Abstract
The precise nature of how genetic and environmental risk factors influence changes in alcohol use (AU) over time has not yet been investigated. Therefore, the aim of the present study is to examine the nature of longitudinal changes in these risk factors to AU from mid-adolescence through young adulthood. Using a large sample of male twins, we compared five developmental models that each makes different predictions regarding the longitudinal changes in genetic and environmental risks for AU. The best-fitting model indicated that genetic influences were consistent with a gradual growth in the liability to AU, whereas unique environmental risk factors were consistent with an accumulation of risks across time. These results imply that two distinct processes influence adolescent AU between the ages of 15–25. Genetic effects influence baseline levels of AU and rates of change across time, while unique environmental effects are more cumulative.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Initiation of alcohol use (AU) typically occurs during adolescence (Wagner and Anthony 2002). Over 70% of US high school students report having consumed at least one alcoholic beverage (Wechsler 2012), while 34% of high school seniors report drinking alcohol to intoxication (Bachman et al. 1998). Excessive AU among adolescents is associated with leading causes of death as a result of motor vehicle accidents and homicides, as well as other risky behaviors, such as physical fighting (Boekeloo and Novik 2011). Thus, it is important to understand how patterns of AU change from adolescence through early adulthood to inform intervention and prevention efforts aimed at reducing AU-related risks and negative outcomes.
Developmental studies of AU have consistently shown that AU increases linearly throughout adolescence (Duncan and Duncan 1996; Duncan et al. 1998, 2006, 1997; Scheier et al. 2000), with heavy drinking peaking among individuals in their early twenties, and subsequently decreasing (Costanzo et al. 2007). Although these studies have been successful in identifying common trajectories of AU, they have not tested competing developmental models describing the possibly different processes that may be driving patterns of AU change over time.
Twin and family studies have demonstrated that AU is influenced by genetic and environmental factors, with genetic risk factors explaining 50–60% of AU variance (Kendler et al. 1994; Reich et al. 1998; Verhulst et al. 2015). Genetic influences have been shown to become relatively more prominent over time, whereas shared environmental factors become less salient over time (Edwards et al. 2015; Kendler et al. 2011, 2008; van Beek et al. 2012). Previous longitudinal analyses of the same data used in the present study also found that genetic variation increased over time (Kendler et al. 2011, 2008). Further, Van Beek and colleagues found evidence of a single, stable set of genetic risk factors (van Beek et al. 2012). In contrast, another report found that genetic risks were due to two significant, dynamic genetic factors (i.e., different genetic factors influencing AU during different time periods) (Edwards and Kendler 2013). One of these genetic risk factors declined in young adulthood, while the second increased in salience during young adulthood.
However, these genetically informative reports were limited in so far as they were largely atheoretical. The methods did not leverage the classical twin design within a defined, developmental framework, but instead relied on Cholesky decompositions, which make no theoretical prediction about possibly different etiological processes involved in changes over time (Kendler et al. 2011, 2008). Another limitation is that they did not specify and test competing models representing different developmental hypotheses (van Beek et al. 2012; Wichers et al. 2013).
It is plausible that genetic and environmental risk factors increase over time as predicted by latent growth models (LGMs) (Duncan and Duncan 1991; Duncan et al. 1994; McArdle 1986; McArdle and Epstein 1987; Nesselroade and Baltes 1974). In LGMs, the rates of change (slope) from baseline levels (intercept) may be linear or non-linear. These processes have been referred to as an “unfolding” of effects across time (Gillespie et al. 2015). Alternatively, there may be an accumulation of random genetic or environmental effects as predicted by autoregressive models (ARMs) (Boomsma et al. 1989; Boomsma and Molenaar 1987; Eaves et al. 1986). It is also possible that both processes act jointly on the risk of AU as predicted by dual change score (DCS) models (McArdle 2009; McArdle and Hamagami 2003; McArdle et al. 2004). This hybrid approach is mathematically and statistically equivalent to a random coefficient, multilevel, or hierarchical linear model (Bryk and Raudenbush 1987; McArdle and Hamagami 1992; McArdle et al. 1991; Mehta and West 2000; Miyazaki and Raudenbush 2000). Costanzo and colleagues (Costanzo et al. 2007) have used this approach to examine rates of change in AU (i.e., latent growth effects) and changes in the probability of heavy drinking relative to the previous probability (i.e., autoregressive effects). Although based on genetically uninformative data, their results showed that heavy drinking was most common in the early 1920s, but decreased thereafter, and that for a subset of individuals, heavy drinking persisted into later adulthood.
