Abstract
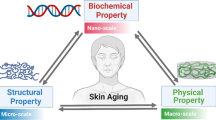
Skin is a rather complex, yet useful organ of our body. Besides, skin aging is a complicated process that gains a growing interest as mediates many molecular processes in our body. Thus, an efficient skin model is important to understand skin aging function as well as to develop an effective innovative product for skin aging treatment. In this mini review, we present in vitro methods for assessments of skin aging in an attempt to pinpoint basic molecular mechanisms behind this process achieving both a better understanding of aging function and an effective evaluation of potential products or ingredients that counteract aging. Specifically, this study presents in vitro assays such as 2D or 3D skin models, to evaluate skin aging-related processes such as skin moisturization, photoaging, wound healing, menopause, and skin microbiome as novel efforts in the designing of efficacy assessments in the development of skincare products.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Skin is a complex organ which provides a protective barrier between the organism and the environment preventing of invasion of pathogens, physical or chemical assaults as well as a loss of water or other substances essential for skin function (Proksch et al. 2008; Yousuf et al. 2018). It consists of an outer epidermal layer, an inner dermal layer and hypodermis.
Skin function depends on the cellular compartments of these three layers. Specifically, epidermis is a multi-layered epithelium that consists mainly of keratinocytes which maintain an equilibrium of proliferation in basal layer and undergo differentiation which strengthens the cytoskeleton, establishes an intercellular diffusion barrier and results in a specialized form of programmed cell death, known as cornification (Eckhart and Zeeuwen 2018). The aging effect on epidermis layer, is the thinning and the impairment of epidermal barrier recovery after damage which suggests that the re-supplementation chain from the stem cell pool becomes less efficient over time (Gruber et al. 2020). Apart from keratinocytes the epidermis consists of a network of melanocytes and Langerhans cells which depends on epidermis homeostasis to function properly. Any disturbance of intercellular coordination between the different cell types contributes to the age-related decline in epidermal immune function and pigment regularity (Rognoni and Watt 2018). On the other hand, dermis consists mainly of extracellular matrix (ECM) related compartments such as proteins and fibroblasts which are the dominant cells. Dermis can be divided into the papillary layer which is densely populated by fibroblasts and reticular dermis which contains collagen fibres. The effect of aging to this layer is the disorganization of ECM which depends mainly on the alteration of ECM’s proteins turnover or the accumulation of post-translational modifications (Driskell et al. 2013). Moreover, wrinkles and sagging of skin are mainly due to changes in the dermal compartments in aging, such as decreased synthesis and increased remodeling or degradation of dermal matrix components. In chronologically aged skin, the fibrillar ordering of dermal elastin is impaired (Rittie and Fisher 2015). Similarly, type I collagen, the most abundant dermal extracellular matrix protein is a target of both chronological- and photo-aging which both promote degradation, lack of ordering and reduced production of this protein (Rittie and Fisher 2015). Also, the dermis contains nerve endings and Schwann cells, endothelial cells organized in vessels, pericytes, mast cells, tissue macrophages and other cells of the immune system (Lai-Cheong and McGrath 2017). Beneath the dermis is a layer of white adipose tissue, the hypodermis, which contains adipocytes that massively accumulate lipids upon their maturation. The effect of aging is the thinning of hypodermis layer (Rittie and Fisher 2015).
Skin aging, like other organs, is characterized by a progressive loss of functionality and regenerative potential. More than other organs, skin aging process is the outcome of two biological factors, intrinsic aging where alterations occur over a lifetime, and extrinsic aging where skin alterations are attributed to external factors such as environmental and lifestyle (Farage et al. 2008; Rittie and Fisher 2015). On the other hand, the systematic hallmarks of aging (López-Otín et al. 2013) apply to the various compartments and cell types of the skin (Tigges et al. 2014), but cells which reside for a long time span in the tissue appear to be affected more severely by loss of cellular maintenance and repair mechanisms than highly proliferative cells that are replaced frequently (Sukseree et al. 2018a, b). With the advent of biotechnology, the effect of aging on skin function is gaining a growing interest in the developed countries as many consumers opt for multifunctional products to defend aging (Rincón-Fontán et al. 2020; Duan et al. 2020). Besides, the growing concern regarding the potential ecotoxicity of cosmetics or personal care products has led many consumers to opt for products with proven scientific efficacy and shreds of evidence for non-ecotoxicity activity (Juliano and Magrini 2017; Vita et al. 2018; Bilal et al. 2020; Letsiou et al. 2020a, b). For these reasons, it is necessary to understand skin function and particularly the aging process and how it influences the mechanical behavior of skin, as these findings can then be implemented in cosmetic development and clinical studies. A combination of methodologies is needed both to understand the aging process as well as to find innovative approaches for skin aging treatment. In vitro skin, models are a useful tool for a better understanding of the skin aging process as they provide an insight into many molecular pathways. Thus, in vitro studies play a fundamental role in understanding whether a newly discovered compound or ingredient could be suitable for clinical trials. In this mini-review, we present recent in vitro based methods related to skin aging to evaluate and understand the aging process as well as to identify the suitable agents for aging treatment. We focused on functions related to the skin aging process such as intrinsic and extrinsic aging, skin moisturization, wound healing, menopause, and skin microbiome.
The intrinsic and extrinsic aging process
Aging is a process that is influenced by both intrinsic and extrinsic factors (Lee et al. 2020a). Skin alterations of intrinsic factors depend on normal biological processes while typical extrinsic factors are UV exposure, IR exposure, smoking, (Kammeyer and Luiten 2015; Newton et al. 2015). Clinical aging signs are wrinkles, fine lines, sagging, wound healing, skin pigmentations, and thin epidermis junctions (DEJ). However, at a molecular level, during the aging process fibroblasts as well as keratinocytes lose their ability of self-renewing and many alterations on the function of sweat glands are observed (Rittié and Fisher 2015). Besides, collagen which is the most representative compartment of the extracellular matrix (ECM), due to age, undergoes gradual fragmentation by the overexpressed matrix metallo proteases (MMPs) leading to loss of skin mechanical properties and dermal cell functions (Rattan 2015; Pittayapruek et al. 2016). Furthermore, photoaging which is based on extrinsic factors causes deep wrinkles, loss of elasticity, dryness, pigmentation disorders as well as cancerous lesions. At the molecular level, the excess reactive oxygen species (ROS) induce the expression of MMPs leading to ECM component degradation such as collagen (Taylor 2005; Kammeyer and Luiten 2015; Newton et al. 2015; Murai et al. 2018). Moreover, the stratum corneum (SC) rate is reduced drastically and damage on the connective tissues is observed (Taylor 2005; Duval et al. 2014; Rittié and Fisher 2015; Kammeyer and Luiten 2015; Löwenau et al. 2017; Damiani et al. 2018).
To begin with, organ culture of human dates back over 50 years (Beaven and Cox 1965), has undergone significant improvements to optimize skin explants suitable for research studies involving therapeutics and formulations. The main benefit of using skin explants is that they can be used in a variety of studies such as investigations of environmental stress, skin aging and skin disease (Lebonvallet et al. 2010; Xu et al. 2012). According to previous reports (Beaven and Cox 1965; Lebonvallet et al. 2010; Park et al. 2015; Vostálová et al. 2018), the main drawback of skin explants is the loss of tissue integrity and keratinocytes differentiation after ~ 14 days. However, a recent study has demonstrated a Human Explant Skin Culture (HESC) which is structurally viable and metabolically active for up to 9 days in culture and can be employed for preclinical testing of delivery and efficacy of skin therapeutics (Neil et al. 2020).
