Abstract
The present study was conducted to find the potential of Lactococcus lactis strains naturally present in raw and fermented milk as probiotics and to evaluate their safety and some technological characteristics. There are numerous studies that evaluated probiotic properties of lactococci, nevertheless, limited studies on the probiotic potential of lactococci isolated from raw milk or dairy products were performed. Strains isolation from raw milk or dairy products and their characterization is important when selection of starter strains for the production of functional dairy foods is performed. Depending on aroma production and acidifying activity, 33 L. lactis strains were selected out of 169 and evaluated for safety, technological and probiotic properties. These strains were screened for antibiotic sensitivity, enzymatic activity, hemolytic and gelatinase activities. The strains were also assessed for resistance to bile salts and acid, growth in bile acids and cholesterol, cell surface hydrophobicity. Based on the obtained results, two strains with the best probiotic potential were selected. These two L. lactis strains, with 51% and 67% survival at low pH and more than 80% resistance to various bile salt concentrations, proved their resistance in vitro to gastric conditions. Also these strains proved to be good acidifiers (the pH of milk was reduced by at least 1 unit in 6 h at 30–37 °C) and can be used in the development of functional dairy foods as starter cultures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, due to the growing interest in food products that has added health benefits, food is used not only to provide the necessary nutrients, but also to provide health-promotion effects to consumers’ health [1, 2]. This raised probiotic food products consumption [3]. Probiotics are defined as non-pathogenic microorganisms which, when administered in sufficient numbers, provide a health benefit to the host [4, 5]. The most common microorganisms applied as probiotics are lactic acid bacteria (LAB)-industrially important microorganisms worldwide for the fermented food production [6]. For a long time dairy products like raw milk served as a major ecological reservoir for isolation of various beneficial microorganisms, because raw milk contains wide microbial diversity, composed mainly of LAB [7]. These microorganisms can improve lactose digestion, stimulate the immune system, prevent and treat diarrhea and provide other health benefits [3]. Lactococcus lactis strains are the majority of LAB that are associated with commercial starter cultures used in the dairy industry for the manufacture and ripening of cheese of both artisanal and commercial origin, fermented milks such as buttermilk, yoghurt and sour cream [8,9,10]. Despite the interest to examine L. lactis in food as starter cultures or biopreservatives for their technological properties, there is a growing tendency to evaluate them for probiotic properties for the production of functional dairy products [11].
World Health Organization (WHO) determined guidelines for probiotics evaluation in food. Among them, some requirements to classify isolates to be potential probiotics are present [4]. Each probiotic candidate strain has to be evaluated for safety (isolation from suitable habitats), functionality (survival to gastrointestinal track conditions) and beneficial properties, to be effectively used [12, 13]. There are two most widely used in vitro tests to evaluate resistance to gastrointestinal environment of the potential probiotic strains and these are resistance to gastric acidity and resistance to bile salts. These tests are based on both survival and growth studies [14]. Clearly, the in vitro assays are rather different from the in vivo conditions as the human gut food matrix pays the protective role for the bacteria. Nevertheless, in vitro tests provide important information and are a helpful tolls for quick screening of the bacteria for probiotic activity [14].
Up to now European Food Safety Authority (EFSA) classify probiotics as food supplements or dietary supplements and discards all health claims made for probiotics [15]. Hence, strains with probiotic potential must meet some industrial requirements and technological properties and should not have any adverse effects on the taste or aroma of the food products [16]. Consumer acceptance is one of the criteria among others for a food product to be considered as probiotic [17].
Regarding its history of safe use in food fermentation for many years, L. lactis is granted Generally Recognized as Safe (GRAS) status by the American Food and Drug Agency (FDA) and Qualified Presumption of Safety (QPS) status by the European Food Safety Authority (EFSA) [18]. Despite this fact, newly isolated L. lactis strains have to be properly characterized for safety features in order to be applied for food production. Antibiotic resistance, hemolytic activity, among other safety properties, remain essential in the selection of strains for application in food production [19, 20].
This study was aimed to characterize probiotic potential of L. lactis strains, previously isolated from food grade samples, through the measure of their safety and in vitro probiotic properties. In addition, it was also evaluated some of the technological characteristics of the L. lactis strains.
