Abstract
Objectives
This work provides to evaluate cholesterol assimilation and folic acid production by determining the probiotic properties of Lactobacillus spp. from raw goat milk with prebiotic properties.
Results
We isolated Lactobacilli from goat milk and identified API 50, CHL, and 16sRNA. Probiotic properties were determined according to bile salt and acidic tolerance, hydrophobicity, hemolytic activity, antibiotic sensitivity, antagonistic effect, and exopolysaccharide production. In addition, the cholesterol assimilation and folate production of cultures were determined.
Conclusions
L. plantarum GM-12 and L. plantarum GM-15 showed the highest folate production and the highest cholesterol assimilation.These two strains are strong candidates for use as potential probiotics and starter cultures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lactic acid bacteria (LAB), which have probiotic qualities, have been utilized in the fermentation of foods for a long time. This bacterial group (LAB) is made up of gram-positive bacteria that create lactic acid, which is employed in a variety of traditional and industrial food fermentations, including probiotic products, all over the world. They're employed in the manufacturing of a variety of dairy products as well as food fermentation. The proteolytic system of LAB transforms proteins into peptides and, as a result, amino acids that are required for bacterial development as well as taste compounds as end products (Moulay et al. 2013). Lactic acid bacteria, particularly those with probiotic capabilities, are characterized as live microorganisms that, when consumed with food at adequate levels, have a beneficial influence on the host's health (Mishra et al. 2021; Markowiak and Śliżewska 2017). All the foods, milk and dairy products are the best probiotics for human health. Although there are yogurt and fermented milk products that contain bacteria, kefir, milk desserts, baby foods, ice cream, butter, and cheese varieties, in recent years, many functional foods have gained popularity in the global market (Tamang et al. 2016). Goat milk is also beneficial to health because it has a high digestion rate, a low allergic effect, and includes useful chemicals and proteins. Goat milk contains a higher concentration of short and medium fatty acid chains, giving it a unique potential to deliver energy, especially to developing children (Turkmen 2017). Goat milk contains prebiotics that helps feed the probiotics in the gut. Prebiotics are special types of fiber that feed probiotics. Leong et al. (2019) reported that the naturally occurring oligosaccharides with prebiotic properties in infant formula prepared from goat milk exhibit strong prebiotic and anti-pathogen adhesion properties and may provide intestinal health benefits to infants.
In the last 20 years, probiotics have begun to attract scientists. In the human digestive system, there are many microorganisms that are both pathogenic and beneficial to the health of the human body. In the gastrointestinal tract, these animals are in a state of equilibrium. When the homeostasis of the system is disturbed for various reasons, pathogenic bacteria multiply and cause damage.
Probiotic bacteria help increase and maintain the balance of the intestinal system by multiplying and reducing the number of harmful bacteria. Cardiovascular illnesses are the world's and our country's leading causes of death. This condition, which is directly linked to the presence of high cholesterol in the blood, is one of the most prevalent and deadly diseases in developed countries. The World Health Organization (WHO) estimates that cardiovascular disease will be a major cause of death by 2030, affecting approximately 23.6 million people worldwide (WHO 2002).
It is known that high cholesterol content in the bloodstream is a risk factor for coronary heart disease in humans (Ahn et al. 2003). Consumption of foods rich in saturated fatty acids and cholesterol plays a role in the increase in heart diseases (Belviso et al. 2009).
Lactic acid bacteria (LAB), a natural member of the human microbiota, has become the focus of intense scientific research around the world for its health-related effects, such as lowering serum cholesterol, stimulating immune responses, preventing cancer, and relieving diarrhea (De Vrese and Schrezenmeir 2008).
In vivo and in vitro studies have shown that probiotic products, especially specific Lactobacillus species, reduce high cholesterol levels in the blood (Kumar et al. 2012; Ooi and Liong 2010).
As is known, probiotic bacteria predominate in the mouths and gastrointestinal tracts of people fed milk and dairy products. Such bacteria affect humans' lives through their metabolites and enzymes as well as the production of vitamins (vitamin K, folic acid, biotin, niacin, pyridoxine, and others) that are important for human life (Rossi et al. 2011).
Folic acid is also called folate. In addition to being a vitamin used in the production of DNA and RNA, folate plays an important role in blood production, the formation of new cells, and the survival of cells. For this reason, folic acid, which the body needs, should also be taken with food. Folate also reduces the risk of heart attack, dementia, and stroke by allowing homocysteine to remain within normal values that cause atherosclerosis (Carmel et al. 2003).