The DCS model has been applied to other complex psychiatric behaviors. For example, Gillespie et al. have used this method to distinguish genetic and environmental mechanisms underlying adolescent depression (Gillespie et al. 2015). They found that environmental risks were best explained with accumulating, autoregressive factors, whereas genetic risks were best explained in terms of latent growth factors that unfold or change at different rates across time. To our knowledge, the DCS method has not been used to examine the underlying genetic and environmental influences underlying adolescent AU.
Given the importance of gaining a more complete understanding of how genetic and environmental influences may be contributing to the etiology of adolescent AU, investigating these developmental features within a genetically informative, developmental framework is needed. This approach has the potential to identify critical time-dependent developmental periods for effective prevention and early intervention efforts. Therefore, the aim of the present study is to examine within a broader developmental framework the nature of longitudinal changes in the contributions of genetic and environmental risk factors to AU from mid-adolescence through young adulthood. Because of the phenotypic and genetic correlations between internalizing disorders and AU (Edwards et al. 2014; Grant et al. 2006; Hasin et al. 2005; Kendler et al. 1993), one hypothesis is that the developmental pattern of genetic and environmental risks will be broadly similar. Accordingly, consistent with the results of Gillespie et al. (2015), we hypothesize that (i) autoregressive effects will better characterize environmental influences on AU, and (ii) latent growth effects will describe genetic risk factors.
Method
Sample
Participants came from the Virginia Adult Twin Study of Psychiatric and Substance Use Disorders (VATSPSUD) (Kendler and Prescott 2006). VATSPSUD consists of Caucasian male, female, and opposite sex twin pairs from the Virginia Twin Registry (now the Mid-Atlantic Twin Registry) born between 1940 and 1974. Between 2000 and 2004, a subsample of the adult same sex male twin pairs were assessed as part of an interview to study the nature and pattern of risk and protective factors for psychoactive substance use and psychoactive substance use disorders across the lifespan. This study was completed by 1794 males, aged 24–62 years (M = 40.3, SD = 9.0), which consisted of 752 complete twin pairs (467 monozygotic and 285 dizygotic) and 290 singletons. Zygosity was determined using a combination of self-report measures, photographs and DNA analysis (Kendler et al. 2000).
Measures
The outcome variable used in the models was alcohol use (AU). AU was assessed using retrospective self-reports of the ages at which changes in AU occurred over the lifespan. A Life History Calendar method (Freedman et al. 1988; Furstenberg et al. 1987; Kessler and Wethington 1991) was used to assess several variables related to AU. This method has shown that although human memory can be relatively poor when attempting to recall past behavior, self-report information may be improved significantly when probed with careful directed questioning involving specific time periods and events. Using this method, participants were asked how old they were when they first started drinking, at what age they drank the heaviest, how much they drank (quantity; drinks per day), and how often they drink (frequency; days drank per month). Twins were asked to report on these consumption variables at the age when their alcohol intake changed. To reduce the number of missing values in the person year data, values were filled in for ages where no change in consumption was reported with the previous change amount. Interviews were administered via telephone or in-person interviews. The calendar alcohol response data was organized into a person year data set with person ages ranging from 0 to 61. The full interview included a number of retrospective assessments to coincide with the timing of major developmental milestones, such as alcohol initiation, leaving the parental home, finishing high school, and college entry and completion. (Gillespie et al. 2007).
A person year change in average number of drinks per month variable was created using a ‘standard’ unit for a drink that equaled one and one-half ounces of spirits, six ounces of wine, or twelve ounces of beer. All longitudinal modeling used this average number of drinks per month variable as the outcome for the selected range of person years 15–25. To adjust for wide ranges of the mean AU, we applied a log transformation to the data.
Statistical analyses
Autoregressive (ARMs), latent growth (LGMs), and dual change score (DCS) developmental models were specified and fit to the AU data to test competing hypotheses regarding the nature of the genetic and environmental risk factors involved in changes in AU over time. ARMs predict an accumulation of time-specific random effects and formally capture the ‘remembering’ or the ‘forgetting’ of time-dependent risk factor influences. Genetic or environmental risks at each time point are a function of new time-specific random effects (“innovations”) plus individual differences expressed from earlier times (“accumulation”). The “innovations” reflect novel, time-dependent genetic effects or environmental influences. Cross temporal correlations within subjects arise because innovations may persist over time and accumulate during development, resulting in developmental increases in the genetic or environmental variances, and increasing correlations between adjacent measures.