On the other hand, the simplest and cost-effective method to simulate the skin aging process is the 2D culture of fibroblasts, keratinocytes, or melanocytes evaluating for different molecular readouts such as MMPs activity, melanin content, tyrosinase activity, mitochondrial integrity, genes expression related to aging (Kovacs et al. 2010; Letsiou et al. 2017, 2020b, e; Nakamura et al. 2018; Yoshimoto et al. 2018; Lago and Puzzi 2019). Moreover, as oxidative stress and cell apoptosis play a major role in aging process, different molecular readouts such as mitochondrial DNA damage, singlet oxygen oxidizes guanine to 8-oxoguanine (8-oxoG), MMPs, glycoproteins, collagen I,III,VII, transcription factor activator protein 1 (AP-1), transforming growth factor beta (TGF-β) (Jenkins 2002; Rinnerthaler et al. 2015; Gu et al. 2020), transcription factor NRF2 and haem-oxygenase (HO-1) (Peter Jorgensen 2014) could be used as targets of skin aging in 2D culture fibroblasts. Additionally, other in vitro biological markers of aging could be β- galactosidase activity, p16 expression, and proliferation rate (Damiani et al. 2018; Gu et al. 2020). Moreover, 2D culture can be used for in vitro bioactivity assessments of natural substances that can counteract the aging process (Peter Jorgensen 2014; Kostyuk et al. 2018; Letsiou et al. 2020a, b, c). According to these studies, the important role of in vitro 2D models in skin aging research is emphasized as these models do not only decipher the molecular mechanism of the aging process but also aids in the investigation of innovative natural substances that counteract the aging process.
However, the 2D in vitro models have some limitations as they lack skin barrier function, 3D models were introduced back in 1970 (Van Wezel 1967; Knazek et al. 1972). From the 1980’s, reconstituted human epithelium (RHE) skin models were developed, in part for human irritancy studies (Botham et al. 1998). These cultures allowed differentiation of an intact stratum corneum in an air–liquid interface that more closely resembled the in vivo human skin barrier. One interesting study (Löwenau et al. 2017) based on the comparison between irradiated and non-irradiated 3D RHE model. Interestingly, irradiated RHE showed some special characteristics compared to normal RHE such as decreased keratinocyte viability, increased permeability of caffeine, testosterone, and nanocarriers, while the release of interleukin-1 (IL-1) and interleukin-8 (IL-8) and increased number of senescence-associated β-galactosidase positive keratinocytes indicated stress-mediated cellular senescence (Löwenau et al. 2017). Moreover, irradiated RHE had thinner stratum corneum possibly due to flattened keratinocytes and/or exfoliated corneocytes (Löwenau et al. 2017). Another interesting model was based on a pigmented skin model comprising a melanocyte-containing epidermis cultured on a living fibroblast embedded-dermal equivalent (Duval et al. 2014). This skin model emphasizes the role of dermal fibroblasts in skin pigmentation in microscopic and macroscopic level (Duval et al. 2014). Moreover, a 3D RHE model was used for phototoxicity assessments as well as for the quantification of free radicals production (Albrecht et al. 2019). Furthermore, another study presented a skin model based on the co-culturing of fibroblasts and keratinocytes on collagen- glycosaminoglycan- chitosan scaffold (Diekmann et al. 2016).
One of the limitations of these 3D models is the fact that they lack immune cells (Nakamura et al. 2018). This observation led to the development of a reconstructed human skin that contained keratinocytes, melanocytes, and Langerhans cells to evaluate sunscreen efficacy and additional parameters that are correlated with the immunosuppression induced by UV-radiation (Duval et al. 2003). Some years later, in similar terms, another group developed a 3D reconstructed human epidermis supplemented with monocytes to study the effect of the aging-induced glycation process in cell differentiation (Pageon et al. 2017).
In principal, in 3D skin models, different biochemical markers such as keratins, lipids (e.g. ceramides, phospholipids), proteins (e.g. involucrin, loricrin), interleukins (e.g. IL6, IL8) can be identified so as to assess skin irritation, phototoxicity and inflammation (Netzlaff et al. 2005). Moreover, the 3D skin models can be used for skin photoaging assessments as they provide interesting findings on different molecular readouts such as MMPs, p53, p21WAF1/Cip1, and Ki-67 which were mainly detected in the basal layers of the histological section of RHE by means of monoclonal antibodies (Torricelli et al. 2017; Karapetsas et al. 2019). On the other hand, under oxidative stress protein carbonyl measurements can evaluate for skin aging in 3D model (Cotovio et al. 2001).
In conclusion, the 3D models were proven useful in the simulation of skin function related to the aging process. There is a wide range of commercially available reconstructed skin models including skin such as ZK 1350 (Liebsch et al. 1995), EpiDerm™ (Líšková 2020), T-skin™ (Bataillon et al. 2019), MelanoDerm™ (Lee et al. 2020b; Park et al. 2020), EpiSkin™ (Chen et al. 2020), SkinEthic™ RHPE (Zeitoun et al. 2020) and The Phenion™ FT Skin model (Pfuhler et al. 2020) that can be used for aging and phototoxicity studies.
Skin moisturization process
Skin moisturization is another key factor related to the skin aging process as it influences physical and mechanical properties (Mojumdar et al. 2017). Skin moisturization is connected to the function of the stratum corneum (SC) which is the outer layer of the epidermis and influences the function of the skin barrier (Mojumdar et al. 2017). The SC consists of corneocytes which are anucleated dead cells filled with keratin filaments and wrapped with cornified envelope (Boncheva et al. 2008; Mojumdar et al. 2017). The corneocytes are associated with SC mechanical properties which are highly influenced by the moisturization process as previous studies supported the notion that water content in SC is of primary importance in SC flexibility. When hydrated SC, water is primarily taken up by the corneocytes. It has been hypothesized that the corneocytes control SC viscoelastic properties through the plasticization of the keratin filament macromolecules by water (Elias 2007; Björklund et al. 2013). At low relative hydration (RH) values, the keratin filaments are present in a rigid state, whereas a recent study has shown that there is a change in the molecular mobility of certain amino acids in the keratin filament upon hydration (Björklund et al. 2013). Corneocytes connect to each other through corneodesmosomes, producing high mechanical strength and creating a physical, chemical, and immunological barrier. Those junctions loosen as corneocytes migrate towards the skin surface leading to desquamation (Elias 2007). Abnormalities of SC are connected with skin diseases such as atopic dermatitis (Watanabe et al. 1991), eczema (Thune 1989), psoriasis (Ghadially et al. 1996), senile xerosis (HORII et al. 1989), and hereditary ichthyosis (Paige et al. 1994). The balance between the sufficient supply of water and the reduction of it which is known as Trans Epidermal Water Loss (TEWL), is regulated by lipids and Natural Moisturizing Factors (NMF) (Verdier-Sévrain and Bonté 2007). NMFs are water-soluble compounds of low molecular weight including urea, lactic acid, and various amino acids (Maeno 2019). Interestingly, UV can damage SC by destroying the skin’s natural moisturizing process. It is important to note that NMFs are found only in SC cells and ensure a wet environment as in SC many hydration-dependent enzymatic reactions take place. Therefore, if water SC content is reduced, many normal enzymatic processes cannot take place leading to the visible appearance of dryness, roughness, scaling, and flaking (Verdier-Sévrain and Bonté 2007).
