Abstract
Context
Dispersal has important fitness consequences for individuals, populations, and species. Despite growing theoretical insights into the evolution of dispersal, its behavioral underpinnings remain empirically understudied, limiting our understanding of the extent and impact of responses to landscape-level heterogeneity of environments, and increasing the risk of inferring species-level responses from biased population sampling.
Objectives
We asked if predictable ecological variation among naturally fragmented arid waterbodies is correlated with disparate dispersal responses of populations of the desert goby Chlamydogobius eremius, which naturally inhabits two habitat “types” (permanent springs, ephemeral rivers), and different levels of hydrological connectivity (high and low) that potentially convey different costs and benefits of dispersal.
Methods
To test for possible behavioral divergence between such populations, we experimentally compared the movement behaviors (correlates of emigration and exploration) of wild-caught fish. We used two biologically relevant spatial scales to test movement relevant to different stages of the dispersal process.
Results
Behavior differed at both spatial scales, suggesting that alternative dispersal strategies enable desert gobies to exploit diverse habitat patches. However, while emigration was best predicted by the connectivity (flood risk) of fish habitats, exploration was linked to their habitat type (spring versus river).
Conclusions
Our findings demonstrate that despite a complex picture of ecological variation, key landscape factors have an overarching effect on among-population variation in dispersal traits. Implications include the maintenance of within-species variation, potentially divergent evolutionary trajectories of naturally or anthropogenically isolated populations, and the direction of future experimental studies on the ecology and evolution of dispersal behavior.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dispersal, the movement of individuals from one habitat patch to another, with the corresponding potential for gene flow, is a crucial component of fitness (Baguette et al. 2013). Animal movement can have a range of ecological and evolutionary consequences, from individual variation in fitness, to the connectivity, demography, and persistence of populations (Bowler and Benton 2005). The three stages of the dispersal process—emigration, transience, and immigration—all impose costs, reflecting that the evolution of dispersal will generally stem from a trade-off between the costs and benefits of leaving a current habitat patch for an unknown alternative (Ronce 2007; Bonte et al. 2012). Decisions about dispersal can thus depend on a number of ecological and demographic factors (Matthysen 2005; Bowler and Benton 2009; Altermatt and Ebert 2010; Gebauer et al. 2013). At landscape levels, environmental variation can affect dispersal (Bowler and Benton 2005), as can heterogeneity in the distribution of habitat patches (Mathias et al. 2001). However, because dispersal has such broad and “intertwined” (Kubisch et al. 2014) ecological and evolutionary consequences, the range of potential scenarios for its evolution is complex, and can be system and taxon-specific.
Despite an emerging wealth of theoretical and molecular insights into dispersal evolution, our knowledge of its behavioral underpinnings remains more limited (Driscoll et al. 2014). This is especially pertinent since modelling approaches are ultimately constrained by deficits in direct, empirical dispersal knowledge. Several authors have recently acknowledged the importance of behavioral insights into dispersal (Duputié and Massol 2013; Driscoll et al. 2014), and highlight the need for increased attention in core areas. First, our understanding of the extent and causes of intraspecific variation in dispersal behavior remains limited, despite evidence that variation between populations can equal that seen among species (e.g. in butterflies: Stevens et al. 2010; Chaine and Clobert 2012; Baguette et al. 2013). In particular, studies have not often considered that spatial variation in the environmental factors shaping dispersal could produce diverse responses within species (but for exceptions, see Hanski et al. 2004; Maes et al. 2013). This is despite the fact that traits underlying movement are traditionally predicted to be highly plastic because dispersal has such broad fitness consequences. In this respect, behavior itself can be highly flexible, and is thus often invoked in an animal’s ability to respond to variation or change in its environment (Candolin and Wong 2012; Driscoll et al. 2014). Second, experimental studies of dispersal behavior remain relatively scarce, and have centred on terrestrial systems over other biomes (Bowler and Benton 2009; Driscoll et al. 2014). For example, while there are noted marine–terrestrial parallels in evolutionary trajectories (Dawson and Hamner 2008), evolutionary contexts for freshwater dispersal are likely to differ because movement pathways may be more structurally defined and constrained.
Here, we asked if habitat variation shapes predictable differences in dispersal behavior using the desert goby (Chlamydogobius eremius, Zietz 1896; Gomon and Bray 2011), a small, benthic fish occurring in naturally fragmented, aquatic habitats of arid central Australia. Three ecological facets make this a powerful setting for studying the processes underlying dispersal evolution. First, habitat variation in this region can be partitioned into (i) permanent, groundwater-fed springs, and (ii) highly variable, rainfall-fed, ephemeral rivers—constituting two characteristic habitat “types” that differ in their permanence, connectivity, and ecological communities (Fig. 1; Fensham et al. 2011). While springs exhibit consistent water chemistry, are permanent sources of water, and fluctuate little in their ecology, the waterholes and pools that represent riverine habitat are notoriously variable with respect to size, water quality, longevity, vegetation, and fish and invertebrate communities: these are dynamic habitat patches that can rapidly appear (with rainfall) and disappear (under hot arid conditions) over space and time. Indeed, while rivers support a dynamic assemblage of up to 12 fish species, including piscovores, desert gobies are usually the sole fish species present in spring environments (Glover 1971; McNeil et al. 2011). Second, locations also differ hydrologically—a particularly important attribute given that hydrology in this arid region is typified by variable surface water flows, which directly influence the availability and structural connectivity of aquatic habitat in river channels (Arthington and Balcombe 2011). Since they are closely tied to rainfall-driven flows, rivers are most often ephemeral and generally have a high connectivity potential (via risk of flooding). In contrast, although hydrological connectivity between springs and other sites is thought to be flood-mediated (Mossop et al. 2015), because their location is independent of the path of river flows, springs vary in their connectivity to other sites. For example, while a number of low-lying springs are more frequently connected to other sites via floods, more elevated sites are also more isolated (McNeil et al. 2011), suggesting that in contrast to rivers, spring habitats could vary substantially in their level of isolation. As a consequence, it is highly feasible that two major factors—the presence of contrasting habitat types, and a distinction between connectivity levels—could importantly alter the costs and benefits of dispersal decisions, and potentially lead to disparate selection regimes on movement. Third, a spatially comprehensive phylogeography (Mossop et al. 2015) of this dispersal-limited species found surprisingly little genetic structuring among populations, suggesting that individuals have strategies to mitigate challenges to their functional connectivity: a property particularly important for persisting in spatially structured environments (Fronhofer et al. 2014).
