Abstract
All eukaryotes have lysosomes which contain hydrolytic enzymes such as protease to degrade waste materials and cellular fragments. As a cellular organelle, lysosomes function as the digestive system of the cell, serving both to degrade material taken up from outside the cell and to digest obsolete components of the cell itself. Conversely, melanin has photochemical functions to protect tissue from the harmful effects of ultraviolet rays. However, too much of melanin leads to problems such as hyperpigmentation, requiring materials to maintain and control the amount of melanin. In this study, we found evidence of correlation between lysosome and melanin in a new eco-friendly material, MelanoDerm, a reconstituted 3D human skin model containing normal melanocytes and keratinocytes. Melanin content assay and cell viability were measured, using 2% kojic acid as positive control, while MelanoDerm was exposed to various concentrations of lysosome. Our results indicate that lysosome may be a useful cosmetic agent for the treatment of hyperpigmentation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lysosomes are cellular organelles that contain acidic enzymes to break down waste materials and cellular debris in all eukaryotes [1, 2]. Lysosomes were reported to contain about 50–60 enzymes and play an important role in a number of biological processes [3]. The degradation pathway of lysosomes generally takes place by endocytosis which provides extracellular materials for digestion [4]. On the other hand, melanin is a multifunctional biopolymer which is the end product of multistep transformations of L-tyrosine. Melanin pigments appear as colored stains in the skin, eyes, and hair and consist of many units linked by carbon–carbon bonds [5]. Melanin is produced from melanosome in melanocytes on the skin, which are found in the basal layer of the epidermis. Also, melanosomes are reported to be specialized organelles as they are lysosome-related organelles [6]. Furthermore, amount of melanin was increased upon sun exposure for protecting the skin against harmful ultraviolet rays [5]. However, this situation causes skin problems such as skin cancer or hyperpigmentation in human [7].
There have been previous research reports regarding lysosome and melanin correlation [6, 8]. Lysosome and melanosome are produced following rough endoplasmic reticulum (RER) structures and vesicles and channels of the trans-Golgi Network (TGN) [9,10,11]. Other research reports demonstrate reduction of melanin by lysosomes [12, 13] and the subsequent melanin reductions that may aid in the treatment of hyperpigmentation or skin cancer. In previous studies, we discussed about melanin reduction by lysosome and analyzed activating lysosomal proteins by melanin treatment [14,15,16]. It means that melanin content is influenced by exposing lysosome and lysosomal activity increased after cells were exposed to a melanin solution.
In this study, we examined the lysosome application in an artificial human skin tissue, MelanoDerm. MelanoDerm was treated with lysosome for 16 days to evaluate the efficiency of melanin reduction by lysosomal function. Additionally, we explored the oxidative stress by hydrogen peroxide (H2O2) before lysosome treatment to further enhance lysosomal function [17]. After lysosomes were exposed to H2O2, lysosomal function increased to reduce melanin amounts. Lysosome appears to be a useful treatment as a cosmetic agent or therapy for hyperpigmentation.
Materials and Methods
Lysosome Isolation from Hen’s Egg White
Lysosomes were isolated from egg white using fraction methods [1, 2]. The yolk was separated from the white component of the eggs and discards. The white component of eggs was released by stirrer until the viscosity of the egg white decreased significantly. To separate unbroken cells and nuclei and lysosomal fraction, egg white was centrifuged for 5 min at 500×g and for 30 min at 20,000×g. The pellet was used to evaluate for skin whitening test for 16 days.
Skin Whitening Test
For skin whitening test, MelanoDerm (MatTek Corp., Asland, MA) was used as a viable reconstituted three-dimensional human skin equivalent containing normal melanocytes and keratinocytes. The cells are derived from African-American (MEL-B), Asian (MEL-A), or white (MEL-C) donors. MEL-B tissues were used in this study and were maintained in the NMM-113 medium as recommended by the manufacturer [18, 19]. The MelanoDerm tissues were fed every other day with 5 ml of fresh NMM-113 and different quantities such as, 1, 5, 10 and 20%, of lysosome every day for 16 days. Additionally, 2% kojic acid was used as positive control.
