Abstract
Lactobacillus helveticus, grown at 37°C in MRS medium supplemented with 3 mM cholesterol, assimilated all the cholesterol in 42 h having 68 U mg−1 of intracellular cholesterol oxidase activity. The strain transformed 1 g cholesterol to 0.05 g of androsta-1, 4-diene-3, 17-dione and 0.04 g of androst-4-ene-3, 17 dione within 48 h at 37°C with extracellular cholesterol oxidase activity at 12 U mg−1 and intracellular oxidase at 0.5 U mg−1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiovascular disease (CVD) is the most important cause of death in the developed countries and it is strongly associated with hypercholesterolemia (American Heart Association 2002). WHO predicted that by the year 2030, CVDs will remain a major cause of death, affecting approximately 23.6 million people around the world even though; a drug-based cost effective combination therapy inhibiting cholesterol synthesis and absorption is in practice (Visseren 2011).
Lactic acid bacteria (LAB) are food-grade, non-toxic generally recognized as safe (GRAS) probiotic organisms. They possess therapeutically useful properties such as synthesis of antimicrobials, alleviation of lactose intolerance, detoxification of potential carcinogens, stimulation of immune response, antitumor effect and antihypertensive compounds (Ahire et al. 2011). Consumption of LAB and fermented dairy products can decrease serum cholesterol (Tok and Aslim 2010). The mechanisms by which the organisms remove cholesterol though are not yet clear. In vitro studies have suggested several possible mechanisms including enzymatic deconjugation of bile salts, microbial transformation of cholesterol to coprostanol, cholesterol binding to the bacterial cell wall, and direct assimilation of cholesterol (Kumar et al. 2011). In this study, growing cells of Lactobacillus helveticus assimilated cholesterol and showed high intracellular cholesterol oxidase-like activity which has not been previously reported.
Cholesterol oxidase is a FAD-dependent enzyme catalyzing conversion of cholesterol into cholest-4-ene-3-one and H2O2 along with side-chain degradation products androsta-1, 4-diene-3, 17-dione (ADD) and androst-4-ene-3, 17 dione (AD), which are major starting materials for the synthesis of anabolic drugs and contraceptive hormones (Chaudhari et al. 2010). Many organisms produce AD and ADD through side-chain degradation reactions of the sterol nucleus when sterols are used as a sole carbon source (Liu et al. 2011), while LAB-mediated biotransformation is less frequently reported (Kumar et al. 2001). Cholesterol oxidase has applications in blood serum and food cholesterol determination, manufacturing of reduced cholesterol diet (Chenfeng et al. 2002). The present investigation was intended to study cholesterol assimilation and biotransformation of L. helveticus under nutritionally rich as well as minimal medium.
Materials and methods
Chemicals and media
Chemicals and media were purchased from Hi-Media. Steroids were from Sigma.
Cholesterol assimilation of strain
Cholesterol assimilation ability of L. helveticus (Ahire et al. 2011) was determined by inoculating 1 ml of 18 h (log10 6.5 cfu ml−1) culture in 100 ml deMan Rogosa Sharpe (MRS) medium containing 3 mM cholesterol (dissolved in 10 ml ethanol). After inoculation, the culture was grown microaerophilically at 37°C and growth was monitored up to 48 h by measuring the cfu ml−1. The cells were separated by centrifugation and supernatant was analysed for cholesterol by HPLC. The assimilation of cholesterol by growing cells was confirmed by disrupting 48 h old cells using ultrasonication at 0°C. The resulting intracellular cell-free supernatant and cell debris were extracted by ethyl acetate and analysed for cholesterol by GC–MS.
Cholesterol oxidase indicator plates
The indicator plates were prepared by adding 1 g cholesterol, 1 g Triton X-100, 0.1 g o-dianisidine and 1000 U horseradish peroxidase to 1 l of MRS agar (pH 6.5). Cells were streaked on it and incubated at 37°C for 24 h to observe formation of intense brown azo compound (Nishiya et al. 1997).
Intracellular enzyme preparation
The cells were grown in MRS medium with 3 mM cholesterol as indicated above, centrifuged (6,000×g for 10 min at 4°C) and washed twice with phosphate buffered saline (PBS; pH 7.3) followed by water. The pellet was resuspended in 5 ml water and disrupted by sonication. Cell debris was removed by centrifugation and resulting supernatant was used for assay. Cfu values were determined prior to preparation of crude enzyme.
