Abstract
The rhodophyte seaweed genus Gracilaria is fast replacing Gelidium in global agar trade. Recently, Gracilaria dura from Indian waters has emerged as species of choice due to its quality agarose. The triphasic life cycle provides an opportunity for selecting an elite cultivar. Here we report diversity pertaining to functional traits over 3 months (January, February and March) among two distinct life phases, the tetrasporophyte and the female gametophyte. It was evident that functional trait related to growth was distinct in tetrasporophytes than in female gametophytes; while those of survival (antioxidant, proximate composition and pigments) were prominent in female gametophytes. Both the lowest 3.42 ± 0.38% day−1 (female gametophytes) and highest 5.17 ± 0. 21% day−1 (terasporophytes) growth rates were in January with 33% higher growth in the latter. Of the survival traits, antioxidant potential ranged from 0.51 ± 0.08 to 1.1 ± 0.03 mg g−1 FW ascorbic acid equivalent. Furthermore, female gametophytes collected in March reported 53.60%, 28.40% and 50.40% higher activity than the tetrasporophytes of January, February and March, respectively. The female gametophyte in March showed 23% and 19.40% higher protein content than the tetrasporophytes of January and February, respectively. Sixty-one metabolites were identified of which 29 were common, with sugars as the major portion (77.69% in February to 89.38% in January) and amine derivatives the least (0.30% in January to 0.51% in February). Studies of bio-ecophysiology of this alga are currently being undertaken by our group.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Food and Agricultural Organisation of the United Nations started tropical open-water cultivation of Gracilaria in Penang, Malaysia and Vedalai and Chinnapalarn, India under its seven-country initiative named Bay of Bengal Program (FAO 1991). The cultivation of Gracilaria edulis although abandoned in both villages due to non-availability of mature seed-stock, in-adequate water quality and abundance of grazers had set a tone for experimental farming. Further studies reported a yield of 3.5 to 4 kg fresh weight m−1 length of rope using various Gracilaria spp. either at experimental or pilot-scale farming (Raju and Thomas 1971; Umamaheswara Rao 1974; Subbaramaiah and Thomas 1990; Oza et al. 1994; Kaladharan et al. 1996). However, cultivation of Gracilaria was never been undertaken as a commercial venture and wild harvesting of the resource is still relevant for industrial production. The establishment of the Gulf of Mannar Marine National Park in 1986 strongly affected the industrial production of agar due to stringent conservation measures enforced by Department of Forest, Government of Tamil Nadu (Marirajan et al. 2012). There is thus the opportunity for undertaking commercial Gracilaria farming in India.
The genus Gracilaria is the second most diverse seaweed genus comprising around 28 species and 7 varieties in Indian waters (Oza and Zaidi 2001). The studies pertaining to G. dura (Meena et al. 2007), G. edulis [now Hydropuntia edulis], G. crassa [now G. canaliculata], G. foliifera, G. corticata (Meena et al. 2008) and G. debilis and G. salicornia (Mehta et al. 2010) confirmed the availability of superior indigenous feedstock, but industrial exploitation is limited only to G. edulis (Ganesan et al. 2019). The prospect of cottage-scale agar production in India has remained unchanged as industrial production is often family-run enterprises, with manufacturing protocols heavily relying on traditional energy-intensive processes and the absence of national standard specifications with respect to gel strength (Mantri et al. 2019). Amidst these shortcomings, G. dura is considered a potential species with high commercial prospects. Besides robust growth, its applications such as a plant bio-stimulant, imparting drought tolerance, pigments and high-value agarose obtained from patented technology, have attracted considerable industrial attention (Meena et al. 2014; Baghel et al. 2015; Veeragurunathan et al. 2015a, b; Mantri et al. 2020; Sharma et al. 2019). Furthermore, this species has been reported as the potential source of agar from Mediterranean region (Murano et al. 1992; Marinho-Soriano et al. 2005). The haploid-diploid life cycle in which free-living tetrasporophytes alternate with independent female as well as male gametophytes is not much investigated. The Polysiphonia-type or triphasic life cycle consists of identical gametophytes and sporophytes, with physiological (biochemical) and genetic (molecular) differences (Gupta et al. 2011). The phenology of this species in nature is reported with dis-proportionate abundance (Mantri 2010); therefore, it is worthwhile to ascertain differences in life cycle stages related to functional traits of economic importance.
The applied aspects pertaining to Gracilaria farming in India have so far focused on elucidating effects of growth-promoting substances, tissue culture, protoplast fusion and developing molecular markers (Oza 1971; Kumar et al. 2007; Baghel et al. 2011; Gupta et al. 2011). Experimental or pilot-scale farming of Gracilaria has been attempted in Indian waters (reviewed in Ganesan et al. 2017), and the National Fisheries Development Board has recently implemented two missions, related to giving impetus to commercial farming of Gracilaria both at the south-east coast (Tamil Nadu) and north-west coast (Gujarat) India, respectively (Mantri et al. 2020). This has necessitated supply of elite germplasm to the commercial growers.