Melanin Content Assay
Melanin content was measured following previously reported protocols [20]. The skin tissue was taken out of the culture dish after 16 days. The tissue was placed in an Eppendorf tube and homogenized for 1 min in lysis buffer (20 mM Tris–Cl pH 7.4, 1 mM EDTA pH 8.8, 150 mM, 1 mM EGTA pH 8.5, 1% v/v Triton X-100). After homogenization, 20 μl protease K (5 mg/ml) was added and incubated at 45 ℃ overnight. 20 μl protease K was placed into the sample the next day for 4 h at RT. 500 mM Na-carbonate 50 μl and 30% H2O2 10 μl were added and incubated at 80 ℃ for 30 min. All tissues were significantly degraded in this mixture and 100 μl of a 2 chloroform:1 MtOH mixture was added with centrifugation performed for 10,000×g, 10 min. Supernatant from this sample was used to determine the melanin content by spectrophotometer based on absorbance at 405 nm.
Cell Viability
Cell viability for MelanoDerm was measured following a previously published protocol [18, 19]. After washing twice by DPBS, 300 μl MTT solution was added to the dishes. All tissue samples were covered to protect from light and incubated at 37 ℃ for 3 h. Tissue samples were moved to other new plate after cleaning by sterilized gauze. Isopropanol and 0.04 M HCl were added to the tissue, and samples were stirred at 120 rpm without air in the anaerobic bag at RT for 2 h. All solutions were moved to an Eppendorf tube and measured by ELASA plate reader on 96-well plates based on absorbance at 570 nm.
Histological Analysis
Histological staining of formalin-fixed tissue sections with hematoxylin and eosin (HE) or Masson’s trichrome and immuno-histochemical staining for α-SMA was performed [21]. This analysis was measured following previously published protocols [17, 20, 22]. Tested MelanoDerm samples were fixed with 10% formalin (Sigma-Aldrich) at 4 °C overnight. Skin samples were observed under phase-contrast microscopy. To visualize the melanin distribution throughout the tissue, all paraffin-embedded tissue samples were processed as described previously.
Results
Decreased Melanin Amount on the MelanoDerm
We confirmed the melanin reduction effect of lysosomes in the last study, so we conducted an applied study in this study [21]. A single membrane surrounds the lysosome, with lysosomal enzyme contained within the lysosome organelle. While we have previously verified the enzyme ability to decrease melanin, demonstration of in vivo lysosomal function required further examination. MelanoDerm were cultured using manufacturer’s protocol and treated with lysosome and lysosomal enzymes. Two methods were used: (1) lysosomes treated once at the first day and cultured for 16 days and (2) lysosomes treated every day for 16 days. Table 1 shows the melanin amount detected and the microscopic images of the artificial tissue after treatment with lysosome and lysosomal enzymes at different concentrations.
Table 1 shows the optimal top-view observation of skin tissue among the various conditions of melanin amount and tissue toxicity (shaded gray). In case of lysosome treatment by method 1 (treated once), melanin remained 89.7% during exposure to 5% lysosome on the tissue (Table 1A-shaded gray), whereas melanin amounts were critically decreased until 54.72% during exposure to 5% lysosome by method 2 (treated every day) (Table 1B-shaded gray). It means that method 2 is more effective in decreasing the melanin amount. Additionally, tissue toxicity was also measured after treatment with different concentrations of lysosomes because lysosome treatment should not have toxicity on tissue. No tissue toxicity was determined for the treatment all concentrations of lysosomes.