To test the possibility of landscape-level differences in selection on dispersal, we experimentally compared the behavior of wild-caught fish from desert water bodies to explore the potential divergence of dispersal-relevant movement behaviors. Such an investigation mirrors that previously conducted for a limited number of other biological contexts (e.g. levels of forestation and Calopteryx damselflies, Jonsen and Taylor 2000; effect of a fragmentation gradient in Pardosa monticola wolf spiders, Bonte et al. 2006). Thus, in controlled conditions we examined correlates of (a) emigration and (b) exploratory behavior to investigate adaptive differences in habitat use. We hypothesised that due to their impermanence and thus a higher risk of local extinction, river patches should favour heightened dispersal because the ability to track favourable habitat over space (and escape being trapped in drying habitats), or to bet-hedge in a temporally changing habitat, should be advantageous (Ronce 2007). In comparison, we predicted that animals in stable, oasis-like springs should experience a high “cost of leaving” and thus be less dispersive. An alternative hypothesis centers on the importance of hydrological connectivity: we predicted that fish from “high” connectivity sites would be less dispersive than those from “low” connectivity sites. This outcome could reflect the possible net benefits of avoiding dispersal when connections are relatively frequent, such as staying and reproducing in a current habitat, and avoiding the risk of a failed or otherwise costly emigration attempt. Finally, we predicted that exploratory behavior in a novel environment would match the direction of emigration-level differences.
Materials and methods
Study populations and animal collection
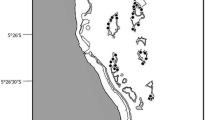
In April 2013, adult desert gobies (n = 400) were captured from spring and river sites of the Lake Eyre region in central Australia (Fig. 2). Seven populations were selected for behavioral study, subject to the limitations of a naturally dynamic system in which the density and number of local populations vary over time and space. Field water conditions differ consistently between springs and rivers, with the latter characterised by higher and more variable salinity and turbidity levels (Costelloe et al. 2005; Fensham et al. 2011; our unpublished data). Mossop et al. (2015) recently elucidated the range-wide phylogeography of the desert goby; hence, sampling also used this spatially comprehensive framework to apply a genetic context for study populations and their broad connectivity. This was particularly important for one of the southern springs, Blanche Cup, as the population here was likely founded by a known anthropogenic translocation in 1970 of fish from Johnson’s Bore (Glover 1971), a site that falls in the genetically distinct north of the species’ range (Fig. 2). Given the desert goby’s short (6 month) generation time and Blanche Cup’s isolation from any northern localities, a contemporary population with a Northern genetic signature would represent up to 80 in situ generations (Glover 1971). Table 1 details site information for the three rivers and four spring-like environments sampled for behavioral and genetic variation.
Desert goby populations in the Lake Eyre region, with the distribution of the two genetic groups (Northern, in grey; Southern, in dark grey) provided for phylogeographic context (Mossop et al. 2015). Circles denote spring populations; triangles denote river populations. The seven populations sampled in the current study are denoted by filled symbols, but Blanche Cup and The Bubbler are represented by pie charts showing the breakdown of Cytochrome b mitochondrial DNA haplotypes (white fill Northern type, dark grey fill Southern type, grey fill other type). ALGE Algebuckina, FIN Finniss Creek, WAR Warriner Creek, JOHN Johnson’s Bore, BLA Blanche Cup, BUB The Bubbler, COW Coward Springs). Inset location of study area in Australia
Housing of animals for behavioral experiments
Wild-caught fish were transported using previously published methods (Wong and Svensson 2009) to Monash University, Melbourne, and housed for a pre-experimental period in mixed sex aquaria (80–120 L), during which time they were fed fish food (Otohime EP1 Hirame extruded pellet, 1.5 mm, Marubeni Nisshin Feed Co. Ltd, Japan) daily. As different field locations varied with regard to aspects of water quality and population density, over the following week tanks were gradually standardised such that all fish were subsequently maintained under consistent densities and sex ratios and a 12:12 light:dark cycle. Conditions of 23–25 °C and a salinity of 3.9 ± 0.2 ppk (using Ocean Fish Marine Salt, PRODAC International s.r.l) were used to reflect meaningful field water parameters. Tanks contained a shallow layer of 2 mm gravel, artificial plants and ceramic flower pot halves as shelter sites. Fish were maintained in standardised conditions for between 39 and 185 days prior to their use in experiments.