There is limited work in in vitro assays related to the skin moisturization process. Specifically, the 2D models based on cell cultures model of normal human epidermal keratinocytes (NHEK) (Sugiyama et al. 2014), skin explant (Del Carmen Velazquez Pereda et al. 2009; Sundaram et al. 2016) and 3D reconstructed human epidermis (RHE) (Régnier et al. 1992; Barbotteau et al. 2005; Capallere et al. 2018) models are proposed for in vitro assessments in skin moisturization. Specifically, the 2D cell cultures based on keratinocytes provide an insight into the skin hydration process targeting the transcripts of genes that encode proteins important in this process such as aquaporin 3(AQP3) which together with hyaluronate receptor CD44 and intercellular adhesion protein E-cadherin are important regulators of skin moisturization (Chaudhuri and Bojanowski 2017; Choi et al. 2019). Moreover, studies focusing on skin explant demonstrated the efficacy of moisturizing agents (Del Carmen Velazquez Pereda et al. 2009; Sundaram et al. 2016). Also, focusing on in vitro 3D Reconstructed Human Epidermis (RHE) model, which is composed of normal human dermal fibroblasts (NHDF) and NHEK cell types, it is a short-term clinical dimension of skin hydration since this model is considered to be similar to human skin as far as differentiation markers, morphology, and functional characteristics are concerned. RHE is metabolically and mitotically active while it exists in many stages of maturity (Poumay and Coquette 2006). Since RHE models can be efficiently used to mimic in vitro skin functions there are two commercially available—SkinEthic™ RHE and EPISKIN™ RHE- which are widely used by the chemical, pharmaceutical, and cosmetic industry (Green et al. 1979; Prunieras 1979). Lastly, recent studies have shown that tape stripping in combination or no with confocal microscopy can be another way to assess skin moisturization and skin barrier function (Davies et al. 2015, 2017; Olesen et al. 2019). The 3D models seem to contribute more on the understanding of skin moisturization process/mechanism compared to other models.
Wound healing process
Wound healing is a physiological, complicated, age-dependent, and evolutionary process that aims to maintain skin integrity after an injury (Wang et al. 2018). Wound healing has three main and partially overlapping phases: inflammation, cell proliferation, and tissue remodeling and it is regulated by both chemicals (e.g. growth factors) and biomechanical stimuli (stresses/strains) (Flanagan 2013; Kim et al. 2019). Both keratinocytes and fibroblasts have an important role during this process. Fibroblasts migrate into the wound in response to transforming growth factor beta 1 (TGF-β1), platelet-derived growth factor (PDGF), and fibroblasts growth factor (FGF), where they proliferate and produce a new ECM . Some fibroblasts differentiate into myofibroblasts and are responsible for wound contraction and the deposition of additional matrix. Moreover, epidermal keratinocytes migrate to the wound to cover it with new epidermis (Martin 1997; Wang et al. 2018; Kim et al. 2019). Interestingly, the migration of keratinocytes is related to an Epithelial-Mesenchymal Transition (EMT) process giving to epithelial cells a migratory ability (Haensel and Dai 2018). During the tissue remodeling phase of wound healing, keratinocytes stop their migration and reverse their EMT-like phenotype. Moreover, myofibroblasts become apoptotic, angiogenesis stops, a cellular scar is formed, collagen type III is gradually dominated by collagen type I, and the disorganized collagen fibers are rearranged and aligned (Wang et al. 2018; Kim et al. 2019).
Although wound healing is a complicated process, there have been some in vitro studies that tried to decipher the molecular mechanism. These studies entailed single cell and co-culture models as well as organotypic multicellular constructs (Ashrafi et al. 2018). To start with, the simplest, fastest, and most inexpensive method is the ‘scratch assay” based on the 2D single-cell culture of keratinocytes or fibroblasts (Buisson et al. 1996; Cha et al. 1996; Gottrup et al. 2000; Henemyre-Harris et al. 2008; Demirovic and Rattan 2011; Kim et al. 2013; Ud-Din and Bayat 2017a; Letsiou et al. 2020d). The migration of the cells can be visualized by microscopy (Calderon et al. 1996). Additionally, other parameters that can be evaluated are protein production, protein secretion, viability, gene expression, and differentiation (Ud-Din and Bayat 2017a).
Moreover, skin explants have been used to study wound repair as well as inflammation in the skin besides testing the effects of different therapeutics (Cho et al. 2013; Ud-Din and Bayat 2017a). The main advantage of skin explants is that provide a 3D-structure that show intercellular interaction between keratinocytes and fibroblasts. Specifically, the micro-environment of cells as well as cell–matrix interaction which are integral in wound healing can be shown in this model (Nayak et al. 2013; Cho et al. 2013; Sami et al. 2019). Even though a variety of wound types have been studied with skin explants (Nayak et al. 2013; Sami et al. 2019) the main disadvantage is that these models lack innervation which is significant for the understating of skin repair, scar formation and desquamation of cells cannot be observed as well as standardization and consistency (Cho et al. 2013; Ud-Din and Bayat 2017b).
Recently, a viable 3D skin model was reported to evaluate in vitro wound healing process (Iyer et al. 2018). In this model keratinocytes and fibroblasts are seeded in a collagen scaffold. The results showed that cell migration was increasing as keratinocytes and fibroblasts were in proximity. Also, the most significant keratinocyte migration was seen in the constructs with small cell growth proposing that fibroblasts promote keratinocyte migration over proliferation in the wound healing process (Iyer et al. 2018).
On the other hand, a 3D Transwell system or Boyden chamber assay with the co-culture of keratinocytes and fibroblasts overcome the limitations of the monolayer in vitro systems (Butler et al. 2008; Iyer et al. 2018). In more detail, in this assay a two-layered chamber of two different cell cultures separated by a porous material that allows cell migration to occur. The migratory cells can be stained and visualized with imaging (Pastar et al. 2017). Additionally, another novel construction was a de-cellularized epidermis that was re-seeded with keratinocytes and fibroblasts to monitor the wound healing process (Xie et al. 2010). Moreover, another study presented a 3D tissue-engineered construct of porous sericin matrix by co-culturing keratinocytes on the upper surface and fibroblasts and the lower surface which showed high potential as skin equivalent to monitor in vitro wound healing process (Nayak et al. 2013).
The use of organotypic skin equivalent is rather interesting for the evaluation of in vitro wound healing process. It came into the light after the observation of the significant interaction between fibroblasts and keratinocytes acts as an important regulator for the formation as well as the function of the ECM (Ghaffari et al. 2009). Generally, the organotypic skin equivalents consist of immortalized human keratinocytes which are grown on surfaces coated with type I collagen and fibroblasts (Pastar et al. 2017). To this, one study used an organotypic skin equivalent known as the raft model, which consists of a normal adult, neonatal, and keloid fibroblasts and keratinocytes, to evaluate the effectiveness of photodynamic therapy in the context of keloid scars (Chiu et al. 2005). An updated version of organotypic skin equivalents was proposed by Van den Broek et al. (2012) who developed a model based on adipose-derived mesenchymal stem cells. This model had many potentials in the therapeutics field as it represents the hypertrophic scar both macroscopically and microscopically while maintaining the characteristics of the epidermal and dermal scar. In a similar study (Bellemare et al. 2005) it was developed a fully differentiated epidermis by using keratinocytes that were obtained from hypertrophic scars and were seeded on a matrix that consisted of fibroblasts. This structure presented abnormal scar characteristics such as dermal and epidermal thickness (Bellemare et al. 2005). Last but not least, the novel organ-on-chip technology seems to be promising in the field of skin biology and especially in the biology of the wound healing process. In more detail, the organ-on-chip technology consists of a microfluidic device that can support the culture of many different cell lines, in different layers under a controlled environment highly mimicking the multilevel organ functions. Recently, Biglary et al. (2019) used a wound-on-a-chip model to mimic a wound and evaluate the inflammatory milieu of the wound microenvironment upon application of different active compounds. It is important to highlight, that this microfluidic device has three interconnected channels and co-cultured fibroblasts, endothelial cells, and macrophages (Biglari et al. 2019). Besides, a recent study assessed cell migration related to the wound healing process based on Electric cell-substrate impedance sensing (ECIS) (Letsiou et al. 2020d). This method allowed the dynamics of scratch repair to be observed in real-time.
