Abstract
The ability to tolerate novel herbivores is widely considered to influence plant invasion success. For clonal plants that have reduced capacity to evolve in response to novel herbivores, legacy effects of herbivory on parental plants might be translated to offspring ramets, resulting in pre-adaptation to tolerate herbivory for new vegetative growth. Using the invasive clonal plant Alternanthera philoxeroides, we first exposed plants to herbivory by Planococcus minor, a widespread and generalist piercing-sucking insect. Herbivory decreased above- and below-ground plant biomass by approximately 50% with a concomitant 134% increase in root N concentration but no changes in concentrations of soluble sugars, starch or non-structural carbohydrates related to herbivory tolerance. Offspring ramets were then exposed to herbivory by three different herbivore species: (1) P. minor, (2) the specialist leaf-beetle Agasicles hygrophila, and (3) the stenophagous tortoise-beetle Cassida piperata. There was no evidence of interactive effects between herbivory on parental plants and herbivory on offspring plants on growth, biomass allocation patterns, or physiological responses, suggesting that pre-adaptation to herbivory did not occur in A. philoxeroides with these herbivores. There were, however, species-specific herbivore tolerance responses. In the offspring generation, herbivory by A. hygrophila strongly suppressed growth and biomass allocation, but patterns were generally weaker for other herbivores. Tolerance effects could be explained by stimulatory effects of grazing by C. piperata and P. minor on taproot biomass along with idiosyncratic increases of starch and non-structural carbohydrate concentration in some storage organs. Our results highlight the importance of A. hygrophila in controlling the aboveground spread of A. philoxeroides. However, herbivory by other species was largely tolerated and accompanied by increased allocation to underground storage organs and altered physiological reserves, both of which could allow this invasive plant to tolerate herbivory and successfully invade new areas in the face of new herbivore pressure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plant invasion is considered a component of global change, threatening biodiversity and ecosystem functioning (D’Antonio et al. 1996; Pimentel et al. 2000). The invasion success of some exotic plants is attributed to the release from co-evolved enemies in introduced ranges and to the shift between resistance and tolerance to herbivores (Agrawal and Kotanen 2003; Keane and Crawley 2002; Colautti et al. 2004; Morrison and Hay 2011). More precisely, due to the reduced pressure from co-evolved herbivores, exotic plants in introduced ranges may alter their adaptive mechanisms from herbivore-specific defense by producing high-cost chemicals (e.g., carbon-based secondary metabolites) to broad-spectrum tolerance by improving regrowth and/or reproduction capacity in response to local herbivores (Joshi and Vrieling 2005; Huang et al. 2010). An exotic plant with a high tolerance ability is likely to exhibit compensatory or over-compensatory growth after herbivore damage, and thus to possess a high potential for invasion (Ashton and Lerdau 2008; Wang et al. 2017). Therefore, assessing tolerance of exotic species to herbivores in introduced ranges may help understand mechanisms underlying successful plant invasions (Maron and Vilà 2001; Colautti et al. 2004).
Traits that help host plants to tolerate herbivory are closely related to not only primary metabolite production but also resource allocation (Dam and Baldwin 2001; Agrawal 2002; Steets and Ashman 2010; Lu and Ding 2012; Dong et al. 2017). To tolerate herbivory, non-structural carbohydrates (NSC, including soluble sugars and starch) can be remobilized from storage organs or ungrazed parts of damaged plants to support subsequent regrowth (Schwachtje et al. 2006; Babst et al. 2010; Lapointe et al. 2010; Machado et al. 2017). Storage organs such as taproots often function as carbohydrate pools that govern bud dormancy, latent meristems and clonal reproduction of plants (Jia et al. 2009; Dong et al. 2017). Moreover, allocation of nitrogen (N) among different organs of plants may be rapidly modified following herbivory, which not only influences the feeding preference of herbivores by, e.g., decreasing tissue nutritive values (Schoonhoven et al. 2005; Agrawal and Weber 2015), but also enhances compensatory growth of damaged plants (Polley and Detling 1988; Newingham et al. 2007). These changes in allocation pattern of NSC and/or N in different plant organs may alleviate the herbivory-induced decline in plant fitness.
Herbivory-induced tolerance responses may persist across multiple generations via sexual or asexual (clonal) reproduction (i.e., legacy effects of herbivory), which may alter the ability of offspring generations to tolerate herbivory (Herman and Sultan 2011; Holeski et al. 2012). Legacy effects of herbivory are often considered adaptive, particularly when they trigger the pre-adaptation of offspring to similar herbivory that parent plants have experienced (Herman and Sultan 2011; Holeski et al. 2012). For instance, the history of exposure to herbivory increased seed mass of Raphanus raphanistrum (Agrawal 2002), shortened seeding emergence time of Impatiens capensis (Steets and Ashman 2010), and facilitated compensatory growth of Alternanthera philoxeroides (Lu and Ding 2012; Dong et al. 2017). However, most studies that documented legacy effects of herbivory have focused on one specific herbivore, and few have tested such effects on plant responses to a diverse array of herbivores (Agrawal 2000; Ali and Agrawal 2015). Furthermore, no study has tested whether the legacy effect of herbivory caused by one herbivore depends on whether the subsequent herbivory is by the same herbivore or not.
