Abstract
Environmentally induced transgenerational effects can increase success of offspring and thereby be adaptive if offspring experience conditions similar to the parental environment. The ecological and evolutionary significance of these effects in plants have been considered overwhelmingly in the context of sexual generations. We investigated whether drought stress and jasmonic acid, a key hormone involved in induction of plant defenses against herbivores, applied in the parental generation, trigger transgenerational effects in clonal offspring of Trifolium repens and whether these effects are adaptive. We found that drought stress experienced by parents significantly affected phenotypes of offspring ramets. Offspring ramets were bigger if they were produced in the parental water regime (control/drought). Repeated application of jasmonic acid to parents increased the subsequent growth of offspring ramets produced by stolons after they were disconnected from the parental clone. However, these offspring ramets experienced similar herbivory by the generalist Spodoptera littoralis caterpillar as did control offspring ramets, indicating that this jasmonic acid application in the parental generation did not result in a transgenerational effect comprising increased herbivory resistance. We conclude that, overall, environmental interaction in the parental generation can trigger transgenerational effects in clonal plants and some of these effects can be adaptive. Moreover, transgenerational effects in clonal plants that significantly influence their growth and behavior can ultimately affect the evolutionary trajectories of clonal populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
If the experience of a particular environment by parents results in modification of offspring phenotype, this represents transgenerational effects (Roach and Wulff 1987; Jablonka and Lamb 1995; Rossiter 1996; Mousseau and Fox 1998; Galloway 2005). Thus, the growth, photosynthesis and reproduction of offspring might be influenced by the nutrients, light or water availability experienced by parents independently of the genes that the offspring inherited (Galloway and Etterson 2007; Latzel et al. 2009; 2010; Sultan et al. 2009). Transgenerational effects can be adaptive in cases in which offspring have higher fitness in environments resembling parental conditions (Galloway 2005; Galloway and Etterson 2007; Latzel et al. 2014; Chen et al. 2014). Adaptive transgenerational effects can be mediated by differences in seed quality (Roach and Wulff 1987) or by epigenetic modification of DNA (Rossiter 1996; Boyko et al. 2010) and are considered as potentially important players in the ecology and evolution of plant populations (Riska 1989; Räsänen and Kruuk 2007).
Our knowledge of the ecological and evolutionary roles of adaptive transgenerational effects is derived almost exclusively from studying them across sexual generations. Such studies have shown, for example, that offspring of Campalunastrum americanum can have more than three times higher fitness if planted in a parental than in a non-parental light environment (Galloway and Etterson 2007). Similarly, plants of Plantago lanceolata can store about 30 % more carbohydrates if they are experiencing parental instead of non-parental soil nutrients levels (Latzel et al. 2014). Such adaptive transgenerational effects may potentially facilitate establishment of plant populations in new environments by enabling adaptation to local conditions much more rapidly than natural selection would, provided that the new environment is sufficiently similar to the original source environment. Indeed, adaptive transgenerational effects hold the potential to alter not only population (Plaistow et al. 2006) but also evolutionary dynamics (Räsänen and Kruuk 2007). Moreover, given that many ecosystems are dominated by plants that rely mostly on clonal reproduction (van Groenendael and de Kroon 1990; Klimeš et al. 1997; Klimešová and de Bello 2009), with local genetic diversity and opportunities for natural selection thereby constrained, adaptive transgenerational effects may play a large and overlooked role in the evolutionary dynamics of asexually reproducing plant species.
A clonal plant genet can comprise a large web of interconnected, genetically identical and potentially independent ramets, and because most environmental resources are heterogeneous in space and time, it is virtually inevitable that individual ramets within such a connected genet encounter different environments. Thus, ramets within a connected genet (which, in its totality, we refer to as “a clonal plant” or as “a clone”) might differ in their phenotypes and/or functions. However, the phenotypes of individual ramets of a clonal plant are not fully independent from each other (de Kroon et al. 2005). Therefore, the growth of a clonal plant can involve a complex process of evaluation of actual conditions across the whole plant. Indeed, the decisions of a clonal plant as to where to place its offspring ramets can take into account local variation in soil nutrient levels between older interconnected ramets of a clone (Louâpre et al. 2012). The phenotypic response of a ramet to light may depend on the light heterogeneity experienced by the whole connected clone (Dong 1995). Ramets can also differentiate their functions to better exploit environmental heterogeneity, displaying division of labour (Magyar et al. 2007). Each ramet can be disconnected from the parental plant (in which case, we refer to it as a “maternal ramet”) and can establish a separate clone, independent from the parent plant. However, we do not know whether the growth and behaviour of the emerging clonal plant is altered by transgenerational effects and thus influenced by environmental interactions of the parental plant.
