10530-011-0128-2
Clough S, Ladle M (1997) Diel migration and site fidelity in a stream-dwelling cyprinid, Leuciscus leuciscus. J Fish Biol 50:1117–1119. doi:10.1111/j.1095-8649.1997.tb01635.x
Cooke SL, Hill WR (2010) Can filter-feeding Asian carp invade the Laurentian Great Lakes? A bioenergetic modelling exercise. Freshw Biol 55:2138–2152. doi:10.1111/j.1365-2427.2010.02474.x
Coulter AA, Keller D, Amberg JJ, Bailey EJ, Goforth RR (2013) Phenotypic plasticity in the spawning traits of bigheaded carp (Hypophthalmichthys spp.) in novel ecosystems. Freshw Biol 58:1029–1037. doi:10.1111/fwb.12106
Croft DP, Albanese B, Arrowsmith BJ, Botham M, Webster M, Krause J (2003) Sex-biased movement in the guppy (Poecilia reticulata). Oecologia 137:62–68. doi:10.1007/s00442-003-1268-6
Crook DA, Macdonald JI, Morrongiello JR, Belcher CA, Lovett D, Walker A, Nicol SJ (2014) Environmental cues and extended estuarine residence in seaward migrating eel (Anguila australis). Freshw Biol 59:1710–1720. doi:10.1111/fwb.12376
Dahl J, Dannewitz J, Karlsson L, Petersson E, Löf A, Ragnarsson B (2004) The timing of spawning migration: implications of environmental variation, life history, and sex. Can J Zool 82:1864–1870. doi:10.1139/Z04-184
Davidson AM, Jennions M, Nicotra AB (2011) Do invasive species show higher phenotypic plasticity than native species and if so, is it adaptive? A meta-analysis. Ecol Lett 14:419–431. doi:10.1111/j.1461-0248.2011.01596.x
DeGrandchamp KL, Garvey JE, Colombo RE (2008) Movements and habitat selection by invasive Asian carps in a large river. Trans Am Fish Soc 137:45–56. doi:10.1577/T06-116.1
Deters JE, Chapman DC, McElroy B (2013) Location and timing of Asian carp spawning in the Lower Missouri River. Environ Biol Fish 96:617–629. doi:10.1007/s10641-012-0052-z
Diana JS (1995) Biology and ecology of fishes. Cooper Publishing Group LLC, Carmel
Frank HJ, Mather ME, Smith JM, Muth RM, Finn JT, McCormick SD (2009) What is “fallback”?: metrics neede to assess telemetry tag effects on anadromous fish behavior. Hydrobiologia 635:237–249
Fraser DF, Gilliam JF, Daley MJ, Le AN, Skalski GT (2001) Explaining leptokurtic movement distributions: intrapopulation variation in boldness and exploration. Am Nat 158:124–135
Fraser DF, Gilliam JF, Albanese BW, Snider SB (2006) Effects of temporal patterning of predation threat on movement of a stream fish: evaluating an intermediate threat hypothesis. Environ Biol Fish 76:25–35. doi:10.1007/s10641-006-9004-9
Gammon JR (1998) The Wabash River ecosystem. Indiana University Press, Bloomington
Gilliam JF, Fraser DF (1987) Habitat selection under predation hazard: test of a model with foraging minnows. Ecology 68:1856–1862
Gilliam JF, Fraser DF (2001) Movement in corridors: enhancement by predation threat, disturbance, and habitat structure. Ecology 82:258–273. doi:10.1890/0012-9658(2001)082[0258:MICEBP]2.0.CO;2
Gowan C, Fausch KD (1996) Mobile brook trout in two high-elevation Colorado streams: re-evaluating the concept of restricted movement. Can J Fish Aquat Sci 53:1370–1381
Hanson KC, Hasler CT, Suski CD, Cooke SJ (2007) Morphological correlates of swimming activity in wild largemouth bass (Micropterus salmoides) in their natural environment. Comp Biochem Phys A 148:913–920
Hanson KC, Hasler CT, Cooke SJ, Suski CD, Philipp DP (2008) Intersexual variation in the seasonal behaviour and depth distribution of a freshwater temperature fish, the largemouth bass. Can J Zool 86:801–811
Harvey BC, Stewart AJ (1991) Fish size and habitat depth relationships in headwater streams. Oecologia 87:336–342
Hayer C, Graeb BDS, Bertrand KN (2014) Adult, juvenile and young-of-year bighead, Hypophthalmichthys nobilis (Richardson, 1845) and silver carp, H. molitrix (Valenciennes, 1844) range expansion on the northwestern front of the invasion in North America. BioInvasions Rec 3:283–289. doi:10.3391/bir.2014.3.4.10
Hill J, Grossman GD (1993) An energetic model of microhabitat use for rainbow trout and rosyside dace. Ecology 74:685–698
Hoggatt RE (1975) Drainage areas of Indiana streams. U.S. Department of the Interior Geological Survey, Water Resources Division, Indianapolis
Hutchings JA, Gerber L (2002) Sex–biased dispersal in a salmonid fish. Proc R Soc Lond [Biol] 269:2487–2493. doi:10.1098/rspb.2002.2176