Materials and Methods
Bacterial Strains
In our previous study [20] we isolated and identified 181 Lactococcus lactis strains from various local food sources including raw and fermented cow and goat milk, fermented buckwheat and wheat. Twelve of these strains were nisin producers and were further characterized. In this study, we evaluated the other 169 L. lactis strains for the best sensory, safety characteristics and also for probiotic potential. All strains used in this study were stored at -80 °C in M17 broth (Merck, Germany) in the presence of 30% glycerol until further analysis. Before conducting any experiments, strains were revitalized in MRS broth (Biolife, Milano, Italy) by growing for 18 h at 30 °C.
Aroma Evaluation and Acid Production
Aroma evaluation was carried out for 169 L. lactis strains. 1% of each revitalized L. lactis culture was added to 50 mL of low-fat UHT milk (1.5% fat) and incubated at 30 °C for 24 h. Aroma evaluation was performed by 10 trained panelists. The intensity of aroma acceptability was scored from 0 to 10 ranging from no or very low acceptability (score 0–1), medium acceptability (score 4–7) to an excellent one (score 10). If the unpleasant aroma was detected-these strains were excluded from further studies.
The acidifying activity of L. lactis strains was measured by the change in pH values after 6 h. The cultures were considered as high (the change in pH is more than 1 unit), medium (the change in pH is from 0.5 to 1 units) or weak (the change in pH is less than 0.5 unit) acidifiers [20].
Depending on aroma production and acidifying activity, only 33 L. lactis strains were selected for further characterization. These strains were isolated from Lithuanian raw and fermented goat and cow milk (see Table 1).
Diacetyl Production
Revitalized L. lactis strains (1%) were inoculated in 10 mL of UHT milk and incubated at 30 °C for 24 h. 1 mL of each culture was then mixed with 0.5 mL of 1% (v/v) α-naphtol (Sigma-Aldrich) and 16% (w/v) KOH and incubated at 30 °C for 10 min. Diacetyl production was observed by the formation of a red ring at the top of the test tubes [21].
Salt Tolerance
Revitalized L. lactis strains were inoculated into MRS broth (Biolife, Milano, Italy) with different sodium chloride (NaCl) concentrations (4%, 6.5% and 10%) and incubated at 37 °C for 24 h. Salt tolerance was evaluated by visual observation comparing blank MRS broth sample with inoculated sample to see if any turbidity has formed. The formation of turbidity was evaluated as positive result (Table 2).
Antibiotic Sensitivity
Antibiotic susceptibility was evaluated using MIC Test Strips (Liofilchem, Roseto degli Abruzzi, Italy) and following the manufacturer’s instructions. The antibiotics tested were chloramphenicol, clindamycin, streptomycin, gentamicin, tetracycline, erythromycin, ampicillin, vancomycin, kanamycin. Minimum Inhibitory Concentrations (MIC) were determined from the MIC reading scale and expressed in µg/mL.
Enzymatic Activities
Enzymatic activities of enzymes listed in Table 3 were evaluated using the API ZYM kit (bioMerieux, Marcy-l’Étoile, France) for each of the selected L. lactis strain. The experiment with API ZYM strips was performed according to the manufacturer’s instructions. Each well of the API ZYM strip was inoculated with 65 µl of the McFarland 5 standard suspension of overnight cultures of the strains and incubated at 30 °C for 4 h. After incubation, ZYM-A and ZYM-B reagents were added to each well and then incubated at 30 °C for 5 min. Results were evaluated according to the manufacturer’s instructions. Changes of color were graded from 0 to 5 based on color formation intensity. Color reaction grade 0 was interpreted to correspond to a negative reaction, grades 1 and 2 corresponded to a weak reaction (5 to < 20 nmol substrate metabolized) and grades 3, 4, and 5 corresponded to a strong reaction (> 20 nmol substrate metabolized).
Hemolytic and Gelatinase Activities
Hemolytic activity was examined using agar plates containing sheep blood (Oxoid, UK). After incubation for 48 h at 30 °C hemolytic activity was recorded as β-hemolysis, α-hemolysis and γ-hemolysis represented as clear zones, green zones or halos around the colonies respectively [22].