Thus, the focus of this research is the new isolate Lactobacillus spp. To investigate how new lactic acid bacteria with probiotic properties assimilate cholesterol and produce folic acid (folic acid) and their antibacterial effects against bacteria pathogenic to humans.
This article aims to evaluate cholesterol assimilation and folic acid production by determining the probiotic properties of Lactobacillus spp. isolated from raw goat milk offered for public consumption in Izmir province.
Materials and methods
Isolation and identification of bacteria
Raw goat milk (GM) samples were grown on De-Man Rogosa Sharpe (MRS) agar under sterile conditions. Cell morphology, Gram stain, cell morphology, and catalase tests were performed on bacteria grown on MRS agar. The carbohydrate fermentation profile from these isolates was then determined by commercial API 50 CHL strips and API 50 CHL Medium (Bio Meriux, SA 6928 Marcy L’Etoile, France) and defined at the species and/or subspecies level.
Molecular identification was done by 16S rDNA homology using a pair of bacteria-specific universal primers; forward 5'-AGT TGA TCC TGG CTC AG-3′ and reverse 5'-CCG TCA ATT CCT TTG AGT TT-3′ (Beasley SS, Saris PE 2004). DNA amplifications were done by using HotStarTaq Master Mix (Qiagen, USA) in a Techne-TC.512 thermocycler (Techne, England) under the following conditions: 94 ºC for 2 min, 30 cycles of 94 ºC for 45 s, 55 ºC for 1 min, and finally 72 ºC for 10 min. A database search was performed by using the BLAST program. The identified lactobacilli isolates were stored in cryogenic tubes (Cryobank, Mast Diagnostics, France) at − 20 °C. Here, Staphylococcus aureus ATCC 25923, Pseudomonas aeruginosa ATCC 27853, Escherichia coli ATCC 25922, and Streptococcus pyogenes ATCC 19615 bacteria were used as indicators.
Acid and bile salt tolerance
MRS broth was adjusted to pH 2.0 (1 N HCl and 10% NaOH), and 0.3% bile salt (Oxcall) was added to MRS broth and sterilized in an autoclave. From the active cultures, approximately 100 µl (log 9.0 cfu) were inoculated into 5 ml of pH 2.0 MRS broth and MRS broth 0.3% bile salt tubes. Cultures were incubated at 37 °C for 3 h and then seeded on MRS agar and allowed to grow for 24 h at 37 °C in a 5% CO2 incubator. Later, the developing colonies were determined as Log CFU/ml (Tulumoğlu et al. 2014).
Hydrophobicity
The pellet obtained from centrifugation of cultures (10,000g × 15 min) was washed twice with phosphate buffer, and the cell suspension was adjusted to A560 nm 1.0. After this stage, 3 ml of cell suspension was taken, and 0.6 ml of n-hexadecane was added and vortexed for 120 s. Two phases were formed in the suspension that was suspended at 37 °C. In these phases, the A560 value was measured on the spectrophotometer with careful consideration of the aqueous phase. The hydrophobicity was calculated according to the following formula (Vinderola and Reinheimer 2003).
A0 :A560 value of culture, A560 value of the n-Hexadecan applied sample.
Antibiotic susceptibility
Antibiotic susceptibility of Lactobacillus bacteria was performed according to the disc diffusion method (Charteris et al. 1998). AKN amikacin, CHL chloramphenicol, CIP ciprofloxacin, CMN clindamycin, ERN erythromycin, FAD fusidic acid, GMN gentamicin, NTM methylmisin, PNG penicillin, RIF rifampicin, TMN tobramycin, and discs were used. Resistance and sensitivity were determined according to NCLS 2015 criteria.
Haemolytic activity
A single colony was planted from active cultures to 5% sheep blood agar and was allowed to develop in a 5% CO2 incubator at 37 °C for 24 h. Haemolysis in the vicinity of the colonies was evaluated as alpha (green, semi-haemolysis), beta (transparent, complete haemolysis) and gamma (non-haemolysis) (Maragkoudakis et al. 2006). As the control strain, Streptococcus pyogenes ATCC 19615 was used.