A maternal ramet can carry a broad spectrum of information on environmental interactions it experienced before detaching from the rest of the parental clone, which can potentially alter future behaviour of the successive clone. Such information can be in the forms of molecular messengers that were circulating in the parental plant (Stuefer et al. 2004), retained resources or other organic or inorganic chemical compounds or their residua or epigenetically modified gene expression reflecting interactions of the parental clone with its environment (Gao et al. 2010; Richards et al. 2012). All of this information can potentially alter early growth and behaviour of the growing clonal plant, and shape its ultimate destiny.
Our understanding, however, of the role of transgenerational effects in clonal reproduction is very limited. We therefore investigated whether drought and simulated herbivory experienced by parental plants of the common clonal herb Trifolium repens can trigger transgenerational effects and, if so, whether they are adaptive in clonal offspring. In particular, by conducting a reciprocal transplant experiment on maternal ramets, we tested whether offspring ramets perform better in the parental than in the non-parental soil water regime (wet vs. dry) and whether offspring ramets are more resistant to herbivory if parental plants were treated with jasmonic acid, a key hormone activating production of defences against insect herbivores (Thaler et al. 1996; McConn et al. 1997; Baldwin 1998; Cipollini and Redman 1999).
To get an idea of the genotypic specificity of transgenerational effects we worked with four genotypes. Additionally, in order to indirectly test whether potential transgenerational effects are driven by heritable epigenetic variation, we treated some parental plants with the demethylating agent 5-azacytidine. This agent is a cytosine analogue that blocks methylation of DNA in eukaryotes (Cihák 1974), which is one of the epigenetic mechanism involved in regulation of gene expression. Previous studies that applied 5-azacytidine successfully demonstrated the roles of epigenetic variation in plant transgenerational adaptation to stress (Boyko et al. 2010), plant phenotypic plasticity (Bossdorf et al. 2010) and control of inbreeding depression (Vergeer and Ouborg 2012).
Methods
Plant material
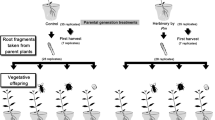
Trifolium repens L. is a widespread stoloniferous monopodial clonal plant occurring in a variety of grasslands and pastures differing in soil quality, nutrient level and soil humidity (Burdon 1983). Each phytomer of T.repens consisting of a node, internode, subtending leaf, axillary bud and two nodal root initials is usually considered as a ramet (Hay et al. 2001). Nevertheless, the monopodial growth style of T. repens means that every stolon elongates along its main axis by producing new phytomers within which resources and information flow is not restricted. In contrast, the side branches produced by axillary buds of the main stolon are more independent from the main stolon because their connection to the main stolon is not permanent, with resources and information exchange with the main stolon thus more limited. Therefore, the growth of side branches should be more independent on the physiological state of the main stolon. Therefore, we define the main stolon as the maternal ramet that is elongating along its main axis, with the side branches produced by axillary buds considered as a subsequent generation, i.e. offspring ramets (see Fig. 1 for a description of maternal and offspring clonal generations considered in the study). Such a conservative approach provides us confidence that we can consider potential observed environmental effects to be transgenerational and ecologically relevant. In 2014, we collected four genotypes (cuttings) of Trifolium repens occurring a minimum of 100 meters apart from each other in the vicinity of the Institute of Botany in Průhonice, Czech Republic, and propagated them vegetatively in a greenhouse of the Institute.
Parental treatments
In February 2015, we created 14 cuttings of each genotype, each cutting consisting of 7 phytomers and an apical end and planted them individually in 30 × 40 × 8 cm trays filled with standardized potting soil (Trávníkový substrát AGRO CS, mixture of sand, compost and peat) in the greenhouse of the Institute, with a 16 h/8 h day/night regime. In March 2015, we randomly assigned these trays to the drought treatment (4 trays per genotype), induction by jasmonic acid (4 trays per genotype) or control treatment (6 trays per genotype). Plants in the drought treatment were watered individually only when the plant showed significant drought stress response, i.e. most leaves wilting. Induction by jasmonic acid (JA) was achieved by spraying plants with a 50 µM solution of JA (Sigma-Aldrich) once a week. Such an approach was chosen to mimic repeated herbivore attacks. Half the plants from each treatment, including controls, were randomly selected for spraying with a 50 µmol solution of 5-azacytidine (referred to hereafter as 5-azaC) every fourth day starting in March 2015. This method was developed in our earlier study on the same species, in which it resulted in about a 4 % average decrease in global methylation level of DNA relative to control plants (González et al. unpublished). The 5-azaC was applied on different days than JA. All treatments, including application of 5-azaC, were terminated in June 2015. Plants from the drought treatment experienced, on average, 14 pulses of water stress. JA-induced plants were sprayed 18 times with the JA solution during the induction period. Plants treated with 5-azaC were sprayed 26 times with the 5-azaC solution. All plants were grown in control conditions for another 20 days after termination of all treatments in order to reduce potential negative physiological aspects of stress treatments on ramet growth and to allow parental plants to produce about four phytomers under unstressed conditions before the start of the transgenerational adaptation tests. To avoid uncontrolled effects of rhizobia symbionts on plant performance we watered all plants with a solution of tetracycline (20 mg per tray) every third week during the study including the phase of pre-cultivation of plant material and transgenerational tests. The absence of rhizobia symbionts was confirmed during harvest of the study.