Determination of cholesterol oxidase
This was assayed by measuring generated H2O2 accompanying the oxidation of cholesterol (Allain et al. 1974). In brief, 125 μl crude enzyme was incubated with 2.5 ml reaction mixture (64 mM sodium cholate, 0.34% (v/v) Triton X-100, 1.4 mM 4-aminoantipyrine, 21 mM phenol, 0.9 mM cholesterol, 1.5 U peroxidase ml−1) at 37°C for 5 min followed by the measurement of increase in absorbance at 500 nm. The 1 U of enzyme activity was defined as the formation of 1 μM H2O2 min−1.
Biotransformation study
One ml of 18 h old LAB (log10 6.9 cfu ml−1) was grown as before in 100 ml transformation medium (1 g NH4NO3, 0.25 g MgSO4 7H2O, 0.25 g K2HPO4, 0.001 g FeSO4, 0.005 g NaCl, 2 ml Tween 80, 1 g cholesterol and 0.05 g 2,2′-dipyridyl in 1 l water, pH 7.2) for 120 h. Cholesterol transformation was analysed daily by HPLC.
Analytical methods
HPLC was performed on LC-8A system using a C18 reverse phase column (100–5, 250 × 4.6 mm) with acetonitrile/water (80: 20, v/v) as mobile phase at 0.7 ml min−1. The elute was monitored at 230 nm.
GC–MS was performed on a QP 2010 system (Shimadzu) interfaced to a mass detector and equipped with an Ultra-2 cross-linked capillary column (5% phenyl–95% methylpolysiloxane bonded phase; 25 m × 0.20 mm, 0.11 μm film thickness). The injector, interface and ion source were at 260, 300 and 230°C, respectively. Helium was used as the carrier gas at 0.5 ml min−1. Samples were introduced in split-injection mode (10:1) and the oven was set initially at 240°C (1 min) and then increased to 300°C (over 10 min).
Results and discussion
Cholesterol assimilation of strain
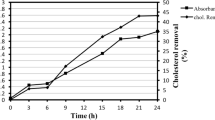
Lactobacillus helveticus assimilated cholesterol when grown in MRS medium. The degree of assimilation increased with respect to the duration of incubation at 37°C (Supplementary Fig. 1). 100% cholesterol assimilation was observed after 42 h (Table 1). During assimilation, the cfu increased with respect to the time of incubation (Fig. 1), indicating that cholesterol was not toxic to growing cells. Cholesterol assimilation by L. helveticus was 20 and 40% higher than other strains of LAB (Tok and Aslim 2010; Lee et al. 2010).
Intracellular cholesterol oxidase-like activity (filled square) and log10 cfu ml−1 (filled triangle) of L. helveticus at 37°C for 0–48 h incubation in MRS medium supplemented with 3 mM cholesterol. The enzyme activity was determined by measuring amount of H2O2 formed during oxidation of cholesterol at 500 nm. The values represent the means of three independent experiments (mean ± standard error)
To determine if the assimilated cholesterol was present in the cells, a cell-free extract and cell debris of a 48 h old culture were extracted with ethyl acetate and analysed by GC–MS. No peak was found in either extract which corresponded to cholesterol (Supplementary Fig. 2). Therefore, the possible presence of a cholesterol oxidase-like enzyme was evaluated.
Cholesterol oxidase indicator plate assay
The organism was negative for extracellular synthesis of cholesterol oxidase, while the growth on indicator plate showed its ability to survive on the medium containing cholesterol (data not shown). These results pointed to the presence of an intracellular enzyme responsible for degradation of cholesterol.
Intracellular enzyme production
The culture showed 68 U intracellular cholesterol oxidase activity/mg protein after 42 h at 37°C (Fig. 1), which was higher than recombinant strains of L. plantarum, L. casei (Kiatpapan et al. 2001). However, the presence of a cholesterol oxidase-like enzyme in LAB cells has not been explored. Kim et al. (2008) reported an intracellular 12 kDa proteinaceous compound in L. acidophilus ATCC 43121 responsible for in vitro and in vivo cholesterol reduction. Subsequently; Lee et al. (2010) demonstrated membrane-associated proteins responsible for cholesterol reduction process. In the present study, we report an intracellular cholesterol oxidase-like activity of LAB responsible for removal of cholesterol from medium.
On the basis of above experiments, we hypothesize that L. helveticus was able to assimilate cholesterol from nutritionally rich MRS medium. Subsequently, the assimilated cholesterol was degraded into small fragments by intracellular cholesterol oxidase-like enzyme, which deserves potential in lowering of cholesterol.