We conducted a greenhouse experiment to test the legacy effect of herbivory of parental plants by a generalist herbivore Planococcus minor on growth and physiology of clonal offspring of a creeping, invasive plant Alternanthera philoxeroides. We also assessed the direct effect of herbivory of clonal offspring by three different herbivores (P. minor, a specialist herbivore Agasicles hygrophila and a stenophagous herbivore Cassida piperata) and its interaction with the legacy effect. We hypothesized (1) that there are legacy effects of herbivory, i.e., herbivory on parental plants can alter growth and physiology of clonal offspring of A. philoxeroides, (2) that such legacy effects of herbivory are context-dependent and can pre-adapt clonal offspring to the similar herbivory situation that parental plants have experienced, and (3) that herbivory on parental plants and offspring plants can alter resource allocation pattern of A. philoxeroides. We used multiple herbivores in the offspring generation to test also the hypothesis (4) that the direct and legacy effects of herbivory vary with herbivores.
Materials and methods
Plant and insect species
Alternanthera philoxeroides (Mart.) Griseb (Amaranthaceae) is a perennial clonal herb native to South America (Holm et al. 1997; Sainty et al. 1998; Yu et al. 2009). The species is listed as one of the most noxious invasive weeds in China and other regions, including North America and Australia (Julien et al. 1995; Holm et al. 1997; Wang et al. 2009). In China, A. philoxeroides exhibits extremely low genetic diversity (Xu et al. 2003; Ye et al. 2003), but has a broad geographic distribution because of clonal reproduction by stem and root fragments. The species can colonize both terrestrial and aquatic habitats and form dense monospecific stands, thereby causing severe ecological and environmental problems (Sainty et al. 1998; Wang et al. 2009).
Agasicles hygrophila Selman and Vogt (Coleoptera: Chrysomelidae) is a host-specific leaf beetle native to South America (Spencer and Coulson 1976). The species was first introduced into China in 1986 as a biological control agent for A. philoxeroides (Lu and Ding 2012). Adults of A. hygrophila are approximately 5.7–7 mm long with black elytra marked with two yellow stripes. Males are generally smaller than females, and have an abdominal tip that is entirely covered by the elytra. Both adults and larvae feed on leaves and buds of A. philoxeroides using their chewing mouthparts (Spencer and Coulson 1976). A. hygrophila has been reported to efficiently control populations of A. philoxeroides in aquatic habitats, but has little impact on terrestrial populations (Sainty et al. 1998).
Cassida piperata Hope (Coleoptera: Cassididae) is a stenophagous tortoise beetle native to eastern Asia, and feeds on leaves of some plants in Amaranthaceae and Chenopodiaceae (Dai et al. 2014; Nagasawa and Matsuda 2015). This beetle is oval, pale green to chartreuse, with a brown spot at the center of the base of the notum (Dai et al. 2014). It is widely distributed in many provinces in southern China, and has been evaluated as a potential candidate for a biological control agent of A. philoxeroides (Lu and Ding 2012).
Planococcus minor (Maskell) (Pseudococcidae: Hemiptera) is a polyphagous insect that is native to Asia and widely distributed in subtropical and tropical regions (Cox 1989). Female adults are soft-bodied, wingless, covered with waxy filaments, and relatively sedentary, whereas male adults are tiny, winged and ephemeral (Roda et al. 2013). During their entire life cycle, females feed on the phloem sap of host plants by inserting their piercing and sucking mouthparts into plant tissues, whereas males feed on host plants only during the nymphal stages (Roda et al. 2013). The species is considered a serious pest that causes severe defoliation of over 250 wild and cultivated host plants in nearly 80 families, leading to stunted growth and even death (Francis et al. 2012).
Plants of A. philoxeroides were collected from several populations in a riparian agricultural area in Zhejiang Province (28.87°N, 121.01°E), China. All sampled plants were mixed and cultivated via clonal propagation for five years in a greenhouse at the Forest Science Co., Ltd., of the Beijing Forestry University. A. hygrophila was personally provided, C. piperata was collected in Wuhan Botanical Garden, Chinese Academy of Sciences, and P. minor was collected in a greenhouse of the Beijing Forestry University.
Experiment and harvest
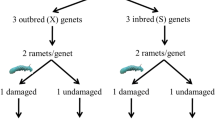
The overall experimental design consisted of two clonal generations of A. philoxeroides. The parental generation experiment consisted of two herbivory levels, i.e., the control (without herbivory) or herbivory by the generalist P. minor. The offspring generation experiment employed a two-way factorial design, i.e., herbivory history of parent plants crossed with herbivory of offspring plants. For herbivory of offspring, offspring plants were randomly subjected to one of the four herbivory treatments, i.e., a control without herbivory or herbivory by A. hygrophila, C. piperata or P. minor. For herbivory history of parents, half of the plants in each of the four herbivory levels of offspring plants were taken from parent plants subjected to herbivory by P. minor; the other half were taken from parent plants not subjected to herbivory (Fig. 1). In this study, a parent and its offspring were not termed in the genetic sense as they share the same genotype. Instead, they represented two generations of clonal individuals, i.e. parental ramets and their clonal offspring ramets.