Lactococcus lactis strains were tested for gelatinase activity according to Perin et al. [11]. 10 µL of fresh L. lactis cultures were spotted on Luria Bertani agar (Liofilchem, Italy) supplemented with 5% (w/v) of gelatin and incubated anaerobically at five different temperatures (37 °C for 48 h, 42 °C for 48 h, 25 °C for 72 h, 10 °C for 10 days, 15 °C for 10 days). After incubation the plates were examined for possible formation of opaque halos around the colonies. The existence of such halos demonstrates gelatinase production.
Growth in Bile Acids and Cholesterol
Growth of L. lactis under bile acids and cholesterol was determined according to Choi and Chang [6]. L. lactis grown overnight in MRS were inoculated (1%) into MRS broth (Biolife, Italy) supplemented with 0.3% (w/v) oxgall (Sigma-Aldrich, Italy) and 0.1 g/L of water-soluble cholesterol, 0.5% (w/v) oxgall and 0.1 g/L of water-soluble cholesterol, 0.3% (w/v) sodium salt taurodeoxychalic acid (TDCA, Sigma-Aldrich, Italy) and 0.1 g/L of water-soluble cholesterol, and 0.5% (w/v) TDCA and 0.1 g/L of water-soluble cholesterol. Growth of L. lactis in MRS broth without bile was used as a control. Initial pHs of the prepared media were pH 6.2–6.4. After incubation anaerobically for 24 h at 37 °C, the absorbance at 600 nm was measured.
Resistance to Bile Salts
The ability of L. lactis strains to grow in the presence of bile salts was determined according to Belicova et al. [23]. L. lactis isolates were inoculated (2% w/v mass of the solution) into MRS broth supplemented with 0.3%, 0.5% and 1% (w/v) of bile (Liofilchem, Italy). Strains were incubated for 24 h at 37 °C after which the absorbance at 560 nm was measured.
Resistance to Acid
Tested strains were incubated in MRS broth at 30 °C for 18 h. 1 mL of each culture was transferred into 9 mL of phosphate buffered saline (PBS, Merck, Germany) adjusted to pH 2.5 with 5 M HCl and incubated at 30 °C. The number of viable bacteria was counted on MRS agar plates after 0 h and 3 h of incubation periods [24].
Auto-Aggregation Activity
Ability of L. lactis to aggregate (auto-aggregation) was determined according to Han et al. [25]. The auto-aggregation was calculated according to the following equation:
where At stands for absorbance at determined interval (1 h) and and A0 stands for the absorbance at the beginning of the assay (0 h).
Cell Surface Hydrophobicity
The method described by Lee and Puong [26] for the determination of cell surface hydrophobicity was used. The decreased absorbance in the aqueous phase was considered as a measure of cell surface hydrophobicity that was calculated according to the following equation:
where A0 and A1 are the absorbance values before and after the extraction with n-hexadecane, respectively.
Evaluation of Isolated L. lactis Carbohydrate Metabolism
Carbohydrate fermentation profiles of selected L. lactis strains were evaluated using the API 50 CH test (BioMerieux, Marcy-l'Etoile, France) and following manufacturer’s instructions. The results were interpreted as strong growth (yellow color of the test), moderate growth (green color), weak growth (dark green) and no growth (blue color).
Statistical Analysis
All the experiments were performed in triplicate. All data analysis was performed by SPSS statistical package (Chicago, SPSS Inc., SPSS 24). Data were analyzed using Descriptive Statistics (Explore) and One-way Analysis (ANOVA) methods. The differences were considered reliable when P < 0.05.
Results and Discussion
Aroma Development and Acidification Activity
Flavor and aroma are the most important attributes besides consistency for consumers in food products [27]. Since tested L. lactis strains were intended to be used for dairy food production, which are the most common probiotic foods [28], their ability to develop desirable aroma was evaluated in milk. Obtained results are presented in Table 1. Half of the tested 169 L. lactis strains produced unpleasant aroma according to sensory evaluation. Only 33 L. lactis strains were selected for further evaluation based on the intensity of pleasant aroma which varied from low to high. However, all 33 selected L. lactis strains were considered as high acidifiers as were able to reduce the pH of milk by more than 1 unit in 6 h.
Technological Characterization
Only 12% (strains LL9, LL11, LL76 and LL57A) out of 33 tested L. lactis strains produced diacetyl (Table 1). Other authors, that isolated L. lactis strains from artisanal Pico cheese, found that 33% of lactococci were producers of diacetyl [19]. These results are also in accordance with authors that examined diacetyl production in Lactococcus strains isolated from raw cow milk [29]. Diacetyl is a volatile compound linked to good aroma formation in dairy food products. Therefore, strains displaying diacetyl production can be used as starter cultures in dairy food production.