Behavioral experiments
Two laboratory experiments were used to investigate behaviors relevant to the aquatic dispersal process in an arid landscape. Movement behavior was measured by examining coarse (“emigration” responses) and fine (“exploratory” responses to a novel environment) scale movement. Here, we use “spatial scale” (coarse or fine) as a relative term, and to reflect our aim of isolating behavioral mechanisms relevant for the dispersal process, given the small body size, frequently small habitat patches, and bottom-dwelling, hopping locomotion that characterises desert goby movement. The two scales of movement were chosen as biologically-relevant proxies for movement mechanisms relevant for emigration and inter-patch/immigration phases of dispersal, since experimental studies have frequently not accounted for the multiple stages or spatial scales at which dispersal-relevant movement may occur (Bowler and Benton 2005). Fish were tested individually to control for confounding social effects, the order of the two experiments was randomised (Bell 2013), and although individual fish spent varying durations in holding conditions prior to experiments, there was no temporal difference among experimental groups. Experiments used adult individuals (mean total length ± SEM: 53.4 ± 0.7 mm; range: 36.4–69.5 mm), since desert goby fry adopt a benthic habit within days of hatching (K.D. Mossop, unpublished data), are poor swimmers, and are physiologically vulnerable to extremes of water quality, suggesting that adult dispersal is the main means of successful movement and gene flow. Further, within an individual’s lifetime, the temporally intermittent opportunities for arid dispersal (which are not necessarily associated with reproductive activity) are more likely to occur during later life-history stages, simply because the larval and juvenile stages of development are relatively short. Prior to their use in experiments, individual animals (males and females) were transferred from holding tanks into individual, 3 L compartments of a recirculating holding system (AHT3-2E3-Shelf Benchtop, Aquatic Habitats, Florida U.S.A.) for a minimum of 24 h. This allowed the reliable identification of individuals throughout the testing period. During the testing period, there was no difference in size (weight or length) between spring and river fish (F1,133 = 0.19, P = 0.66) or between connectivity levels (F1,131 = 0.61, P = 0.44).
Experiment 1: emigration
As emigration is a critical component of the dispersal process, Experiment 1 tested an individual’s propensity to leave a “current” habitat patch by constructing a series of pools to mimic discrete pools of water connected intermittently by flooding (Fig. 3a). This experimental set-up mirrored published methods for investigating movement in fishes (Rehage and Sih 2004; Cote et al. 2010a) and was used as a correlate of the intention to disperse. River habitats in the Lake Eyre region frequently occur as discrete, shallow, ephemeral pools of water that are sporadically connected during increased flow events by narrow riffle habitats. Similarly, most spring environments are characterised by shallow wetlands, which change over time and can experience temporary connections with the surrounding landscape in larger flood events. Thus, a scenario of movement corridors between pools was an ecologically relevant test of an individual’s response to a temporary dispersal opportunity (Moran et al. 2016), and presented individuals with the choice to move actively into an adjacent corridor.
a The experimental recirculating emigration system, consisting of a series of connected pools. Individual test fish were allowed to move from Pool 1 to a maximum endpoint of Pool 4. b The exploratory novel maze set-up (modified from Ward 2012, based on Chapman et al. 2010). Experimental fish exited a refuge and were allowed to move through the maze channels. “Edge” grid squares are shaded; “central” grid squares are white
Experimental pools were black plastic tubs measuring 127 × 84 cm, containing a 2 cm layer of gravel, and filtered tap water to a depth of 15 cm. Similar dimensions are observed in the field in both spring wetlands and in river environments, and thus form a reasonable experimental scale that is feasible but retains ecological relevance; previous studies have also worked within logistical limitations to examine movement behaviors in controlled settings (e.g. Stevens et al. 2004; Janin et al. 2012; O’Sullivan et al. 2014). Water was aged for 24–48 h in barrels and held at ~4 ppk, which is realistic for spring and river environments. Aquarium heaters (Heto brand, MAS, Coburg) maintained the water temperature at 23.5 ± 0.5 °C, and half ceramic pots and artificial plants were placed within the tubs to create habitat complexity. White PVC piping was inserted into the “downstream” ends of pools to create channels that allowed water to flow from one pool to the next; their internal surfaces were painted black with non-toxic paint (AquaPro Pondomastic Pond Sealer, Aquatex Equipment, WA, Australia) and coated with 2 mm gravel to standardise experimental substrates. Silicone sealant (Selleys brand) was used to make the joins watertight, and all materials were soaked in fresh water for 1 week prior to use. CCTV cameras positioned with tripods allowed us to film the downstream end of each channel and detect the timing of dispersal “events” (i.e. the movement of fish from one pool into the next) with a Swann 4-Channel DVR (DVR4-2000, Swann Communications Pty. Ltd., Melbourne). Aquarium lights (Heto brand, Hengtong Aquarium Co. Ltd, Guangdong, China) were positioned above tanks to provide lighting. A Heto brand submerged, inline pump in Pool 4 (behind a barrier) recirculated water from the end of the system back to the start to achieve a flow rate of 370.4 ml/s, which is ecologically relevant for the low-flow connections that can occur between arid water bodies, but is easily resisted by desert gobies. Due to the ecologically realistic scale of the system and the size of fish, it was not possible to track both upstream and downstream movement. Hence, the experimental pools were elevated from ground level at staggered heights to prevent fish moving back up channels in an “upstream” direction, and to facilitate some gravity-fed flow of water.
To begin a trial, an individual fish was acclimated for 40 min in Pool 1; a mesh barrier at the exit point prevented premature movement of fish while still allowing water to flow through the system. The barrier was then removed and filming commenced for a period of 3 hours, as pilot experiments showed this was sufficient to allow movement of gobies. It is worth noting that to exit a pool, individuals had to approach the end of the pool (which was many times the length of individual animals), actively swim up to the channel opening from the bottom, and cross over the raised lip of the channel edge before they could enter the channel. At the conclusion of a trial, the location of the fish within the system was recorded and the animal returned to its individual holding compartment. The trial footage was analysed using iSpy motion detection software to determine the times of movement events. Measurements included the emigration “distance” (i.e. the number of pools entered, ranging from 0 to 3), and the time to begin emigrating (min).