Experimental design. The parental generation experiment had two herbivory levels, i.e., the control (without herbivory) and herbivory by Planococcus minor. The offspring generation experiment employed a two-way factorial design, i.e., herbivory on offspring plants [the control (without herbivory) or herbivory by Agasicles hygrophila, Cassida piperata or P. minor] crossed with herbivory on parent plants (i.e., offspring plants originated from parent plants subjected to the control or herbivory by P. minor). Ah, Cp and Pm stand for A. hygrophila, C. piperata and P. minor, respectively
On 6 December 2015, 70 6-cm-long root fragments of A. philoxeroides were randomly selected from the mixed, cultivated populations. Each root fragment was planted in a plastic pot (14 cm in diameter and 12 cm in height) filled with an 1:1 (v:v) mixture of quartz sand (0.5–1 mm particle size) and peat (Pindstrup Seedling; Pindstrup Mosebrug A/S, Pindstrup, Denmark), along with 2 g L−1 slow-release fertilizer (16 N: 9P: 12 K: 2 Mg; Osmocote Exact Standard 3–4 M, Scotts, Marysville, Ohio, USA). Plants initially originated from these root fragments were treated as the parental generation, and grown without herbivory for the first five months. On 10 April 2016, half of the parent plants were infested with adult female P. minor (density: six adults per plant), whereas the remaining half were not infested and served as the control. To avoid the escape of P. minor in the herbivory treatment and the infestation by P. minor in the control, each parent plant was placed in a cage (25 cm long × 25 cm wide × 50 cm high) covered with a 0.25-mm nylon mesh. There were 35 replicates of parent plants for each of the parental generation treatments. On 7 July 2016, i.e., three months after the start of the treatments, we harvested seven replicate parent plants from each of the parental generation treatments to test the effects of herbivory by P. minor on growth, biomass allocation and root physiological traits of parent plants. Leaves, stems, taproots and fine roots of each parent plant were separated and weighted after drying at 70 °C for 48 h. The 28 remaining parent plants in each of the two treatments were used as the sources for the offspring generation experiment.
A 6-cm-long fragment of a secondary root was cut from each of the remaining parent plants, weighted to obtain fresh mass, and then each grown in a plastic pot filled with the same substrate as the parental generation experiment. Plants originated from these root fragments were termed as the offspring plants. On 19 August 2016, of the 28 offspring plants derived from each of two treatments of the parental generation, seven were randomly assigned to one of four herbivory treatments, i.e., the control or herbivory by A. hygrophila (density: three female and three male adults per plant), C. piperata (density: six adults per plant) or P. minor (density: six female adults per plant). All plants in the offspring generation were placed in cages. The offspring generation experiment was ended on 9 September 2016 and lasted only three weeks. This was because herbivory by A. hygrophila was highly intensive and most of leaves of A. philoxeroides had been eaten within these three weeks. At harvest, we counted number of ramets and number of leaves of A. philoxeroides. Leaves, stems, taproots and fine roots of each plant were separated, dried at 70 °C for 48 h, and weighed. Root fragments from the parental generation were excluded from harvest. The mean temperature and relative humidity during the offspring generation experiment were 24.6 ± 0.4 °C and 70.9 ± 2.0%, respectively.
Measurements of non-structural carbohydrates
Water-soluble sugars and starch are major components of non-structural carbohydrates (NSC). Water-soluble sugars are considered an indicator of plant vigor, and starch is an important carbohydrate reserve in plants (Briske and Richards 1994; Machado et al. 2017). NSC can be easily remobilized and used by plants for compensatory growth after herbivory (Schwachtje et al. 2006; Gómez et al. 2012).
Dried leaves, stems, taproots and fine roots were finely ground using a Retsch MM400 Mixer Mill (Retsch GmbH, Haan, Germany). Subsamples of approximately 50 mg were analyzed for concentrations of soluble sugars and starch using the perchloric acid/anthrone method (Luo et al. 2014; Dong et al. 2017). Briefly, the soluble sugars were extracted in 80% ethanol at 80 °C for 30 min, the extracts were centrifuged at 4000× for 10 min, and then the supernatants of three successive extractions were pooled and thoroughly mixed. The concentration of soluble sugars was determined by measuring the absorbance at 620 nm in a spectrophotometer. The starch in the pellet was reacted with perchloric acid, extracted, and analyzed using the anthrone reaction with the method described for measuring soluble sugars. The concentration of total NSC was the sum of the concentrations of soluble sugars and starch. In the offspring generation experiment, only five replicates of plants were used for NSC measurement in each of the eight treatments.
Measurements of total C and N
Total N in plant tissues is a key element relevant to physiological responses of host plants to herbivory-induced stress (Schoonhoven et al. 2005; Agrawal and Weber 2015). C/N is often used to indicate the ability of host plants to tolerate herbivory and is also an indicator of food quality for herbivores (Fan et al. 2016). After combustion, concentrations of total C in leaves, stems, taproots and fine roots were determined using a total organic carbon analyzer (Multi N/C 3100, Analytik Jena, Germany). The concentration of total N in each plant part was determined using a continuous-flow injection auto-analyzer (Technicon AA3-HR; SEAL Analytical, Germany). In the offspring generation experiment, five replicates of plants were used for C and N measurements in each treatment.
