Abstract
The growth and biochemical profile of the red seaweed Gracilaria corticata var. cylindrica was evaluated after in vitro cultivation with effluent from a shrimp Litopenaeus vannamei farming pond. Seaweeds were cultivated for 21 days in sterilized seawater enriched with shrimp effluent with 5 different concentrations (0.1, 1, 10, 50, and 100 mL L−1). Fragments with 50 and 100 mL L−1 showed a higher significant growth rate of 3.53 ± 0.08% and 3.7 ± 0.55% respectively compared to sterilized seawater as control (2.5 ± 0.15%). Survival and regeneration of fragments were higher in the treatments 10, 50 and 100 mL L−1. A 100% survival was observed in 10 and 100 mL L−1, while 100-mL L−1 showed 100% regeneration in fragments. The number of branchlets developed per fragment was observed higher (2.33 ± 0.52) and (2.23 ± 0.05) in 50 and 100 mL L−1 respectively. A 50 mL L−1 showed the highest (124.45 ± 9.28 µg g−1) chlorophyll-a, and 100 mL L−1 showed the highest (54.5 ± 5.42 µg g−1) chlorophyll-b content. Agar yield (2.8 ± 0.32% FW), total sugar (72.6 ± 2.7%), and 3,6-anhydrogalactose (8.77 ± 0.63%) was observed highest in 100 mL L−1 treatment of shrimp effluent, while sulphate content (1.5 ± 0.8%) found higher in 50 mL L−1. Results showed that effluent treatments positively affected G. corticata var. cylindrica growth and biochemical composition, especially 50 and 100 mL L−1, which could be considered a good bio-stimulator for the making of the seedling. This work presents the potential use of effluents from shrimp cultivation as fertilizer, improving the growth and seedling production for sustainable cultivation of endemic red seaweed Gracilaria corticata var. cylindrica.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gracilaria species contributed about 10.67% of the total world aquaculture production of aquatic algae (FAO 2020). This species is the main source of agar production especially food-grade agar because it has been successfully cultivated in Chile, Indonesia (Qin 2018) and India (Veeragurunathan et al. 2015). Agar manufacturing industries produced 14,500 t of agar globally including bacteriological and technical grade agar (Porse and Rudolph 2017). Gracilaria is cultivated on large scale in countries like China, Malaysia, Indonesia, the Philippines, Spain, Portugal, Chile, Peru, and Argentina (Bixler and Porse 2011). Krishnamurthy (1991) reported 32 species of Gracilaria in the Indian region. Gracilaria corticata var. cylindrica is endemic to the Indian coast. Native agar reported from Gracilaria corticata showed gel strength below 100 ± 6.19 g cm−2 (Meena et al. 2008) which makes them extremely important for pharmaceutical applications where low gel strength agar is needed. The agars with low gel strength are used for medical formulations and preparation of special food for cancer survivals and also to impart slimy texture to the products. (Dawange and Jaiswar 2020). The global dysphagia diet thickening agents market was worth US$ 415 million in 2018 and is expected to witness a CAGR (compound annual growth rate) of 5% from 2019 to 2029. The prevalence of dysphagia is relatively high among the geriatric population, and it has been estimated that around 10 − 20% of the geriatric population across the globe have swallowing difficulties (Persistence Market Research, 2022). The extremely low gel strength agarophytes are thus crucial, but their existence in nature is limited, and thus there is an immediate requirement to develop cultivation practices for such species. Since Gracilaria biomass is propagated through vegetative means, development of elite seed material for large-scale cultivation has substantial importance.
Marine shrimp farming is well known aquaculture business; presently, shrimp farming is mainly cultivated in brackishwater for human consumption. Though traditional shrimp farming activity has been carried out in Asia for more than centuries, actual commercial shrimp farming began in the 1970s, and production grew steeply, to match the market demands of region like USA, Japan, and Western Europe. If we see in terms of global trade, China and the USA are the top-most importing countries of shrimp, whereas India is the largest exporting country. More than 40% of all shrimp imported into the USA came from India worth 4426.19 USD million during 2020 − 2021 (https://mpeda.gov.in/?page_id=438). During the full harvest, wastewater is generated which significantly pollutes the water (Ge et al. 2019). Deprived farm management practices such as littering the farm with wastes resulting from dead shrimps can lead to pollution load into the cultured water. The major problem of this cause is the discharged water effluent from the shrimp farms. The discharged water effluent contains a huge load of nutrients and organic matter. If this water is left untreated, it can cause serious spoiling to the surrounding water bodies and expressively reduce the environmental carrying capacity (https://thefishsite.com/articles/how-to-manage-water-effluent-from-shrimp-farms). Since shrimp farm effluents contain a high amount of nutrients, they could be effectively used for rearing the small filaments of Gracilaria corticata var. cylindrica and also reduce an environmental load of pollution. The use of shrimp farm effluent as a growth medium for seaweed gives remarkable growth and quality to plants (Martins et al. 2020). The present study aims to evaluate the effect of shrimp farm effluents as a source of fertilizer for the development of seedling material and also its evaluation by studying growth, survival, regeneration, pigments, and agar contents.