Antagonistic activity
Antagonistic activity was performed according to the agar well diffusion method (Harris et al. 1989). Lactobacillus cultures were cultivated in MRS broth and incubated at 37 °C for 24 h in a 5% CO2 incubator. A 5000 RPC (4C core, Turkey) free cell supernatant was obtained by centrifugation. Supernatants were sterilized by passing through a 0.22 micron filter (Milipor). S. aureus ATCC 25923, P. aeruginosa ATCC 27853, and E. coli ATCC 25922 MH Agar (BD, USA) were sown to the plates. On the plates, 10 mm diameter wells were opened on the plates and 100 µl of free cell supernatant was added into them. It was incubated at room temperature for 2 h and then incubated at 37 °C for 24 h. Zones formed around the wells were measured in mm. In order to detect bacteriocin and similar structures, free cell supernatants were neutralized by adjusting pH 6.5 with 10% NaOH and then sterilized by passing through 0.22 microporous. The above procedures were carried out exactly. Zones formed around the wells were measured in mm. Those that are 0–0.5 mm were not evaluated.
Exopolysaccharide production (EPS)
Inoculated with 100 µl of Lactobacillus (log 9.0 cfu) were taken and inoculated with 5 ml of liquid MRS medium. Distributions from 100 µl of vaccinated MRS to 96-well microplates were made and allowed to develop at 37 °C for 24 h in a 5% CO2 incubator. The microplate was washed 3 times with pure water, covered with crystal violet and kept for 24 h. Again, the plate was washed three times with pure water and allowed to dry. EPS production according to the blue color intensity formed in the plate was (−) colorless (no production), ( +) less blue (production less), (+ +) medium blue (production medium) and (+ + +) very dark blue (production high).
Colesterol assimilation
The active Lactobacillus was vaccinated in an MRS broth containing 0.3% oxalate and then allowed to develop in a 5% CO2 environment for 24 h. Samples were centrifuged at 5000*g for 10 min. An enzymatic kit was used to determine the cholesterol level of the supernatant. Tulumolu et al. (2014) used an enzymatic kit [SYNCHRON® Systems (Beckman Coulter, USA)] and a Unicell DxC800 model autoanalyser (Beckman Coulter, USA) to determine the cholesterol level in the supernatant.
Folic acid production
From 100 µl of active culture incubated overnight, it was inoculated into 5 ml of MRS medium and allowed to grow for 5 h at 37 °C in a 5% CO2 incubator. At 5000*g, 2 ml of supernatant was taken from the top and folate was determined as ng/dl by measuring 600 nm with the Abbott brand Architect i1000sr Immuno Thetics Analyzer (Tulumoğlu et al. 2014). All studies were independently repeated three times.
Statistical analysis
Correlation coefficients and regression analyses on the data were computed using MINITAB statistical analysis software.
Results and discussion
Goat milk could be a good source of lactic acid bacteria (LAB) that could be employed as co-cultures to boost the quality and safety of dairy products. Natural LAB strains can be found in raw milk (from cows, goats, sheep, and camels) and traditional fermented foods, which can be utilized as starters or preservative cultures. It is believed that the goat milk-derived lactobacilli with probiotic qualities tested in this research provide perfect candidate for the manufacturing of dairy products (Belarbi et al. 2022). According to the findings of a resarch conducted in the western provinces of Turkey (Balıkesir, Çanakkale and İzmir), farmers sell 85.38% of goat milk to companies that process goat milk (Engindeniz and Uçar 2016). Because goat milk is highly digestible, has a minimal allergy effect, and includes useful chemicals and proteins, it is also beneficial to human health. Furthermore, ice cream made with goat milk has been demonstrated to meet higher quality standards and have better sensory qualities (Paz et al. 2014; Zenebe et al. 2014; Lad et al. 2017).
Tolerance to acid and bile salts
The main characteristics of probiotics are their natural presence in the large intestinal flora, being non-pathogenic, resistant to gastric acid and bile salts, attaching to the gastrointestinal tract and benefiting the host by colonizing the intestinal epithelium (Dixit et al. 2013).
Today, probiotics have been used as an alternative in a wide range of applications, from the prevention of antibiotic-induced diarrhea to preventing atopic dermatitis in children and for therapeutic purposes (Gorbach 2002).
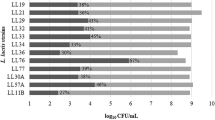
Among the bacteria used here, it was found that (pH 2.0), 10.11%–65.93% and bile salt (0.3% oxcall), 0.0%–97.27% tolerances were determined in a low acid environment (Figs. 1 and 2). In bile salt (97.27%), L. plantarum GM-11 (65.93%), L. plantarum GM-12 (58.42%), L. casei GM-5 and L. plantarum GM-8 (55%), and L. paracasei GM-2 (55%), L. gasseri GM-6 (71.11%), L. casei GM-9 (66.37%), and L. casei GM-10 (58.62%) strains showed the highest tolerance to the acidic environment.