LGMs predict that developmental change in a phenotype is a function of unfolding, random risks in baseline levels (intercept) and rates of change (slope) over time that can be decomposed into genetic and environmental sources of variance. Rates of change can be linear or non-linear. These models correspond with special cases of the latent factor model in which factor loadings for the baseline levels and change factors are functions of the coefficients of a priori contrasts on the ages at which the measures were taken.
DCS models are hybrid models specifying that change within the genetic or environmental risk factors is a function of both autoregressive and latent growth factors. The DCS model is a more complex developmental model, as it combines the LGM (linear and quadratic rates of changes) with first-order ARM effects for both genes and environment. The diagram in Fig. 1 summarizes the principal features of this model and how these two types of developmental processes are formally represented and integrated. For simplicity, the Figure only considers the elements of the model without distinction between genetic and environmental components, although our analysis evaluates both independently to allow for the possibility that different processes underlie genetic and environmental components of developmental change.
Path diagram of a structural model for the developmental changes in alcohol use for autoregressive and latent growth curve effects for ages 15 through 25. I intercept (constant), S slope (linear rate of change), Q quadratic (nonlinear rate of change), β—first-order, autoregressive coefficients (the accumulation of risks due to the constant and change factors including latent residual error over time), Ψ—item-specific or residual variances, ϵ—error variances for the latent AU factors. This model can be applied to genetic or environmental developmental change, or both
Normalized orthogonal contrasts were used for the latent growth factor loadings for the intercept (I), linear slope (S), and quadratic (Q) latent growth factors in the saturated DCS model. These values are shown in Supplementary Table 1. In Fig. 1, the first-order, autoregressive coefficients are denoted by β and reflect the accumulation of risks due to the constant and change factors including latent residual error over time. These were set to be equal across ages. The item-specific or residual variances in the observed AU frequencies are represented by Ψ, and finally, the error variances for the latent AU factors at each age (ages 15–25) not explained by the constant and change factors are represented by ϵ.
The variances in autoregressive innovations, as well as the intercept and slope from the growth processes, can be decomposed into additive (A) genetic, shared (C), and unique (E) environmental variance components using standard biometrical genetic methods for twin data (Jinks and Fulker 1970; Neale and Cardon 1992). Because monozygotic (MZ) twin pairs are genetically identical, while dizygotic (DZ) twin pairs share on average half of their segregating genes, the expected twin pair correlations for the genetic (A) effects are 1.0 and 0.5, respectively. An important assumption in twin modeling is that the common environments (C) are equal in MZ and DZ twin pairs, and because non-shared environments (E) are by definition uncorrelated, E must also reflect measurement error. The developmental models were fitted and compared using structural equation modeling within the R-based OpenMx software package using Full Information Maximum Likelihood (FIML) (Boker et al. 2011; Neale et al. 2006; R Development Core Team 2013).
The full DCS model (Model 1) can be modified by removing the LGM component to produce a pure ARM (Model 2), or by removing the ARM, resulting in a pure LGM (Model 3). Within the twin model, the structure can then be further simplified. Model 4 removed the effect of shared environmental influences for both the LGM and ARM components. Model 5 removed the genetic ARM component and the unique environmental LGM component. Thus, Model 5 predicted that LGM effects account for genetic risk factors, while ARM effects account for unique environmental influences on AU.
The best-fitting model was identified by examining the lowest Akaike Information Criterion (AIC) (Akaike 1987), Bayesian Information Criterion (BIC), and sample-size adjusted BIC (sBIC) (Schwarz 1978), which are information-based parsimony indices. Selecting a best-fitting model based solely on log-likelihoods can be misleading due to ‘over-fitting’ since modeldata misfit will decrease simply by adding more parameters to a model. Therefore, the advantage of parsimony indices is that they penalize models with larger numbers of parameters, thereby providing an index of each model’s efficiency to explain the patterning in observed data when balanced against model complexity. Our rationale for including BIC and sBIC is also based on simulations (Nylund et al. 2007), which have shown that these indices outperform AICs (Schwarz 1978). Information based indices are appropriate when model comparisons are to be made for models that are not all nested, as is the case here. Under Model 5, the means for the unshared environmental (E) ARM component were necessarily modeled on the latent true scores for each observed phenotype, as opposed to the intercept, slope and quadratic in the full DCS model (Model 1).