Furthermore, all 33 L. lactis strains showed good tolerance to 4% NaCl concentration, but seven strains were not able to tolerate 6.5% NaCl concentration. At 10% NaCl concentration no growth was observed (Table 1). LAB from milk environment, usually are able to survive 1–9% NaCl concentrations. This is a desirable feature for potential LAB probiotics [30]. Our results are also in agreement with those reported by de Almeida Junior et al. [31]. In their study 9 out of the 13 L. lactis strains were able to grow at 6.5% NaCl. NaCl could be applied up to 6% in dairy food products [32], therefore ability of strains to tolerate salt concentrations up to 6% is of big importance in order for them to survive in the product.
Safety Assays of L. lactis Strains
Antibiotic resistance evaluation revealed a high variation among L. lactis strains (Table 2). Eight L. lactis strains showed antibiotic resistance above the breakpoint provided by European Food Safety Authority (EFSA 2012) to streptomycin. The breakpoint for L. lactis to streptomycin suggested by EFSA is 32 µg/mL, whereas strain LL27 had minimum inhibitory concentration of 96 µg/mL, strains LL36A, LL55A, LL61A had minimum inhibitory concentrations of 512 µg/mL and strains LL9, LL11, LL48A, LL51A had minimum inhibitory concentrations above 1024 µg/mL. Some L. lactis strains showed resistance to tetracycline. The breakpoint for L. lactis to tetracycline suggested by EFSA is 6 µg/mL, whereas strains LL51A, LL56A and LL61A had minimum inhibitory concentrations of 12 µg/mL, strain LL55A had minimum inhibitory concentration of 24 µg/mL and strains LL36A, LL48A had minimum inhibitory concentrations of 32 µg/mL. In general five L. lactis strains showed resistance to both tetracycline and streptomycin and in total nine L. lactis strains were excluded from further studies. Antibiotic resistance alone will not cause risk in probiotic LAB candidates, however capability to transfer the antibiotic resistance genes is the real cause of risk and needs to be investigated prior commercial applications [33].
Regarding enzymatic activity, strains should not produce harmful enzymes like β-glucosidase or β-glucuronidase if they are intended to be used in the food industry [34]. These two enzymes attracts special attention as they are known to be potential mediators of colon carcinogenesis [19]. The activity of these enzymes was tested using API ZYM kit and is presented in Table 3. Four strains (LL17, LL18, 44A and 58A) were found to produce strong and two strains (LL78 and LL42A) were found to produce weak β-glucosidase activity. Also, two L. lactis strains (LL10 and LL13) were found to produce strong β-glucuronidase activity which was also reported by other authors [19]. Due to activity of these two enzymes, these L. lactis strains were eliminated from further studies. Likewise, activities of α-chymotrypsin and N-acetyl-β-glucosaminidase are associated with intestinal diseases [35, 36] and strains possessing activities of these enzymes should be avoided in food products. In this study, no activity of α-chymotrypsin was detected, though three strains (LL12, LL36 and LL14C) were found to produce strong N-acetyl-β-glucosaminidase activity and were also eliminated from further studies. In contrast, β-galactosidase activity is considered an advantageous criterion in the selection of probiotic strains as activity of this enzyme is helpful in improving lactose tolerance in the gut [3, 36]. However, five strains (LL18, LL42A, LL44A, LL58A and LL14C) possessing activity of this enzyme also displayed activity of unfavorable enzymes and only one strain (LL16) showed weak activity of β-galactosidase activity. In general, after enzymatic activity evaluation only thirteen strains were characterized further.
Hemolytic activity is a typical feature of pathogenic bacteria. This harmful effect may only happen if the ingested bacteria end up in the blood; however, this is an unlikely situation. Nevertheless this test provides an important information about tested strain’s pathogenicity [37]. None of the tested thirteen L. lactis strains presented hemolytic activity, therefore the safety of selected L. lactis strains concerning hemolytic activity is not a concern. Also, none of the tested thirteen L. lactis strains presented gelatinase activity. The absence of this feature supports the safety of selected L. lactis strains.