The 2D model as well as a wound-on-a-chip and ECIS-based models seems to contribute more on the understanding of wound healing mechanism, while the 3D models seem contribute more on the outcome of wound healing process, serving as screening tools for the identification of an effective compound which promote cell migration, cell proliferation related to wound healing process.
Menopause process
Menopause is an aging-related factor associated mostly with the intrinsic mechanism (Affinito et al. 1999; Remoué et al. 2013; Reus et al. 2020). This physiological process is a result of changes to ovarian follicular activity and has effects on the skin. Specifically, menopause can cause severe skin-related problems in women such as alopecia, skin dryness, loss of skin elasticity, and skin atrophy leading to increased anxiety, reduced self-esteem, and low quality of life (Affinito et al. 1999; Remoué et al. 2013; Reus et al. 2020). Therefore, many pharmacological and cosmetic companies proceed on the development of innovative products to improve skin quality. To this attempt, in vitro models represent an essential and interesting tool for the discovery as well as the efficacy assessment of bioactive compounds that counteract menopause-related skin disorders.
An interesting study was conducted by Remoue et al. (Remoué et al. 2013) based on the 2D cell culture of primary human dermal fibroblasts. Cells were cultured in four different ways so as to simulate nonmenopausal and menopausal conditions. Under these conditions, cell proliferation, matrix metalloproteinase-1, and metalloproteinase-3 (MMPs) release, collagen deposition, and procollagen gene expression were evaluated by using cellular and molecular approaches. The main outcome of this study was that the results reflect most of the effects that are observed in vivo.
In 2016, Bogdanowicz (Bogdanowicz et al. 2016) proposed a study based on in vitro and ex vivo models to evaluate the antielastase and antiglycation MMP-12 potential of glycylglycine oleamide (GGO), as glycation is a consequence of ageing and happens especially in dermal proteins such as type I collagen and elastin. Even though this kind of study is far from an ideal model for menopause, it is indeed a viable approach for the screening of potential antiglycation and antielastase substances, which are claims that are becoming more common among skin care cosmetics.
In addition, Yoshimoto et al. (2018) proposed a method using normal human fibroblasts from foreskin exposed to UVA irradiation. After irradiation, cells were evaluated regarding different characteristics related to aging and senescence, such as the senescence marker β-galactosidase, reactive oxygen species (ROS) evaluation, and p16 expression. The results showed that repeated UVA radiation to cells led to typical senescence markers, such as increased SA-β-galactosidase staining, flattening and larger cells with a larger diameter ratio, higher levels of ROS, yellowish coloration (accumulation of oxidized proteins, carbonylated proteins, and advanced glycation end products) and increased p16 expression. Therefore, this model could represent an additional and complementary approach for evaluating menopause aged skin.
Furthermore, Mainzer et al. (2018) proposed an epidermis model (reconstructed human epidermis—RHE) with IGF-1R knockdown. Cell proliferation, colony forming assay, adhesion assay, immunolabelling, quantification of epidermal thickness, gene expression analysis by real-time PCR and protein expression analysis by Western blotting were evaluated by this model. The results showed that RHE with IGF-1R knockdown had a loss of function of the stratum basale. However, this model is interesting as it mimics the function of epidermis under hormonal decline that appears in menopause making a really useful tool for the investigation of menopausal effects in skin.
All of the aforementioned models mimic well the function of epidermis under hormonal changes related to menopause. However, further work is needed to decipher better this process.
Skin microbiome
Skin microbiota is an important part of skin barrier as it regulates inflammation and immune response processes (Zhai et al. 2018). Skin pathological state occurs in compositional imbalance of skin microbiome as a result of different factors such as aging (Kong et al. 2012; Schommer and Gallo 2013; Prescott et al. 2017; Tett et al. 2017; Rocha and Bagatin 2018). Skin aging is a process that entails the changing of the dominant bacteria of skin (Grice and Segre 2011; Shibagaki et al. 2017).
The advent of next-generation sequencing technologies has revolutionized our view of human-associated microbial communities. Using DNA sequencing methodology, we are now able to characterize and analyze microbiomes with greater precision and accuracy, and less bias compared to culture-based approaches. A common approach used to identify bacterial populations is based on sequencing of the small subunit bacterial 16S ribosomal RNA (rRNA) gene. Most of the studies based on DNA sequencing investigated different skin disorders such as acne and rosacea, body odor and atopic dermatitis, psoriasis, skin immunity (Grice and Segre 2011; Grice 2014; Byrd et al. 2018). Although, skin aging is accompanied by an alteration on skin microbiome little is known about how the composition of these changes during the course of aging are related to skin microbes. A recent study (Li et al. 2020) based on 16S ribosomal DNA and internal transcribed spacer ribosomal DNA sequencing revealed that skin microbiome plays a significant regulatory role in skin aging -related functions such as immune response, resistance to ultraviolet, and biosynthesis of age-related substances. In addition, other studies supported that specific bacteria such as Lactobacillus and Cutibacterium (formerly Propionibacterium) are associated with skin hydration (Grice and Segre 2011; Byrd et al. 2018). More studies are needed to associate skin aging signs such as wrinkles ore sagging with skin microbiome alterations.
Conclusions
According to the literature cited in this work, there is evidence that some in vitro assays are proven more effective than others however, its necessary to identify the most effective in vitro approaches before the conduction of clinical assessments. Nevertheless, additional research is needed to understand more the skin aging-related molecular processes, this mini-review indicates the need to develop integrated and standardized in vitro assays that enable to the identification and understanding of important molecular skin aging processes in addition to assessments of cosmetic raw materials efficacy.
The criteria established by the most important international organizations, allied with the alternative biotechnological methods currently available, point toward establishing a routine analysis of skin aging impact, allowing the selection or replacement of raw materials according to their skin aging impact and thus generating cosmetic/personal care products that are more sustainable and effective for skin.