Materials and methods
Collection of samples
Healthy growing samples of Gracilaria corticata var. cylindrica were collected from the intertidal area of the Mithi Viradi coast of Gujarat (21° 47 ′11.28″ N, 72° 24′ 56.69″ E). Samples were immediately brought to the CSMCRI laboratory in seawater. Samples were cleaned with filtered seawater to remove and clean the attached unwanted particles, sand, and epiphytes. Samples were acclimatized for 5 days in controlled laboratory conditions (35 PSU salinity, 25 °C temperature, 40 µmole light intensity, and 12:12 (L:D) photoperiod) (Dawange and Jaiswar 2020). Litopenaeus vannamei shrimp effluent was collected from a farm pond located at Mannar (21°24′55.3″N 72°10′08.3″E) village (Supplementary Fig. 1). The effluent was brought to the laboratory in sampling bottles and filtered through glass microfiber filter (GFC, Whatman, 1.2 μm pore size). It was stored in cool condition for further use.
Study of daily growth rate, survival, and regeneration
Approx. 0.5 cm fragments were excised randomly and acclimatized in seawater for a day. Ten fragments were placed in a 500-mL flask (Stocking density 5 g/L) containing one set of sterilized seawater as control (n = 3) and 4 sets (n = 3) with different concentrations of filtered Shrimp farm effluent (SFE), i.e., 0.1, 1.0, 10, 50, and 100 mL per liter in sterilized filter seawater with 35 PSU salinity. Standard controlled laboratory conditions were maintained with temperature (25 ˚C), Light (40 µmole photons m−2 s−1) and photoperiod 12:12 h (light:dark). Media was replenished every 5 days interval, and fragments were cleaned with a soft brush to reduce fouling. The experiment was carried out in 3 replicates (n = 3). After 21 days, fragments were weighed and the daily growth rate (DGR) (% d−1) was estimated by using the formula: \(\mathrm{DGR}\left(\%\;\mathrm d^{-1}\right)=\mathrm{In}\;\left[\frac{\frac{W_2}{W_1}}{t}\right]\ast100\), where W1 is the initial weight recorded, W2 is the final weight recorded, and t is the total number of days (Dawes et al. 1993).
The survival rate was calculated by the number of initial fragments and final remaining fragments. Regeneration was calculated manually by counting the number of fragments developing new branchlets and the number of branchlets.
Estimation of pigments
R-phycoerythrin (PE) and R-phycocyanin (PC) contents were determined by homogenizing 100 mg fresh sample in mortar and pestle using liquid nitrogen. A 0.8 mL 0.1 M phosphate buffer (6.8 pH) was added to the homogenate and incubated overnight at 4 ˚C. After incubation, the homogenate was vortex, centrifuged at 13,000 rpm at 4 ˚C, and supernatant was collected in a 1.5-mL tube. To the pellet, 0.2 mL phosphate buffer was added for the complete extraction of pigments. Absorbance scan was recorded on a dual-beam UV spectrophotometer (EPOCH/2 Biotek, USA). For extraction of chlorophyll-a, a similar process was carried out using 90% acetone as a solvent. The amount of R-PE and R-PC content was calculated according to Sampath-Wiley and Neefus (2007), while Chl-a content was calculated according to Jeffrey and Humphrey (1975).
Agar characterization
Extraction of agar
Native agar was extracted as per the process described by Sambhwani et al. (2022). A 5 g of fresh biomass was dipped in 50 mL of distilled water and kept overnight. It was then cooked at 121 °C for 1 h. The cooked solution was then filtered using a muslin cloth. The filtrate was added to the 2 volumes of prechilled isopropanol for the precipitation of agar. Precipitated agar was collected, then dried, and crushed to a fine powder with liquid nitrogen. The agar was collected in the pre-weighed tube for estimation of total agar content. Total sugar, 3,6-anhydro-galactose and sulphate content was estimated using the native agar.
