Abstract
Skin aging is attributed to extrinsic and intrinsic factors which intersect, leading to macro- and microscopic alterations. Histologically, there is a loss of dermal collagen and decreased lipid production, leading to a thinning of the skin. This process is exacerbated by the effect of cumulative sun exposure and oxidative damage caused by pollution, stress, and smoking. These changes manifest as wrinkles, loss of elasticity, dryness, and texture changes in mature skin.
Based on this scenario and considering the growing search for reduction of the effects of time, cosmeceuticals appeared. Cosmeceuticals are topical products which, when coming into contact with the skin, can cause structural and/or functional changes. Not intended for therapeutic use, cosmeceuticals work to prevent and are not exclusively limited to beautification. Cosmeceuticals are still the most popular option to improve the appearance of the skin and counteract the effects of aging. Although there are many examples of mere cosmetic effect, successful manufacturers of cosmeceuticals have been doing research on the aging process, and the knowledge thus acquired has been converted into formulations that may, from a cellular point of view, make a difference.
In this chapter, we present to you several classes of cosmeceuticals and discuss how these may work on the skin in the postmenopause phase.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Estrogen Receptor
- Postmenopausal Woman
- Androgen Receptor
- Luteinizing Hormone
- Hormone Replacement Therapy
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 The Postmenopause
On average, women live about 30 years beyond postmenopause. Menopause is defined as the period of 12 months of amenorrhea after the last menstrual cycle. About 60–70 % of the visits to general practitioners and specialists are of people over the age of 60, mostly women [1]. Thus, hormonal influence in the skin aging process has drawn more and more attention. Several functions of the skin are known to be hormone dependent and, although the effects of estrogen on skin are not fully understood, the decline in estrogen is known to be associated with various skin disorders, many of which can be reversed through hormone replacement treatment [2, 3].
Studies involving postmenopausal women indicate that the decline in estrogen is associated with atrophy, fine wrinkles, scarring, and hot flushes. Other incidences may be the thinning of the epidermis, decrease of dermal collagen, sagging, and impaired wound healing [3]. The decline in estrogen reduces the mitotic activity of the basal layer of the epidermis and also modifies its lipid synthesis, causing xerosis [4]. Hormone replacement therapy (HRT) has beneficial effects on some of the lost properties of the skin and can slowdown the intrinsic aging process. The phenomenon of hormone-related skin aging has been demonstrated; however, to distinguish it from the effects of senescence, photoaging, and genetic and environmental aggressions is difficult [5]. It is our objective to present to you in this chapter some of the most important changes.
2 The Skin
The skin is the largest organ in the body, accounting for approximately 15 % of the total body weight of a human adult. It performs several vital functions to protect the body against environmental aggressions. This is made possible owing to the skin’s elaborate structure, the ectodermal and mesodermal origin of its tissues, and its organization in three layers: the epidermis (and its appendages), the dermis, and the hypodermis [6].
Most dermal fibers (over 90 %) are made of interstitial collagen, mainly types I and III. The collagen fibers are responsible for the mechanical strength of the skin. Collagen is responsible for 98 % of the total dry weight of the dermis. On the other hand, the elastic fibers, which are responsible for the shrinking properties of the skin, are composed of elastin, an insoluble protein which is surrounded by a variable number of microfibrils. Viewed under electronic microscope, the elastic fibers show variations depending on age and exposure study area (whether exposed to the sun or not). The reticulin fibers consist of a group of biochemically fine collagen type I and III fibers, as well as fibronectin [6].
The effects of estrogen deficiency in postmenopausal women include: atrophy, decrease in collagen content and hydration, reduction in sebaceous secretions, loss of elasticity, and manifestations of hyperandrogenism (Fig. 2.1). The cumulative effects of estrogen deficiency contribute to poor wound healing in older patients and accelerated skin aging; however, it is difficult to distinguish between changes that occur specifically with age and those that occur as a result of estrogen deprivation [7].
3 Estrogen
Estrogen, the main steroid responsible for the secondary gender characteristics in females, affects most systems in the body. The skin is the largest nonreproductive estrogen target area [3].