Results
Descriptive statistics
The full MZ and DZ twin correlation matrices by age are presented in Supplementary Tables 2 and 3, respectively. Generally, the MZ twin correlations showed an increase until peaking at age 21 (r = 0.50), after which the correlations stabilize or decline slightly, ranging between 0.46 and 0.48. Conversely, the DZ within-pair twin correlations are more modest, ranging between 0.17 and 0.37. Despite a slight increase from age 15 through age 18, the correlations subsequently decrease steadily. The size of the MZ correlations are greater than the DZ correlations at all time points, but the DZ correlations are also greater than twice the MZ correlations. This suggests that familial aggregation is likely attributable to a combination of additive genetic and shared environmental risk factors.
Developmental models
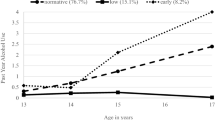
Table 1 shows a summary of the fit indices for the different model comparisons. As hypothesized, the best-fitting developmental model as determined by the AIC, BIC, and sBIC was Model 5, which predicted that LGM effects account for genetic risk factors, whereas an ARM better characterizes how unique environmental influences operate on AU. Figure 2a shows the expected means for AU change by age as predicted by Model 5. There is an approximately linear increase in changes in AU from age 15 through age 21, at which point changes in consumption stabilize. Figure 2b shows the patterns of change in the genetic, unique environmental, and total phenotypic unstandardized variances. The pattern of mean changes in AU roughly corresponds to the patterns of change in the variance of AU across the ages measured. There is a marked increase in the total phenotypic variance from age 15 to age 18, at which time the effect stabilizes followed by relatively small changes through age 25. The average contribution of the unique environment also shows a sharp increase from age 15 to age 18, but then shows a decline. The genetic variance, however, increases fairly linearly with age.
The key model parameter estimates from the best-fitting model are presented in Table 2. The top panel of the table presents the variances of the latent growth factors on the diagonal and their inter-correlations on the off diagonal. The middle panel presents the means of the latent growth parameters. The bottom panel presents the autoregressive parameter. As can be seen in the table, the mean of the latent intercept (1.95, 95% CI 1.81–2.04) represents the average level of reported changes in AU across the age range. The relatively large variance in the intercept indicates that there is considerable heterogeneity in the level of AU. The small and statistically non-significant linear slope parameter suggests a lack of linear change in reported changes in AU across this time period in the aggregate. However, although relatively smaller, the presence of variance in the linear random slope effects implies that there were individual differences in the rate of linear change over time. The mean of the quadratic slope is larger, negative, and statistically significant, indicating a nonlinear curvature aspect to the expected trajectory of changes in AU. The smaller variance for the quadratic slope suggests there is less heterogeneity in the nonlinear curvature. Finally, the autoregressive parameter, β (0.77, 95% CI 0.75–0.79), represents the consistency of changes in AU over time.
Notably, the genetic correlations between the intercept and the linear slope and the intercept-quadratic slope correlations were relatively small and not statistically significant. However, the correlation between the linear and quadratic slopes is much larger and statistically significant, but caution should be used when interpreting this association because the magnitude of the variances of the two slopes (and hence the covariance) is quite small.
Discussion
We investigated the nature of longitudinal contributions of genetic and environmental risk factors to changes in AU from mid-adolescence through young adulthood. While a cursory inspection of the twin correlations may suggest that familial aggregation in AU is potentially attributable to a combination of additive genetic and shared environmental risk factors, we were able to drop the shared environmental variance components with no statistically significant reduction in model fit. This type of global parameter testing is often performed first to determine if a more parsimonious model fits the data better. Therefore, on the basis of parsimony, we chose the best fitting model to be Model 5. Despite this, these patterns underscore the need for follow-up inspection of the shared environment as part of the developmental process.
With model 5 as the best fitting model, the way genetic risk factors contribute to changes in AU over time between ages 15 and 25 were best described by expectations from a quadratic LGM (i.e., an unfolding). In other words, genes were found to affect baseline levels of AU and rates of change across time. The main implication of the LGM best describing how genes influence changes in AU is that the same genes may be expressed at different stages of development, although their effects may increase with age. In contrast, unshared environmental influences were best described by expectations from an ARM (i.e., an accumulation), suggesting that the impact of idiosyncratic aspects of the environment is cumulative. Thus, an adverse or protective event occurring at age 15, such as a break-up with a significant other or becoming involved with a sport, can continue to influence AU at later ages, in addition to other life events.