Estimation of total sugar
Approximately 10 mg native agar was added to 250 µL of concentrated sulphuric acid and kept for 30 min. After incubation, the volume was made up to 7 mL by adding distilled water and was kept at 121 °C for 1 h. The solution was neutralized by adding calcium carbonate till the effervescence stopped. The solution was centrifuged at 7500 rpm for 10 min. The collected supernatant was used for further total sugar estimation. To the sample, 5% phenol and concentrated sulphuric acid was added and after incubation of 25 min, OD was measured at 390 nm and plotted against glucose as standard (Dubois et al 1956).
Estimation of 3,6-anhydrogalactose
Approximately 10 mg agar was taken to estimate the 3,6-anhydrogalactose content with fructose as standard. In an ice bath, samples were cooled, and a resorcinol acetal mixture was added to it. The mixture was then heated at 80 °C for 10 min before transferring to an ice bath. The violet-pink color developed was read at 555 nm. Conversion of fructose to 3,6-anhydrogalactose, the final values were multiplied by a factor of 1.087 (Yaphe and Arsenault 1965).
Estimation of sulphate content
Approximately 10 mg agar was hydrolyzed in 2.0 N HCl at 100 °C for 1 h in a sealed tube, the hydrolysate was centrifuged at 7000 rpm for 20 min, and the supernatant was used for analysis. Four percent TCA was added to each aliquot followed by the addition of barium chloride (BaCl2)-Gelatin solution. Potassium sulphate (K2SO4) was used as a standard and UV absorbance was measured at 360 nm (Sambhwani et al. 2022).
Statistical analysis
All the experiments were performed in triplicates and the values are expressed as mean ± standard deviation. The data analysis was carried out by one-way analysis of variance (ANOVA) with Tukey as a post hoc test that was applied at the significance difference level of confidence (α = 0.05) with the help of software Infostat (Di Rienzo et al. 2018). Correlation between DGR and survival, regeneration, and no. of branchlet per segment of different concentrations of SFE was carried out using the parametric Pearson’s coefficient correlation, with a minimum significance level of P < 0.05 being accepted.
Results
Growth, survival, and regeneration
After 21 days of culture, the growth rate (DGR), survival, and regeneration were observed. Highest DGR (3.7 ± 0.55% d−1) was observed in the samples with the treatment of 100 mL L−1 of shrimp farm effluent compared to seawater (2.54 ± 0.15% d−1) culture medium while no significant difference was found in 0.1 mL L−1,1 mL L−1, and 10 mL L−1 treatments (Fig. 1).
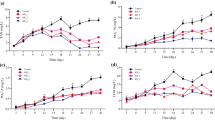
Considering the survival of the fragments, there was no significant difference observed in all the treatments including the control. One hundred percent survival rate was found in treatments 10 mL L−1 and 100 mL L−1 (Fig. 2a).
No significant difference was observed in the regeneration of new fragments. The 10 mL L−1 and 100 mL L−1 showed the highest regeneration with 98.33 ± 2.88% and 100% regeneration respectively (Fig. 2b). The number of branchlets developed per segment was found significantly higher in treated fragments compared to seawater. The highest (2.28 ± 0.45%) branchlets per segment were observed in treatment with 50 mL L−1 while lowest (1.33 ± 0.15%) in 0.1 mL L−1 (Fig. 2c). Correlation between DGR and survival, regeneration, and no. of branchlet per segment of different concentrations of SFE during the study was significant. Estimation of chlorophyll-a, chlorophyll-b, R-phycoerythrin, R-phycocyanin, total agar yield, total sugar content, 3,6-anhydrogalactose content, and sulphate content of native agar was performed for concentration 50 mL L−1 and 100 mL L−1 as it shows highest growth rate.
Pigment analysis
Pigment analysis of Chl-a, Chl-b, R-phycocyanin (R-PC), and R-phycoerythrin (R-PE) pigments was performed. No significant difference was observed in Chl-a content, while a significant difference was observed in Chl-b content. Chl-a ranged from 98.57 ± 29.5 to 124.45 ± 9.28 µg g−1, while Chl-b found ranging 14.11 ± 8.9 to 54.50 ± 5.42 µg g−1. The highest Chl-a content was observed in 50 mL L−1, while the highest Chl-b content was observed in samples treated with 100 mL L−1 effluent (Fig. 3).