Experiment 2: exploration
Given that successful dispersal can rely on an individual’s behavior during transience and upon arrival in a new habitat patch (Cote et al. 2010b), Experiment 2 tested the responses of individual fish to a novel environment (in which fine-scale movements could correlate with the likelihood of successfully finding resources such as food and mates, or avoiding predators). This used a maze design adapted from open field tests of exploratory behavior (Chapman et al. 2010; Ward 2012) and of dispersal tendency (Myles-Gonzalez et al. 2015). The novel test arena was a 75 × 45 cm aquarium, in which five maze corridors were delineated by internal acrylic “walls” (Acrylico Displays, Melbourne, Australia) that extended two-thirds of the distance between the aquarium sides (Fig. 3b). This functioned to create a longitudinal route and inhibit a continuous line of sight. The external sides of the tank were covered with opaque white foam to prevent disturbance, and an enclosed 15 × 15 cm acrylic box in one corner provided a “refuge” site, in which fish were acclimated for 15 min before a remotely controlled pulley door was opened to begin a trial. The base area of the arena was covered with the outline of a 5 x 5 cm grid (on clear acrylic sheet), which was subsequently covered with a thin layer (~4 mm) of 2 mm gravel to provide a consistent substrate. While a larger grid dimension of 15 × 15 cm was also investigated, a grid size of 25 cm2 proved most informative in measuring the fine scale space use, which should be important for a benthic fish exploring a novel environment.
Trials were videorecorded from above with CCTV cameras positioned 1 m above the arena to allow remote viewing by experimenters. A trial was initiated by opening the pulley door. We recorded the time individuals took to emerge and the time they spent inside the refuge following emergence. Every time the fish entered a grid square (defined as 50% of body length inside the square perimeter) was noted. Accordingly, we used two measures to capture how animals used their environment: total activity (the total number of times a fish entered any square, a measure correlated with the number of different squares entered); and the activity that occurred in “central” versus “edge” parts of the maze (Fig. 3b). Variables relating to this “edge effect” were chosen to reflect the potential preferential use of edge areas of freshwater habitats, especially by prey taxa (Ishiyama et al. 2012). Exploratory trials were terminated if an individual fish failed to emerge within 15 min, or ran for 10 min following a successful emergence.
Mitochondrial DNA sequencing
Using allozyme and mitochondrial DNA data, Mossop et al. (2015) found the presence of two main genetic groups in desert gobies, distributed in the north and south of the species’ distribution (Fig. 2). For the current study, two of the sampled populations (The Bubbler and Blanche Cup) were additional to those previously presented. Hence, their phylogeographic status was characterised by sequencing recently collected individuals for variation in a 560 bp fragment of the Cytochrome b gene (Table 1). Methods for the preparation and analysis of molecular data are provided in Mossop et al. (2015).
Statistical analyses
For use in analyses, sex, habitat (spring or river) and connectivity level (high or low) of fish origin were categorical predictor variables. Phylogeographic context (genetic group of individuals) was also included in analyses, but dropped from final models if it yielded P values >0.25 (Quinn and Keough 2002). Unless otherwise noted, all analyses were conducted in SYSTAT 13 (Systat Inc. 2009).
Emigration responses could take one of four values (ranging from 0, no dispersal, to 3, maximum dispersal), and hence represented a categorical variable. In practice however, most (89.5%) individuals either failed to emigrate, or dispersed the maximum distance. Hence, we converted this variable into a binary disperse/no disperse response, and used a generalized linear model with logit link function for binary data, in R (version 0.99.447) to test for differences between connectivity levels and between habitats in emigration distance. Since high connectivity sites could be either permanent or ephemeral), we included a “permanence” covariate in analyses that tested for a main effect of connectivity. However, this was neither significant in itself, nor did it improve the fit of models. We included weight (as a proxy for size, since weight and length are tightly correlated in desert gobies; F1,133 = 1295.0, P < 0.001, K. D. Mossop unpub. data) as a continuous covariate. “Site” was investigated as either a fixed or random factor, however was unimportant in improving the fit of models and was thus dropped from analyses. As the latency to emigrate data were right-censored, we used a stratified, Kaplan–Meier survival analysis to explore the same comparisons (populations from alternative connectivity or habitat types) and to visualise the outcomes using GraphPad Prism 6 (GraphPad Software, Inc. 2015).
For exploratory variables (activity and edge use), data were transformed where necessary to achieve normality, before being used in general linear models. Analyses of activity levels included, on an a priori basis, a “time in end corridor” or “linear length of maze explored” covariate to control for the fact that the maze design necessarily had an endpoint, and thus precluded infinite forward movement. As with emigration latency, emergence latency in the exploratory experiment was examined with Kaplan–Meier survival analyses, a non-parametric method for estimating survival experience (time to an event of interest, such as emigration or emergence) that can importantly accommodate the fact that not all individuals emerged within the observation period (i.e. data were right censored). The resulting survival curves show the percentage of individuals yet to experience the event for each plotted time on the X axis, and log-rank tests in GraphPad Prism 6 (GraphPad Software, Inc. 2015) allowed us to compare the survival probabilities of groups of interest.
Results
Experiment 1: emigration
Emigration behavior was explained not by habitat, but by a connectivity distinction: fish originally from low connectivity sites emigrated more quickly (χ2 = 9.17, P = 0.002; Fig. 4a) and dispersed further (z = −2.8, P = 0.005; Fig. 4b) than did fish from high connectivity sites. In contrast, a simple spring–river contrast did not consistently predict emigration responses (latency to begin emigrating: mean (springs) = 141.87, mean (rivers) = 161.65, χ2 = 3.07, P = 0.08; emigration distance: z = 1.3, P = 0.2).
There was a non-significant tendency for emigration (but not exploration) responses of Blanche Cup fish to vary with their genetic group (Fig. S1): a covariate that differed within this site based on the discovery of a genetically mixed population in the molecular sequencing results (Fig. 2; Table 1). Although this sample size was limited, it showed that genetically Northern fish emigrated shorter distances and took longer to do so, than did fish from the local Southern group (Fig. S1). Further, Northern Blanche Cup individuals did not differ from Johnson’s Bore fish (data not shown).