Kahler TH, Roni P, Quinn TP (2001) Summer movement and growth of juvenile anadromous salmonids in small western Washington streams. Can J Fish Aquat Sci 58:1947–1956. doi:10.1139/cjfas-58-10-1947
Keefer ML, Moser ML, Boggs CT, Daigle WR, Peery CA (2009) Variability in migration timing of adult pacific lamprey (Lampetra tridentate) in the Columbia River, U.S.A. Environ Biol Fish 85:253–264. doi:10.1007/s10641-009-9490-7
Kocovsky PM, Chapman DC, McKenna JE (2012) Thermal and hydrologic suitability of Lake Erie and its major tributaries for spawning of Asian carps. J Great Lakes Res 38:159–166. doi:10.1016/j.jglr.2011.11.015
Koel TM, Peterka JJ (2003) Stream fish communities and environmental correlates in the Red River of the north, Minnesota and North Dakota. Environ Biol Fish 67:137–155. doi:10.1023/A:1025699512619
Kolar CS, Lodge DM (2001) Progress in invasion biology: predicting invaders. Trends Ecol Evol 16:199–204. doi:10.1016/S0169-5347(01)02101-2
Kolar CS, Chapman DC, Courtenay WR, Housel CM, Williams JD, Jennings DP (2007) Bigheaded carps: a biological synopsis and environmental risk assessment. American Fisheries Society Special Publication 33, Bethesda, Maryland
Lamer JT, Dolan CR, Petersen JL, Chick JH, Epifanio JM (2010) Introgressive hybridization between bighead carp and silver carp in the Mississippi and Illinois Rivers. N Am J Fish Manage 30:1452–1461
Lindström T, Brown GP, Sisson SA, Phillips BL, Shine R (2013) Rapid shifts in dispersal behavior on an expanding range edge. Proc Natl Acad Sci USA 110:13452–13456. doi:10.1073/pnas.1303157110
Liu C, Chen Y, Olden JD, He D, Sui X, Ding C (2015) Phenotypic shifts in life history traits influence invasion success of goldfish in the Yarlung Tsangpo River, Tibet. Trans Am Fish Soc 144:602–609. doi:10.1080/00028487.2014.996668
Lucas MC, Batley E (1996) Seasonal movements and behaviour of adult barbel Barbus barbus, a riverine cyprinid fish: implications for river management. J Appl Ecol 33:1345–1358
Manion PJ (1977) Downstream movement of fish in a tributary of southern Lake Superior. Prog Fish Cult 39:14–16
Marentette JR, Wang G, Tong S, Sopinka NM, Taves MD, Koops MA, Balshine S (2011) Laboratory and field evidence of sex-biased movement in the invasive round goby. Behav Ecol Sociobiol 65:2239–2249
Matheney MP IV, Rabeni CF (1995) Patterns of movement and habitat use by northern hog suckers in an Ozark stream. Trans Am Fish Soc 124:886–897
McNeil DG, Closs GP (2007) Behavioral responses of a southeast Australian floodplain fish community to gradual hypoxia. Freshw Biol 52:412–420. doi:10.1111/j.1365-2427.2006.01705.x
Meyers LS, Thuemler TF, Kornely GW (1992) Seasonal movements of brown trout in northeast Wisconsin. N Am J Fish Manage 12:433–441
Mia MY, Taggart JB, Gilmour AE, Gheyas AA, Das TK, Kohinoor AHM, Rahman MA, Sattar MA, Hussain MG, Mazid MA, Penman DJ, McAndrew BJ (2005) Detection of hybridization between Chinese carp species (Hypophthalmichthys molitrix and Aristichthys nobilis) in hatchery broodstock in Bangladesh, using DNA microsatellite loci. Aquaculture 247:267–273. doi:10.1016/j.aquaculture.2005.02.018
Morrissey MB, Ferguson MM (2011) Individual variation in movement throughout the life cycle of a stream-dwelling salmonid fish. Mol Ecol 20:235–248. doi:10.1111/j.1365-294X.2010.04921.x
Ovidio M, Philippart JC (2002) The impact of small physical obstacles on upstream movements of six species of fish. Hydrobiologia 483:55–69
Pagon N, Grignolio S, Pipia A, Bongi P, Bertolucci C, Apollonio M (2013) Seasonal variation of activity pattern in roe deer in a temperate forested area. Chronobiol Int 30(6):772–785. doi:10.3109/07420528.2013.765887
Perkins TA, Phillips BL, Maskett ML, Hastings A (2013) Evolution of dispersal and life history interact to drive accelerating spread of an invasive species. Ecol Lett 16:1079–1087. doi:10.1111/ele.12136
Perrin N, Mazalov V (2000) Local competition, inbreeding, and the evolution of sex-biased dispersal. Am Nat 155:116–127
Peters LM, Pegg MA, Reinhardt UG (2006) Movements of adult radio-tagged bighead carp in the Illinois River. Trans Am Fish Soc 135:1205–1212. doi:10.1577/T05-162.1
Petty JT, Grossman GD (2007) Size-dependent territoriality of mottled sculpin in a southern Appalachian stream. Trans Am Fish Soc 136:1750–1761. doi:10.1577/T06-034.1
Pusey A (1987) Sex-biased dispersal and inbreeding avoidance in birds and mammals. Trends Ecol Evol 2:295–299