Data analysis
For the parental generation of A. philoxeroides, independent t-tests were conducted to examine effects of herbivory by P. minor on growth (total mass, shoot mass, taproot mass and fine root mass), biomass allocation (root to shoot ratio), and root physiological traits (concentrations of soluble sugars, starch, total NSC, total C and total N and C/N) of parent plants. For the offspring generation of A. philoxeroides, two-way ANCOVAs were used to test effects of herbivory history of parent plants (legacy effect of herbivory; the control vs. herbivory by P. minor) and current herbivory of offspring plants (direct effect of herbivory; the control vs. herbivory by A. hygrophila, C. piperata or P. minor) on growth (total mass, leaf mass, stem mass, taproot mass, fine root mass, number of leaves and number of ramets), allocation (root to shoot ratio) and physiology (concentrations of soluble sugars, starch, total NSC and total N and C/N) of clonal offspring plants. The initial fresh mass of the root fragments was treated as a covariate to exclude the potential effect of differences in initial size. Because mass of leaves and fine roots of plants subjected to herbivory by A. hygrophila were insufficient for chemical measurements, the effects from herbivory by A. hygrophila on leaf and fine-root chemicals were not considered in ANCOVAs. In addition, one plant in the control group died during the experiment and was excluded from the analyses. Linear contrasts were used to examine the difference between the four herbivory treatments on offspring across the herbivory treatments on parents. Before analysis, fine root mass was transformed to square root to meet the assumptions of normality and homogeneity of variances. Data analyses were conducted using SPSS 22.0 (SPSS, Inc., Chicago, IL, USA).
Results
Direct effects of herbivory on parent plant tolerance
Grazed parent plants of A. philoxeroides produced significantly less total mass, shoot mass and taproot mass and had a higher root to shoot ratio than control plants (Table 1a). Grazed parent plants also had a lower C concentration, a higher N concentration in roots and thus a lower C/N ratio than control plants, but there were no significant differences in concentrations of soluble sugars, starch or NSC (Table 1b).
Legacy effects of herbivory across clonal generations
A legacy effect of herbivory by P. minor in the parental generation was only found on fine root growth and concentrations of starch and NSC in offspring stems of A. philoxeroides (Tables 2, 3, 4 in “Appendix”). Offspring taken from grazed parents produced significantly less fine root mass and had lower concentrations of starch and NSC in stems than those taken from ungrazed parents (Figs. 2h, 3e, f, Tables 2, 3 in “Appendix”). A significant interaction effect between herbivory history of parents and current herbivory of offspring was detected on none of the traits measured, suggesting that the legacy effect of herbivory was independent of the direct effect of herbivory (Tables 2, 3, 4 in “Appendix”).
Effects of herbivory of parent and offspring plants on growth measures and biomass allocation of Alternanthera philoxeroides. Mean ± SE are adjusted based on ANCOVA. Different letters indicate a significant difference between the herbivory treatments on offspring (linear contrasts, P < 0.05). CK, Ah, Cp and Pm represent control (without herbivory) and herbivory by A. hygrophila, C. piperata and P. minor, respectively
Effects of herbivory of parent and offspring plants on concentrations of soluble sugars, starch and total NSC in each organ of Alternanthera philoxeroides. Mean ± SE are adjusted based on ANCOVA. Different letters indicate a significant difference between the herbivory treatments on offspring (linear contrasts, P < 0.05). CK, Ah, Cp, and Pm represent control (without herbivory) and herbivory by A. hygrophila, C. piperata and P. minor, respectively. Data are not available for leaves and fine roots of plants under Ah due to insufficient mass for chemical analysis
Species-specific direct effects of herbivores on offspring plant tolerance
Current herbivory on offspring significantly affected all growth measures and biomass allocation of offspring plants of A. philoxeroides (Fig. 2, Table 2 in “Appendix”). Offspring grazed by A. hygrophila produced significantly less total mass, leaf mass, stem mass and fine root mass than control plants, but herbivory by C. piperata or P. minor had no significant effects on biomass (Fig. 2a, e, f, h). Offspring grazed by C. piperata and P. minor produced more taproot mass than control plants, but herbivory by A. hygrophila had no significant effect (Fig. 2g). Irrespective of herbivore identity, grazed offspring had a higher root to shoot ratio than control plants (Fig. 2d, Table 2 in “Appendix”). Offspring produced the fewest ramets and leaves when grazed by A. hygrophila, the greatest when not grazed or grazed by P. minor, and intermediate when grazed by C. piperata (Fig. 2b, c).
Current herbivory influenced NSC and N of stems and taproots of offspring plants, but not NSC or N of leaves or fine roots (Table 3 in “Appendix”). Compared to control plants, offspring plants grazed by P. minor had a significantly higher concentration of starch in stems and total NSC in taproots, but herbivory by A. hygrophila or C. piperata had no significant effects (Fig. 3e, i). Compared to control plants, offspring plants grazed by A. hygrophila had a significantly higher concentration of sugars in stems and N in stems and taproots, and a lower C/N in stems and taproots, but herbivory by C. piperata or P. minor had no significant effects (Figs. 3d, 4c–f).
Effects of herbivory of parent and offspring plants on total N concentration and C/N in plant organs of Alternanthera philoxeroides. Mean ± SE are adjusted based on ANCOVA. Different letters indicate a significant difference between the herbivory treatments on offspring (linear contrasts, P < 0.05). CK, Ah, Cp and Pm represent control (without herbivory) and herbivory by A. hygrophila, C. piperata and P. minor, respectively. Data are not available for leaves and fine roots of plants under Ah due to insufficient mass for chemical analysis
Discussion
Direct effects of herbivory on parent plant tolerance
Not surprisingly, approximately three months of herbivory by P. minor in the parental generation significantly reduced growth of parent plants of A. philoxeroides (Table 1), and total C concentration in roots (Table 1). These results suggest that a relative long period of herbivory by a generalist herbivore imposed a detrimental impact on growth of clonal plants (Schooler et al. 2006; Dong et al. 2017; Wang et al. 2017). This may be because infestation by P. minor accelerated the defoliation of leaves of host plants, and thus reduced their potential for photosynthesis and yield (Cox 1989; Venette and Davis 2004). However, the relative long period of herbivory by P. minor did not influence the concentration of non-structural carbohydrates and even increased the concentration of N in roots. These results indicate that A. philoxeroides may maintain the similar or even a higher quality of internal resources in the underground storage organ when it encounters aboveground herbivore damage. Such a resource allocation pattern may not only alleviate the ongoing herbivory pressure on the growth of A. philoxeroides, but also guarantee the regrowth potential of the vegetative (clonal) propagules that originate from root fragments (Jia et al. 2009; Dong et al. 2017).