Experiment 2: exploration
In contrast to emigration, activity rates of gobies in the maze experiment were related to whether they came from a river or a spring: river fish were more active than those from springs (mean grid squares entered/second for river fish: 0.66 ± 0.03; for spring fish: 0.52 ± 0.03; F1,121 = 11.37, P = 0.001); in contrast, fish from different connectivity levels had comparable activity scores (F1,122 = 1.7, P = 0.19). Although river fish were also more likely than spring fish to use edge rather than central squares of the maze (F1,100 = 13.62, P < 0.001), a “connectivity” predictor in fact produced a slightly more powerful model, largely because, like river populations, fish from Johnson’s Bore were also behaviorally more edge-associated (F1,124 = 20.11, P < 0.001, Fig. 5). Unlike emigration responses, exploratory behaviors were unrelated to genetic group (e.g. activity: F1,116 = 0.44, P = 0.51; edge ratio: F1,120 = 0.24, P = 0.63). Spring and river fish had largely comparable emergence latencies (χ2 = 3.43, P = 0.06), as did those sourced from different connectivity levels (χ2 = 0.02, P = 0.89). Refuge use was also unrelated to either variable (habitat type: F1,121 = 0.06, P = 0.80; connectivity: F1,119 = 0.12, P = 0.73).
Propensity of wild-caught desert gobies to use central versus edge squares of a novel maze experiment. A lower central: edge value indicates greater use of the edge areas of the arena. Data are presented by population (abbreviations as per Fig. 2) and by the presence or absence of other fish species
Finally, sex, weight, duration of holding time (i.e. days in captivity), and the permanence of sites were consistently unrelated to any of the measured emigration or exploratory variables (all P > 0.05), and hence were dropped from analyses.
Discussion
Our results indicated that predictable sources of landscape variation did indeed affect desert goby movement at two different spatial scales (chosen for their differing ecological relevance). A main implication is that behavioral mechanisms could shape outcomes at multiple stages of the dispersal process, and that such outcomes can differ predictably with heterogeneity of habitat patches across the landscape. However, the factors predicting population-level differences differed for emigration and exploratory behaviour in desert gobies, reflecting the likely role of contrasting ecological drivers (hydrological connectivity versus habitat type) at different scales of movement.
Emigration
The hydrological connectivity levels of source habitats was the main predictor of coarse scale movement: while we saw little support for a simple spring-river contrast, desert gobies from high-connectivity sites (rivers, Johnson’s Bore, and genetically “Northern” Blanche Cup fish) were indeed less dispersive than those from more isolated sites (elevated springs), in line with our prediction.
A parsimonious explanation for this result is that in high-connectivity habitats, the costs of dispersing away from a current habitat patch, regardless of impermanence, outweigh the benefits of leaving a known habitat in which water and prospective mates are available. Thus, rather than favouring increased dispersal (as in our “habitat” hypothesis), frequent, flood-mediated connections could in fact lead to increased resistance to coarse scale movements. Interestingly, although connectivity and permanence are frequently related, we saw no evidence that permanence per se affected emigration response, suggesting that by imposing divergent regimes of dispersal opportunity, structural connectivity is the overarching driver of dispersal strategies. Divergence in movement responses were also seen between Calopteryx maculata damselflies originating from different habitats: individuals native to forested patches were more likely to move away from streams than those from clear or partially forested landscapes (Jonsen and Taylor 2000). In our system, such a scenario aligns with the ecological niche of desert gobies within riverine fish communities, in which the species differs from most other fishes in its strategy of high resistance (tolerance of extreme conditions through physiological and behavioral adaptations), rather than resilience (rapid and large scale re-colonisation of habitat following drought via high dispersal potential) to environmental extremes (Crook et al. 2010). Indeed, surveys across multiple time-points found that desert gobies become particularly and rapidly abundant in drying, high-salinity waterholes (McNeil et al. 2011). While not permanent, these high-salinity sites are important for persistence in the medium term because they exclude predators and most competitors, allow the maintenance of viable populations, and critically, facilitate immediate reproduction once conditions improve and habitat expands.
Why then should individuals leave stable, albeit isolated, springs, in which both permanent habitat and potential mates are guaranteed? An opposing constraint on reproductive success could explain an otherwise counterintuitive outcome ( Travis 2001; Hof et al. 2012). Although we expected that the high mortality risks of leaving hydrologically isolated springs should disfavour dispersal, a heightened dispersal propensity points to greater-than-anticipated costs of staying in permanent springs. In fact, while benign in some ways, spring environments are also space- and resource-limited, are potentially highly competitive, and may frequently be at carrying capacity. Increased levels of competition are broadly important for dispersal behavior (Ronce 2007), while resource limitation (e.g. nest sites) could rapidly limit reproductive opportunities, particularly since desert gobies have a resource-defence based mating system (Wong and Svensson 2009). For springs that are set apart from river channels, escape via dispersal will rely largely on floodwaters that create temporary movement corridors, as is seen in other spring fauna (Worthington Wilmer et al. 2008). Thus, despite the inhospitable landscape surrounding many springs, it could be advantageous for individuals to disperse opportunistically during movement windows (i.e. flooding) which are particularly sporadic and short-lived for isolated sites.
Alternatively, the non-random sorting of dispersal phenotypes over space could also explain the increased dispersal propensity of poorly connected populations. The difficulty of reaching isolated spring environments could itself filter out individuals with low dispersal propensity. In this case, isolated habitats could act as “islands” for increased dispersal, so that even if strong dispersers also leave such patches, the remaining breeding population, and any new immigrants, will still comprise more dispersive phenotypes than the background population (Shine et al. 2011). When this spatial sorting persists over time (thus concentrating in space any alleles underlying behavior), it can also increase mean dispersal ability in subsequent generations, even in the absence of selective advantage (Lee 2011).