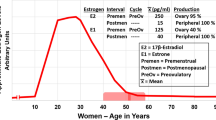
Estrogen production is regulated by the axle involving the hypothalamus, pituitary gland, and ovary. The pulsatile release of the gonadotropin-releasing hormone from the hypothalamus stimulates the pituitary gland to secrete luteinizing hormone (LH) and follicle-stimulating hormone (FSH). In the ovary, LH stimulates the theca cells to produce androstenedione, while FSH stimulates the follicular cells to convert androstenedione to estradiol (Fig. 2.2). The increase in serum estradiol results in the negative feedback to the production of LH and FSH from the hypothalamus, maintaining serum estradiol levels of around 10–20 mIU/mL. Consequently, with a decrease in estradiol production during postmenopause, negative feedback is lost, and there is an increase in serum levels of FSH and LH. The absence of menstruation associated with high levels of FSH and LH is a possible diagnosis of ovarian failure [3].
4 Estrogen and Postmenopause
After postmenopause, estrogen production by the ovaries becomes negligible. However, women maintain detectable levels of estradiol (E2) and estrone (E1) throughout their lives. This occurs as a result of the ability of the peripheral tissues to convert (aromatize) androgens produced by the adrenal glands and ovaries [8, 9].
Estrone is the primary estrogen after postmenopause and is derived from the peripheral conversion of androgens in the muscle, liver, and brain tissue, as well as in the adipose tissue, since the aromatase enzyme displays greater expression in the adipose tissue [8, 9] (Fig. 2.3). Estradiol continues to be produced after postmenopause through the peripheral conversion of estrone; however, it presents in much lower levels than during reproductive life and also in levels lower than those of estrone. The changes in estrogen levels are responsible for most of the morbidity in women after postmenopause, such as osteoporosis, cardiovascular diseases, vulvovaginal atrophy (VVA), and sagging, among others [8, 9].
5 Hormone Receptors and Skin
Several functions of the skin have proved to be hormone dependent. Sex steroids clearly play a key role in the skin aging process, as evidenced by the rapid decline in skin appearance noticeable from onset of the premenopausal years. These changes have not been studied thoroughly, although the histological work has demonstrated the presence of estrogen and progesterone receptors in the skin and a relative decrease in expression from the onset of postmenopause [10].
The concentration of estrogen receptors in nuclear and cytoplasmic skin is relatively low. However, surgically induced hypoestrogenemia resulted in a significant decrease in the expression of these receptors [10]. Similarly, studies have revealed a gradient coloration of such specific receptors in the epidermis, with darker color in the granular layer. Similar staining has been observed in the hair follicles and sebaceous glands. Although the presence of estrogen receptors on the skin does not confirm their role on the skin, the even distribution of color suggests that the skin is a major target organ of sex steroids [10].
Based on the levels of the concentrations observed in the female genital tract, the highest concentration of estrogen receptors is found in the vaginal epithelium. Androgen receptors are also expressed on the skin of the external female genitalia. Immunohistochemistry studies identified a greater proportion of estrogen receptors in the vagina compared to androgen receptors, and this ratio in the vulva is reversed where an increased number of androgen receptors are found, with an accompanying decrease of estrogen receptors and progesterone [11] (Fig. 2.4).
During the postmenopausal period, changes occur in the following indexes; while the concentration of estrogen receptors in the pubic skin remains stable before and after postmenopause, the concentration of androgen receptors, in relation to estrogen receptors, decreases by almost 40 % in the postmenopausal period, and there is also a significant reduction in the level of progesterone receptors [2]. These changes may be relevant for purposes of treatment of dermatoses in the vulvovaginal region in postmenopausal women [10].
6 Skin Hydration
The dermis contributes to water retention by way of its hydrophilic content contained in the glycosaminoglycans. The glycosaminoglycans have a negative ionic charge, transporting water to the dermis and, therefore, contributing to skin turgor and tissue protection against excessive compression [12]. Glycosaminoglycans decrease with aging, and this contributes to skin dryness, atrophy, and rhytids [3].
Estrogen enhances the hygroscopic quality of the dermis, most probably by increasing the synthesis of the hyaluronic acid [12, 13]. Studies with animals confirm the hormonal role in skin hydration, showing a significant increase in glycosaminoglycans after estrogen-based therapies [13].
7 Skin Thickness and Postmenopause
The thinning of the dermis, clinically recognized by easy bruising and tearing, always occurs with aging. Most studies suggest that the loss of collagen is more closely linked to the postmenopausal period and less to the chronological age, hence hormonal influence [3].