Transgenerational adaptation tests
Transgenerational adaptation to drought stress
In the beginning of June 2015 we created 14 standardized cuttings from each genotype and treatment group (control, control + 5-azaC, drought, drought + 5azaC), each consisting of 4 phytomers along with the apical end, recorded their lengths, labeled each apical end with a plastic ring and planted them individually into 18 × 10 × 6 cm trays filled with the same potting soil mixture used in the parental generation (Fig. 1). A random half of the transplanted cuttings from each treatment were assigned either to the parental or non-parental water regime; these treatments are further referred to as offspring environments. Thus, we grew 112 plants in their parental water regimes [4 genotypes × 2 water regimes × 2 methylation levels (5-azaC) × 7 replicates] and 112 plants in non-parental water conditions. A total of 22 plants were excluded during the study due to their mortality or aberrant growth. The drought treatment started 14 days after transplantation and followed the same procedure as for the maternal generation. Plants that were assigned to drought treatment received on average seven drought pulses. Each transplanted cutting was represented by a main stolon that we consider as an elongating maternal ramet, with branches arising from it constituting offspring ramets (see also Fig. 1).
Transgenerational effects of jasmonic acid
At the same time that we began the drought-stress experiment, we created 15 standardized cuttings (each consisting of 4 phytomers and including the apical end) from each genotype and treatment group (control, control + 5-azaC, JA, JA + 5-azaC), recorded their lengths, labeled each apical end with a plastic ring and planted them individually into trays filled with standardized potting soil. We grew 240 plants in total in the control environment (4 genotypes × 2 JA levels × 2 methylation levels × 15 replicates). After 8 weeks we randomly selected eight plants from each treatment group and genotype and collected one fully developed leaf from the oldest and one from the youngest offspring ramet produced by each plant. These leaves were used in an herbivore preference test (see below). The remaining seven plants from each treatment group and genotype were harvested simultaneously with the plants from the drought experiment (see below).
Preference test
For the herbivore preference test, we used the generalist herbivore, Egyptian cotton leafworm Spodoptera littoralis Boisduval (Lepidoptera: Noctuidae), which is known to develop on plants of at least 40 plant families (Brown and Dewhurst 1975) including Fabaceae (Maffei et al. 2004). Its extreme polyphagy makes it an excellent bioassay species for experiments onleaf palatability (Kempel et al. 2011). This species originates from (sub)tropical Africa, and thus it may not share a coevolutionary history with our study species. Therefore, its feeding ability on Trifolium repens reflects general palatability of the plants (Kempel et al. 2011).
The Spodoptera caterpillars we used originated from laboratory stock (Laboratory of Quarantine Organisms, Department of Entomology, Crop Research Institute in Prague, CZ). The caterpillars were bred at the Crop Research Institute at 21 ± 1 °C with a relative humidity of 40–60 % and a 16:8 (L:D) photoperiod and were fed on Stonefly Heliothis (artificial) Diet (Wards Natural Science Inc., USA). The individual caterpillars used in the experiment were all of identical age (hatched within the same day) and were 16 days old when the experiment was initiated.
At the beginning of August 2015, we set up pairs of leaves for preference testing, with both leaves in each pair being of the same offspring ramet cohort, i.e. oldest with oldest and youngest with youngest, but with one leaf in each pair coming from an offspring ramet of a control maternal plant that had been subjected to the control treatment and the other from an offspring ramet of a plant that had undergone the JA- induction treatment. Additionally, the leaf pairs were always from offspring of parent plants that had the same 5-azaC treatment status (i.e., either treated or untreated). Altogether, this design yielded a total of 16 pair combinations (oldest JA vs. oldest control, youngest JA vs. youngest control × 4 genotypes × 2 statuses of 5-azaC treatment). Each pair combination was represented by a total of eight replicates, each in a Petri dish (9 cm in diameter and 1 cm in height) with wet filter paper at the bottom. These were placed in the laboratory at 21 ± 1 °C, relative humidity 40–60 %, and a 16:8 (L:D) photoperiod. Single Spodoptera caterpillars were placed in the middle of the Petri dishes, between the two leaves and allowed to feed for 24 h. Before and after the feeding experiment, each leaf was photographed and measured. Based on examination of the photographs, the degree of leaf damage was estimated similarly as in Münzbergová and Skuhrovec (2013) (with leaf damage levels of 0, <10, <25, <50, <75 and >75 %).
Harvest
Two months after establishing the transgenerational experiments (drought stress and jasmonic acid induction), we harvested the above-ground plant parts that had developed after transplantation (i.e., without the original transplanted cutting that had developed before transplantation). We divided every harvested plant into the maternal ramet (main stolon) and offspring ramets (branches, see Fig. 1), measured the lengths of all ramets and then dried them at 70 °C for 24 h and weighed them.