R-PE and R-PC content was found significantly different. Higher R-PE (481.64 ± 18.6 µg g−1) and R-PC (248.96 ± 12.11 µg g−1) were observed in the control compared to fragments treated with shrimp effluent. A decrease in red pigment content was observed as the effluent concentration was increased (Fig. 4).
Agar yield, total sugar, 3,6-anhydrogalactose, and sulphate content
No significant differences were observed in the agar yield and its biochemical analysis. Highest native agar content (2.85 ± 0.8%FW) (Fig. 5a), total sugar (72.65 ± 7.6%) (Fig. 5b), and 3,6-anhydrogalactose content (7.8 ± 0.6%) (Fig. 5c) were observed in the fragments with 100 mL L−1 treatment. Sulphate content was higher (1.5 ± 0.8%) in treatment with 50 mL L−1 compared to the control (Fig. 5d).
Total agar yield (a), total sugar content (b), 3,6-anhydrogalactose content (c), and Sulphate content (d) of native agar from Gracilaria corticata var. cylindrica over 21 days under the laboratory condition. of 25 °C temperature, 40 μmol photons m−2 s.−1 light intensity, 12-h light period, and 35 PSU salinity. Data shown are the mean ± SD (n = 3)
Discussion
The results confirmed that the effect of shrimp farm effluent on the growth and development of Gracilaria corticata var. cylindrica was significant. Seaweeds are known to use nitrogen and phosphorous as primary nutrients for their growth and development (Harrison and Hurd 2001). These two important nutrients were present in sufficient quantity in SFE. Seaweeds have an excellent capacity as biofilters because of their ability to uptake and convert dissolved nutrients for somatic growth (Xu et al. 2008). Gracilaria tenuistipitata growth, survival performance, and immune potential were carried out in a biofloc-based Recirculating Aquaculture System (RAS) (Das et al. 2022). Nutrients removal efficiency from aquafarm discharge waters by Gracilaria verrucosa was studied by Devi and Gowri (2007). Macroalgae were found to effectively treat fishpond effluents producing useful algal biomass and recorded a daily growth rate of 1.17 ± 0.4% and 1.05 ± 0.25% for Gracilaria crassa and Ulva reticulata respectively (Msuya and Neori 2002). Highest DGR was found in the plant with the highest concentration of SFE; this may be due to the ability to absorption of a high rate of nitrogen from the medium as in the case of G. changii (Badraeni et al. 2020). Marinho-Soriano et al. (2002) studied SFE as a source of nutrients for the growth of Gracilaria sp., growth rate varied from 8.8% day−1 to 1.8% day−1, whereas in our study, it ranged between 2.38% day−1 and 4.15% day−1 with 42.65% increased as compared to control and this was statistically significant. Marinho-Soriano et al. (2009) again studied the utilization of shrimp farming wastewater as nutrients for evaluation of the growth rate in Gracilaria caudata; his observation revealed that the highest growth rate of 2.28% day−1 which was almost 50% less as compared to the growth of Gracilaria corticata var. cylindrica in our study; however, they were able to achieve the growth rate of 3.32% day−1 by using the tubular net methodology of cultivation. The low growth rate in control fragments shows the requirement of nutrients for the seaweed. Under laboratory conditions, commercially available medium is widely used for the growth and development of seaweed; costly medium does not permit the rearing of seedling on large scale; therefore, use of SFE could be an effective alternative for the development of nursery culture for sustainable seedling availability for large scale seaweed cultivation. The observation of a higher growth rate in fragments treated with SFE indicates that seaweed was probable with low contents of nitrogen and phosphorous internally. The nutritional state, light intensity, biomass density, water motion, age of the seaweed, structure and surface area of the thallus, initial nutrient concentration, etc. depend on the nutrient absorption by the seaweed to determine the uptake rate at a range of concentrations (Hurd et al. 2014; DeBusk et al. 1986; Zhou et al. 2006; Mao et al. 2009); therefore, seaweed with low nitrogen and phosphorous contents rapidly absorb those nutrients from the surrounding area and consequently exhibited fast growth during the growing period. The efficiency of SFE increased with increasing dose due