The skin tends to become thinner as postmenopause progresses, and this can be confirmed by high-frequency ultrasound (22.5 MHz). Ultrasound can also be used to measure the thickness of the epidermis and dermis. Several studies using ultrasound have showed that the dermal skin thickness increases with estrogen replacement therapy, such as the finding in a double-blind placebo-controlled randomized study by Maheux et al. The aforementioned study displayed a significant increase in skin thickness, measured at the level of the greater trochanter in postmenopausal women who received conjugated estrogens. This finding was confirmed by both an ultrasound scan and a skin biopsy [14].
8 Skin Collagen and Postmenopause
In 1941, Albright et al. observed that postmenopausal women with osteoporosis had a remarkably thin skin, suggesting that the atrophy was more comprehensive than that found in the bone matrix [15]. Brincat et al. demonstrated a similar decrease in the skin thickness and collagen content, corresponding to a reduction of bone mineral density measured throughout the years following postmenopause [15]. In 1983, Brincat et al. concluded in their study that postmenopausal women on hormone replacement therapy with estrogen and testosterone had a collagen content 48 % higher compared to the content measured in untreated women, who were grouped by age [16].
The decrease in collagen levels on the skin occurs at an accelerated rate immediately after postmenopause and subsequently becomes more gradual. Approximately 30 % of skin collagen is lost during the first 5 years after postmenopause, with an average decrease of 2.1 % per year in postmenopausal women over a period of 20 years [10].
9 Skin Looseness and Wrinkling
The aging of the skin on the face is characterized by a progressive increase in stretch, associated with reduced elasticity. The loss of tonicity is accompanied by a progressive aggravation of facial wrinkles [17]. Bolognia et al., in a double-blind, placebo-controlled study, observed that young women in early menopause have an accelerated increase in degeneration of elastic fibers in the dermis [18]. Histologically, severe degenerative changes of elastic fibers have been noticed, including the coalescence of cystic spaces into lacunae, peripheral fragmentation, granular degeneration, and the splitting of the fibers into strands. These changes were noticed in individuals 20 years older than said patients. This suggests a close association between estrogen deprivation and changes in elastic fibers [18].
10 Wound Healing in Postmenopause
Healing of the skin is first characterized by an inflammation, followed by the formation of granulation tissue, after reepithelialization, and finally, the remodeling of tissues. Delayed wound healing usually occurs in the elderly, and estrogen has been shown to play a crucial role in wound healing [3].
Elastase is capable of degrading a wide range of functional and structural proteins, such as proteoglycans, fibronectin, and collagen. Fibronectin is essential for the healing process by influencing reepithelialization, collagen deposition, and wound contraction. With age, the amount of fibronectin decreases and is degraded secondary by elastase, which is high due to the increased number of neutrophils found in old wounds (Fig. 2.5). Therapies aimed at decreasing elastase, such as those aiming at reducing the number of neutrophils, can enhance the healing process [19, 20].
Ashcroft et al. in a randomized double-blind placebo-controlled study investigated the effects of topical estrogen on wound healing in healthy elderly men and women and reported the results of this therapy in terms of inflammatory response and elastase levels during the healing process. Compared to placebo, the treatment with estrogen increased the levels of collagen and fibronectin and resulted in a decrease in elastase levels, secondary to a reduction in the number of neutrophils, with consequent reduction in the degradation of fibronectin. The data thus obtained suggest that the delay in wound healing of the elderly can be improved by topical estrogen therapy [21]. Following the same reasoning, recent studies have shown that hormone replacement therapies prevent the onset of ulcers and venous stasis in postmenopausal women [14].
More recently, Ashcroft et al. conducted a study on mice, the results of which suggesting that estrogen also has a role in regulating the migratory inhibitory factor (MIF) of macrophages. Estrogen acts as a proinflammatory agent and has been implicated in the formation of aberrant scarring and in the alteration of inflammatory response in vivo. The estrogen-deficient mice showed a marked increase in the MIF in wound healing, while mice lacking the MIF gene showed no delayed wound healing, despite their estrogen deficiency. We may, therefore, confirm the role of estrogen in the regulation of the MIF and its role in wound healing [22].