References
Affinito P, Palomba S, Sorrentino C et al (1999) Effects of postmenopausal hypoestrogenism on skin collagen. Maturitas. https://doi.org/10.1016/S0378-5122(99)00077-8
Albrecht S, Elpelt A, Kasim C et al (2019) Quantification and characterization of radical production in human, animal and 3D skin models during sun irradiation measured by EPR spectroscopy. Free Radic Biol Med. https://doi.org/10.1016/j.freeradbiomed.2018.12.022
Ashrafi M, Hague A, Baguneid M et al (2018) Wound healing and cutaneous scarring models of the human skin. Skin tissue models. Elsevier, Amsterdam
Barbotteau Y, Gontier E, Barberet P et al (2005) Reconstructed human epidermis: a model to study the barrier function. Nucl Instrum Methods Phys Res B. https://doi.org/10.1016/j.nimb.2005.01.072
Bataillon M, Lelièvre D, Chapuis A et al (2019) Characterization of a new reconstructed full thickness skin model, t-skinTM, and its application for investigations of anti-aging compounds. Int J Mol Sci. https://doi.org/10.3390/ijms20092240
Beaven EP, Cox AJ (1965) Organ culture of human skin. J Invest Dermatol 44:151–156
Bellemare J, Roberge CJ, Bergeron D et al (2005) Epidermis promotes dermal fibrosis: role in the pathogenesis of hypertrophic scars. J Pathol. https://doi.org/10.1002/path.1737
Biglari S, Le TYL, Tan RP et al (2019) Simulating inflammation in a wound microenvironment using a dermal wound-on-a-chip model. Adv Healthc Mater. https://doi.org/10.1002/adhm.201801307
Bilal M, Mehmood S, Iqbal HMN (2020) The beast of beauty: environmental and health concerns of toxic components in cosmetics. Cosmetics 7:13. https://doi.org/10.3390/cosmetics7010013
Björklund S, Nowacka A, Bouwstra JA et al (2013) Characterization of stratum corneum molecular dynamics by natural-abundance 13C solid-state NMR. PLoS ONE 8:e61889. https://doi.org/10.1371/journal.pone.0061889
Bogdanowicz P, Haure M-J, Ceruti I et al (2016) Results from in vitro and ex vivo skin aging models assessing the antiglycation and anti-elastase MMP-12 potential of glycylglycine oleamide. Clin Cosmet Investig Dermatol. https://doi.org/10.2147/CCID.S98633
Boncheva M, Damien F, Normand V (2008) Molecular organization of the lipid matrix in intact Stratum corneum using ATR-FTIR spectroscopy. Biochim Biophys Acta - Biomembr 1778:1344–1355. https://doi.org/10.1016/j.bbamem.2008.01.022
Botham PA, Earl LK, Fentem JH et al (1998) Alternative methods for skin irritation testing: the current status. Altern Lab Anim 26:195–211. https://doi.org/10.1177/026119299802600205
Buisson AC, Zahm JM, Polette M et al (1996) Gelatinase B is involved in the in vitro wound repair of human respiratory epithelium. J Cell Physiol. https://doi.org/10.1002/(SICI)1097-4652(199602)166:2%3c413::AID-JCP20%3e3.0.CO;2-A
Butler PD, Ly DP, Longaker MT, Yang GP (2008) Use of organotypic coculture to study keloid biology. Am J Surg. https://doi.org/10.1016/j.amjsurg.2007.10.003
Byrd AL, Belkaid Y, Segre JA (2018) The human skin microbiome. Nat Rev Microbiol 16:143–155. https://doi.org/10.1038/nrmicro.2017.157
Calderon M, Lawrence WT, Banes AJ (1996) Increased proliferation in keloid fibroblasts wounded in vitro. J Surg Res. https://doi.org/10.1006/jsre.1996.0127
Capallere C, Plaza C, Meyrignac C et al (2018) Property characterization of reconstructed human epidermis equivalents, and performance as a skin irritation model. Toxicol In Vitro. https://doi.org/10.1016/j.tiv.2018.07.005
Cha D, O’Brien P, O’Toole EA et al (1996) Enhanced modulation of keratinocyte motility by transforming growth factor-α (TGF-α) relative to epidermal growth factor (EGF). J Invest Dermatol. https://doi.org/10.1111/1523-1747.ep12345083
Chaudhuri RK, Bojanowski K (2017) Improvement of hydration and epidermal barrier function in human skin by a novel compound isosorbide dicaprylate. Int J Cosmet Sci. https://doi.org/10.1111/ics.12405
Chen L, Li N, Liu Y et al (2020) A new 3D model for genotoxicity assessment: EpiSkinTM micronucleus assay. Mutagenesis. https://doi.org/10.1093/mutage/geaa003
Chiu LL, Sun CH, Yeh AT et al (2005) Photodynamic therapy on keloid fibroblasts in tissue-engineered keratinocyte-fibroblast co-culture. Lasers Surg Med. https://doi.org/10.1002/lsm.20213
Cho H, Won CH, Chang SE et al (2013) Usefulness and limitations of skin explants to assess laser treatment. Med Lasers 2:58–63. https://doi.org/10.25289/ML.2013.2.2.58
Choi E, Kang YG, Hwang SH et al (2019) In vitro effects of dehydrotrametenolic acid on skin barrier function. Molecules. https://doi.org/10.3390/molecules24244583
Cotovio J, Onno L, Justine P et al (2001) Generation of oxidative stress in human cutaneous models following in vitro ozone exposure. Toxicol In Vitro 15:357–362. https://doi.org/10.1016/S0887-2333(01)00036-4
Davies DJ, Heylings JR, McCarthy TJ, Correa CM (2015) Development of an in vitro model for studying the penetration of chemicals through compromised skin. Toxicol In Vitro. https://doi.org/10.1016/j.tiv.2014.09.012
Davies DJ, Heylings JR, Gayes H et al (2017) Further development of an in vitro model for studying the penetration of chemicals through compromised skin. Toxicol In Vitro. https://doi.org/10.1016/j.tiv.2016.10.004
Damiani E, Brugè F, Cirilli I et al (2018) Modulation of oxidative status by normoxia and hypoxia on cultures of human dermal fibroblasts: how does it affect cell aging? Oxid Med Cell Longev. https://doi.org/10.1155/2018/5469159
Del Carmen Velazquez Pereda M, de Campos Dieamant G, Eberlin S et al (2009) Effect of green Coffea arabica L. seed oil on extracellular matrix components and water-channel expression in in vitro and ex vivo human skin models. J Cosmet Dermatol 8:56–62. https://doi.org/10.1111/j.1473-2165.2009.00425.x
Demirovic D, Rattan SIS (2011) Curcumin induces stress response and hormetically modulates wound healing ability of human skin fibroblasts undergoing ageing in vitro. Biogerontology 12:437–444. https://doi.org/10.1007/s10522-011-9326-7
Diekmann J, Alili L, Scholz O et al (2016) A three-dimensional skin equivalent reflecting some aspects of in vivo aged skin. Exp Dermatol. https://doi.org/10.1111/exd.12866
Driskell RR, Lichtenberger BM, Hoste E et al (2013) Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature 504:277–281. https://doi.org/10.1038/nature12783
Duan W, Qiao S, Zhuo M et al (2020) Multifunctional platforms: metal-organic frameworks for cutaneous and cosmetic treatment. Chem. https://doi.org/10.1016/j.chempr.2020.11.018