Exploratory behavior
At a fine spatial scale, river fish were more active than those from springs, and showed an increased preference for edge habitat (Fig. 5): both likely effects of differences in predation risk. Small-scale movement can reflect an animal’s propensity to investigate both inter-patch environments and new habitat patches (Debeffe et al. 2013), but should also respond to predation risk, since movement can influence encounter rates with predators (Richardson 2001). Although activity levels could diverge with differences in dietary environments, activity is sensitive to predator presence in a broad range of taxa (e.g. rock lizards, Martín et al. 2009; fish, Hartman and Lawler 2014; limpets, Manzur et al. 2014). In Lake Eyre rivers, fish assemblages vary over time, but can include predatory spangled perch (Leiopotherapon unicolor, Michelangeli and Wong 2014) and golden perch (Macquaria sp., McNeil et al. 2011). However, increased turbidity and water depth in rivers potentially relax the costs of conspicuous, frequent movements by reducing their visibility (Ajemian et al. 2015), with previous work finding that desert goby courtship behavior is sensitive to both turbidity and predator presence (Michelangeli and Wong 2014; Michelangeli et al. 2015). Although springs are free of aquatic predators, the lower activity of these fish may be a conserved response to predation risk (Herczeg and Välimäki 2011), an outcome potentially supported by aerial (bird) predation operating in the very clear, shallow waters of springs.
A predator-avoidance hypothesis is cautiously supported by the fact that unlike for activity levels, the Johnson’s Bore population resembled closely the mean behavior of riverine fish for edge preference (Fig. 5). While this location is in many respects analogous to springs, in which gobies are usually the only fish species present, Johnson’s Bore is atypical in that it also contains the introduced mosquitofish (Gambusia holbrooki). This species represents competition for resources but also direct harm through fin-nipping, chasing, and egg predation (Wager and Unmack 2000). Thus, an increased association with habitat edges aligns with that suggested for other freshwater taxa (e.g. guppies: Luyten and Liley 1985; Kodric-Brown and Nicoletto 2005; crayfish: Ishiyama et al. 2012), in which the preferential use of edge habitat could function to reduce predation risk by allowing prey to exploit increased vegetation cover, or shallower depths that exclude pelagic predators (Rincon et al. 2002).
For both scales of movement, a potentially compelling explanation for divergent strategies is developmental plasticity, in which environmental conditions might vary between generations, but are constant within an individual’s lifetime. Strong differences in multiple, dispersal-relevant behaviors support this scenario over a model of reversible plasticity, which carries increased costs such as higher neural investment, and thus should only be favoured when costly phenotype-environment mismatches are likely (Snell-Rood 2013). For example, in nine-spined sticklebacks (Pungitius pungitius), wild fish from pond (predator-free) populations were more exploratory than those from marine (predators present) habitats under perceived predation risk, but their common-garden F1 progeny were equally plastic in the same assays (Herczeg and Välimäki 2011). In the desert goby, additional tests of a common-garden raised F1 generation of a subset (n = 4) of the seven wild populations (N.P. Moran, unpublished data) will provide further insights into the relative contributions of environmental and genetic mechanisms. On this note, the possible behavioral divergence between genetic groups in the “common garden” Blanche Cup site flags that a potential genetic basis for emigration behavior or its plasticity could be a fruitful line of inquiry.
Conclusions
Our findings of among-population divergence in movement responses highlight the key importance of behavioral processes in dispersal ecology. We demonstrate that at two different spatial scales, dispersal-relevant behaviors were associated with predictable, landscape-level sources of environmental variation. However, the direction and drivers of responses differed: higher activity rates were not associated with high emigration rates, and they responded to different components of habitat variation. Interestingly, although variation characterises many ecological facets of this system, at the population level, key drivers appear to have an overarching effect. This suggests that populations might differ in predictable ways, even when environmental heterogeneity is complex or difficult to characterise: findings that have implications for the maintenance of within-species phenotypic variation (Hanski et al. 2004) and the ability of experimental studies to identify important behavioral variation. A caveat for the current findings is that this system has a high prevalence of stochastic (i.e. in addition to spatial and temporal autocorrelation) variation, which may mitigate the role of traditionally important predictors of dispersal (e.g. sex, age; Bowler and Benton 2009). Thus, it is possible that the stochasticity of a system—by affecting the adaptive value of cue use in decision making—could be an important consideration for ecologists looking to sample likely drivers of variation in dispersal (Mathias et al. 2001; Cheptou and Massol 2009). Studies taking an experimental approach, which address within-species variation in movement, and those that redress current taxonomic and biome deficits, will make valuable contributions to the study and application of dispersal ecology and evolution.