As it is understood from these studies, acidic and bile salt resistance among the bacteria varies widely. For this reason, probiotics should be resistant to these conditions to survive. Sabir et al. (2010) reported that the lactobacilli strains tolerate 0.3% of the bile in a ratio ranging from 72 to 92%. They also reported that the viability of bacteria in an acidic environment such as pH 3.5 had a percentage of 85–96%.
Hydrophobicity
The (attachment) hydrophobicity is another distinguishing feature for determining probiotic properties. In our research, the hydrophobicity of Lactobacillus bacteria was found to be between 24 and 56%. The highest hydrophobicity was shown by the L. plantarum GM-12 (56%), L. paracasei GM-3 (54%), and L. casei GM-9 (50%) bacteria (Fig. 3).
According to Savage (1992), the hydrophobicity of lactic acid bacteria obtained from human, calf, mouse, rat, and swine digestive systems was determined as 35% for human-induced L. acidophilus strains, 44% for calf origin L. reuteri, 54.6% for mouse-induced L. fermentum, 81% for rat-induced L. murinus, and 17% for pig acid L. acidophilus and L. fermentum. An additional investigation on hydrophobicity, the hydrophobicity of lactic acid bacteria isolated from pickles and olives varied between 30.32% and 80.07%. L. plantarum has the highest hydrophobicity (80.07%) (Karasu et al. 2010). As it is understood from the results of these studies, there are differences in the hydrophobicity of bacteria. Probiotic strains with high hydrophobicity are preferred.
Antibiotic susceptibility
In order to ensure that the normal microflora is not affected by antibiotic treatments, resistance to antibiotics is an important criterion. It is emphasized that the preservation of microflora can be continued by using probiotics during antibiotic applications (Verraes et al. 2013). All isolated bacteria were found to be 100% resistant to fusidic acid and tobramycin. On the other hand, they were found to be 100% sensitive to rifampicin, penicillin, erythromycin, clindamycin, and chloramphenicol (Table 1).
Antibiotics can be passed to dangerous bacteria through bacteria used as probiotics or starter cultures. Lactobacillus spp. (L. plantarum/pentosus, L. rhamnosus, L. paracasei, L. sakei, L. curvatus, and species of the Lactobacillus acidophilus group: L. johnsonii, L. crispatus, L. gasseri, and L. acidophilus) were used to determine susceptibility levels to 25 antibiotics. The findings of the studies revealed that antimicrobial susceptibility varies depending on the species. The findings of the E-tests revealed that susceptibility to antimicrobial drugs varies depending on the species. Vancomycin, teicoplanin, tetracycline, norfloxacin, ciprofloxacin, fusidic acid, and clindamycin susceptibility varied by several orders of magnitude between species (Danielsen et al. 2003).
Hemolytic activity
None of the Lactobacillus bacteria have beta-hemolytic activity in blood agar. All showed alpha-type hemolysis. Similarly, Maragkoudakis et al. (2006) found that lactic acid bacteria did not produce beta-hemolysis.
Antagonistic activity
Organic acids (lactic acid, acetic acid, citric acid, etc.), hydrogen peroxide, bacteriocin and similar products produced by lactic acid bacteria inhibit the development of pathogenic bacteria or cause death. The strongest effect here is the organic acids, which provide the acidification of the medium. When compared to neutralized supernatants, Lactobacillus supernatants demonstrated an antagonistic effect between the indicator bacteria S. aureus ATCC 25923, P. aeruginosa ATCC 27853 and E. coli ATCC 25922 against 10–16 mm (Table 2). According to the research done by Tulumolu et al. (2014), organic acids were the source of this impact, which had varying degrees of antagonistic action.
Exopolysaccharide production
Exopolysaccharides (EPS) are polysaccharides synthesized outside of the cell by microorganisms, and the EPSs bound to the cell wall are capsular exopolysaccharides. In recent years, the use of microorganisms capable of producing EPS has become increasingly widespread. Since the EPS is passed through the digestive system without being digested, it has probiotic properties. In addition, EPS has positive effects on health (Freitas et al. 2011).