Duval C, Schmidt R, Regnier M et al (2003) The use of reconstructed human skin to evaluate UV-induced modifications and sunscreen efficacy. Exp Dermatol. https://doi.org/10.1034/j.1600-0625.12.s2.10.x
Duval C, Cohen C, Chagnoleau C et al (2014) Key regulatory role of dermal fibroblasts in pigmentation as demonstrated using a reconstructed skin model: impact of photo-aging. PLoS ONE 9:e114182. https://doi.org/10.1371/journal.pone.0114182
Eckhart L, Zeeuwen PLJM (2018) The skin barrier: epidermis vs environment. Exp Dermatol 27:805–806. https://doi.org/10.1111/exd.13731
Elias PM (2007) The skin barrier as an innate immune element. Semin Immunopathol. https://doi.org/10.1007/s00281-007-0060-9
Farage MA, Miller KW, Elsner P, Maibach HI (2008) Intrinsic and extrinsic factors in skin ageing: a review. Int J Cosmet Sci 30:87–95. https://doi.org/10.1111/j.1468-2494.2007.00415.x
Flanagan M (2013) Wound healing and skin integrity. Wiley, Hoboken
Ghadially R, Reed JT, Elias PM (1996) Stratum corneum structure and function correlates with phenotype in psoriasis. J Invest Dermatol. https://doi.org/10.1111/1523-1747.ep12582813
Ghaffari A, Kilani RT, Ghahary A (2009) Keratinocyte-conditioned media regulate collagen expression in dermal fibroblasts. J Invest Dermatol. https://doi.org/10.1038/jid.2008.253
Gottrup F, Ågren MS, Karlsmark T (2000) Models for use in wound healing research: a survey focusing on in vitro and in vivo adult soft tissue. Wound Repair Regen 8:83–96
Green H, Kehinde O, Thomas J (1979) Growth of cultured human epidermal cells into multiple epithelia suitable for grafting. Proc Natl Acad Sci USA. https://doi.org/10.1073/pnas.76.11.5665
Grice E (2014) The skin microbiome: potential for novel diagnostic and therapeutic approaches to cutaneous disease. Semin Cutan Med Surg 33:98–103. https://doi.org/10.12788/j.sder.0087
Grice EA, Segre JA (2011) The skin microbiome. Nat Rev Microbiol 9:244–253. https://doi.org/10.1038/nrmicro2537
Gruber F, Kremslehner C, Eckhart L, Tschachler E (2020) Cell aging and cellular senescence in skin aging—Recent advances in fibroblast and keratinocyte biology. Exp Gerontol 130:110780. https://doi.org/10.1016/j.exger.2019.110780
Gu Y, Han J, Jiang C, Zhang Y (2020) Biomarkers, oxidative stress and autophagy in skin aging. Ageing Res Rev 59:101036. https://doi.org/10.1016/j.arr.2020.101036
Haensel D, Dai X (2018) Epithelial-to-mesenchymal transition in cutaneous wound healing: where we are and where we are heading. Dev Dyn. https://doi.org/10.1002/dvdy.24561
Henemyre-Harris CL, Adkins AL, Chuang AH, Graham JS (2008) Addition of epidermal growth factor improves the rate of sulfur mustard wound healing in an in vitro model. Eplasty 8:e16
Horii I, Nakayama Y, Obata M, Tagami H (1989) Stratum corneum hydration and amino acid content in xerotic skin. Br J Dermatol. https://doi.org/10.1111/j.1365-2133.1989.tb08190.x
Iyer K, Chen Z, Ganapa T et al (2018) Keratinocyte migration in a three-dimensional in vitro wound healing model co-cultured with fibroblasts. Tissue Eng Regen Med. https://doi.org/10.1007/s13770-018-0145-7
Jenkins G (2002) Molecular mechanisms of skin ageing. Mech Ageing Dev 123:801–810. https://doi.org/10.1016/S0047-6374(01)00425-0
Juliano C, Magrini G (2017) Cosmetic ingredients as emerging pollutants of environmental and health concern. A mini-review. Cosmetics 4:11. https://doi.org/10.3390/cosmetics4020011
Kammeyer A, Luiten RM (2015) Oxidation events and skin aging. Ageing Res Rev 21:16–29. https://doi.org/10.1016/j.arr.2015.01.001
Karapetsas A, Voulgaridou G-P, Konialis M et al (2019) Propolis extracts inhibit UV-induced photodamage in human experimental in vitro skin models. Antioxidants 8:125. https://doi.org/10.3390/antiox8050125
Kim WS, Lee JS, Bae GY et al (2013) Extract of Aneilema keisak inhibits transforming growth factor-β-dependent signalling by inducing Smad2 downregulation in keloid fibroblasts. Exp Dermatol. https://doi.org/10.1111/exd.12063
Kim HS, Sun X, Lee JH et al (2019) Advanced drug delivery systems and artificial skin grafts for skin wound healing. Adv Drug Deliv Rev. https://doi.org/10.1016/j.addr.2018.12.014
Knazek RA, Gullino PM, Kohler PO, Dedrick RL (1972) Cell culture on artificial capillaries: an approach to tissue growth in vitro. Science. https://doi.org/10.1126/science.178.4056.65
Kong HH, Oh J, Deming C et al (2012) Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res 22:850–859. https://doi.org/10.1101/gr.131029.111
Kostyuk V, Potapovich A, Albuhaydar AR et al (2018) Natural substances for prevention of skin photoaging: screening systems in the development of sunscreen and rejuvenation cosmetics. Rejuvenation Res. https://doi.org/10.1089/rej.2017.1931
Kovacs D, Cardinali G, Aspite N et al (2010) Role of fibroblast-derived growth factors in regulating hyperpigmentation of solar lentigo. Br J Dermatol. https://doi.org/10.1111/j.1365-2133.2010.09946.x
Lago JC, Puzzi MB (2019) The effect of aging in primary human dermal fibroblasts. PLoS ONE 14:e0219165. https://doi.org/10.1371/journal.pone.0219165
Lai-Cheong JE, McGrath JA (2017) Structure and function of skin, hair and nails. Medicine (Baltimore) 45:347–351. https://doi.org/10.1016/j.mpmed.2017.03.004
Lebonvallet N, Jeanmaire C, Danoux L et al (2010) The evolution and use of skin explants: potential and limitations for dermatological research. Eur J Dermatol 20:671–684. https://doi.org/10.1684/ejd.2010.1054
Lee CM, Watson REB, Kleyn CE (2020a) The impact of perceived stress on skin ageing. J Eur Acad Dermatol Venereol 34:54–58. https://doi.org/10.1111/jdv.15865
Lee JY, Lee J, Min D et al (2020b) Tyrosinase-targeting gallacetophenone inhibits melanogenesis in melanocytes and human skin-equivalents. Int J Mol Sci. https://doi.org/10.3390/ijms21093144
Letsiou S, Bakea A, Le GG et al (2020a) Marine fungus Aspergillus chevalieri TM2-S6 extract protects skin fibroblasts from oxidative stress. Mar Drugs 18:460. https://doi.org/10.3390/md18090460
Letsiou S, Bakea A, Holefors A, Rembiesa J (2020b) In vitro protective effects of Paeonia mascula subsp. hellenica callus extract on human keratinocytes. Sci Rep 10:19213. https://doi.org/10.1038/s41598-020-76169-0
Letsiou S, Bakea A, Le Goff G et al (2020c) In vitro protective effects of marine-derived Aspergillus puulaauensis TM124-S4 extract on H2O2-stressed primary human fibroblasts. Toxicol In Vitro 66:104869. https://doi.org/10.1016/j.tiv.2020.104869
Letsiou S, Félix RC, Cardoso JCR et al (2020d) Cartilage acidic protein 1 promotes increased cell viability, cell proliferation and energy metabolism in primary human dermal fibroblasts. Biochimie 171–172:72–78. https://doi.org/10.1016/j.biochi.2020.02.008