11 Urogenital and Menopausal Problems
The epitheliums of the vulvar, vaginal, and urinary tracts show a relatively high number of estrogen receptors and are, therefore, sensitive to decreased levels of circulating estrogens. Usual urogenital changes that occur during postmenopause occur as the result of a combination of physiological aging and low estrogen levels [23].
Lower levels of estradiol lead to numerous adverse effects, including changes in the lower urinary tract. The main change is vaginal atrophy: the mucosa becomes thinner and drier. This may lead to vaginal discomfort, dryness, stinging, pruritus, and dyspareunia (Fig. 2.6). The vaginal epithelium can become inflamed, thus contributing to urinary symptoms, such as increased urinary frequency and urgency, dysuria, incontinence, and recurring infections. Furthermore, it has been suggested that reduced estrogen levels can affect the periurethral tissues and contribute to loosening and pelvic incontinence. In association with hypoestrogenemia, these changes in pH and vaginal flora may predispose postmenopausal women to urinary tract infection [24, 25].
12 Atrophic Vaginitis
Atrophic vaginitis is inflammation of the vagina that develops when there is a significant decrease in estrogen levels. Estradiol plays a vital role in maintaining the vaginal tissue healthy and lubricated, and decreased levels of estradiol may cause the vaginal epithelium to become atrophic, thin, dry, and wrinkled. Common conditions of low estrogen levels that result in atrophic vaginitis include postmenopause, breastfeeding, surgical removal of the ovaries in young women, and medication used to decrease estrogen levels in conditions such as uterine fibroids or endometriosis [24, 25].
Diagnosis is possible by way of physical examination; however, the diagnosis has to be confirmed by cytological examination. Due to the fact that estrogen stimulates the maturation of the vaginal epithelium from basal to superficial cells, in general a postmenopausal smear will act initially and advance from a decreased number of superficial cells to being completely clear eventually. A predominance of basal cells associated with a relative absence of superficial cells is indicative of atrophy [5].
The treatment may be conducted topically with estrogen, and this method of treatment should not influence the success of treatment. The topical estrogen lowers the vaginal pH, induces the maturation of vaginal and urethral mucosa, and decreases the frequency of urinary tract infections (Fig. 2.7). Atrophy is quickly reversed after 1–2 weeks. Many treatments are suggested, and generally, those with estriol and estradiol creams are the treatments most frequently recommended. The appropriate duration of therapy is unknown [5, 23].
13 Menopausal Flushing
During postmenopause, 70–80 % of women experience transient flushing and sweating that may be associated with palpitations, anxiety symptoms, and sleep disorders and 25 % reported flushes by up to 5 years [5, 10]. The blush seems to cause vasodilatation in the papillary dermis and subcutaneous tissue and occurs mainly in the face, neck, chest, palms, and soles [10]. Rosacea is known to be a complication of flushing, which is more commonly detected in postmenopausal women than in men of the same age [26]. The prevalence of this condition during the early years of postmenopause may in part be explained by virtue of the loss of the peripheral vascular control seen in association with estrogen deficiency, which is correctable with hormone replacement therapy [27]. Studies show that low doses of a combination of androgen and estrogen are as effective as high doses of estrogen on its own. However, persistent flushing during the use of HRT may occur due to the increase of sex hormone-building globulin (SHBG), which decreases the bioavailability of testosterone. In this case, HRT can be exchanged from oral to patch, gel, or implant, leading to a reduction of SHBG in 6–8 weeks [28]. An empirical study conducted to analyze the changes in peripheral vascular control in postmenopausal women induced by topical estrogen cream suggested that both estrogen and its metabolites have a direct effect on neurotransmitters and their neurovascular control [29].
14 Hormone Replacement Therapy
Estrogen HRT, with or without progesterone, has been used to treat menopausal symptoms and prevent long-term illnesses such as osteoporosis and cardiovascular disease [3]. Observational studies have found lower rates of coronary heart disease in women taking estrogen after postmenopause, compared with women who did not receive this therapy. This association has been reported as a means of secondary prevention in women with coronary artery disease, with hormone users experiencing 35–80 % fewer recurring events than nonusers. If this association is causal, estrogen therapy may be an important method for the prevention of coronary heart disease in postmenopausal women. However, the observed association between estrogen therapy and the reduced risk of coronary events can be attributed to a selection bias, if the women who choose to take hormones are healthier and have a more favorable cardiovascular profile than those who do not. Observational studies have not been able to resolve this uncertainty [30]. In addition, the American Heart Association does not recommend the use of HRT for the secondary prevention of cardiovascular disease [31].