to the presence of nitrate and phosphate contents at high concentrations (Table 1) which was clearly demarcated in survival, regeneration, and number of branchlets per segment observations. This may be due to the ability to absorb nitrates in water as stated by Badraeni et al. (2020). Since Gracilaria corticata var. cylindrica shows a good growth rate and effective utilization of nutrients from SFE, it could be efficiently utilized in IMTA (Integrated Multitrophic Aquaculture) approach based on this species. In the present study formation of the new branchlet was observed during regeneration on the 5th day, whereas the formation of a new axis in the same seaweed was observed on the 3rd day itself by applying Ascophyllum marine plant extract powder (Dawange and Jaiswar 2020). The regeneration in seaweed is found to be in the form of filamentous callus or bud or branchlet formation. The direct regeneration from the mother tissue makes the propagation of studies plant more cost-effective and economical as it avoids the process of callus induction followed by the requirement of excising and further culturing procedures. It may be noted that the number of Branchlets per segment was highest in 50 mL L−1 and 100 mL L−1 of SFE, and it was statistically significant. The plant obtained by this technique prefers to have a cost-effective strategy for large-scale cultivation. The concentration of chlorophyll a and b pigments increased in the plant treated with SFE since the frequency of nutrients concentration increased by making nitrogen available in the cultivation environment similar results were revealed by De Martino et al. (2021) and this was also observed by Hayashi et al. (2008) and made hypothesis stronger that the enriched thalli accumulated nutrients in chlorophyll form (Dodds et al. 1997), however, it was decreased in case of R-Phycoerythrin and R-Phycocyanin this may be due to the high amount of sodium in SFE similar observation was made by Mihriban et al. (2018) in case of Gracilaria gracilis were PE and PC contents were decreased in higher concentration of salinity. Under laboratory conditions, the chlorophyll concentration was stable with no significant differences. Although the reduction in phycobiliproteins was significant as compared to the control (seawater), it did not affect the seaweed’s photosynthetic ability, as it was observed in respective daily growth rates. The average agar yield was observed higher at 100 mL L−1 of SFE as compared to the control; however, it was not statistically different from control and 50 mL L−1. The quality of agar is an important aspect, but it remains unchanged in our study; nevertheless, agar yield was increased SFE treated plant which would be considered for economic profitability while considering the seaweed for large-scale cultivation. Since Gracilaria corticata var. cylindrica was reported to produce low gel strength agar, their yield was also less compared to other agarophytes like Gelidium elegans grown in shrimp broodstock effluent (SBE) that reported higher yield of agar which was significantly (p < 0.05) higher than that in the seawater (Rabiei et al. 2016). It is worth mentioning that the agar contents in seaweeds varies with species, location, season, extraction methods, and environmental condition during culture (Martín et al. 2013; Santelices 1987). Higher sulphate content and higher 3,6-anhydrogalactose value was an indication that it contains inferior quality of agar whereas lower sulphate content (2.51 ± 0.21%), and higher 3,6-anhydrogalactose value (31.21 ± 2.02%) was reported from Gracilaria dura known for the superior quality of agar (Sambhwani et al. 2022).
Conclusion
Finally, as per the results of this study, we can conclude that development of cultivation technology by applying SFE as nutrients for early seedling development. However, the growth and performance of seedlings need to be assessed at open sea cultivation to confirm the feasibility of using endemic red seaweed Gracilaria corticata var. cylindrica for large-scale cultivation. Effective utilization of SFE leads to the development of low-cost treatment techniques and conversion of wastewater into resource. However, further research needs to be done for the essential elemental composition and make necessary adjustments before utilizing SFE in cultivation technique. If we could able to cultivate this species on large scale, it would be a surprisingly add-on to the requirement of low gel strength agar for the formulation of special food for people suffering from dysphasia.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Badraeni, Azis HY, Tresnati J, Tuwo A (2020) Seaweed Gracilaria changii as a bioremediator agent for ammonia, nitrite and nitrate in controlled tanks of Whiteleg Shrimp Litopenaeus vannamei. IOP Conf Ser Earth Environ Sci 564(1):012059