Letsiou S, Kapazoglou A, Tsaftaris A (2020e) Transcriptional and epigenetic effects of Vitis vinifera L. leaf extract on UV-stressed human dermal fibroblasts. Mol Biol Rep 47:5763–5772. https://doi.org/10.1007/s11033-020-05645-7
Letsiou S, Kalliampakou K, Gardikis K et al (2017) Skin protective effects of nannochloropsis gaditana extract on H2O2-stressed human dermal fibroblasts. Front Mar Sci. https://doi.org/10.3389/fmars.2017.00221
Li Z, Bai X, Peng T et al (2020) New insights into the skin microbial communities and skin aging. Front Microbiol. https://doi.org/10.3389/fmicb.2020.565549
Liebsch M, Döring B, Donelly TA et al (1995) Application of the human dermal model skin2 ZK 1350 to phototoxicity and skin corrosivity testing. Toxicol In Vitro. https://doi.org/10.1016/0887-2333(95)00042-7
Líšková A (2020) Evaluation of phototoxic and cytotoxic potential of TiO2 nanosheets in a 3D reconstructed human skin model. Altex. https://doi.org/10.14573/altex.1910012
López-Otín C, Blasco MA, Partridge L et al (2013) The hallmarks of aging. Cell 153:1194–1217. https://doi.org/10.1016/j.cell.2013.05.039
Löwenau LJ, Zoschke C, Brodwolf R et al (2017) Increased permeability of reconstructed human epidermis from UVB-irradiated keratinocytes. Eur J Pharm Biopharm 116:149–154. https://doi.org/10.1016/j.ejpb.2016.12.017
Maeno K (2019) Direct quantification of natural moisturizing factors in stratum corneum using direct analysis in real time mass spectrometry with inkjet-printing technique. Sci Rep. https://doi.org/10.1038/s41598-019-54454-x
Mainzer C, Remoué N, Molinari J et al (2018) In vitro epidermis model mimicking IGF-1–specific age-related decline. Exp Dermatol. https://doi.org/10.1111/exd.13547
Martin P (1997) Wound healing—aiming for perfect skin regeneration. Science. https://doi.org/10.1126/science.276.5309.75
Mojumdar EH, Pham QD, Topgaard D, Sparr E (2017) Skin hydration: interplay between molecular dynamics, structure and water uptake in the stratum corneum. Sci Rep 7:15712. https://doi.org/10.1038/s41598-017-15921-5
Murai M, Tsuji G, Hashimoto-Hachiya A et al (2018) An endogenous tryptophan photo-product, FICZ, is potentially involved in photo-aging by reducing TGF-β-regulated collagen homeostasis. J Dermatol Sci 89:19–26. https://doi.org/10.1016/j.jdermsci.2017.10.002
Nakamura M, Haarmann-Stemmann T, Krutmann J, Morita A (2018) Alternative test models for skin ageing research. Exp Dermatol. https://doi.org/10.1111/exd.13519
Nayak S, Dey S, Kundu SC (2013) Skin equivalent tissue-engineered construct: co-cultured fibroblasts/keratinocytes on 3D matrices of sericin hope cocoons. PLoS ONE. https://doi.org/10.1371/journal.pone.0074779
Neil JE, Brown MB, Williams AC (2020) Human skin explant model for the investigation of topical therapeutics. Sci Rep 10:21192. https://doi.org/10.1038/s41598-020-78292-4
Netzlaff F, Lehr C-M, Wertz PW, Schaefer UF (2005) The human epidermis models EpiSkin®, SkinEthic® and EpiDerm®: an evaluation of morphology and their suitability for testing phototoxicity, irritancy, corrosivity, and substance transport. Eur J Pharm Biopharm 60:167–178. https://doi.org/10.1016/j.ejpb.2005.03.004
Newton VL, Mcconnell JC, Hibbert SA et al (2015) Skin aging: molecular pathology, dermal remodelling and the imaging revolution. G Ital Dermatol Venereol 150:665–674
Olesen CM, Fuchs CSK, Philipsen PA et al (2019) Advancement through epidermis using tape stripping technique and reflectance confocal microscopy. Sci Rep 9:12217. https://doi.org/10.1038/s41598-019-48698-w
Pageon H, Zucchi H, Rousset F et al (2017) Glycation stimulates cutaneous monocyte differentiation in reconstructed skin in vitro. Mech Ageing Dev. https://doi.org/10.1016/j.mad.2017.02.001
Paige DG, Morse-Fisher N, Harper JI (1994) Quantification of stratum corneum ceramides and lipid envelope ceramides in the hereditary ichthyoses. Br J Dermatol. https://doi.org/10.1111/j.1365-2133.1994.tb08452.x
Park G-H, Chang SE, Bang S et al (2015) Usefulness of skin explants for histologic analysis after fractional photothermolysis. Ann Dermatol 27:283. https://doi.org/10.5021/ad.2015.27.3.283
Park DJ, Jeon G, Bang SH et al (2020) Cellular lysosomes’ activity for melanin reduction on artificial skin tissue. Mol Biotechnol. https://doi.org/10.1007/s12033-019-00235-w
Pastar I, Liang L, Sawaya AP et al (2017) Preclinical models for wound-healing studies. Skin tissue models. Elsevier, Amsterdam
Peter Jorgensen SRI (2014) Extracellular matrix modulates morphology, growth, oxidative stress response and functionality of human skin fibroblasts during aging in vitro. J Aging Sci. https://doi.org/10.4172/2329-8847.1000122
Pfuhler S, Pirow R, Downs TR et al (2020) Validation of the 3D reconstructed human skin comet assay, an animal-free alternative for following-up positive results from standard in vitro genotoxicity assays. Mutagenesis. https://doi.org/10.1093/mutage/geaa009
Pittayapruek P, Meephansan J, Prapapan O et al (2016) Role of matrix metalloproteinases in photoaging and photocarcinogenesis. Int J Mol Sci. https://doi.org/10.3390/ijms17060868
Poumay Y, Coquette A (2006) Modelling the human epidermis in vitro: tools for basic and applied research. Arch Dermatol Res 298:361–369. https://doi.org/10.1007/s00403-006-0709-6
Prescott SL, Larcombe D-L, Logan AC et al (2017) The skin microbiome: impact of modern environments on skin ecology, barrier integrity, and systemic immune programming. World Allergy Organ J 10:29. https://doi.org/10.1186/s40413-017-0160-5
Proksch E, Brandner JM, Jensen J-M (2008) The skin: an indispensable barrier. Exp Dermatol 17:1063–1072. https://doi.org/10.1111/j.1600-0625.2008.00786.x
Prunieras M (1979) Epidermal cell cultures as models for living epidermis. J Invest Dermatol. https://doi.org/10.1111/1523-1747.ep12556751
Rattan SIS (2015) Aging and Senescence of skin cells in culture. Textbook of aging skin. Springer, Berlin, pp 1–8
Régnier M, Caron D, Reichert U, Schaefer H (1992) Reconstructed human epidermis: a model to study in vitro the barrier function of the skin. Skin Pharmacol Physiol. https://doi.org/10.1159/000211017
Remoué N, Molinari J, Andres E et al (2013) Development of an in vitro model of menopause using primary human dermal fibroblasts. Int J Cosmet Sci. https://doi.org/10.1111/ics.12075
Reus TL, Brohem CA, Schuck DC, Lorencini M (2020) Revisiting the effects of menopause on the skin: functional changes, clinical studies, in vitro models and therapeutic alternatives. Mech Ageing Dev 185:111193. https://doi.org/10.1016/j.mad.2019.111193