Potential Probiotic Properties In Vitro
Table 4 presents tolerance results of selected L. lactis strains to bile salts. Tolerance to bile salts is an important property for any potential probiotic bacteria and is one of the criteria for a strain to be used as probiotic culture [38]. All L. lactis isolates were resistant at 0.3% and 0.5% bile salt concentrations with resistance of more than 90%. Also, all L. lactis strains were resistant at 1% bile salt. Two L. lactis strains (LL 16 and LL19) showed resistance of 82% at this bile concentration by expressing growth decrease from 8.96 ± 0.01 to 7.38 ± 0.27 Log10 CFU/mL and from 9.15 ± 0.07 to 7.49 ± 0.14 Log10 CFU/mL, respectively. All other L. lactis strains showed resistance of more than 90%. Physiological concentration of human bile ranges from 0.3% to 0.5%. It is well known, that bile salts dissolve membrane lipids leading to the cell’s death because of the leakage of the cell contents [6], therefore it is important to evaluate the ability of potential probiotic cultures to survive in the presence of bile in order for probiotic strain to arrive alive to the small intestine or colon [39, 40]. Our results indicate that all L. lactis strains showed good bile tolerance. These results are in accordance with García-Ruiz et al. [41] and Kumar and Kumar [38]. They detected good bile resistance to a variety of LAB strains.
The ability of L. lactis strains to survive the combination of bile acids (oxgall and TDCA) and cholesterol after 24 h of incubation at 37 °C is shown in Table 5. Two L. lactis strains (LL19 and LL11B) did not grow in the presence of 0.3% oxgall + cholesterol and six L. lactis strains (LL19, LL21, LL32, LL34, LL36 and LL11B) did not grow in the presence of 0.5% oxgall + cholesterol showing no resistance to this toxic effect. All tested L. lactis strains grew well in the presence of TDCA-containing media showing resistance of 98–100% and expressing good resistance. In general, oxgall showed a higher toxic effect on L. lactis strains than TDCA. These results are in accordance to Choi and Chang [6]. They detected that oxgall showed a slightly higher toxic effect on LAB growth than TDCA.
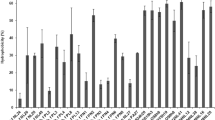
Figure 1 presents tolerance results of L. lactis strains to acid. The survival of LAB in low pH of stomach is important for tolerating the initial acid stress [38]. The pH in the human stomach ranges from 1.5 to 4.5 and it has been reported before that acidity has the most negative effect on bacterial growth and viability [34]. Only two L. lactis strains (LL16 and LL76) were resistant to acid pH value 2.5 and expressed resistance above 50%. Highest resistance was observed in strain LL76 where after 3 h of incubation at 30 °C under acidic condition the growth of the isolate decreased from 8.70 ± 0.01 to 5.90 ± 0.00 Log10 CFU/mL expressing resistance of 67%. This strain was isolated from fermented cow milk. Strain LL16 expressed growth decreased from 9.40 ± 0.00 to 4.80 ± 0.03 Log10CFU/mL showing resistance of 51%. This strain was isolated from raw cow milk. Other L. lactis strains expressed resistance lower than 50%, thus demonstrating weak tolerance and were eliminated from further studies.
Lactococcus lactis strains LL16 and LL76 displayed auto-aggregation properties of 76 and 55%, respectively. This is considered a positive attribute as auto-aggregation ability enables bacteria to persist in the intestinal mucosa and promote their beneficial effects to the host. Also, cell surface hydrophobicity is an important beneficial property as it is the ability of bacteria to present interactions with mucosal cells [3]. In our study, both tested L. lactis strains (LL16 and LL76) showed good hydrophobicity of 55 and 70%. These results are in accordance with other authors, who determined good hydrophobicity for Lactobacillus [42] and Pediococcus [43] strains.
Carbohydrate Metabolism of Isolated L. lactis Strains
Two L. lactis strains, possessing the best bile and acid tolerance (LL16 and LL76) were tested for carbohydrate metabolism. Both strains were able to ferment d-ribose, d-galactose, d-glucose, d-fructose, d-manose, N-acetylglucosamine, arbutin, esculin ferrin citrate, salicin, d-cellobiose, d-maltose, d-lactose (bovine origin), d-saccharose (sucrose). Only L. lactis strain LL16 was able to ferment d-xylose, amygdalin and glycogen. Likewise, only L. lactis strain LL76 was able to ferment starch and gentiobiose.