Bixler HJ, Porse H (2011) A decade of change in the seaweed hydrocolloids industry. J Appl Phycol 23:321–335
Das RR, Sarkar S, Saranya C, Esakkiraj P, Aravind R, Saraswathy R et al (2022) Co-culture of Indian white shrimp, Penaeus indicus and seaweed, Gracilaria tenuistipitata in amended biofloc and recirculating aquaculture system (RAS). Aquaculture 548:737432
Dawange P, Jaiswar S (2020) Effects of Ascophyllum marine plant extract powder (AMPEP) on tissue growth, proximate, phenolic contents, and free radical scavenging activities in endemic red seaweed Gracilaria corticata var. cylindrica from India. J Appl Phycol 32:4127–4135
Dawes CJ, Luisma AD, Trono CG (1993) Clonal propagation of Eucheuma denticulatum and Kappaphycus alvarezii for Philippine seaweed farms. Hydrobiologia 260:379–438
DeBusk TA, Blakeslee M, Ryther JH (1986) Studies on the outdoor cultivation of Ulva lactuca L. Botanica Marina 29:381–386
Di Rienzo JA, Casanoves F, Balzarini MG, Gonzalez L, Tablada M, Robledo CW, Infostat version (2018) Cetnro de Transferencia Infostat, Faculted de Ciencias Agropecuarias, Universidad Nacional de Cordoba, Argentina
De Martino R, Mariot LV, da Silva FZ, Simioni C, do Amaral Carneiro MA, Oliveira ER, Hayashi L (2021) Effects of biofloc effluent in different regimes as a fertilizer for Kappaphycus alvarezii: indoor maintenance and sea cultivation. J Appl Phycol 33:3225–3237. https://doi.org/10.1007/s10811-021-02539-4
Devi IRP, Gowri VS (2007) Biological treatment of aquaculture discharge waters by seaweeds. I Control Pollut 23(1):135–140
Dodds WK, Smith VH, Zander B (1997) Developing nutrient targets to control benthic chlorophyll levels in streams: a case study of the Clark Fork River. Water Res 31:1738–1750
Dubois M, Gilles KA, Hamilton JK, Rebers PT, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
FAO (2020) The state of world fisheries and aquaculture 2020. In: Sustainability in Action. FAO, Rome, p 32
Ge J, Zhang Y, Heo YJ, Park SJ (2019) Advanced design and synthesis of composite photocatalysts for the remediation of wastewater: a review. Catalysts 9(2):122. https://doi.org/10.3390/catal9020122
Harrison PJ, Hurd CL (2001) Nutrient physiology of seaweeds: application of concepts to aquaculture. Cah Biol Mar 42:71–82
Hayashi L, Yokoya NS, Ostini S, Pereira RTL, Braga ES, Oliveira EC (2008) Nutrients removed by Kappaphycus alvarezii (Rhodophyta, Solieriaceae) in integrated cultivation with fishes in recirculating water. Aquaculture 277:185–191
https://mpeda.gov.in/?page_id=438. (Accessed on 16 Nov 2022)
https://www.persistencemarketresearch.com/market-research/dysphagia-diet-thickening-agents-market.asp. (Accessed on 28 Jun 2022)
https://thefishsite.com/articles/how-to-manage-water-effluent-from-shrimp-farms. (Accessed on 16 Nov 2022)
Hurd CL, Harrison PJ, Bischof K, Lobban CS (2014) Seaweed ecology and physiology, ed. 2. Cambridge University Press, Cambridge, UK. 551 pp
Jeffrey ST, Humphrey GF (1975) New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem Physiol Pfanz 167:191–194
Krishnamurthy V (1991) Gracilaria resources of India with particular reference to Tamil Nadu Coast. Seaweed Resour Utilisat 14:1–7
Mao Y, Yang H, Zhou Y, Ye N, Fang J (2009) Potential of the seaweed Gracilaria lemaneiformis for integrated multi-trophic aquaculture with scallop Chlamys farreri in North China. J Appl Phycol 21(6):649–656
Marinho-Soriano E, Panucci RA, Carneiro MAA, Pereira DC (2009) Evaluation of Gracilaria caudata J. Agardh for bioremediation of nutrients from shrimp farming wastewater. Bioresour Technol 100(24):6192–6198. https://doi.org/10.1016/j.biortech.2009.06.102
Marinho-Soriano MC, Moreira WSC (2002) Cultivation of Gracilaria (Rhodophyta) in shrimp pond effluents in Brazil. Aquaculture Res 33:1081–1086