Legacy effects of herbivory across clonal generations
Contrary to our expectation (1st hypothesis), we detected a significant legacy effect only on three out of the 28 study traits related to growth, biomass allocation pattern or physiological responses of A. philoxeroides (Figs. 2, 3, 4). This result suggests that there was little legacy effect of herbivory in the clonal offspring generation of A. philoxeroides. However, in some sexually reproducing plant species, legacy effects of herbivory appeared to be much greater so that they reduced seed mass and vigor (Obeso 1993) and subsequent fitness of offspring plants (Mueller et al. 2005). One possible explanation for lack of legacy effects in A. philoxeroides is that for clonal plants vegetative propagules such as stolon, rhizome or root fragments have an apparent size and quality advantage over seeds (Latzel and Klimešová 2009). Such a propagule advantage may benefit the early growth and establishment of offspring plants and thus buffer them against potential legacy effects. Another possible explanation is that the legacy effect of herbivory may be delayed in subsequent generations and tends to occur during the late period of plant development (Dong et al. 2017). For instance, our previous work showed that a negative legacy effect of herbivory became significant only after offspring of A. philoxeroides had been grazed for 16 weeks (Dong et al. 2017).
A legacy effect of herbivory often arises when the parental and offspring environments are similar (Mousseau and Fox 1998; Galloway 2005), and has been reported under stressful conditions such as nutrient deficiency (Latzel et al. 2014) and drought (González et al. 2016). In our study, however, none of the few detected legacy effects depended on the current herbivory conditions that offspring plants experienced, suggesting that pre-adaption to herbivory did not occur in the clonal offspring of A. philoxeroides. Our results thus do not support the 2nd hypothesis. The likely reason is lack of evolutionary history between A. philoxeroides and P. minor in introduced ranges (Lu and Ding 2012). In other words, the temporary plant–herbivore interaction constructed in our experiment could not stimulate a rapid evolution of tolerance responses of A. philoxeroides across two vegetative generations. On the contrary, a long-term history (approximately 10–20 years) of exposure to herbivory by A. hygrophila or C. piperata in introduced ranges was found to improve the compensatory ability of offspring ramets of A. philoxeroides to tolerate the similar herbivores (Lu and Ding 2012). Therefore, future studies testing herbivory tolerance of invasive species could consider the evolutionary relationship between introduced plants and local herbivores.
Species-specific direct effects of herbivores on offspring plant tolerance
In the offspring generation, three weeks of herbivory by the specialist herbivore A. hygrophila significantly reduced growth of clonal offspring plants of A. philoxeroides (Fig. 2). The result agrees with findings of many other studies (Schooler et al. 2006; Lu et al. 2013; Fan et al. 2016; Dong et al. 2017), suggesting that specialist herbivory can impose a strikingly detrimental impact on growth of clonal plants (Schooler et al. 2006; Dong et al. 2017; Wang et al. 2017). However, three weeks of herbivory by C. piperata or P. minor had no negative impact on biomass accumulation of clonal offspring plants of A. philoxeroides (Fig. 2). These results partly support the 4th hypothesis, suggesting that effects of herbivory on growth of A. philoxeroides are species specific and that specialist herbivores may impose more negative effects than other herbivores. The results also suggest that A. hygrophila could potentially control the aboveground expansion of A. philoxeroides at least in a short period, whereas the other two herbivores might not. Thus, the results are consistent with the fact that A. hygrophila is considered a successful biological control agent for aquatic populations of A. philoxeroides and has been intentionally released in the introduced ranges, including China (Lu et al. 2013), the USA (Spencer and Coulson 1976) and Australia (Julien et al. 1995; Schooler et al. 2006).
Our results also support the third hypothesis that herbivory can alter resource allocation of host plants and such impacts vary with herbivores. Herbivory by A. hygrophila did not significantly reduce growth of taproots (underground storage organ) of A. philoxeroides, and herbivory by C. piperata and P. minor even stimulated it (Fig. 2g). Consequently, aboveground herbivory enhanced relative resource allocation to the underground parts of damaged plants (Fig. 2d), agreeing with previous findings on the same plant (Huang et al. 2016). Damaged plants of A. philoxeroides seemed not to directly compensate for the reduction of light-harvesting structures (leaves) caused by aboveground herbivory, but allocated relatively more resources to organs for vegetative reproduction (i.e., taproots). The biomass allocation pattern of A. philoxeroides under aboveground herbivory does not seem to agree with the optimal partitioning theory which predicts that plants allocate relatively more resources to the organ that acquires the most limiting resources (Bloom et al. 1985; Mccarthy and Enquist 2007). One likely explanation is that taproots functioned as an important pool of vegetative buds during the entire developmental period of A. philoxeroides (Jia et al. 2009; Dong et al. 2017). Consequently, increasing resource storage in taproots could ensure that sufficient resources could be re-mobilized and utilized for regrowth of A. philoxeroides to alleviate potential detrimental effects of disturbance such as herbivory (Wilson et al. 2007; Jia et al. 2009). Such a resource allocation pattern may have resulted in a low efficiency of using herbivores to control terrestrial populations of A. philoxeroides that have accumulated a much larger amount of taproots than its aquatic populations. Our results are also consistent with the fact that the release of A. hygrophila could only delay, but not completely terminate the establishment and expansion of terrestrial populations of A. philoxeroides in introduced ranges (Sainty et al. 1998; Lu et al. 2014; Fan et al. 2016).