Conclusion
In this study, two L. lactis strains (LL6 and LL76) isolated from food grade samples—raw and fermented cow milk demonstrated good probiotic potential. These L. lactis strains exhibited an important resistance in vitro to gastrointestinal tract conditions (bile salts and acid). Likewise, strains showed good auto-aggregation activity and cell surface hydrophobicity. Moreover, these two strains displayed some beneficial technological properties like salt tolerance up to 6% and good acidifying activity. Regarding antibiotic susceptibility, hemolytic, gelatinase and enzymatic activities, strains showed no activities of undesirable traits and proved to be safe. These results suggest that these two probiotic candidates can be used in the development of functional dairy foods as starter cultures due to their acidifying activity, also one strain (LL76) exhibited diacetyl production, which is a desirable trait for a starter culture.
References
Linares DM, Gómez C, Renes E et al (2017) Lactic acid bacteria and bifidobacteria with potential to design natural biofunctional health-promoting dairy foods. Front Microbiol 8:846. https://doi.org/10.3389/fmicb.2017.00846
Ruiz-Moyano S, Gonçalves dos Santos MTP, Galván AI et al (2019) Screening of autochthonous lactic acid bacteria strains from artisanal soft cheese: probiotic characteristics and prebiotic metabolism. LWT-Food Sci Technol. https://doi.org/10.1016/j.lwt.2019.108388
Colombo M, Castilho NPA, Todorov SD, Nero LA (2018) Beneficial properties of lactic acid bacteria naturally present in dairy production. BMC Microbiol 18:219. https://doi.org/10.1186/s12866-018-1356-8
FAO (2001) Probiotics in food: Health and nutritional properties and guidelines for evaluation. Food Nutr Pap. https://doi.org/10.1201/9781420009613.ch16
Hossain MI, Mizan MFR, Ashrafudoulla M et al (2020) Inhibitory effects of probiotic potential lactic acid bacteria isolated from kimchi against Listeria monocytogenes biofilm on lettuce, stainless-steel surfaces, and MBECTM biofilm device. LWT 118:108864. https://doi.org/10.1016/j.lwt.2019.108864
Choi EA, Chang HC (2015) Cholesterol-lowering effects of a putative probiotic strain Lactobacillus plantarum EM isolated from kimchi. LWT Food Sci Technol 62:210–217. https://doi.org/10.1016/j.lwt.2015.01.019
Rastogi S, Mittal V, Singh A (2019) In vitro evaluation of probiotic potential and safety assessment of Lactobacillus mucosae strains isolated from donkey’s lactation. Probiot Antimicro Prot. https://doi.org/10.1007/s12602-019-09610-0
Tidona F, Meucci A, Povolo M et al (2018) Applicability of Lactococcus hircilactis and Lactococcus laudensis as dairy cultures. Int J Food Microbiol 271:1–7. https://doi.org/10.1016/j.ijfoodmicro.2018.02.015
Cavanagh D, Fitzgerald GF, McAuliffe O (2015) From field to fermentation: The origins of Lactococcus lactis and its domestication to the dairy environment. Food Microbiol 47:45–61. https://doi.org/10.1016/j.fm.2014.11.001
Song AAL, In LLA, Lim SHE, Rahim RA (2017) A review on Lactococcus lactis: From food to factory. Microb Cell Fact 16:55. https://doi.org/10.1186/s12934-017-0669-x
Perin LM, Miranda RO, Todorov SD et al (2014) Virulence, antibiotic resistance and biogenic amines of bacteriocinogenic lactococci and enterococci isolated from goat milk. Int J Food Microbiol 185:121–126. https://doi.org/10.1016/j.ijfoodmicro.2014.06.001
García-Hernández Y, Pérez-Sánchez T, Boucourt R et al (2016) Isolation, characterization and evaluation of probiotic lactic acid bacteria for potential use in animal production. Res Vet Sci 108:125–132. https://doi.org/10.1016/j.rvsc.2016.08.009
FAO/WHO (2002) Guidelines for the evaluation of probiotics in food. Food and Agriculture Organization of the United Nations and World Health Organization Group Report.(London Ontario, Canada). FAO Food Nutr Pap 85