Alternatively, different concentrations of lysosomal enzyme were used to treat MelanoDerm (Table 1C, D). When 630 μg/μl lysosomal enzymes were used to treat once on the first day, melanin amount measured was 86.6%. To decrease melanin amount further, lysosomal enzyme was needed at high concentration. Furthermore, melanin amount was measured at 63.51% and 58.1% while melanoderm was exposed to lysosomal enzyme 1260 μg/μl and 2520 μg/μl on every day, respectively (Table 1D). However, tissue toxicity increased dramatically compared to method 1 lysosomal enzyme treatment. All concentrations of lysosomal enzyme have toxicity of about 20% to 102%. In fact, lysosomal enzyme appears to be toxic to MelanoDerm, except in the case of 2520 μg/μl lysosomal enzyme treatment. The highest concentration of lysosomal enzyme did not show toxicity to the skin tissue, even as melanin amount decreased. In addition, microscopic images of MelanoDerm after lysosome and lysosomal enzyme treatment, are summarized in Table 1.
Alteration of Appearance by Lysosome Treatment on MelanoDerm for 16 Days
The 5% lysosome concentration showed the optimal melanin reduction on skin tissue (Table 1B). Altered appearance of tissue images was observed after 5% lysosome daily treatment for 16 days, with appearance changing significantly at Day 9 (Fig. 1).
Additionally, we observed the appearance of MelanoDerm by histological analysis. After 16 days, melanin amount decreased by 5% lysosome (Fig. 2). In Fig. 2, the arrows indicate melanin component and we confirmed by microscopic images that melanin amount was decreased.
Increased Activity of Lysosomal Function to Decrease Melanin Amount After Lysosome Exposure to Hydrogen Peroxide (H2O2)
100 μM Hydrogen peroxide (H2O2) is known to increase lysosomal activity [17]. Top-view observation by microscope of the 1, 5, 10, and 20% lysosome treatment of MelanoDerm for 16 days are shown in Table 2. In order to decrease melanin amount, lysosome was prepared in different concentrations and treated for 16 days. Table 2 shows the appearance of skin tissue upon treatment with two different types of lysosomes. When we compared microscopic images of skin tissue treated with normal lysosomes and lysosomes exposed to 100uM H2O2, the latter case appeared to be more effective in decreasing melanin amount over 16 days.
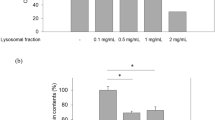
Melanin amounts were measured by protocol and reported in Fig. 3, with kojic acid as a positive control. Melanin levels evaluated after treatment with 1 and 5% showed a decrease of melanin. 1% lysosome not exposed to H2O2 demonstrates 78.3% melanin level but 1% lysosome exposed to H2O2 decreased melanin level to 40.5%. The 5% lysosome concentration not exposed to H2O2 demonstrated a 54.7% melanin level, but 5% lysosome exposed to H2O2 demonstrated a further reduction of melanin to 39.1%. Results showed that when lysosome was exposed to H2O2,, an increased activity of lysosomal function is observed, which decreases melanin.
Melanin amount is compared after treatment of MelanoDerm with normal lysosome and lysosome activated by hydrogen peroxide (H2O2). Both methods of lysosome application decrease melanin amount over 16 days. The lysosome activated by H2O2 is more effective in decreasing melanin amount. During exposure with 1% lysosome, melanin levels decreased about 37.8%, and 5% lysosome works to decrease melanin amount by 15.6%
Discussion
This experiment is extension research of application by lysosome treatment for decreasing melanin amount in skin tissue. Previously, we suggested that lysosome is a useful material for decreasing melanin amount [14,15,16] using lysosomal functions to degrade macromolecules by various enzymes [1, 2, 9, 23]. Lysosome was used to treat commercial artificial skin tissue called “MelanoDerm.” When treated for 16 days, lysosome was demonstrated to decrease melanin on the skin tissue, the microscopic image of which are shown in Table 1.This result shows that 5% lysosome influenced after treated to the skin for daily with no toxicity. When compared between treated once and treated daily, melanin amount is so far different about 35%. High concentrations of lysosome were shown to work slowly on the skin due to agglomeration to form larger, less reactive clusters. These agglomerations are suggested to have difficulty penetrating the skin. Melanin showed decreasing activity with increasing concentrations of lysosomal enzyme, 126, 630, 1260, and 2520 μg/μl. However, lysosomal enzymes showed harmful effects at 20–30% on the skin tissue (Table 1C, D). It means that lysosomal enzyme causes stress to the skin.