After three weeks of herbivory, grazed offspring plants of A. philoxeroides had higher concentrations of sugars, starch or total NSC in storage organs (stems or taproots) than control plants, although herbivory had no effect on any of these measures in resource-acquiring organs (leaves and fine roots; Fig. 3). Thus, even a short period of herbivory could already activate NSC-related tolerance responses, and damaged plants accumulated more resources in storage organs for subsequent regrowth (Wilson et al. 2007; Dong et al. 2017). Moreover, such physiological responses to herbivory varied with herbivores (Fig. 3e, i) and were especially beneficial for damaged plants attacked by P. minor. When attacked by P. minor, A. philoxeroides accumulated higher NSC concentrations in taproots that could be directly used for subsequent regrowth. In contrast, when attacked by A. hygrophila and C. piperata, NSC concentrations in plant organs was little changed (Fig. 3). These results suggest again that tolerance responses of A. philoxeroides to herbivory are species specific.
N and C/N in plant organs are often considered key indicators for physiological status of plants subjected to herbivory (Schoonhoven et al. 2005; Agrawal and Weber 2015; Dong et al. 2017). Our work provides evidence that the allocation pattern of N could be closely related to herbivore types. Intensive herbivory by the chewing herbivore A. hygrophila resulted in an increase in N concentration by 48.7–80.5% and thus a decrease in C/N by 38.2–46.8% in taproots compared to the control or less intensive herbivory by C. piperata or P. minor (Fig. 4). We speculate that intensive herbivory by chewing herbivores may block the translocation of N-based chemicals from roots to leaves or shoot tips, thereby resulting in the accumulation of N in storage organs (Thornton et al. 1993; Thornton and Millard 1997; Newingham et al. 2007). Meanwhile, intensive herbivory may also induce damaged plants to remobilize N toward undamaged organs, reducing the foliar N concentration and consequently leaf palatability (Schoonhoven et al. 2005; Fan et al. 2016). Such changes in N concentrations in damaged plants caused by aboveground herbivory may be of great importance to damaged plants, allowing them to achieve higher compensatory growth (Thornton et al. 1993; Millard et al. 2010).
Conclusions
Our results highlight the importance of A. hygrophila in controlling the aboveground spread of A. philoxeroides. However, herbivory by other species was largely tolerated and accompanied by increased allocation to underground storage organs and altered physiological reserves. Both of them could allow this invasive plant to tolerate herbivory and successfully invade new areas in the face of new herbivore pressure.
References
Agrawal AA (2000) Specificity of induced resistance in wild radish: causes and consequences for two specialist and two generalist caterpillars. Oikos 89:493–500
Agrawal AA (2002) Herbivory and maternal effects: mechanisms and consequences of transgenerational induced plant resistance. Ecology 83:3408–3415
Agrawal AA, Kotanen PM (2003) Herbivores and the success of exotic plants: a phylogenetically controlled experiment. Ecol Lett 6:712–715
Agrawal AA, Weber MG (2015) On the study of plant defence and herbivory using comparative approaches: how important are secondary plant compounds. Ecol Lett 18:985–991
Ali JG, Agrawal AA (2015) Asymmetry of plant-mediated interactions between specialist aphids and caterpillars on two milkweeds. Funct Ecol 28:1404–1412
Ashton IW, Lerdau MT (2008) Tolerance to herbivory, and not resistance, may explain differential success of invasive, naturalized, and native North American temperate vines. Divers Distrib 14:169–178
Babst BA, Ferrieri RA, Thorpe MR, Orians CM (2010) Lymantria dispar herbivory induces rapid changes in carbon transport and partitioning in Populus nigra. Entomologia Exp Appl 128:117–125
Bloom AJ, Chapin FS, Mooney HA (1985) Resource limitations in plants-an economic analogy. Annu Rev Ecol Evol S 16:363–392
Briske DD, Richards JH (1994) Physiological responses of individual plants to grazing: current status and ecological significance. In: Vavra M, Laycock WA, Pieper RD (eds) Ecological implications of herbivory in the west. Society for Range Management, Denver, pp 147–176
Colautti RI, Ricciardi A, Grigorovich IA, Macisaac HJ (2004) Is invasion success explained by the enemy release hypothesis? Ecol Lett 7:721–733
Cox JM (1989) The mealybug genus Planococcus (Homoptera: Pseudococcidae). B Brit Mus Entomol 58:1–78
Dai H, Lu XM, Zhang J, Ding JQ (2014) Responses of a native beetle to novel exotic plant species with varying invasion history. Ecol Entomol 39:118–124
Dam NMV, Baldwin IT (2001) Competition mediates costs of jasmonate-induced defences, nitrogen acquisition and transgenerational plasticity in Nicotiana attenuata. Funct Ecol 15:406–415