Zielińska D, Rzepkowska A, Radawska A, Zieliński K (2015) In vitro screening of selected probiotic properties of Lactobacillus strains isolated from traditional fermented cabbage and cucumber. Curr Microbiol 70:183–194. https://doi.org/10.1007/s00284-014-0699-0
de Simone C (2019) The unregulated probiotic market. Clin Gastroenterol Hepatol 17(5):809–817. https://doi.org/10.1016/j.cgh.2018.01.018
de Melo Pereira GV, de Oliveira CB, Magalhães Júnior AI et al (2018) How to select a probiotic? A review and update of methods and criteria. Biotechnol Adv 36(8):2060–2076. https://doi.org/10.1016/j.biotechadv.2018.09.003
Yerlikaya O (2019) Probiotic potential and biochemical and technological properties of Lactococcus lactis ssp. lactis strains isolated from raw milk and kefir grains. J Dairy Sci 102:124–134. https://doi.org/10.3168/jds.2018-14983
Andreoletti O, Baggesen DL, Bolton D et al (2012) Scientific Opinion on the maintenance of the list of QPS biological agents intentionally added to food and feed (2013 update). EFSA J 11:3449. https://doi.org/10.2903/j.efsa.2013.3449
Domingos-Lopes MFP, Stanton C, Ross PR et al (2017) Genetic diversity, safety and technological characterization of lactic acid bacteria isolated from artisanal Pico cheese. Food Microbiol 63:178–190. https://doi.org/10.1016/j.fm.2016.11.014
Kondrotiene K, Kasnauskyte N, Serniene L et al (2018) Characterization and application of newly isolated nisin producing Lactococcus lactis strains for control of Listeria monocytogenes growth in fresh cheese. LWT Food Sci Technol 87:507–514. https://doi.org/10.1016/j.lwt.2017.09.021
Dal Bello B, Cocolin L, Zeppa G et al (2012) Technological characterization of bacteriocin producing Lactococcus lactis strains employed to control Listeria monocytogenes in Cottage cheese. Int J Food Microbiol 153:58–65. https://doi.org/10.1016/j.ijfoodmicro.2011.10.016
Maragkoudakis PA, Mountzouris KC, Psyrras D et al (2009) Functional properties of novel protective lactic acid bacteria and application in raw chicken meat against Listeria monocytogenes and Salmonella enteritidis. Int J Food Microbiol 130:219–226. https://doi.org/10.1016/j.ijfoodmicro.2009.01.027
Belicová A, Mikulášová M, Dušinský R (2013) Probiotic potential and safety properties of Lactobacillus plantarum from Slovak Bryndza cheese. Biomed Res Int 2013:760298. https://doi.org/10.1155/2013/760298
Thirabunyanon M, Boonprasom P, Niamsup P (2009) Probiotic potential of lactic acid bacteria isolated from fermented dairy milks on antiproliferation of colon cancer cells. Biotechnol Lett 31:571–576. https://doi.org/10.1007/s10529-008-9902-3
Han Q, Kong B, Chen Q et al (2017) In vitro comparison of probiotic properties of lactic acid bacteria isolated from Harbin dry sausages and selected probiotics. J Funct Foods 32:391–400. https://doi.org/10.1016/j.jff.2017.03.020
Lee Y-K, Puong K-Y (2002) Competition for adhesion between probiotics and human gastrointestinal pathogens in the presence of carbohydrate. Br J Nutr 88:101–108. https://doi.org/10.1079/bjn2002635
Allam MGM, Darwish AMG, Ayad EHE et al (2017) Lactococcus species for conventional Karish cheese conservation. LWT Food Sci Technol 79:625–631. https://doi.org/10.1016/j.lwt.2016.11.032
Abdollahzadeh SM, Zahedani MR, Rahmdel S et al (2018) Development of Lactobacillus acidophilus-fermented milk fortified with date extract. LWT Food Sci Technol 98:577–582. https://doi.org/10.1016/j.lwt.2018.09.042
Franciosi E, Settanni L, Cavazza A, Poznanski E (2009) Biodiversity and technological potential of wild lactic acid bacteria from raw cows’ milk. Int Dairy J 19:3–11. https://doi.org/10.1016/j.idairyj.2008.07.008
Reuben RC, Roy PC, Sarkar SL et al (2019) Isolation, characterization, and assessment of lactic acid bacteria toward their selection as poultry probiotics. BMC Microbiol 19:253. https://doi.org/10.1186/s12866-019-1626-0