Our results based on the exhaustive, hybrid DCS model are broadly consistent with previous results using the same data (Kendler et al. 2008). These results showed that genetic variation increased over time, which we also found. However, as mentioned in the introduction, Kendler et al. (2008) used a Cholesky decomposition to obtain their results, which is useful in describing how genetic effects act over time, but provides no information about the underlying mechanisms contributing to the changes in the genetic and environmental variance. The DCS model goes beyond the Cholesky decomposition to elucidate how these underlying mechanisms are changing over time.
Although DCS models have been applied to a variety of complex behaviors, to our knowledge, this is the first study to specify and compare a broader set of competing longitudinal developmental models within the context of a twin design to characterize the sources of genetic and environmental risks involved in changes in AU from mid-adolescence to young adulthood. Previously, Gillespie and colleagues compared the fits of competing developmental models to investigate longitudinal change in adolescent depression (Gillespie et al. 2015). Similar to our findings, environmental risks were best explained as an accumulating, autoregressive process, and genetic risks were best explained as an unfolding, latent growth process. One possible explanation for why both complex behaviors appear to follow similar genetic and environmental models for change is that they are modestly correlated (Buckner et al. 2007; Conner et al. 2009). One proposed pathway to problematic alcohol use is through internalizing disorders (Hussong et al. 2011; King et al. 2004). Thus, it may be that the genes and the environment that influence adolescent depression operate broadly in similar ways as do the processes influencing adolescent AU.
Another study that is relevant to the current findings is the report by Costanzo and colleagues (Costanzo et al. 2007). Although they did not use genetically informative data, they used a similar modeling approach to examine rates of change in AU and changes in the probability of heavy drinking. They showed that heavy drinking is most common in the early 20s, but decreases thereafter, and that for a subset of individuals, heavy drinking persists into later adulthood. This is consistent with our findings that the mean changes in AU increased through age 21. Also of interest is that our MZ twin correlations and pattern of genetic variance increased steadily until age 21, when they peaked, and then stabilized thereafter. Accordingly, it is possible that genetic factors underlie the pattern of drinking behavior shown in the Costanzo et al. (2007) study.
These results are also broadly consistent with the notion of there being two discernable genetic risk factors involved in changes in AU over time: an adolescent-limited genetic risk factor and an adult-onset genetic risk factor (Edwards and Kendler 2013). Because externalizing disorders and alcohol problems are genetically correlated during adolescence (Hicks et al. 2007), it may be that the adolescent-limited genetic risk factor is broadly capturing liability to externalizing disorders, which includes AU, while the adult-onset genetic risk factor is capturing liability specifically for alcohol use disorder (Edwards and Kendler 2013).
Limitations
The findings of the present study should be considered in the context of three limitations. First, our sample only consisted of white male twins, and therefore, our results may not generalize beyond this population. However, white males were targeted because they have significantly higher rates of AU than other populations (Grant et al. 2015; Hasin et al. 2007). Previous analyses have shown that this sample is broadly representative of white U.S. males and do not differ from the general population in rates of psychopathology, drug use, and abuse (Kendler et al. 2000).
Second, these analyses were carried out on retrospectively assessed data, which may be subject to various degrees of recall bias. A Life History Calendar (LHC) method (Belli 1998) was used to improve recall accuracy when assessing the twins by providing multiple cues to improve recall (Belli 1998). The reliability of retrospective recall of AU using the LHC method is good (Czarnecki et al. 1990; Koenig et al. 2009) and previous studies suggest that retrospective assessments might suffer less from underreporting than prospective assessments of AU (Czarnecki et al. 1990; Koenig et al. 2009). The sampling time frame was also limited to age 25. It is unclear how genetic and environmental risks will continue to impact AU at later ages.
Third, because values were filled in for ages where no change in consumption was reported with the previous change amount, it is possible that participants’ use was not constant in between the reported ages. To determine if this possibility would change the best-fitting model, we fitted the same sequence of models using only the actual reported change data. Because of the large amount of missing data, model solutions and parameters estimates were unable to reliably converge.