In 2002, the Women’s Health Initiative reported results of a randomized controlled trial involving 16,608 postmenopausal women, comparing the effects of estrogen and progesterone with placebos with regard to the risk of chronic disease, and confirmed that the combination of estrogen and progesterone increases the risk of invasive breast cancer. However, this treatment did not alter the risk of endometrial cancer and decreased the chance/risk of colon cancer and osteoporosis [32]. In 2003, Rowan et al. confirmed the observation in relation to the risk of breast cancer, showing that estrogen combined with short-term progesterone relatively increased the incidence of breast cancer diagnosed at a more advanced stage, compared to the use of a placebo, and also substantially increased the percentage of women with abnormal mammograms. These results suggest that estrogen and progesterone can stimulate the growth of breast cancer and make its diagnosis more difficult [33].
With regard to the skin, the treatment with estrogen in postmenopausal women has been proved to increase, on several occasions, the content of collagen, dermal thickness, and elasticity. Studies also illustrated the role of estrogen in wound healing, showing its beneficial effects [34]. Topical estrogen creams applied locally on confined surface areas (such as facial and vaginal application) appear to be safe, without significant systemic absorption [3].
References
Al-Azzawi F. Endocrinological aspects of the menopause. BMJ. 1992;48:262–75.
Schmidt JB, Lindmaier A, Spona J. Hormone receptors in pubic skin of premenopausal and postmenopausal females. Gynecol Obstet Invest. 1990;30(2):97–100.
Hall G, Phillips TJ. Estrogen and skin: the effects of estrogen, menopause, and hormone replacement therapy on the skin. J Am Acad Dermatol. 2005;53:555–68.
Blume-Peytavi U, Atkin S, Gieler U, Grimalt R. Skin Academy: hair, skin, hormones and menopause – current status/knowledge on the management of hair disorders in menopausal women. Eur J Dermatol. 2012;22(3):310–8.
Wines N, Willsteed E. Menopause and skin. Australas J Dermatol. 2001;42:149–60.
Kanitakis J. Anatomy, histology and immunohistochemistry of normal human skin. Eur J Dermatol. 2002;12(4):390–401.
Calleja-Agius J, Brincat M. The effect of menopause on the skin and other connective tissues. Gynecol Endocrinol. 2012;28(4):273–7.
Nelson LR, Bulun SE. Estrogen production and action. J Am Acad Dermatol. 2001;45(Suppl):S116–24.
Edman CD, MacDonald PC. Effect of obesity on conversion of plasma androstenedione to estrone in ovulatory and anovulatory young women. Am J Obstet Gynecol. 1978;130:456–61.
Raine-Fenning NJ, Brincat MP, Muscat-Baron Y. Skin aging and menopause. Am J Clin Dermatol. 2003;4(6):371–8.
MacLean AB, Nicol LA, Hodgins MB. Immunohistochemical localization of estrogen receptors in the vulva and vagina. J Reprod Med. 1990;35(11):1015–6.
Danforth DN, Veis A, Breen M, Weinstein HG, Buckingham JC, Manalo P. The effect of pregnancy and labor on the human cervix: changes in collagen, glycoproteins, and glycosaminoglycans. Am J Obstet Gynecol. 1974;120(5):641–51.
Grosman N, Hvidberg E, Schou J. The effect of estrogenic treatment on the acid mucopolysaccharide pattern in skin of mice. Acta Pharmacol Toxicol. 1971;30:458–64.
Maheux R, Naud F, Rioux M, Grenier R, Lemay A, Guy J, et al. A randomized, double-blind, placebo-controlled study on the effect of conjugated estrogens on skin thickness. Am J Obstet Gynecol. 1994;170(2):642–9.
Holland EF, Studd JW, Mansell JP, Leather AT, Bailey AJ. Changes in collagen composition and cross-links in bone and skin of osteoporotic postmenopausal women treated with percutaneous estradiol implants. Obstet Gynecol. 1994;83(2):180–3.