Lactobacillus has generally produced EPS (except GM-11 and GM-13 strains). GM-3 and GM-15 strains produced the highest EPS (+ + +). EPS production in the GM-11 and GM-13 strains was either absent or very weak (Table 3).
Cholesterol assimilation
Table 3 presents the cholesterol assimilation capabilities of the cultures. Cholesterol assimilation ability was found to be the lowest in the GM-2 and GM-11 (8 mg/dl) strains, while GM-12 (60 mg/dl) and GM-15 (58 mg/dl) showed the highest. The investigation has revealed that the assimilation capacities of the strains differed.
Tulumoğlu et al. (2018) found that Lactobacillus had cholesterol assimilation of between 25 and 59 mg/dl. As shown in Table 3, there is a relationship between EPS production and cholesterol removal. Tok and Aslım (2010) determined a correlation between cholesterol removal and EPS production. According to research, lactobacilli help with intracellular breakdown and have a reducing effect on cholesterol (Ahire et al. 2012).
Folic acid production
As it is understood from Table 3, it is seen that Lactobacillus strains produce folate at different levels. The strains L. gasseri GM-6 (13 ng/dl) and L. casei GM-5 (17 ng/dl) produced the least. On the other hand, L. plantarum GM-15 (160 ng/dl) and L. plantarum GM-12 (170 ng/dl) bacteria performed the highest production.
Similar research on the synthesis of folate found that S. thermophilus strains produced more folate than L. delbrueckii sp. bulgaricus strains, which produced little to no folate (Laio et al. 2012). Lactococcus sbp cremoris CM22 at 12.5 ng/ml and Lactococcus lactis sbp lactis CM28 at 14.2 ng/ml were found to produce folic acid in a similar experiment (Gangadharan et al. 2010).
In all of the Lactobacillus bacteria used in the examination, different levels of folate production were determined. L. plantarum GM-12 (179 ng/dl) and L. plantarum GM-15 (160 ng/dl) had the maximum folate production. Salvucci et al. (2016) discovered that L. pentosus ES124, 62 ng/ml, L. plantarum ES137 ng/ml, and P. acidilactici generated folate in MRS broth. Masuda et al. (2012) discovered that L. plantarum CN-49 108 g/L, L. sakei CN-28 107 g/L, and L. sakei CN-3 101 g/L produced folic acid in another experiment.
Conclusions
The probiotic properties of raw goat milk with prebiotic properties induced by Lactobacillus were looked into in this research. As for the potential probiotic, in L. plantarum GM-12, it was determined that exopolysaccharide production was moderately (+ +), folate production was the highest (179 ng/dl), and cholesterol assimilation was high (60 mg/dl), while in L. plantarum GM-15, exopolysaccharide production was high (+ + +), folate production was high (160 ng/dl), and cholesterol assimilation was high (58 mg/dl). These strains have positive effects on health through the production of EPS and contribute to the reduction of cholesterol. In addition, it is understood that these strains will play a very important role due to their characteristics like preventing cardiovascular diseases by cholesterol assimilation and cell regeneration by producing folic acid and their role in DNA and RNA production. Furthermore, it demonstrates that Lactobacilli isolated from raw goat milk, which have probiotic qualities, have anti-infective capabilities and can protect humans from gastrointestinal infections.