References
Ajemian MJ, Sohel S, Mattila J (2015) Effects of turbidity and habitat complexity on antipredator behavior of three-spined sticklebacks (Gasterosteus aculeatus). Environ Biol Fishes 98:45–55
Altermatt F, Ebert D (2010) Populations in small, ephemeral habitat patches may drive dynamics in a Daphnia magna metapopulation. Ecology 91:2975–2982
Arthington AH, Balcombe SR (2011) Extreme flow variability and the ‘boom and bust’ ecology of fish in arid-zone floodplain rivers: a case history with implications for environmental flows, conservation and management. Ecohydrology 4(5):708–720
Baguette M, Blanchet S, Legrand D, Stevens VM, Turlure C (2013) Individual dispersal, landscape connectivity and ecological networks. Biol Rev 88:310–326
Bell A (2013) Randomized or fixed order for studies of behavioral syndromes? Behav Ecol 24:16–20
Bonte D, Vanden Borre J, Lens L, Maelfait JP (2006) Geographical variation in wolf spider dispersal behaviour is related to landscape structure. Anim Behav 72:655–662
Bonte D , Van Dyck H, Bullock JM, Coulon A, Delgado M, Gibbs M, Lehouck V, Matthysen E, Mustin K, Saastamoinen M, Schtickzelle N, Stevens VM, Vandewoestijne S, Baguette M, Barton K, Benton TG, Chaput-Bardy A, Clobert J, Dytham C, Hovestadt T, Meier CM, Palmer SCF, Turlure C, Travis JMJ (2012) Costs of dispersal. Biol Rev 87:290–312
Bowler DE, Benton TG (2005) Causes and consequences of animal dispersal strategies: relating individual behavior to spatial dynamics. Biol Rev 80:205–225
Bowler DE, Benton TG (2009) Variation in dispersal mortality and dispersal propensity among individuals: the effects of age, sex and resource availability. J Anim Ecol 78:1234–1241
Candolin U, Wong BBM (2012) Behavioral responses to a changing world: mechanisms and consequences. Oxford University Press, Oxford
Chaine AS, Clobert J (2012) Dispersal. In: Candolin U, Wong BBM (eds) Behavioral responses to a changing world: mechanisms and consequences. Oxford University Press, Oxford, pp 63–79
Chapman BB, Morrell LJ, Krause J (2010) Unpredictability in food supply during early life influences boldness in fish. Behav Ecol 21:501–506
Cheptou PO, Massol F (2009) Pollination fluctuations drive evolutionary syndromes linking dispersal and mating system. Am Nat 174:46–55
Costelloe JF, Grayson RB, McMahon TA, Argent RM (2005) Spatial and temporal variability of water salinity in an ephemeral, arid-zone river, central Australia. Hydrol Process 19:3147–3166
Cote J, Clobert J, Brodin T, Fogarty S, Sih A (2010a) Personality-dependent dispersal: characterization, ontogeny and consequences for spatially structured populations. Philos Trans R Soc B 365:4065–4076
Cote J, Fogarty S, Weinersmith K, Brodin T, Sih A (2010b) Personality traits and dispersal tendency in the invasive mosquitofish (Gambusia affinis). Philos Trans R Soc B 277:1571–1579
Crook DA, Reich P, Bond NR, McMaster D, Koehn JD, Lake PS (2010) Using biological information to support proactive strategies for managing freshwater fish during drought. Mar Freshw Res 61:379–387
Dawson MN, Hamner WM (2008) A biophysical perspective on dispersal and the geography of evolution in marine and terrestrial systems. J R Soc Interface 5:135–150
Debeffe L, Morellet N, Cargnelutti B, Lourtet B, Coulon A, Gaillard JM, Bon R, Hewison AJM (2013) Exploration as a key component of natal dispersal: dispersers explore more than philopatric individuals in roe deer. Anim Behav 86:143–151
Driscoll DA, Banks SC, Barton PS, Ikin K, Lentini P, Lindenmayer DB, Smith AL, Berry LE, Burns EL, Edworthy A, Evans MJ, Gibson R, Heinsohn R, Howland B, Kay G, Munro N, Scheele BC, Stirnemann I, Stojanovic D, Sweaney N, Villasenor NR, Westgate MJ (2014) The trajectory of dispersal research in conservation biology. PLoS One 9(4):e95053
Duputié A, Massol F (2013) An empiricist’s guide to theoretical predictions on the evolution of dispersal. Interface Focus 3(6):20130028
Fensham RJ, Silcock JL, Kerezsy A, Ponder W (2011) Four desert waters: setting arid zone wetland conservation priorities through understanding patterns of endemism. Biol Conserv 144:2459–2467
Fronhofer EA, Stelz JM, Lutz E, Poethke HJ, Bonte D (2014) Spatially correlated extinctions select for less emigration but larger dispersal distances in the spider mite Tetranychus urticae. Evolution 68:1838–1844
Gebauer K, Dickinson KJM, Whigham PA, Seddon PJ (2013) Matrix matters: differences of grand skink metapopulation parameters in native tussock grasslands and exotic pasture grasslands. PLoS ONE 8:e76076
Glover C (1971) The taxonomy and biology of Chlamydogobius eremius (Zietz, 1896). Master’s Thesis, Department of Zoology, University of Adelaide
Gomon MF, Bray DJ (2011) Desert goby, Chlamydogobius eremius in fishes of Australia. Accessed 10 Dec 2014
GraphPad Software, Inc (2015) GraphPad Prism 6 (Computer software). GraphPad Software, La Jolla
Hanski I, Eralahti C, Kankare M, Ovaskainen O, Siren H (2004) Variation in migration propensity among individuals maintained by landscape structure. Ecol Lett 7:958–966
Hartman R, Lawler S (2014) Evidence for contemporary evolution of behavioral responses to introduced fish. Anim Behav 97:213–220
Herczeg G, Välimäki K (2011) Intraspecific variation in behavior: effects of evolutionary history, ontogenetic experience and sex. J Evol Biol 24:2434–2444
Hof C, Brandle M, Dehling DM, Munguia M, Brandl R, Araujo MB, Rahbek C (2012) Habitat stability affects dispersal and the ability to track climate change. Biol Lett 8:639–643
Ishiyama N, Nagayama S, Akasaka T, Nakamura F (2012) Habitat use by endangered Japanese crayfish (Cambaroides japonicus) in low-gradient streams of southern Hokkaido, Japan: reach and microhabitat-scale analysis. Hydrobiologia 686:257–266
Janin A, Lena JP, Joly P (2012) Habitat fragmentation affects movement behavior of migrating juvenile common toads. Behav Ecol Sociobiol 66(9):1351–1356
Jonsen ID, Taylor PD (2000) Fine-scale movement behaviors of calopterygid damselflies are influenced by landscape structure: an experimental manipulation. Oikos 88(3):553–562
Kodric-Brown A, Nicoletto PF (2005) Courtship behavior, swimming performance, and microhabitat use of Trinidadian guppies. Environ Biol Fishes 73:299–307