Furthermore, Fig. 1 shows artificial skin tissue changing in brightness and melanin decreased over 16 days. During exposure to 5% lysosome for 16 days, the skin tissue did not have harmful effects and melanin amount decreased. Melanin component is slowly decreased during 16 days and we analyzed artificial skin tissue by histological analysis after treatment of 5% lysosome for 16 days. As shown in Fig. 2, the appearance of skin tissue showed decreasing melanin amount on the skin tissue.
In order to activate lysosomal function, lysosome has been exposed to H2O2 [17]. If lysosomal function increases without harmful effects to humans, addition of H2O2 may be an easy way to control melanin amount in the skin. This comparison of normal lysosome and exposed H2O2 condition showed a melanin reduction effect on 1 and 5% lysosome concentrations (Table 2). Normal lysosome reduced melanin amounts from 54.7 to 39.1%, while addition of H2O2 reduces melanin amounts from 78.3 to 40.5% (Fig. 3). In fact, lysosome exposed to H2O2 worked rapidly even at low concentration of lysosome. However, 10% and 20% lysosome did not show effective decrease of melanin amount. High concentration of lysosome is too difficult to handle in order to decrease melanin. Therefore, 5% lysosome is the ideal concentration to reduce melanin amount and H2O2 may be used to significantly activate lysosomal function. Our results suggest that lysosome may be a potential material for cosmeceutical skin lighting and for therapeutic treatment to cure harmful diseases such as hyperpigmentation.
References
Yoon, J., Park, J. M., Jung, S. K., Kim, Y. H., & Min, J. (2009). Characterization of antimicrobial activity of the lysosomes isolated from Saccharomyces cerevisiae. Current Microbiology, 59, 48–52.
Yoon, J., Park, J. M., Kim, K. J., Kim, Y. H., & Min, J. (2009). Antimicrobial activity of the cell organelles, lysosomes, isolated from egg white. Journal of Microbiology Biotechnology, 19, 1364–1368.
Anderson, R. R., & Parrish, J. A. (1981). The optics of human skin. Journal of Investigative Dermatology, 77, 13–19.
Marusa, H. C., Katarina, P., Ales, S., & Boris, T. (2012). Lysosomal pathways to cell death and their therapeutic applications. Experimental Cell Research, 318, 1245–1251.
Andrzej, S., Desmond, J. T., Shigeki, S., & Jacobo, W. (2004). Melanin pigmentation in mammalian skin and its hormonal regulation. Physiological Reviews, 84, 1155–1228.
Seth, J. O. (1995). Melanosomes are specialized members of the lysosomal lineage of organelles. Journal of Investigative Dermatology, 105, 3–7.
Yochida, Y., Hachiya, A., Sriwiriyanont, P., Ohuchi, A., Kitahara, T., Takema, Y., et al. (2007). Functional analysis of keratinocytes in skin color using a human skin substitute model composed of cells derived from different skin pigmentation types. FASEB Journal, 21, 2829–2839.
Klaus, W., & Herbert, H. (1972). Are melanosome complexes lysosomes? Journal of Investigative Dermatology, 59, 170–176.
Michael, S. M., & Miguel, C. S. (2001). The melanosome: Membrane dynamics in black and white. Molecular Cell Biology, 2, 738–748.
Raposo, G., Tenza, D., Murphy, D. M., Derson, J. F., & Marks, M. S. (2001). Distinct protein sorting and localization to premelanosomes, melanosomes and lysosomes in pigmented melanocytic cells. The Journal of Cell Biology, 152, 809–823.