Conclusions
Using a large sample of male twins, we formally tested and compared longitudinal twin models to investigate the nature of how genetic and environmental influences contribute to changes in AU over a developmentally relevant period of mid-adolescence through young adulthood. Modeling fitting results showed that genetic influences were consistent with an unfolding, growing pattern of risks as predicted by a latent growth model, while unshared environmental factors were best described by an accumulating pattern of risk as predicted by autoregressive effects. These findings add to our understanding of how genetic and environmental risk factors may operate to influence changes in AU across time. The results of this study will inform gene identification efforts and ultimately help to identify critical developmental periods for effective prevention and early intervention efforts.
References
Akaike H (1987) Factor analysis and AIC. Psychometrika 52(3):317–332
Bachman A, Johnston LD, O’Malley PM (1998) Alcohol use among adolescents. Alcohol Health Res World 22(2):85
Belli RF (1998) The structure of autobiographical memory and the event history calendar: potential improvements in the quality of retrospective reports in surveys. Memory 6(4):383–406
Boekeloo BO, Novik MG (2011) Clinical approaches to improving alcohol education and counseling in adolescents and young adults. Adolesc Med State Art Rev 22(3):631–648, xiv
Boker S, Neale M, Maes H, Wilde M, Spiegel M, Brick T, Spies J, Estabrook R, Kenny S, Bates T, Mehta P, Fox J (2011) OpenMx: an open source extended structural equation modeling framework. Psychometrika 76(2):306–317
Boomsma DI, Molenaar PC (1987) The genetic analysis of repeated measures. I. Simplex models. Behav Genet 17:111–123
Boomsma DI, Martin NG, Molenaar PC (1989) Factor and simplex models for repeated measures: application to two psychomotor measures of alcohol sensitivity in twins. Behav Genet 19:79–96
Bryk AS, Raudenbush SW (1987) Application of hierarchical linear models to assessing change. Psychol Bull 101(1):147–158
Buckner JD, Keough ME, Schmidt NB (2007) Problematic alcohol and cannabis use among young adults: the roles of depression and discomfort and distress tolerance. Addict Behav 32(9):1957–1963
Conner KR, Pinquart M, Gamble SA (2009) Meta-analysis of depression and substance use among individuals with alcohol use disorders. J Subst Abuse Treat 37(2):127–137
Costanzo PR, Malone PS, Belsky D, Kertesz S, Pletcher M, Sloan FA (2007) Longitudinal differences in alcohol use in early adulthood. J Stud Alcohol Drugs 68(5):727–737
Czarnecki DM, Russell M, Cooper ML, Salter D (1990) Five-year reliability of self-reported alcohol consumption. J Stud Alcohol 51(1):68–76
Duncan TE, Duncan SC (1991) A latent growth curve approach to investigating developmental dynamics and correlates of change in children’s perceptions of physical competence. Res Q Exerc Sport 62(4):390–398
Duncan SC, Duncan TE (1996) A multivariate latent growth curve analysis of adolescent substance use. Struct Equ Modeling 3(4):323–347
Duncan TE, Duncan SC, Hops H (1994) The effects of family cohesiveness and peer encouragement on the development of adolescent alcohol use: a cohort-sequential approach to the analysis of longitudinal data. J Stud Alcohol 55(5):588–599
Duncan TE, Duncan SC, Alpert A, Hops H, Stoolmiller M, Muthen B (1997) Latent variable modeling of longitudinal and multilevel substance use data. Multivar Behav Res 32(3):275–318
Duncan SC, Duncan TE, Biglan A, Ary D (1998) Contributions of the social context to the development of adolescent substance use: a multivariate latent growth modeling approach. Drug Alcohol Depend 50(1):57–71
Duncan SC, Duncan TE, Strycker LA (2006) Alcohol use from ages 9 to 16: A cohort-sequential latent growth model. Drug Alcohol Depend 81(1):71–81
Eaves LJ, Long J, Heath AC (1986) A theory of developmental change in quantitative phenotypes applied to cognitive development. Behav Genet 16:143–162
Edwards AC, Kendler KS (2013) Alcohol consumption in men is influenced by qualitatively different genetic factors in adolescence and adulthood. Psychol Med 43(9):1857–1868
Edwards AC, Heron J, Dick DM, Hickman M, Lewis G, MacLeod J, Kendler KS (2014) Adolescent alcohol use is positively associated with later depression in a population-based UK cohort. J Stud Alcohol Drugs 75(5):758–765