Statistical analyses
The effects of genotype, water regime in the parental and offspring generations and the application of 5-azaC on the length and biomass of the maternal ramet, number of offspring ramets, total length and biomass of offspring ramets, mean offspring biomass produced by maternal ramet (expressed as a total offspring biomass divided by the number of offspring ramets produced by every maternal ramet), total biomass of a clone (maternal ramet plus offspring ramets) were analyzed using analysis of variance (ANOVA) with a four-way factorial design. All factors were considered as fixed effects. The effects of genotype, application of JA and application of 5-azaC on the length and biomass of the maternal ramet and offspring ramets were analyzed using ANOVA with three-way factorial design. To account for potential effects of the initial size of the transplanted cutting and the size of the maternal ramet that had developed after transplantation on offspring ramet number and biomass, we included transplanted cutting length and maternal ramet biomass that developed after transplantation as covariates in the statistical model when analysing the offspring generation. We included transplanted cutting initial size when analysing maternal ramet biomass. All variables were log-transformed prior to analyses to meet the assumptions of homoscedasticity and normality. Whenever we found a significant interaction, we would use a Tukey HSD post hoc test to compare significant differences of means of main effects (genotype, parental and offspring environment).
Herbivore preference tests were analyzed using paired t tests first for the cumulative dataset, i.e., comparison of preference across all genotypes, ramet cohorts and application statuses of 5-azaC, and then we applied multiple paired t test for each cohort (oldest and youngest offspring ramet), genotype and 5-azaC treatment status separately. All statistical analyses were performed using JMP statistical software (JMP 10, SAS Inst.).
Results
Because lengths of maternal as well as offspring ramets were positively correlated with their biomass and statistical results were similar for both parameters, we hereafter refer only to results regarding biomass.
The length of transplanted standardised cutting, which we included as a covariate in the statistical model, was the most significant predictor of the subsequent growth of both the maternal ramet (elongating transplanted cutting) and offspring ramets (Table 1).
Transgenerational adaptation to drought
Maternal ramet after transplantation
Plant genotypes differed in all measured criteria (genotype, Table 1). Offspring drought increased biomass of maternal ramets (Table 1, mean biomass and SE in control: 0.456 ± 0.006 g, mean biomass in drought: 0.473 ± 0.006 g) but the effect of offspring drought was genotype-specific (offspring treatment × genotype, Table 1, Fig. 2a): offspring drought environment increased biomass of maternal ramets of genotypes A, B and C, but reduced biomass in genotype D.
Biomass (a) of maternal ramets and number of offspring ramets (b) of four genotypes grown in control or drought environments after transplantation; and (c) the number of offspring ramets of four genotypes in relation to parental induction with jasmonic acid. Means and standard errors are shown. Asterisks indicates significant differences within individual genotypes
Application of 5-azaC to parental plants reduced growth of maternal ramets after transplantation (mean biomass of control maternal ramets and SE: 0.479 ± 0.006 g, mean biomass and SE of 5-azaC-treated main stolons: 0.450 ± 0.006 g).
Offspring ramets
Drought experienced by the parental generation increased the number of offspring ramets produced by maternal ramets after transplantation (mean number and SE of offspring ramets produced by maternal ramets from parental control treatment: 3.71 ± 0.04, mean number and SE of offspring ramets produced by maternal ramets from parental drought treatment: 4.23 ± 0.04). The number of offspring ramets was also affected, depending on the genotype used, by the offspring drought treatment (genotype × offspring treatment, Table 1; Fig. 2b): offspring drought increased the number of offspring ramets in genotype D and decreased it in the other three genotypes.
Mean offspring biomass also differed among plants experiencing parental versus non-parental drought treatment (interaction parental × offspring treatment, Table 1; Fig. 3a). Offspring ramets were considerably smaller if parental plants experienced drought and maternal ramets were subsequently transplanted to the control treatment in comparison to offspring of parents from the control treatment. On the other hand, the parental water regime had no effect on the mean offspring biomass if the plants had been transplanted to the offspring drought treatment (Fig. 3a). Application of 5-azaC in the parental generation reduced differences in mean offspring biomass caused by parental treatments (interaction parental treatment × 5-azaC, Table 1; Fig. 3b).
Mean offspring biomass, cumulative offspring biomass and total biomass developed after transplantation of maternal ramets to control or drought environment, shown in relation to parental drought and control environments. Means and standard errors are shown. Asterisks indicates significant differences within offspring and parental environments
Offspring drought treatment reduced cumulative biomass of offspring ramets (mean cumulative offspring biomass and SE in control treatment: 0.51 ± 0.02 g, in drought treatment: 0.41 ± 0.02 g), however the degree of the biomass reduction was affected by an interaction with the parental treatment (interaction parental × offspring treatment, Table 1; Fig. 3c). Maternal ramets produced higher cumulative offspring biomass if they were transplanted to parental environment than if they experienced non-parental conditions.