References
Ahire JJ, Bhat AA, Thakare JM, Pawar PB, Zope DG, Jain RM, Chaudhari BL (2012) Cholesterol assimilation and biotransformation by Lactobacillus helveticus. Biotechnol Lett. https://doi.org/10.1007/s10529-011-0733-2
Ahn YT, Kim GB, Lim KS, Baek YJ, Kim HU (2003) Deconjugation of bile salts by Lactobacillus acidophilus isolates. Int Dairy J. https://doi.org/10.1128/aem.33.1.15-18.1977
Beasley SS, Saris PE (2004) Nisin-producing Lactococcus lactis strains isolated from human milk. Appl Environ Microbiol. https://doi.org/10.1128/AEM.70.8.5051-5053.2004
Belarbi AY, de Almeida OGG, Gatto V, Torriani S, Del Rio B, Ladero V, Redruello B, Bensalah F, Alvarez MA (2022) Investigating the biotechnological potential of lactic acid bacteria strains isolated from different Algerian dairy and farm sources. Arch Microbiol. https://doi.org/10.1007/s00203-022-02828-7
Belviso S, Giordano M, Zeppa PDG (2009) In vitro cholesterol-lowering activity of Lactobacillus plantarum and Lactobacillus paracasei strains isolated from the italian castelmagno pdo cheese. Dairy Sci Technol. https://doi.org/10.1051/dst/2009004
Carmel R, Green R, Rosenblatt DS, Watkins D (2003) Update on cobalamin, folate, and homocysteine. Hematol Am Soc Hematol Educ Program. https://doi.org/10.1182/asheducation-2003.1.62
Charteris WP, Kelly MP, Morelli L, Collins KJ (1998) Antibiotic susceptibility of potentially probiotic Lactobacillus species. J Food Protect. https://doi.org/10.4315/0362-028x-61.12.1636
Danielsen M, Wind A (2003) Susceptibility of Lactobacillus spp. to antimicrobial agents. Int J Food Microbiol. https://doi.org/10.1016/s0168-1605(02)00254-4
De Vrese M, Schrezenmeir J (2008) Probiotics, prebiotics, and synbiotics advances. Adv Biochem Eng Biotechnol. https://doi.org/10.1007/10_2008_097
Dixit G, Samarth D, Tale V, Bhadekar R (2013) Comparative studies on potential probiotic characteristics of Lactobacillus acidophilus strains. Eurasia J Biosci. https://doi.org/10.5053/ejobios.2013.7.0.1
Engindeniz S, Ucar K (2016) Goat milk production and marketing in Turkey. J Global Agr Ecol 5:240–245
Freitas F, Alves VD, Reis MA (2011) Advances in bacterial exopolysaccharides from production to biotechnological applications. Trends Biotechnol. https://doi.org/10.1016/j.tibtech.2011.03.008
Gangadharan D, Sivaramakrishnan S, Pandey A, Nampoothiri KM (2010) Folate-producing lactic acid bacteria from cow’s milk with probiotic characteristics. Int J Dairy Technol. https://doi.org/10.1111/j.1471-0307.2010.00590.x
Gorbach SL (2002) Probiotics in the third millennium. Digest Liver Dis. https://doi.org/10.1016/s1590-8658(02)80155-4
Harris LJ, Daeschel MA, Stiles ME, Klaenhammer TR (1989) Antimicrobial activity of lactic acid bacteria against Listeria monocytogenes. J Food Prot. https://doi.org/10.4315/0362-028X-52.6.384
Karasu N, Şimşek Ö, Çon AH (2010) Technological and probiotic characteristics of Lactobacillus plantarum strains isolated from traditionally produced fermented vegetables. Ann Microbiol. https://doi.org/10.1007/s13213-010-0031-6
Kumar M, Nagpal R, Kumar R, Hemalatha R et al (2012) Cholesterol-lowering probiotics as potential biotherapeutics for metabolic diseases. Exp Diabetes Res. https://doi.org/10.1155/2012/902917
Lad SS, Aparnathi KD, Mehta B, Velpula S (2017) Goat milk in human nutrition and health—a review. Int J Curr Microbiol App Sci. https://doi.org/10.20546/ijcmas.2017.605.194
Laiño JE, Leblanc JG, Savoy de Giori G (2012) Production of natural folates by lactic acid bacteria starter cultures isolated from artisanal Argentinean yogurts. Can J Microbiol. https://doi.org/10.1139/w2012-026
Leong A, Liu Z, Almshawit H, Zisu B, Pillidge C, Rochfort S, Gill H (2019) Oligosaccharides in goats’ milk-based infant formula and their prebiotic and anti-infection properties. Br J Nutr. https://doi.org/10.1017/S000711451900134X
Maragkoudakis PA, Zoumpopoulou G, Miaris C, Kalantzopoulos G, Pot B, Tsakalidou E (2006) Probiotic potential of Lactobacillus strains isolated from dairy products. Int Dairy J. https://doi.org/10.1016/j.idairyj.2005.02.009
Markowiak P, Śliżewska K (2017) Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients. https://doi.org/10.3390/nu9091021
Masuda M, Ide M, Utsumi H, Niiro T, Shimamura Y, Murata M (2012) Production potency of folate, vitamin B12, and thiamine by lactic acid bacteria isolated from Japanese pickles. Biosci Biotech Biochem. https://doi.org/10.1271/bbb.120414