de Almeida Júnior WLG, da Ferrari Í et al (2015) Characterization and evaluation of lactic acid bacteria isolated from goat milk. Food Control 53:96–103. https://doi.org/10.1016/j.foodcont.2015.01.013
Schirru S, Todorov SD, Favaro L et al (2012) Sardinian goat’s milk as source of bacteriocinogenic potential protective cultures. Food Control 25:309–320. https://doi.org/10.1016/j.foodcont.2011.10.060
Divisekera DMWD, Samarasekera JKRR, Hettiarachchi C et al (2019) Lactic acid bacteria isolated from fermented flour of finger millet, its probiotic attributes and bioactive properties. Ann Microbiol 69:79–92. https://doi.org/10.1007/s13213-018-1399-y
Ji K, Jang NY, Kim YT (2015) Isolation of lactic acid bacteria showing antioxidative and probiotic activities from kimchi and infant feces. J Microbiol Biotechnol 25:1568–1577. https://doi.org/10.4014/jmb.1501.01077
Zommiti M, Connil N, Ben HJ, Ferchichi M (2017) Probiotic characteristics of Lactobacillus curvatus DN317, a strain isolated from chicken ceca. Probiotics Antimicrob Proteins 9:415–424. https://doi.org/10.1007/s12602-017-9301-y
Abouloifa H, Rokni Y, Bellaouchi R et al (2019) Characterization of probiotic properties of antifungal Lactobacillus strains isolated from traditional fermenting green olives. Probiotics Antimicrob Proteins. https://doi.org/10.1007/s12602-019-09543-8
Miquel S, Beaumont M, Martín R et al (2015) A proposed framework for an appropriate evaluation scheme for microorganisms as novel foods with a health claim in Europe. Microb Cell Fact 14:48. https://doi.org/10.1186/s12934-015-0229-1
Kumar A, Kumar D (2015) Characterization of Lactobacillus isolated from dairy samples for probiotic properties. Anaerobe 33:117–123. https://doi.org/10.1016/j.anaerobe.2015.03.004
Kim SE, Kim YH, Lee H et al (2012) Probiotic properties of lactic acid bacteria isolated from Mukeunji, a long-term ripened kimchi. Food Sci Biotechnol 21:1135–1140. https://doi.org/10.1007/s10068-012-0148-4
Banwo K, Sanni A, Tan H (2013) Technological properties and probiotic potential of Enterococcus faecium strains isolated from cow milk. J Appl Microbiol 114:229–241. https://doi.org/10.1111/jam.12031
García-Ruiz A, González de Llano D, Esteban-Fernández A et al (2014) Assessment of probiotic properties in lactic acid bacteria isolated from wine. Food Microbiol 44:220–225. https://doi.org/10.1016/j.fm.2014.06.015
Caggia C, De Angelis M, Pitino I et al (2015) Probiotic features of Lactobacillus strains isolated from Ragusano and Pecorino Siciliano cheeses. Food Microbiol 50:109–117. https://doi.org/10.1016/j.fm.2015.03.010
Vidhyasagar V, Jeevaratnam K (2013) Evaluation of Pediococcus pentosaceus strains isolated from Idly batter for probiotic properties in vitro. J Funct Foods 5:235–243. https://doi.org/10.1016/j.jff.2012.10.012
Acknowledgement
This research was funded by the European Regional Development Fund according to the supported activity ‘Research Projects Implemented by World-class Researcher Groups' under Measure No. 01.2.2-LMT-K-718.
Author information
Authors and Affiliations
Contributions
KK drafted the manuscript, conducted the research. LL performed statistical analysis. MM and LS were responsible for supervison, revision of the manuscript. VA and DS were responsible for editing. NK conducted the research.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kondrotiene, K., Lauciene, L., Andruleviciute, V. et al. Safety Assessment and Preliminary In Vitro Evaluation of Probiotic Potential of Lactococcus lactis Strains Naturally Present in Raw and Fermented Milk. Curr Microbiol 77, 3013–3023 (2020). https://doi.org/10.1007/s00284-020-02119-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-020-02119-8