Anthony, J. T., & Alison, G. (1998). Does α-MSH have a role in regulating skin pigmentation in human? Pigment Cell Research, 11, 265–274.
Jan, B., & Milan, E. (2003). Melanosome degradation: Fact or fiction. Pigment Cell Research, 16, 280–286.
Ohtaki, N., & Seiji, M. (1971). Degradation of mlanosomes by lysosomes. Journal of Investigative Dermatology, 57, 1–5.
Park, D. J., Sekhon, S. S., Yoon, J., Kim, Y. H., & Min, J. (2016). Color reduction of melanin by lysosomal and peroxisomal enzymes isolated from mammalian cells. Molecular and Cellular Biochemistry, 413, 119–125.
Park, D. J., Sekhon, S. S., Ahn, J. Y., Yoon, H., Ko, J. H., Lee, L., et al. (2016). Proteomic analysis of lysosomal proteins in Melanocyte B16F10 exposed to Melanin. Toxicology and Environmental Health Sciences, 8, 7–11.
Park, D. J., Sekhon, S. S., Ahn, J. Y., Yoon, H., Lee, L., Ko, J. H., et al. (2016). Different expression patterns of lysosomal proteins exposed to Melanin in HeLa cells. Toxicology and Environmental Health Sciences, 7, 1–5.
Yoon, J., Bang, S. H., Park, J. S., Chang, S. T., Kim, Y. H., & Min, J. (2011). Increased in vitro lysosomal function in oxidative stress-induced cell line. Applied Biochemistry and Biotechnology, 163, 1002–1011.
Fischer, A. H., Jacobson, K. A., Rose, J., & Zeller, R. (2008). Hematoxylinand eosin staining of tissue and cell sections. Cold Spring Harbor Protocols. https://doi.org/10.1101/pdb.prot4986.
Claudine, T., Patrice, C., Helene, A., Thierry, L., & Nathalie, A. A. (2006). Lysosomes and lysosomal proteins in cancer cell death. Biochimica et biophysica Acta, 1765, 101–125.
Li, N. K., Tong, C. X., Chen, G., Brindzei, N., & Orlow, S. J. (2008). Identification of quinolones that inhibit melanogenesis by altering tyrosinase family trafficking. Molecular Pharmacology, 74, 1576–1586.
Ni, X., Canuel, M., & Morales, C. R. (2006). The sorting and trafficking of lysosomal proteins. Histology and Histopathology, 21, 899–913.
Yoon, T. J., Lei, T. C., Yamaguchi, Y., Wolber, R., & Hearing, V. J. (2003). Reconstituted 3-dimensional human skin of various ethnic origins as a vitro model for studies of pigmentation. Analytical Biochemistry, 318, 260–269.
Bessou-Touya, S., Picardo, M., Maresca, M., Surlève-Bazeille, J., Catherine, P., & Alain, T. (1998). Chimeric human epidermal reconstructs to study the role of melanocytes and keratinocytes in pigmentation and photoprotection. Journal of Investigative Dermatology, 111, 1103–1108.
Acknowledgements
This research was supported by the Ministry of Trade, Industry & Energy (MOTIE) and Korea Institute for Advancement of Technology (KIAT) through the Encouragement Program (P0006145) for The Industries of Economic Cooperation Region.
Author information
Authors and Affiliations
Contributions
DJP, GJ, SHB, YHK, and JM designed the experiments and analyzed the results. SYK analyzed the experimental data. JHW conducted the artificial tissue analysis. All authors contributed to writing of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declared that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Park, D.J., Jeon, G., Bang, S.H. et al. Cellular Lysosomes’ Activity for Melanin Reduction on Artificial Skin Tissue. Mol Biotechnol 62, 185–191 (2020). https://doi.org/10.1007/s12033-019-00235-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-019-00235-w