Edwards AC, Maes HH, Prescott CA, Kendler KS (2015) Multiple mechanisms influencing the relationship between alcohol consumption and peer alcohol use. Alcohol Clin Exp Res 39:324–332
Freedman D, Thornton A, Camburn D, Alwin D, Young-demarco L (1988) The life history calendar: a technique for collecting retrospective data. Sociol Methodol 18(1):37–68
Furstenberg FF Jr, Brooks-Gunn J, Morgan SP (1987) Adolescent mothers in later life. Cambridge University Press, Cambridge
Gillespie NA, Kendler KS, Prescott CA, Aggen SH, Gardner CO Jr, Jacobson K, Neale MC (2007) Longitudinal modeling of genetic and environmental influences on self-reported availability of psychoactive substances: alcohol, cigarettes, marijuana, cocaine and stimulants. Psychol Med 37(7):947–959
Gillespie NA, Eaves LJ, Maes H, Silberg JL (2015) Testing models for the contributions of genes and environment to developmental change in adolescent depression. Behav Genet 45(4):382–393
Grant BF, Stinson FS, Dawson DA, Chou S, Dufour M, Compton W, Pickering R, Kaplan K (2006) Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders. Alcohol Res Health 29(2):107–120
Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, Pickering RP, Ruan WJ, Smith SM, Huang B (2015) Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on alcohol and related conditions III. JAMA psychiatry 72(8):757–766
Hasin DS, Goodwin RD, Stinson FS, Grant BF (2005) Epidemiology of major depressive disorder: results from the National Epidemiologic Survey on alcoholism and related conditions. AMA Arch Gen Psychiatry 62(10):1097–1106
Hasin DS, Stinson FS, Ogburn E, Grant BF (2007) Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on alcohol and related conditions. AMA Arch Gen Psychiatry 64(7):830–842
Hicks BM, Blonigen DM, Kramer MD, Krueger RF, Patrick CJ, Iacono WG, McGue M (2007) Gender differences and developmental change in externalizing disorders from late adolescence to early adulthood: a longitudinal twin study. J Abnorm Psychol 116(3):433–447
Hussong AM, Jones DJ, Stein GL, Baucom DH, Boeding S (2011) An internalizing pathway to alcohol use and disorder. Psychol Addict Behav 25(3):390–404
Jinks JL, Fulker DW (1970) Comparison of the biometrical genetical, MAVA, and classical approaches to the analysis of human behavior. Psychol Bull 73(5):311–349
Kendler KS, Prescott CA (2006) Genes, environment, and psychopathology. Guilford, New York
Kendler KS, Heath AC, Neale MC, Kessler RC, Eaves LJ (1993) Alcoholism and major depression in women. A twin study of the causes of comorbidity. Arch Gen Psychiatry 50(9):690–698
Kendler KS, Neale MC, Heath AC, Kessler RC, Eaves LJ (1994) A twin-family study of alcoholism in women. Am J Psychiatry 151(5):707–715
Kendler KS, Karkowski LM, Neale MC, Prescott CA (2000) Illicit psychoactive substance use, heavy use, abuse, and dependence in a US population-based sample of male twins. AMA Arch Gen Psychiatry 57(3):261–269
Kendler KS, Schmitt E, Aggen SH, Prescott CA (2008) Genetic and environmental influences on alcohol, caffeine, cannabis, and nicotine use from early adolescence to middle adulthood. AMA Arch Gen Psychiatry 65:674–682
Kendler KS, Gardner C, Dick DM (2011) Predicting alcohol consumption in adolescence from alcohol-specific and general externalizing genetic risk factors, key environmental exposures and their interaction. Psychol Med 41(7):1507–1516
Kessler RC, Wethington E (1991) The reliability of life event reports in a community survey. Psychol Med 21:723–738
King SM, Iacono WG, McGue M (2004) Childhood externalizing and internalizing psychopathology in the prediction of early substance use. Addiction 99(12):1548–1559
Koenig LB, Jacob T, Haber JR (2009) Validity of the lifetime drinking history: a comparison of retrospective and prospective quantity-frequency measures. J Stud Alcohol Drugs 70(2):296–303
McArdle JJ (1986) Latent variable growth within behavior genetic models. Behav Genet 16:163–200
McArdle JJ (2009) Latent variable modeling of differences and changes with longitudinal data. Annu Rev Psychol 60:577–605
McArdle JJ, Epstein D (1987) Latent growth curves within developmental structural equation models. Child Dev 1:110–133