Brincat M, Moniz CF, Studd JW, Darby AJ, Magos A, Cooper D. Sex hormones and skin collagen content in postmenopausal women. Br Med J (Clin Res Ed). 1983;287(6402):1337–8.
Henry F, Pierard-Franchimont C, Cauwenbergh G, Pierard GE. Age-related changes in facial skin contours and rheology. J Am Geriatr Soc. 1997;45:220–2.
Bolognia JL, Braverman IM, Rousseau ME, Sarrel PM. Skin changes in menopause. Maturitas. 1989;11:295–304.
Herrick SE, Ashcroft GS, Ireland G, Horan MA, McCollum C, Ferguson MWJ. Up-regulation of elastase in acute wounds of healthy aged humans and chronic venous leg ulcers is associated with matrix degradation. Lab Invest. 1996;77:281–8.
Ashcroft GS, Horan MA, Ferguson MWJ. Ageing is associated with reduced deposition of specific extracellular matrix components, an up-regulation of angiogenesis, and an altered inflammatory response in a murine incisional wound healing model. J Invest Dermatol. 1997;108:430–7.
Ashcroft GS, Greenwell-Wild T, Horan MA, Wahl SM, Ferguson MW. Topical estrogen accelerates cutaneous wound healing in aged humans associated with an altered inflammatory response. Am J Pathol. 1999;155:1137–46.
Ashcroft GS, Mills SJ, Lei KJ, Gibbons L, Jeong MJ, Taniguchi M, et al. Estrogen modulates cutaneous wound healing by downregulating macrophage migration inhibitory factor. J Clin Invest. 2003;111:1309–18.
Schaffer J, Fantil JA. Urogenital effects of the menopause. Clin Obstet Gynaecol. 1996;10:401–17.
Castelo-Branco C, Cancelo MJ, Villero J, Nohales F, Juliá MD. Management of postmenopausal vaginal atrophy and atrophic vaginitis. Maturitas. 2005;52(Supp.1):S46–52.
Palacios S, Castelo-Branco C, Cancelo MJ, Vazquez F. Low-dose, vaginally administered oestrogens may enhance local benefits of systemic therapy in the treatment of urogenital atrophy in post-menopausal women on hormone therapy. Maturitas. 2005;50:98–104.
Bergfield WF. A lifetime of healthy skin: implications for women. Int J Fertil Womens Med. 1999;44(2):83–95.
Ginsburg J, Hardiman P, O’Reilly B. Peripheral blood flow in menopausal women who have hot flushes and in those who do not. BMJ. 1989;298(6686):1488–90.
Simon J, Klaiber E, Wiita B, Bowen A, Yang HM. Differential effects of estrogen-androgen and estrogen-only therapy on vasomotor symptoms, gonadotropin secretion, and endogenous androgen bioavailability in postmenopausal women. Menopause. 1999;6:138–46.
Brincat M, de Trafford JC, Lafferty K, Studd JW. Peripheral vasomotor control and menopausal flushing: a preliminary report. Br J Obstet Gynaecol. 1984;91(11):1107–10.
Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. JAMA. 1998;280:605–13.
Mosca L, Collins P, Herrington DM, Mendelsohn ME, Pasternak RC, Robertson RM, et al. Hormone replacement therapy and cardiovascular disease – a statement for healthcare professionals from the American Heart Association. Circulation. 2001;104(4):499–503.
Chlebowski RT, Hendrix SL, Langer RD, Stefanick ML, Gass M, Lane D, et al. Influence of estrogen plus progestin on breast cancer mammography in healthy postmenopausal women. JAMA. 2003;289(24):3243–53.
Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women – principal results from the Women’s Health Initiative. JAMA. 2002;288(3):321–33.
Brincat MP, Baron YM, Galea R. Estrogens and the skin. Climacteric. 2005;8:110–23.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Pereira, E.S.P., Langen, S.B., Fidelis, M.C., Pereira, M.O., Costa, A. (2015). Skin and Menopause. In: Farage, M., Miller, K., Fugate Woods, N., Maibach, H. (eds) Skin, Mucosa and Menopause. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-44080-3_2
Download citation
DOI: https://doi.org/10.1007/978-3-662-44080-3_2
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-44079-7
Online ISBN: 978-3-662-44080-3
eBook Packages: MedicineMedicine (R0)