Biotransformation
Biotransformation was carried out by growing the cells up to 120 h in minimal medium with 3 mM cholesterol as sole carbon source along with nucleus cleavage inhibitor 2,2′-dipyridyl (0.05 g l−1). HPLC of supernatant showed six peaks of which three matched cholesterol, AD and ADD (Supplementary Fig. 3) while the remaining three were unidentified compounds. At the end of 48 h, 1 g cholesterol l−1 was transformed to 0.04 g AD and 0.05 g ADD (Table 2) with 12 U mg−1 extracellular cholesterol oxidase and 0.5 U mg−1 intracellular oxidase activity at 37°C. The unknown compounds produced at 48 and 96 h were not determined. Transformation at 96 h yielded 0.36 g of AD with 15 U mg−1 extracellular and 0.5 U mg−1 intracellular cholesterol oxidase activity which later remained constant up to 120 h (Fig. 2). This is the first report of cholesterol transformation to AD and ADD by cholesterol oxidase-like enzyme in Lactobacillus.
Intracellular (filled square) and extracellular (filled diamond) cholesterol oxidase-like activity of L. helveticus at 37°C for 0–120 h incubation in minimal medium with 3 mM cholesterol along with 0.05 g l−1 2,2′-dipyridyl. The enzyme activity was determined by measuring amount of H2O2 formed during oxidation of cholesterol at 500 nm. The values represent the means of three independent experiments (mean ± standard error)
Conclusion
Our study demonstrates the assimilation and intracellular degradation as a cholesterol-lowering mechanism in lactobacilli. The presence of cholesterol oxidase-like activity in lactobacillus has potential to bring desirable compositional changes in dairy foods and increase economic value.
References
Ahire JJ, Mokashe NU, Patil HJ et al. (2011) Antioxidative potential of folate producing probiotic Lactobacillus helveticus CD6. J Food Sci Technol. doi:10.1007/s13197-011-0244-0
Allain CC, Poon LS, Chan CSG et al (1974) Enzymatic determination of total serum cholesterol. Clin Chem 20:470–475
American Heart Association (2002) Heart disease and stroke statistics–2002 update. American Heart Association, Dallas
Chaudhari PN, Chaudhari BL, Chincholkar SB (2010) Cholesterol biotransformation to androsta-1, 4-diene-3, 17-dione by growing cells of Chryseobacterium gleum. Biotechnol Lett 32:695–699
Chenfeng L, Yixin T, Longgang W et al (2002) Bioconversion of yolk cholesterol by extracellular cholesterol oxidase from Brevibacterium sp. Food Chem 77:457–463
Kiatpapan P, Yamashita M, Kawaraichi N et al (2001) Heterologous expression of a gene encoding cholesterol oxidase in probiotic strains of Lactobacillus plantarum and Propionibacterium freudenreichii under the control native promoters. J Biosci Bioengi 92:459–465
Kim Y, Whang JY, Whang KY et al (2008) Characterization of the cholesterol-reducing activity in a cell-free supernatant of Lactobacillus acidophilus ATCC 43121. Biosci Biotechnol Biochem 72:1483–1490
Kumar R, Dahiya JS, Singh D et al (2001) Biotransformation of cholesterol using Lactobacillus bulgaricus in a glucose-controlled bioreactor. Bioresour Technol 78:209–2011
Kumar R, Grover S, Batish VK (2011) Hypocholesterolemic effect of dietary inclusion of two putative probiotic bile salt hydrolase-producing Lactobacillus plantarum strains in Sprague–Dawley rats. Br J Nutr 105:561–573
Lee J, Kim Y, Yun HS et al (2010) Genetic and proteomic analysis of factors affecting serum cholesterol reduction by Lactobacillus acidophilus A4. Appl Environ Microbiol 76:4829–4835
Liu Y, Chen G, Fanglan G et al (2011) Efficient biotransformation of cholesterol to androsta-1, 4-diene-3, 17-dione by a newly isolated actinomycete Gordonia neofelifaecis. World J Microbiol Biotechnol 27:759–765
Nishiya Y, Harada N, Teshima S (1997) Improvement of thermal stability of Streptomyces cholesterol oxidase by random mutagenesis and a structural interpretation. Protein Eng 10:231–235
Tok E, Aslim B (2010) Cholesterol removal by some lactic acid bacteria that can be used as probiotic. Microbiol Immunol 54:257–264
Visseren FLJ (2011) Cost-effectiveness of lipid lowering therapy. Neth Heart J 19:59–60
Acknowledgments
Bhushan Chaudhari gratefully acknowledges financial support and Jayesh Ahire is thankful to Research Fellowship in Science for Meritorious Students (RFSMS) from University Grants Commission, New Delhi.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ahire, J.J., Bhat, A.A., Thakare, J.M. et al. Cholesterol assimilation and biotransformation by Lactobacillus helveticus . Biotechnol Lett 34, 103–107 (2012). https://doi.org/10.1007/s10529-011-0733-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-011-0733-2