Total plants biomass (cumulative biomass of maternal together with offspring ramets) also revealed better growth of clones that experienced parental conditions in comparison to clones that were transplanted to non-parental conditions (parental × offspring treatment, Table 1; Fig. 3d).
Transgenerational effects of jasmonic acid
Maternal ramets after transplantation
Plant genotypes differed in all measured criteria (genotype, Table 2). Jasmonic acid previously applied to parental plants increased biomass of the maternal ramets (mean biomass of maternal ramets not previously treated with JA and SE: 0.51 ± 0.0124 g; mean biomass of maternal ramets previously treated with JA and SE: 0.53 ± 0.0119 g) after their transplantation to the control environment. Application of 5-azaC in the parental generation reduced growth of transplanted maternal ramets (5-azaC, Table 2, mean biomass and SE of maternal ramets not treated with 5-azaC: 0.488 ± 0.0121S g, mean biomass and SE of maternal ramets previously treated with 5-azaC: 0.463 ± 0.0124 g).
Offspring ramets
Application of JA had different effects on the number of offspring ramets produced, depending on genotype (G × JA, Table 2, Fig. 2c): application of JA reduced the number of offspring ramets in two genotypes (A and C), increased the number of offspring in genotype B, and had no effect on number of offspring ramets in genotype D. JA treatment also increased the cumulative biomass of offspring ramets (JA, Table 2). Plants from the control parental environment produced 0.488 ± 0.06SE g cumulative offspring biomass, whereas plants from the JA treatment produced 0.747 ± 0.06SE g offspring ramet biomass. Caterpillars consumed on average 28 % of the leaf area in the preference tests, with no statistically significant preference shown between offspring ramets of parents that were treated or not treated with JA.
Discussion
We found that the effect of drought experienced by parental plants can extend to the clonal offspring generation in a manner that is likely adaptive. We also identified transgenerational effects triggered by JA (a defence signalling hormone) applied to parental plants. These effects increased offspring biomass but did not alter the leaf preferences of caterpillars. Results of our study demonstrate that environmental interactions experienced by parental plants can have significant effects on the growth of offspring ramets even after the maternal ramets lose connection with the parental clone. It has been suggested that transgenerational effects can alter both population dynamics (Benton et al. 2005; Plaistow et al. 2006) and evolutionary change in response to directional selection (Wade 1998; Räsänen and Kruuk 2007). In support of this, our study suggests that because the behaviour of clonal plants can be affected by environments that are not currently present, it could affect the phenotypic variation within clonal populations. Thus, if the phenotypes of different individuals reflect the environmental conditions experienced by different generations, this would increase phenotypic variation. Alternatively, if the ability of plasticity to respond to contemporaneous conditions is constrained, it could reduce phenotypic variation, but also make it more adaptive with regard to long-term variation (see below). In any case, transgenerational effects, by influencing plasticity, would have consequences for population dynamics, and ultimately, evolution, especially given the reduced levels of genotypic variation as a source of variation in clonal plants.
Although we observed only slightly better performance of offspring ramets in the drought treatment if their parents had experienced drought (Fig. 3c), we consider this transgenerational effect to be adaptive. Nevertheless, it has been argued that it is not sufficient to consider only benefits to the offspring generation, since these might come at a fitness cost to the parental generation, which could outweigh the benefits, preventing the transgenerational effects from being adaptive (Marshall and Uller 2007; Plaistow et al. 2007). In our study, this was not the case, since the additional biomass of offspring ramets was not at the cost of parental biomass, and thus the transgenerational effects improved the performance of the clone as a whole (see Fig. 3d).
Interestingly, offspring biomass produced by maternal ramets of plants that had experienced drought before transplantation to new environments was similar in control and drought conditions (see Fig. 2c). This suggests that the phenotype of offspring ramets reflected former rather than contemporaneous conditions. Such behaviour might represent transgenerational control of clonal offspring phenotypes and be beneficial in reducing potential costs incurred by phenotypic plasticity that is overly responsive to contemporaneous conditions. Thus, in the case of the temporal environmental variation modelled in our experimental system, if offspring ramets grown in control conditions phenotypically reflect only these conditions, they could be at risk of substantial damage in the event of a drought. Certainly, the constraints imposed by such transgenerational control could also be costly, if for example, they prevented plants from fully taking advantage of favourable conditions such as sufficient moisture to maximize reproductive output. Thus, there are risks and rewards potentially associated with either option—being entirely responsive to contemporaneous conditions versus being entirely constrained by conditions experienced by a previous generation. From an evolutionary perspective, it would seem that in evaluating these risks and rewards, plants should take into account not only the predictability of the environment (Galloway 2005), but also the potential magnitude of these costs and benefits. Thus, for example, the potential cost of drought on overly exuberantly growing plant would seem to be quite high, as this might make it vulnerable to serious injury and increased mortality, whereas, in contrast, overly conservative, drought-tolerant growth might not carry such severe potential consequences even though it could result in decreased output in favourable conditions. This idea is speculative on our part, but could be worthy of further investigation in which potential costs and benefits are experimentally manipulated.