Reynolds LF (1983) Migration patterns of five fish species in the Murray-Darling River system. Mar Freshwater Res 34:857–871. doi:10.1071/MF9830857
Roberts JH, Angermeier PL (2007) Spatiotemporal variability of stream habitat and movement of three fish species. Oecologia 151:417–430. doi:10.1007/s00442-006-0598-6
Rowe DC, Pierce CL, Wilton TF (2009) Fish assemblage relationships with physical habitat in wadeable Iowa streams. N Am J Fish Manag 29:1314–1332. doi:10.1577/M08-192.1
Schmaljohann H, Fox JW, Bairlein F (2012) Phenotypic response to environmental cues, orientation and migration costs in songbirds flying halfway around the world. Anim Behav 84:623–640. doi:10.1016/j.anbehav.2012.06.018
Sharma S, Jackson DA (2007) Fish assemblages and environmental conditions in the lower reaches of northeastern Lake Erie tributaries. J Great Lakes Res 33:15–27. doi:10.3394/0380-1330(2007)33%5B15:FAAECI%5D2.0.CO%3B2
Shaw SL, Chipps SR, Windels SK, Webb MA, McLeod DT (2013) Influence of sex and reproductive status on seasonal movement of Lake Sturgeon in Namakan Reservoir, Minnesota-Ontario. Trans Am Fish Soc 142:10–20
Skalski GT, Gilliam JF (2000) Modeling diffusive spread in a heterogeneous population: a movement study with stream fishes. Ecology 81:1685–1700. doi:10.1890/0012-9658(2000)081%5B1685:MDSIAH%5D2.0.CO%3B2
Stecyk JAW, Farrell AP (2007) Regulation of the cardiorespiratory system of common carp (Cyprinus carpio) during severe hypoxia at three seasonal acclimation temperatures. Physiol Biochem Zool 79:614–627. doi:10.1086/501064
Swan CM, Palmer MA (2000) What drives small-scale spatial patterns in lotic meiofauna communities? Freshwater Biol 44:109–121. doi:10.1046/j.1365-2427.2000.00587.x
Taylor MK, Cooke SJ (2012) Meta-analyses of the effects of river flow on fish movement and activity. Environ Rev 20:211–219
Taylor CM, Winston MR, Matthews WJ (1996) Temporal variation in tributary and mainstem fish assemblages in a Great Plains stream system. Copeia 1996:280–289
United State Army Corps of Engineers (2010) Great lakes and Mississippi river interbasin study: other pathways preliminary risk characterization. U.S. Army Engineer District, Louisville, Kentucky. http://glmris.anl.gov/documents/docs/Other_Pathways_Risk.pdf. Accessed 19 December 2014
Westhoff JT, Paukert C, Ettinger-Dietzel S, Dodd H, Siepker M (2014) Behavioural thermoregulation and bioenergetics of riverine smallmouth bass associated with ambient cold-period thermal refuge. Ecol Freshw Fish. doi:10.1111/eff.12192
Winkler DW, Jørgensen C, Both C, Houston AI, McNamara JM, Levey DJ, Partecke J, Fudickar A, Kacelnik A, Roshier D, Piersma T (2014) Cues, strategies and outcomes: how migrating vertebrates track environmental change. Mov Ecol 2(1):10. doi:10.1186/2051-3933-2-10
Winter JD (1996) Underwater biotelemetry. In: Murphy BR, Willis DW (eds) Fisheries Techniques, 2nd edn. American Fisheries Society, Bethesda, pp 371–395
Acknowledgments
This research was supported by the Indiana Department of Natural Resources with support from the Great Lakes Restoration Initiative. Thanks to B. Pijanowski for securing funding for A.A.C. through the Graduate Assistance in Areas of National Needs Fellowship. Thanks to T. Stefanavage, N. Nye and C. Jansen of the Indiana Department of Natural Resources for their assistance and advice. Additional thanks to the many Purdue University student technicians who have collected data for this project as well as members of the Goforth lab who were invaluable in the completion of this work. Thanks to S. Huck and R. Columbo of Eastern Illinois University for supplying additional tracking data and to the Höök lab, the Goforth lab, and several anonymous reviewers for comments which led to the improvement of this manuscript.
Funding
This study was funded by Indiana Department of Natural Resources (EDS E2-1-D639) awarded to R.R.G.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interest.
Ethical approval
The procedures described in this manuscript were approved by the Purdue University Animal Care and Use Committee under protocol 09-040.
Rights and permissions
About this article
Cite this article
Coulter, A.A., Bailey, E.J., Keller, D. et al. Invasive Silver Carp movement patterns in the predominantly free-flowing Wabash River (Indiana, USA). Biol Invasions 18, 471–485 (2016). https://doi.org/10.1007/s10530-015-1020-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-015-1020-2