Rincón-Fontán M, Rodríguez-López L, Vecino X et al (2020) Potential application of a multifunctional biosurfactant extract obtained from corn as stabilizing agent of vitamin C in cosmetic formulations. Sustain Chem Pharm 16:100248. https://doi.org/10.1016/j.scp.2020.100248
Rinnerthaler M, Bischof J, Streubel M et al (2015) Oxidative stress in aging human skin. Biomolecules 5:545–589. https://doi.org/10.3390/biom5020545
Rittié L, Fisher GJ (2015) Natural and sun-induced aging of human skin. Cold Spring Harb Perspect Med. https://doi.org/10.1101/cshperspect.a015370
Rocha MA, Bagatin E (2018) Skin barrier and microbiome in acne. Arch Dermatol Res 310:181–185. https://doi.org/10.1007/s00403-017-1795-3
Rognoni E, Watt FM (2018) Skin cell heterogeneity in development, wound healing, and cancer. Trends Cell Biol 28:709–722. https://doi.org/10.1016/j.tcb.2018.05.002
Sami DG, Heiba HH, Abdellatif A (2019) Wound healing models: a systematic review of animal and non-animal models. Wound Med 24:8–17. https://doi.org/10.1016/j.wndm.2018.12.001
Schommer NN, Gallo RL (2013) Structure and function of the human skin microbiome. Trends Microbiol 21:660–668. https://doi.org/10.1016/j.tim.2013.10.001
Shibagaki N, Suda W, Clavaud C et al (2017) Aging-related changes in the diversity of women’s skin microbiomes associated with oral bacteria. Sci Rep 7:10567. https://doi.org/10.1038/s41598-017-10834-9
Sugiyama Y, Yamazaki K, Kusaka-Kikushima A et al (2014) Analysis of aquaporin 9 expression in human epidermis and cultured keratinocytes. FEBS Open Bio. https://doi.org/10.1016/j.fob.2014.06.004
Sukseree S, Bergmann S, Pajdzik K et al (2018a) Suppression of epithelial autophagy compromises the homeostasis of sweat glands during aging. J Invest Dermatol 138:2061–2063. https://doi.org/10.1016/j.jid.2018.03.1502
Sukseree S, Bergmann S, Pajdzik K et al (2018b) Suppression of autophagy perturbs turnover of sequestosome-1/p62 in Merkel cells but not in keratinocytes. J Dermatol Sci 90:209–211. https://doi.org/10.1016/j.jdermsci.2018.01.008
Sundaram H, Mackiewicz N, Burton E et al (2016) Pilot comparative study of the topical action of a novel, crosslinked resilient hyaluronic acid on skin hydration and barrier function in a dynamic, three-dimensional human explant model. J Drugs Dermatol 15:434–441
Taylor SC (2005) Photoaging and pigmentary changes of the skin. Cosmetic dermatology. Springer, Berlin, pp 29–51
Tett A, Pasolli E, Farina S et al (2017) Unexplored diversity and strain-level structure of the skin microbiome associated with psoriasis. NPJ Biofilms Microb 3:14. https://doi.org/10.1038/s41522-017-0022-5
Thune P (1989) Evaluation of the hydration and the water-holding capacity in atopic skin and so-called dry skin. Acta Dermato-Venereol 144:133–135
Tigges J, Krutmann J, Fritsche E et al (2014) The hallmarks of fibroblast ageing. Mech Ageing Dev 138:26–44. https://doi.org/10.1016/j.mad.2014.03.004
Torricelli P, Fini M, Fanti PA et al (2017) Protective effects of polypodium leucotomos extract against UVB-induced damage in a model of reconstructed human epidermis. Photodermatol Photoimmunol Photomed 33:156–163. https://doi.org/10.1111/phpp.12297
Ud-Din S, Bayat A (2017a) Non-animal models of wound healing in cutaneous repair: in silico, in vitro, ex vivo, and in vivo models of wounds and scars in human skin. Wound Repair Regen. https://doi.org/10.1111/wrr.12513
Ud-Din S, Bayat A (2017b) Non-animal models of wound healing in cutaneous repair: in silico, in vitro, ex vivo, and in vivo models of wounds and scars in human skin. Wound Repair Regen 25:164–176. https://doi.org/10.1111/wrr.12513
Van Den Broek LJ, Niessen FB, Scheper RJ, Gibbs S (2012) Development, validation, and testing of a human tissue engineered hypertrophic scar model. Altex. https://doi.org/10.14573/altex.2012.4.389
Van Wezel AL (1967) Growth of cell-strains and primary cells on micro-carriers in homogeneous culture. Nature. https://doi.org/10.1038/216064a0
Verdier-Sévrain S, Bonté F (2007) Skin hydration: a review on its molecular mechanisms. JCosmet Dermatol. https://doi.org/10.1111/j.1473-2165.2007.00300.x
Vita NA, Brohem CA, Canavez ADPM et al (2018) Parameters for assessing the aquatic environmental impact of cosmetic products. Toxicol Lett 287:70–82. https://doi.org/10.1016/j.toxlet.2018.01.015
Vostálová J, Cukr M, Zálešák B et al (2018) Comparison of various methods to analyse toxic effects in human skin explants: rediscovery of TTC assay. J Photochem Photobiol B 178:530–536. https://doi.org/10.1016/j.jphotobiol.2017.12.011
Wang PH, Huang BS, Horng HC et al (2018) Wound healing. J Chin Med Assoc. https://doi.org/10.1016/j.jcma.2017.11.002
Watanabe M, Tagami H, Horii I et al (1991) Functional analyses of the superficial stratum corneum in atopic xerosis. Arch Dermatol. https://doi.org/10.1001/archderm.1991.01680100089010
Xie Y, Rizzi SC, Dawson R et al (2010) Development of a three-dimensional human skin equivalent wound model for investigating novel wound healing therapies. Tissue Eng C. https://doi.org/10.1089/ten.tec.2009.0725
Xu W, Jong Hong S, Jia S et al (2012) Application of a partial-thickness human ex vivo skin culture model in cutaneous wound healing study. Lab Investig 92:584–599. https://doi.org/10.1038/labinvest.2011.184
Yoshimoto S, Yoshida M, Ando H, Ichihashi M (2018) Establishment of photoaging in vitro by repetitive UVA irradiation: induction of characteristic markers of senescence and its prevention by PAPLAL with potent catalase activity. Photochem Photobiol. https://doi.org/10.1111/php.12871
Yousuf Y, Amini-Nik S, Jeschke MG (2018) Overall perspective on the clinical importance of skin models. Skin tissue models for regenerative medicine. Elsevier, Amsterdam, pp 39–54
Zeitoun H, Michael-Jubeli R, El Khoury R et al (2020) Skin lightening effect of natural extracts coming from Senegal botanical biodiversity. Int J Dermatol. https://doi.org/10.1111/ijd.14699
Zhai W, Huang Y, Zhang X et al (2018) Profile of the skin microbiota in a healthy Chinese population. J Dermatol 45:1289–1300. https://doi.org/10.1111/1346-8138.14594
Funding
This study was supported by APIVITA SA.
Author information
Authors and Affiliations
Contributions
Sophia Letsiou conceived, planned and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Letsiou, S. Tracing skin aging process: a mini- review of in vitro approaches. Biogerontology 22, 261–272 (2021). https://doi.org/10.1007/s10522-021-09916-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10522-021-09916-z