McArdle JJ, Hamagami F (1992) Modeling incomplete longitudinal and cross-sectional data using latent growth structural models. Exp Aging Res 18:145–166
McArdle JJ, Hamagami F (2003) Structural equation models for evaluating dynamic concepts within longitudinal twin analyses. Behav Genet 33(2):137–159
McArdle JJ, Hamagami F, Elias MF, Robbins MA (1991) Structural modeling of mixed longitudinal and cross-sectional data. Exp Aging Res 17(1):29–52
McArdle JJ, Hamgami F, Jones K, Jolesz F, Kikinis R, Spiro A, Albert MS (2004) Structural modeling of dynamic changes in memory and brain structure using longitudinal data from the normative aging study. J Gerontol B Psychol Sci Soc Sci 59(6):P294–P304
Mehta PD, West SG (2000) Putting the individual back into individual growth curves. Psychol Methods 5(1):23–43
Miyazaki Y, Raudenbush SW (2000) Tests for linkage of multiple cohorts in an accelerated longitudinal design. Psychol Methods 5(1):44–63
Neale M, Cardon L (1992) Methodology for genetic studies of twins and families. Springer Science & Business Media, Berlin
Neale MC, Boker SM, Xie G, Maes HM (2006) Statistical modeling. Richmond, Virginia, Department of Psychiatry
Nesselroade JR, Baltes PB (1974) Adolescent personality development and historical change: 1970–1972. Monogr Soc Res Child Dev 39(1):1–80
Nylund KL, Asparouhov T, Muthén BO (2007) Deciding on the number of classes in latent class analysis and growth mixture modeling: a Monte Carlo simulation study. Struct Equ Modeling 14(4):535–569
R Development Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Reich T, Edenberg HJ, Goate A, Williams JT, Rice JP, Van Eerdewegh P, Foroud T, Hesselbrock V, Schuckit MA, Bucholz K, Porjesz B, Li TK, Conneally PM, Nurnberger JI Jr, Tischfield JA, Crowe RR, Cloninger CR, Wu W, Shears S, Carr K, Crose C, Willig C, Begleiter H (1998) Genome-wide search for genes affecting the risk for alcohol dependence. Am J Med Genet 81(3):207–215
Scheier LM, Botvin GJ, Griffin KW, Diaz T (2000) Dynamic growth models of self-esteem and adolescent alcohol use. J Early Adolesc 20(2):178–209
Schwarz G (1978) Estimating the dimension of a model. Ann Stat 6(2):461–464
van Beek JH, Kendler KS, de Moor MH, Geels LM, Bartels M, Vink JM, van den Berg SM, Willemsen G, Boomsma DI (2012) Stable genetic effects on symptoms of alcohol abuse and dependence from adolescence into early adulthood. Behav Genet 42(1):40–56
Verhulst B, Neale MC, Kendler KS (2015) The heritability of alcohol use disorders: a meta-analysis of twin and adoption studies. Psychol Med 45:1061–1072
Wagner FA, Anthony JC (2002) Into the world of illegal drug use: exposure opportunity and other mechanisms linking the use of alcohol, tobacco, marijuana, and cocaine. Am J Epidemiol 155:918–925
Wechsler H (2012) Youth risk behavior surveillance—United States, 2011. MMWR Surveillence Summary 611–162
Wichers M, Gillespie NA, Kendler KS (2013) Genetic and environmental predictors of latent trajectories of alcohol use from adolescence to adulthood: a male twin study. Alcohol Clin Exp Res 37(3):498–506
Funding
This study was funded by the National Institute of Health grant DA-011287 (to K.S.K).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
E. C. Long, B. Verhulst, S. H. Aggen, K. S. Kendler and N. A. Gillespie declare that they have no conflict of interest.
Ethical approval
Data is no longer being conducted. However, all past procedures performed in studies involving human participants were in accordance with the ethical standards of the Committee for the Conduct of Human Research at Virginia Commonwealth University and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
The Committee for the Conduct of Human Research at Virginia Commonwealth University approved the project. Informed consent was obtained from all participants.
Research involving human and animal rights
This article does not contain any studies with animals performed by any of the authors.
Additional information
N. A. Gillespie and K. S. Kendler are joint senior authors.
Edited by Stephen Petrill.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Long, E.C., Verhulst, B., Aggen, S.H. et al. Contributions of Genes and Environment to Developmental Change in Alcohol Use. Behav Genet 47, 498–506 (2017). https://doi.org/10.1007/s10519-017-9858-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10519-017-9858-y