D’Antonio CM, Loope LL, Westbrooks R (1996) Biological invasions as a global environment change. Am Sci 84:218–228
Dong BC, Fu T, Luo FL, Yu FH (2017) Herbivory-induced maternal effects on growth and defense traits in the clonal species Alternanthera philoxeroides. Sci Total Environ 605–606:114–123
Fan SF, Yu HH, Dong XR, Wang LG, Chen XW, Yu D, Liu CH (2016) Invasive plant Alternanthera philoxeroides suffers more severe herbivory pressure than native competitors in recipient communities. Sci Rep 6:36542
Francis AW, Kairo MT, Roda AL, Liburd OE, Polar P (2012) The passionvine mealybug, Planococcus minor (Maskell)(Hemiptera: Pseudococcidae), and its natural enemies in the cocoa agroecosystem in Trinidad. Biol Control 60:290–296
Galloway LF (2005) Maternal effects provide phenotypic adaptation to local environmental conditions. New Phytol 166:93–100
Gómez S, Steinbrenner AD, Osorio S, Schueller M, Ferrieri RA, Fernie AR, Orians CM (2012) From shoots to roots: transport and metabolic changes in tomato after simulated feeding by a specialist lepidopteran. Entomol Exp Appl 44:101–111
González APR, Dumalasová V, Rosenthal J, Skuhrovec J, Latzel V (2016) The role of transgenerational effects in adaptation of clonal offspring of white clover (Trifolium repens) to drought and herbivory. Evol Ecol 31:345–361
Herman JJ, Sultan SE (2011) Adaptive transgenerational plasticity in plants: case studies, mechanisms, and implications for natural populations. Front Plant Sci 2:102
Holeski LM, Jander G, Agrawal AA (2012) Transgenerational defense induction and epigenetic inheritance in plants. Trends Ecol Evol 27:618–626
Holm LG, Doll J, Holm E, Pancho JV, Herberger JP (eds) (1997) World weeds: natural histories and distribution. Wiley, New York, pp 37–44
Huang W, Siemann E, Wheeler GS, Zuo JW, Carrillo J, Ding JQ (2010) Resource allocation to defence and growth are driven by different responses to generalist and specialist herbivory in an invasive plant. J Ecol 98:1157–1167
Huang W, He MY, Lu XM, Ding JQ (2016) Differences in interactions of aboveground and belowground herbivores on the invasive plant Alternanthera philoxeroides and native host A. sessilis. Biol Invasions 18:3437–3447
Jia X, Pan XY, Li B, Chen JK, Yang XZ (2009) Allometric growth, disturbance regime, and dilemmas of controlling invasive plants: a model analysis. Biol Invasions 11:743–752
Joshi J, Vrieling K (2005) The enemy release and EICA hypothesis revisited: incorporating the fundamental difference between specialist and generalist herbivores. Ecol Lett 8:704–714
Julien MH, Skarratt B, Maywald GF (1995) Potential geographical distribution of alligator weed and its biological control by Agasicles hygrophila. J Aquat Plant Manag 33:55–60
Keane RM, Crawley MJ (2002) Exotic plant invasions and the enemy release hypothesis. Trends Ecol Evol 17:164–170
Lapointe L, Bussières J, Crête M, Ouellet JP (2010) Impact of growth form and carbohydrate reserves on tolerance to simulated deer herbivory and subsequent recovery in Liliaceae. Am J Bot 97:913–924
Latzel V, Klimešová J (2009) Fitness of resprouters versus seeders in relation to nutrient availability in two Plantago species. Acta Oecol 35:541–547
Latzel V, Janeček Š, Doležal J, Klimešová J, Bossdorf O (2014) Adaptive transgenerational plasticity in the perennial Plantago lanceolata. Oikos 123:41–46
Lu XM, Ding JQ (2012) History of exposure to herbivores increases the compensatory ability of an invasive plant. Biol Invasions 14:649–658
Lu XM, Siemann E, Shao X, Huang W, Ding JQ (2013) Climate warming affects biological invasions by shifting interactions of plants and herbivores. Global Change Biol 19:2339–2347
Lu XM, Shao X, Ding JQ (2014) No impact of a native beetle on exotic plant performance and competitive ability due to plant compensation. Plant Ecol 215:275–284
Luo FL, Chen Y, Huang L, Wang A, Zhang MX, Yu FH (2014) Shifting effects of physiological integration on performance of a clonal plant during submergence and de-submergence. Ann Bot 113:1265–1274
Machado RAR, Zhou W, Ferrieri AP, Arce CCM, Baldwin IT, Xu S, Erb M (2017) Species-specific regulation of herbivory-induced defoliation tolerance is associated with jasmonate inducibility. Ecol Evol 7:3703–3712
Maron JL, Vilà M (2001) When do herbivores affect plant invasion? Evidence for the natural enemies and biotic resistance hypotheses. Oikos 95:361–373
Mccarthy MC, Enquist BJ (2007) Consistency between an allometric approach and optimal partitioning theory in global patterns of plant biomass allocation. Funct Ecol 21:713–720
Millard P, Hester A, Wendler R, Baillie G (2010) Interspecific defoliation responses of trees depend on sites of winter nitrogen storage. Funct Ecol 15:535–543