The consistency of transgenerational effects triggered by drought across genotypes in T. repens suggests that these effects are not genotype-specific, but this conclusion should be considered as preliminary because of the relatively few genotypes tested. Interestingly, whereas the cumulative offspring biomass was generally lower in the offspring drought treatment, the maternal ramets usually increased biomass in the drought conditions in comparison to the control environment (Fig. 2a). It has been shown that clonal plants can intensify their growth to escape from unfavourable conditions by growing longer internodes and reducing branching (Sutherland and Stillman 1988; Cain 1994). This was probably the case in our study, as biomass was positively correlated with the length of ramets, and maternal ramets reduced the number of offspring ramets in the drought environment.
Altered phenotypes of offspring ramets of parents treated with JA confirmed that the effects of JA persisted in transplanted clones although it did not increase their resistance to herbivory. The expression of inducible defences can be very variable among genotypes (Bergelson 1994). Because we did not actually test the resistance of parental plants to herbivores after application of JA, we cannot rule out the scenarios that JA did not trigger defence production or more broadly that the genotypes in our study were not producing inducible defences at all. Induced plants could be also producing defences only for a short period after JA application. Thus, a study by Gómez et al. (2010) showed that defence induction in T. repens can last for 28 days after the end of induction, with the defence expression decreasing over time. We performed preference tests approximately 80 days after the final induction; therefore, the potential signal of herbivore attack could already have been absent in maternal as well as offspring ramets. It is also possible that repeated application of JA in the parental generation interacted with other physiological processes of treated plants such as photosynthesis (e.g. Creelman and Mullet 1997), which could result in the observed transgenerational effects that altered phenotypes of offspring ramets without actually inducing plant defences. Finally, artificial absence of rhizobia symbionts in our study could have had negatively affected the ability of plants to produce defences (Kempel et al. 2009). Nonetheless, the finding that jasmonic acid triggers clonal transgenerational effects is novel, and the biological context of this response should be investigated in future research.
The lack of an effect of parental drought on the growth of transplanted maternal ramets suggests that transgenerational effects cannot be ascribed to physiological deprivation of maternal ramets. Given the effect of 5-azaC on mean offspring biomass, and because we did not analyse DNA methylation status of plants at the molecular level, we cannot rule out the possibility that transgenerational effects were at least partly mediated by epigenetic variation. However, we suggest that mechanisms other than DNA methylation likely had a more prominent role in the observed transgenerational effects. Since the biochemical composition of T. repens reflects interaction with the environment (Gómez et al. 2008), it is possible that hormones and/or other biochemical compounds (or their traces) that were involved in the drought response were present in the transplanted maternal ramets, and thus were directly affecting the growth of the offspring ramets.
Adaptive response of clonal plants to differences in their environment is a complex process that employs evaluation of variation in the vicinity of all the interconnected ramets (de Kroon et al. 2005). Several studies have also shown that clonal plants can choose the positions of offspring ramets during clonal growth based on the characteristics of the environment into which they are spreading (Hutchings and de Kroon 1994; van Kleunen and Fischer 2001; Waters and Watson 2015). If transgenerational effects are adaptive in clonal plants, a mother plant’s decisions on where to place offspring ramets likely depends not only on the offspring environmental characteristics but also on the potential favourable and unfavourable interactions of maternal and offspring environments. Therefore, we can speculate that even in the case of more subtle environmental variation than presented in our experiment (i.e., drought vs. sufficient water) genetically identical mother plants (e.g., maternal ramets) can evaluate the suitability of given offspring environment differently depending on their own (i.e., maternal ramet) environmental histories. Thus, the behaviour of clonal plants has the potential to reflect past and current environmental conditions and anticipate (depending upon predictability) future conditions. Such behaviour can alter the dynamics and genetics of clonal populations by affecting the correspondence of genotypes to phenotypes.
Conclusion
We have shown that environmental conditions experienced by clonal plants can affect growth of clonal offspring and some of these transgenerational effects could be adaptive. This is in agreement with the widely accepted theory that adaptive transgenerational plasticity should be evolutionarily promoted particularly in predictable environments (Galloway 2005) because in these conditions offspring can truly benefit from “prepared” phenotypes. It is therefore reasonable to expect that not all environmental factors can trigger adaptive transgenerational effects. Less predictable factors, such as some types of herbivory, might cause no transgenerational effects at all or such effects could actually be maladaptive and therefore selected against. Results of our highly controlled study also raise several interesting and important questions. These would include whether transgenerational effects are ecologically important in natural conditions. Also, considering that many clonal species reproduce via bulbs, tubers or turions that can be spread over variable distances, the question arises whether the role of transgenerational effects differs among species with different types of clonal growth and clonal offspring dispersal (Latzel and Klimešová 2010). Our study considered the special scenario in which the maternal ramet is detached from the parental plant. Therefore, we do not know whether the stress history of ramets would have similar ecological consequences if connection and therefore direct communication between ramets of the parental plants is not interrupted. We should also investigate for how many generations the effects of former environmental interactions of a clonal plant can persist and influence its decision making and therefore its behaviour.