Kubisch A, Holt RD, Poethke HJ, Fronhofer EA (2014) Where am I and why? Synthesizing range biology and the eco-evolutionary dynamics of dispersal. Oikos 123:5–22
Lee MSY (2011) Macroevolutionary consequences of “spatial sorting”. Proc Natl Acad Sci USA 108:E347–E347
Luyten PH, Liley NR (1985) Geographic-variation in the sexual-behavior of the guppy, Poecilia reticulata (Peters). Behaviour 95:164–179
Maes J, Van Damme R, Matthysen E (2013) Individual and among-population variation in dispersal-related traits in Natterjack toads. Behav Ecol 24:521–531
Manzur T, Vidal F, Pantoja JF, Fernaandez M, Navarrete SA (2014) Behavioral and physiological responses of limpet prey to a seastar predator and their transmission to basal trophic levels. J Anim Ecol 83:923–933
Martín J, Lopez P, Polo V (2009) Temporal patterns of predation risk affect antipredator behavior allocation by Iberian rock lizards. Anim Behav 77:1261–1266
Mathias A, Kisdi E, Olivieri I (2001) Divergent evolution of dispersal in a heterogeneous landscape. Evolution 55:246–259
Matthysen E (2005) Density-dependent dispersal in birds and mammals. Ecography 28:403–416
McNeil DG, Schmarr DW, Rosenberger AE (2011) Climatic variability, fish and the role of refuge waterholes in the Neales River Catchment: Lake Eyre Basin, South Australia. South Australian Arid Lands NRM Board, Port Augusta
Michelangeli M, Wong BBM (2014) A recent predatory encounter influences male courtship in a desert-dwelling fish. Behav Ecol 25:928–932
Michelangeli M, Tuomainen U, Candolin U, Wong BBM (2015) Habitat alteration influences male signalling effort in the Australian desert goby. Behav Ecol 26(4):1164–1169
Moran NP, Mossop KD, Thompson RM, Wong BBM (2016) Boldness in extreme environments: temperament divergence in a desert-dwelling fish. Anim Behav 122:125–133
Mossop KD, Adams M, Unmack PJ, Date KLS, Wong BBM, Chapple DG (2015) Dispersal in the desert: ephemeral water drives connectivity and phylogeography of an arid-adapted fish. J. Biogeogr 42:2374–2388
Myles-Gonzalez E, Burness G, Yavno S, Rooke A, Fox MG (2015) To boldly go where no goby has gone before: boldness, dispersal tendency, and metabolism at the invasion front. Behav Ecol 26:1083–1090
O’Sullivan D, Benton TG, Cameron TC (2014) Inter-patch movement in an experimental system: the effects of life history and the environment. Oikos 123(5):623–629
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge
Rehage JS, Sih A (2004) Dispersal behavior, boldness, and the link to invasiveness: a comparison of four Gambusia species. Biol Invasions 6:379–391
Richardson JML (2001) A comparative study of activity levels in larval anurans and response to the presence of different predators. Behav Ecol 12:51–58
Rincon PA, Correas AM, Morcillo F, Risueno P, Lobon-Cervia J (2002) Interaction between the introduced eastern mosquitofish and two autochthonous Spanish toothcarps. J Fish Biol 61:1560–1585
Ronce O (2007) How does it feel to be like a rolling stone? Ten questions about dispersal evolution. Annu Rev Ecol Evol Syst 38:231–253
Shine R, Brown GP, Phillips BL (2011) An evolutionary process that assembles phenotypes through space rather than through time. Proc Natl Acad Sci 108:5708–5711
Snell-Rood EC (2013) An overview of the evolutionary causes and consequences of behavioral plasticity. Anim Behav 85:1004–1011
Stevens VM, Pavoine S, Baguette M (2010) Variation within and between closely related species uncovers high intra-specific variability in dispersal. PLoS ONE 5(6):e11123
Stevens VM, Polus E, Wesselingh RA, Schtickzelle N, Baguette M (2004) Quantifying functional connectivity: experimental evidence for patch-specific resistance in the Natterjack toad (Bufo calamita). Landscape Ecol 19(8):829–842
Systat Software, Inc (2009) Systat 13 (Computer software). Systat Software, Inc, San Jose. http://www.systat.com/
Travis JMJ (2001) The color of noise and the evolution of dispersal. Ecol Res 16(1):157–163
Wager R, Unmack PJ (2000) Fishes of the Lake Eyre catchment of central Australia, Queensland. Department of Primary Industries, Brisbane
Ward AJ (2012) Social facilitation of exploration in mosquitofish (Gambusia holbrooki). Behav Ecol Sociobiol 66:223–230
Wong BBM, Svensson PA (2009) Strategic male signalling effort in a desert-dwelling fish. Behav Ecol Sociobiol 63:543–549
Worthington Wilmer J, Elkin C, Wilcox C, Murray L, Niejalke D, Possingham HP (2008) The influence of multiple dispersal mechanisms and landscape structure on population clustering and connectivity in fragmented Artesian spring snail populations. Mol Ecol 17:3733–3751
Acknowledgements
We acknowledge the traditional owners of the Kati Thanda-Lake Eyre region, and particularly thank Reg Dodd and Dean Ah Chee for valuable natural and cultural insights. Thanks to Matt Simpson for assistance with laboratory trials. Financial support was received from the Nature Foundation SA, the Holsworth Wildlife Endowment, and the Great Artesian Basin Coordinating Committee (to K.D.M.) and the Australian Research Council (DP120103010 to B.B.M.W.). Procedures for field sampling were approved by the Monash University Animal Ethics Committee (BSCI/2012/14), and the South Australian Department of Environment and Natural Resources granted collection permits (9902391, 9902523, and 9902598). We thank Diana Bowler and two additional referees for highly constructive comments on a prior version of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors were involved in conceiving project ideas, K.D.M. and B.B.M.W. obtained funding for the project, K.D.M. and N.P.M. collected and analysed the data, K.D.M. wrote the original manuscript, which was then edited by all other authors.
Corresponding author
Additional information
Data accessibility: Behavioral data, species location data, and information on unique genetic variation not previously archived will be submitted to the appropriate repositories (Dryad and GenBank).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mossop, K.D., Moran, N.P., Chapple, D.G. et al. Connectivity and habitat type shape divergent dispersal behavior in a desert-dwelling fish. Landscape Ecol 32, 1065–1078 (2017). https://doi.org/10.1007/s10980-017-0509-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-017-0509-8