Morrison WE, Hay ME (2011) Herbivore preference for native vs. exotic plants: generalist herbivores from multiple continents prefer exotic plants that are evolutionarily Naïve. PLoS ONE 6:e17227
Mousseau TA, Fox CW (1998) The adaptive significance of maternal effects. Trends Ecol Evol 13:403–407
Mueller RC, Wade BD, Gehring CA, Whitham TG (2005) Chronic herbivory negatively impacts cone and seed production, seed quality and seedling growth of susceptible pinyon pines. Oecologia 143:558–565
Nagasawa A, Matsuda K (2015) Factors determining the host range of two tortoise beetles, Cassida nebulosa L. and C. piperata Hope (Coleoptera: Chrysomelidae) in Japan. Open Entomol J 9:1–6
Newingham BA, Callaway RM, Bassirirad H (2007) Allocating nitrogen away from a herbivore: a novel compensatory response to root herbivory. Oecologia 153:913–920
Obeso JR (1993) Does defoliation affect reproductive output in herbaceous perennials and woody plants in different ways? Funct Ecol 7:150–155
Pimentel D, Lach L, Zuniga R, Morrison D (2000) Environmental and economic costs of nonindigenous species in the United States. Bioscience 50:53–65
Polley HW, Detling JK (1988) Herbivory tolerance of Agropyron smithii populations with different grazing histories. Oecologia 77:261–267
Roda A, Francis AW, Kairo MTK, Culik M, Peña JE (2013) Planococcus minor (Hemiptera: Pseudococcidae): bioecology, survey and mitigation strategies. In: Peña JE (ed) Potential invasive pests of agricultural crops. CAB International, Wallingford, pp 288–300
Sainty G, McCorkelle G, Julien M (1998) Control and spread of alligator weed Alternanthera philoxeroides (Mart.) Griseb., in Australia: lessons for other regions. Wetl Ecol Manag 5:195–201
Schooler S, Baron Z, Julien M (2006) Effect of simulated and actual herbivory on alligator weed, Alternanthera philoxeroides, growth and reproduction. Biol Control 36:74–79
Schoonhoven LM, Loon JJAV, Dicke M (2005) Insect-plant biology, 2nd. Oxford University Press, Oxford
Schwachtje J, Minchin PE, Jahnke S, van Dongen JT, Schittko U, Baldwin IT (2006) SNF1-related kinases allow plants to tolerate herbivory by allocating carbon to roots. P Natl Acad Sci USA 103:12935–12940
Spencer NR, Coulson JR (1976) The biological control of alligatorweed, Alternanthera philoxeroides, in the United States of America. Aquat Bot 2:177–190
Steets J, Ashman TL (2010) Maternal effects of herbivory in Impatiens capensis. Int J Plant Sci 171:509–518
Thornton B, Millard P (1997) Increased defoliation frequency depletes remobilization of nitrogen for leaf growth in grasses. Ann Bot 80:89–95
Thornton B, Millard P, Duff EI, Buckland ST (1993) The relative contribution of remobilization and root uptake in supplying nitrogen after defoliation for regrowth of laminae in four grass species. New Phytol 124:689–694
Venette RC, Davis EE (2004) Mini risk assessment: passionvine mealybug, Planococcus minor (Maskell) (Pseudococcidae: Hemiptera). Department of Entomology, University of Minnesota, St. Paul, pp 1–30
Wang N, Yu FH, Li PX, He WM, Liu J, Yu GL, Song YB, Dong M (2009) Clonal integration supports the expansion from terrestrial to aquatic environments of the amphibious stoloniferous herb Alternanthera philoxeroides. Plant Biol 11:483–489
Wang P, Li H, Pang XY, Wang A, Dong BC, Lei JP, Yu FH, Li MH (2017) Clonal integration increases tolerance of a phalanx clonal plant to defoliation. Sci Total Environ 593–594:236–241
Wilson JRU, Yeates A, Schooler S, Julien MH (2007) Rapid response to shoot removal by the invasive wetland plant, alligator weed (Alternanthera philoxeroides). Environ Exp Bot 60:20–25
Xu CY, Zhang WJ, Fu CZ, Lu BR (2003) Genetic diversity of alligator weed in China by RAPD analysis. Biodivers Conserv 12:637–645
Ye WH, Li J, Cao HL, Cao HL, Ge XJ (2003) Genetic uniformity of Alternanthera philoxeroides in South China. Weed Res 43:297–302
Yu FH, Wang N, Alpert P, He WM, Dong M (2009) Physiological integration in an introduced, invasive plant increases its spread into experimental communities and modifies their structure. Am J Bot 96:1983–1989
Acknowledgements
We thank Jia-Hao Wang and Ting Fu for assistance with the management of the experiment and plant harvest, Dr. Bo-Yi Chen, Dr. Jian-Yu Li, and Dr. Yong-Jian Wang for assistance with insect collection, and Qiao-Qi Sun, the associate editor and two anonymous reviewers for their valuable comments. This work was supported by National Key Research and Development Program of China (2016YFC1202102, 2016YFC1201101), National Natural Science Foundation of China (31500331), and Fundamental Research Funds for the Central Universities (2015ZCQ-BH-01).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dong, BC., Wang, MZ., Liu, RH. et al. Direct and legacy effects of herbivory on growth and physiology of a clonal plant. Biol Invasions 20, 3631–3645 (2018). https://doi.org/10.1007/s10530-018-1801-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-018-1801-5