References
Baldwin IT (1998) Jasmonate-induced responses are costly but benefit plants under attack in native populations. Proc Natl Acad Sci USA 95:8113–8118
Benton TG, Plaistow SJ, Beckerman AP, Lapsley CT, Littlejohns S (2005) Changes in maternal investment in eggs can affect population dynamics. Proc R Soc Lond B 272:1351–1356
Bergelson J (1994) The effects of genotype and the environment on costs of resistance in lettuce. Am Nat 143:349–359
Bossdorf O, Arcuri D, Richards CL, Pigliucci M (2010) Experimental alteration of DNA methylation affects the phenotypic plasticity of ecologically relevant traits in Arabidopsis thaliana. Evol Ecol 24:541–553
Boyko A, Blevins T, Yao Y, Golubov A, Bilichak A, Ilnytskyy Y, Hollander J, Meins F, Kovalchuk I (2010) Transgenerational adaptation of Arabidopsis to stress requires DNA methylation and the function of dicer–like proteins. PLoS ONE 5:e9514
Brown E, Dewhurst C (1975) Genus spodoptera (Lepidoptera, Noctuidae) in Africa and Near East. Bulletin Entom Res 65:221–262
Burdon JJ (1983) Biological flora of the British isles: Trifolium repens. J Ecol 71:307–330
Cain ML (1994) Consequences of foraging in clonal plant species. Ecology 75:933–944
Chen BJW, During HJ, Vermeulen PJ, Anten NPR (2014) The presence of a below-ground neighbour alters within-plant seed size distribution in Phaseolus vulgaris. Ann Bot 114:937–943
Cihák A (1974) Biological effects of 5-azacytidine in eukaryotes. Oncology 30:405–422
Cipollini DF, Redman AM (1999) Age-dependent effects of jasmonic acid treatment and wind exposure on foliar oxidase activity and insect resistance in tomato. J Chem Ecol 25:271–281
Creelman RA, Mullet JA (1997) Biosynthesis and action of jasmonates in plants. Ann Rev Plant Physiol Mol Biol 48:355–381
de Kroon H, Huber H, Stuefer JF, van Groenendael JM (2005) A modular concept of phenotypic plasticity in plants. New Phytol 166:73–82
Dong M (1995) Morphological responses to local light conditions in clonal herbs from contrasting habitats, and their modification due to physiological integration. Oecologia 101:282–288
Galloway LF (2005) Maternal effects provide phenotypic adaptation to local environmental conditions. New Phytol 166:93–99
Galloway LF, Etterson JR (2007) Transgenerational plasticity is adaptive in the wild. Science 318:1134–1136
Gao L, Geng Y, Li B, Chen J, Yang J (2010) Genome-wide DNA methylation alterations of Alternanthera philoxeroides in natural and manipulated habitats: implications for epigenetic regulation of rapid responses to environmental fluctuation and phenotypic variation. Plant Cell Environ 33:1820–1827
Gómez S, Onoda Y, Ossipov V, Stuefer JF (2008) Systemic induced resistance: a risk-spreading strategy in clonal plant networks. New Phytol 179:1142–1153
Gómez S, van Dijk W, Stuefer JF (2010) Timing of induced resistance in a clonal plant network. Plant Biol 12:512–517
Hay MJM, Newton PCD, Robin C, Cresswell A (2001) Branching responses of a plagiotropic clonai herb to localised incidence of light simulating that reflected from vegetation. Oecologia 127:185–190
Hutchings MJ, de Kroon H (1994) Foraging in plants: the role of morphological plasticity in resource acquisition. Adv Ecol Res 25:159–238
Jablonka E, Lamb MJ (1995) Epigenetic inheritance and evolution: the Lamarckian dimension. Oxford University Press, Oxford
Kempel A, Brandl R, Schädler M (2009) Symbiotic soil microorganisms as players in aboveground plant–herbivore interactions: the role of rhizobia. Oikos 118:634–640
Kempel A, Schadler M, Chrobock T, Fischer M, van Kleunen M (2011) Tradeoffs associated with constitutive and induced plant resistance against herbivory. Proc Natl Acad Sci USA 108:5685–5689. doi:10.1073/pnas.1016508108
Klimeš L, Klimešová J, Hendriks R, Van Groenendael J (1997) Clonal plant architecture: a comparative analysis of form and function. In: De Kroon H, Van Groenendael J (eds) The ecology and evolution of clonal plants. Backhuys, Leiden, pp 1–29
Klimešová J, de Bello F (2009) CLO-PLA: the database of clonal and bud bank traits of Central European flora. J Veg Sci 20:511–516
Latzel V, Klimešová J (2010) Transgenerational plasticity in clonal plants. Evol Ecol 24:1537–1543
Latzel V, Hájek T, Klimešová J, Gómez S (2009) Nutrients and disturbance history in two Plantago species: maternal effects as a clue for observed dichotomy between resprouting and seeding strategies. Oikos 118:1669–1678