Mishra S, Acharya S (2021) A brief overview on probiotics the health friendly microbes. Biomed Pharmacol J. https://doi.org/10.13005/bpj/2285
Moulay M, Benlahcen K, Aggad H, Kihal M (2013) Diversity and technological properties of predominant lactic acid bacteria isolated from algerian raw goat’s milk. Adv Environ Biol 7:999–1007
Ooi G, Liong M (2010) Cholesterol-lowering effects of probiotics and prebiotics: a review of in vivo and in vitro findings. Int J Mol Sci. https://doi.org/10.3390/ijms11062499
Paz NF, Oliveira EG, Kairuz MSN, Ramón AN (2014) Characterization of goat milk and potentially synbiotic non-fat yogurt. Food Sci Technol. https://doi.org/10.1590/1678-457x.6409
Rossi M, Amaretti A, Raimondi S (2011) Folate production by probiotic bacteria. Nutrients. https://doi.org/10.3390/nu3010118
Sabir F, Beyatli Y, Cokmus C, Onal-Darilmaz D (2010) Assessment of potential probiotic properties of Lactobacillus spp., Lactococcus spp., and Pediococcus spp., strains isolated from kefir. J Food Sci. https://doi.org/10.1111/j.1750-3841.2010.01855.x
Salvucci E, LeBlanc JG, Gabriela P (2016) Technological properties of Lactic acid bacteria isolated from raw cereal material. LWT-Food Sci Technol. https://doi.org/10.1016/j.lwt.2016.02.043
Savage DC (1992) Growth phase, cellular hydrophobicity, and adhesion in vitro of lactobacilli colonizing the keratinizing gastric epithelium in the mouse. Appl Environ Microbiol. https://doi.org/10.1128/aem.58.6.1992-1995.1992
Tamang JP, Shin DH, Jung SJ, Chae SW (2016) Functional properties of microorganisms in fermented foods. Front Microbiol. https://doi.org/10.3389/fmicb.2016.00578
Tok E, Aslim B (2010) Cholesterol removal by some lactic acid bacteria that can be used as probiotic. Microbiol Immunol. https://doi.org/10.1111/j.1348-0421.2010.00219.x
Tulumoğlu Ş, Kaya Hİ, Şimşek Ö (2014) Probiotic characteristics of Lactobacillus fermentum strains isolated from tulum cheese. Anaerobe. https://doi.org/10.1016/j.anaerobe.2014.09.015
Tulumoğlu Ş, Erdem E, Şimşek Ö (2018) The effects of inulin and fructo-oligosaccharide on the probiotic properties of Lactobacillus spp. isolated from human milk. Z Naturforsch C J Biosci. https://doi.org/10.1515/znc-2018-0001
Turkmen N (2017) The nutritional value and health benefits of goat milk components. In nutrients in dairy and their implications on health and disease, academic press 35:441–449. https://doi.org/10.1016/B978-0-12-809762-5.00035-8
Verraes C, Van Boxstael S, Van Meervenne E, Van Coillie E et al (2013) Antimicrobial resistance in the food chain: a review. Int J Environ Res Public Health. https://doi.org/10.3390/ijerph10072643
Vinderola CG, Reinheimer JA (2003) Lactic acid starter and probiotic bacteria: a comparative in vitro study of probiotic characteristics and biological resistance. Int Food Res J. https://doi.org/10.1016/S0963-9969(03)00098-X
World Health Organization (WHO) (2002) Joint working group report on drafting guidelines for the evaluation of probiotics in food. World Health Organization, Geneva, Switzerland
Zenebe T, Ahmed N, Kabeta T, Kebede G (2014) Review on medicinal and nutritional values of goat milk. Acad J Nutr. https://doi.org/10.5829/idosi.ajn.2014.3.3.93210
Funding
There was no funding for this study.
Author information
Authors and Affiliations
Contributions
The study’s conception and design were provided by all of the writers. Şener Tulumoğlu and Belgin Erdem were in charge of material preparation, data collection, and analysis. The first draft of the manuscript was written by Ergin Kariptaş and Belgin Erdem, and all contributors provided feedback on prior drafts. The final manuscript was read and approved by all writers.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that there are no conflict of interest.
Ethical approval
This article does not contain any study with human participants performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tulumoğlu, Ş., Kariptaş, E. & Erdem, B. Lactobacillus spp. isolated from prebiotic-derived raw goat milk: probiotic characteristics, cholesterol assimilation and folate production. Biotechnol Lett 45, 47–56 (2023). https://doi.org/10.1007/s10529-022-03314-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-022-03314-2