Martín LA, Rodríguez MC, Matulewicz MC, Fissore EN, Gerschenson LN, Leonardi PI (2013) Seasonal variation in agar composition and properties from G racilaria gracilis (G racilariales, R hodophyta) of the Patagonian coast of Argentina. Phycol Res 61(3):163–171
Martins MA, da Silva VF, Tarapuez PR, Hayashi L, Vieira FN (2020) Cultivation of the seaweed Ulva spp. with effluent from a shrimp biofloc rearing system: different species and stocking density. Boletim do Instituto de Pesca 46(3):1–6
Meena R, Prasad K, Ganesan M, Siddhanta AK (2008) Superior quality of agar from Gracilaria species (Gracilariales, Rhodophyta) collected from the gulf of Mannar, India. J Appl Phycol 20:397–402
Msuya FE, Neori A (2002) Ulva reticulata and Gracilaria crassa: macroalgae that can biofilter effluent from tidal fish ponds in Tanzania Western Indian Ocean. J Mar Sci 1:117–126
Ozen M, Kozak A, Dere S, Kizilkaya IT (2018) The effects of different salt concentrations on the biochemical contents of Gracilaria gracilis Greville (Rhodophyta). Celal Bayar Univ J Sci 14:303–307
Porse H, Rudolph B (2017) The seaweed hydrocolloid industry: 2016 updates, requirements, and outlook. J Appl Phycol 29:2187–2200. https://doi.org/10.1007/s10811-017-1144-0
Qin Y (2018) Seaweed bioresources. In: Bioactive seaweeds for food applications. Natural ingredients for healthy diets. Elsevier, pp 3–24. https://doi.org/10.1016/B978-0-12-813312-5.00001-7
Rabiei R, Phang SM, Lim PE, Salleh A, Sohrabipour J, Ajdari D, Zarshenas GA (2016) Productivity, biochemical composition and biofiltering performance of agarophytic seaweed, Gelidium elegans (Red algae) grown in shrimp hatchery effluents in Malaysia. Iran J Fish Sci 15(1):53–74
Santelices B (1987) The wild harvest and culture of the economically important species of Gelidium in Chile. Case studies of seven commercial seaweed resources; FAO Fish Tech Pap (FAO) (281):165–192
Sambhwani K, Mathukiya G, Dawange PS, Sequeira RA, Prasad K, Mantri VA (2022) Analysis of functional traits in Gracilaria dura (Rhodophyta: Gracilariacae) reveals variation in wild and farmed populations. J Appl Phycol 32:1961–1969
Sampath-Wiley P, Neefus CD (2007) An improved method for estimating R-phycoerythrin and R-phycocyanin contents from crude aqueous extracts of Porphyra (Bangiales, Rhodophyta). J Appl Phycol 19:123–129
Veeragurunathan V, Eswaran K, Malarvizhi J, Gobalakrishnan M (2015) Cultivation of Gracilaria dura in the open sea along the southeast coast of India. J App Phycol 27:2353–2365
Xu Y, Fang J, Wei W (2008) Application of Gracilaria lichenoides (Rhodophyta) for alleviating excess nutrients in aquaculture. J Appl Phycol 20:199–203
Yaphe W, Arsenault GP (1965) Effect of acetaldehyde, acetic acid and ethanol on the resorcinol test for fructose. Anal Biochem 13:133–142
Zhou Y, Yang H, Hu H, Liu Y, Mao Y, Zhou H, Xu X, Zhang F (2006) Bioremediation potential of the macroalga Gracilaria lemaneiformis (Rhodophyta) integrated into fed fish culture in coastal waters of north China. Aquaculture 252(2–4):264–276
Acknowledgements
The authors would like to thank the Director, CSIR-Central Salt and Marine Chemicals Research Institute, Bhavnagar for providing facilities. This communication has CSMCRI PRIS approval number 110/2022.
Funding
The authors would like to thank Science and Engineering Research Board (SERB), New Delhi (EEQ/2018/000562) for providing funding for this study.
Author information
Authors and Affiliations
Contributions
Chetna M. Zala conducted the experiments; Pankaj S. Dawange and Nikunj Balar wrote the manuscript and data analysis; Santlal Jaiswar conceptualized and planning of the work, data analysis, interpretation, and manuscript preparation. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethical approval
No animals were used in this study.
Consent for publication
All authors agree to give their consent for publication.
Conflict of interests
The authors declare no competing interests.
Additional information
Handling editor Ronan Sulpice
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jaiswar, S., Dawange, P.S., Zala, C.M. et al. Effect of shrimp farm effluent (SFE) on growth, survival, regeneration, and biochemical composition in indigenous red seaweed Gracilaria corticata var. cylindrica. Aquacult Int 31, 1389–1400 (2023). https://doi.org/10.1007/s10499-023-01055-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-023-01055-1