Latzel V, Klimešová J, Hájek T, Gómez S, Šmilauer P (2010) Maternal effects alter progeny’s response to disturbance and nutrients in two Plantago species. Oikos 119:1700–1710
Latzel V, Janeček Š, Doležal J, Klimešová J, Bossdorf O (2014) Adaptive transgenerational plasticity in the perennial Plantago lanceolata. Oikos 123:41–46
Louâpre P, Bittebière A, Clément B, Pierre J, Mony C (2012) How past and present influence the foraging of clonal plants? PLoS ONE 6:e38288
Maffei M, Bossi S, Spiteller D, Mithofer A, Boland W (2004) Effects of feeding Spodoptera littoralis on lima bean leaves. I. Membrane potentials, intracellular calcium variations, oral secretions, and regurgitate components. Plant Physiol 134:1752–1762
Magyar G, Kun A, Oborny B, Stuefer JF (2007) Importance of plasticity and decision-making strategies for plant resource acquisition in spatio-temporally variable environments. New Phytol 174:182–193
Marshall DJ, Uller T (2007) When is a maternal effect ‘adaptive’? Oikos 116:1957–1963
McConn M, Creelman RA, Bell E, Mullet JE, Browse J (1997) Jasmonate is essential for insect defense in Arabidopsis. Proc Natl Acad Sci USA 94:5473–5477
Mousseau TA, Fox CW (1998) Maternal effects as adaptations. Oxford University Press, New York
Münzbergová Z, Skuhrovec J (2013) Effect of habitat conditions and plant traits on leaf damage in the Carduoideae subfamily. PLoS ONE 8(5):e64639
Plaistow SJ, Lapsley CT, Benton TG (2006) Context dependent intergenerational effects: the interaction between past and present environments and its effect on population dynamics. Am Nat 167:206–215
Plaistow SJ, Clair JJH, Grant J, Benton TG (2007) How to put all your eggs in one basket: empirical patterns of off spring provisioning throughout a mother’s lifetime. Am Nat 170:520–529
Räsänen K, Kruuk LEB (2007) Maternal effects and evolution at ecological timescales. Funct Ecol 21:408–421
Richards CL, Schrey AW, Pigliucci M (2012) Invasion of diverse habitats by few Japanese knotweed genotypes is correlated with epigenetic differentiation. Ecol Lett 15:1016–1025
Riska B (1989) Composite traits, selection response, and evolution. Evolution 43:1172–1191
Roach DA, Wulff RD (1987) Maternal effects in plants. Ann Rev Ecol Syst 18:209–235
Rossiter MC (1996) Incidence and consequences of inherited environmental effects. Ann Rev Ecol Syst 27:451–476
Stuefer JF, Gómez S, van Mölken T (2004) Clonal integration beyond resource sharing: implications for defence signalling and disease transmission in clonal plant networks. Evol Ecol 18:647–667
Sultan ES, Barton K, Wilczek AM (2009) Contrasting patterns of transgenerational plasticity in ecologically distinct congeners. Ecology 90:1831–1839
Sutherland WJ, Stillman RA (1988) The foraging tactics of plants. Oikos 52:239–244
Thaler JS, Stout MJ, Karban R, Duffey SS (1996) Exogenous jasmonates simulate insect wounding in tomato plants (Lycopersicon esculentum) in the laboratory and field. J Chem Ecol 22:1767–1781
van Groenendael JM, de Kroon H (eds) (1990) Clonal growth in plants: regulation and function. SPB Academic Publishing, The Hague
van Kleunen M, Fischer M (2001) Adaptive evolution of plastic responses in a clonal plants. Ecology 82:3309–3319
Vergeer P, Ouborg NJ (2012) Evidence for an epigenetic role in inbreeding depression. Biol Lett 8:798–801
Wade MJ (1998) The evolutionary genetics of maternal effects. In: Mousseau TA, Fox CW (eds) Maternal effects as adaptations. Oxford Univ. Press, New York, p 521
Waters EM, Watson MA (2015) Live substrate positively affects root growth and stolon direction in the woodland strawberry, Fragaria vesca. Front Plant Sci. doi:10.3389/fpls.2015.00814
Acknowledgments
The study was supported financially by the Czech Science Foundation (Grant No. GA14–06802S) and by the institutional long-term research development project RVO 67985939. JS was supported by the Czech Ministry of Agriculture (Mze ČR, Grant No. RO0416). We thank people from the Population Ecology group for their valuable comments on the previous version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
González, A.P.R., Dumalasová, V., Rosenthal, J. et al. The role of transgenerational effects in adaptation of clonal offspring of white clover (Trifolium repens) to drought and herbivory. Evol Ecol 31, 345–361 (2017). https://doi.org/10.1007/s10682-016-9844-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-016-9844-5