Vegetative clones have been exclusively used for this alga in large-scale operations, thereby providing opportunity for selecting elite cultivars. It may be noted that the selection of cultivar also involves confirming the persistence of characters besides its superiority (Santelices 1992). Nevertheless, reliable screening criteria for choosing desired trait for biomass development are not yet practised in Indian agarophytes (Mantri et al. 2020; Veeragurunathan et al. 2019). The present investigation thus presents initial steps in ascertaining the diversity pertaining to functional traits of potential interest over 3 months in G. dura from Indian waters. The detailed characterisation would be helpful for identification, selection and improving the efficiency of germplasm based on different life cycle phases for developing new cultivars.
Materials and methods
Collection and identification of life phases
Individuals of Gracilaria dura were collected at low tide from the coast of Veraval (20.910404° N, 70.351273° E), Gujarat, India, in January, February and March 2019. Healthy live fronds were collected and brought to the laboratory immediately under cool conditions. Although occurrence of this alga along the coast of Veraval was reported during December to May (Mantri 2010), well-developed gametophytic and sporophytic plants only appeared from January to March and this study was restricted to these 3 months. Epiphytes and some adhered particles were removed from the collected samples by cleaning with filtered seawater. The type of individual was identified by viewing transverse section under the light microscope. According to the type of reproductive structures (presence of tetrasporangia for tetrasporophyte and cystocarps for female gametophyte), they were segregated.
Experimental design
The cleaned samples collected in each month were divided into two groups, i.e. one was maintained in modified Erdschreiber seawater (ESS) culture medium (Suto 1959) for growth rate experiment. All the cultures were aerated with ambient air under controlled light intensity (55 μmol photons m−2 s−1), photoperiod (12:12 h L/D), temperature (25 °C ± 1) and salinity (35 psu). The other group was processed immediately for proximate and metabolic analysis.
Determination of growth
Apical segments of size approx. 2 cm were excised from each group of fronds (tetrasporophyte and female gametophyte). Five of these fragments were then selected by mass of approx. 1 g and placed in 500-mL flasks containing ESS medium (35 psu salinity). All the flasks were subjected to 24 h aeration and the mouth of each flask was closed with a sponge (plugged). The experiment was carried out in triplicates (n = 3) under laboratory conditions as described above. The medium was replenished every alternate day during which fronds were cleaned with a brush to prevent fouling. After 2 weeks of experimental setup of each month, the fronds were weighed and their daily growth rate was estimated using the formula \( \left[\left\{{\left(\frac{W\mathrm{f}}{W\mathrm{i}}\right)}^{\frac{1}{t}}\right\}-1\right]\times 100 \) where, t =time (days), Wi = initial mass and Wf = final mass (Gupta et al. 2011).
Total antioxidant activity estimation
To estimate total antioxidant potential, 100 mg of frozen sample was extracted twice sequentially, initially with ultrapure water and then with 70% methanol of volume 1 mL and both the extracts were then pooled and analysed spectrophotometrically according to Prieto et al. (1999). Briefly, the extract was mixed with 1 mL of reagent solution containing 0.6 M H2SO4, 28 mM sodium phosphate and 4 mM ammonium molybdate. Following incubation at 100 °C for 60 min with ascorbic acid as standard, the absorbance was measured at 695 nm.
Pigment analysis
For analysis of R-phycoerythrin (R-PE) and R-phycocyanin (R-PC), 100 mg of fresh algal tissues was homogenised using liquid nitrogen in mortar and pestle. The homogenate was added to 0.8 mL of 0.1 M phosphate buffer (pH 6.8) and incubated overnight at 4 °C. After incubation, homogenised samples were vortexed and centrifuged at 15,000×g for 10 min at 4 °C. The supernatant was collected and the residue was re-extracted using 0.2 mL phosphate buffer ensuring complete pigment extraction. The absorbance scan was recorded using a dual-beam UV-visible spectrophotometer. For the analysis of chlorophyll-a (Chl-a), the same process was applied using 90% acetone as solvent. The contents of R-PE and R-PC were calculated according to Sampath-Wiley and Neefus (2007) and the chlorophyll-a content was determined according to Jeffrey and Humphrey (1975).
Protein extraction
Individual fronds weighing 100 mg were crushed in liquid nitrogen and subjected to alkaline digestion for protein extraction using 1 mL 1 N sodium hydroxide. The solution was incubated at 80 °C for 1 h for easy protein liberation from the biomass and was then cooled at room temperature and centrifuged. The supernatant was neutralised using 6 N HCl. The protein content of the supernatant was determined by the Lowry method with bovine serum albumin (BSA) as standard (Lowry et al. 1951).
Lipid extraction
Lipid extraction was carried out by the Bligh and Dyer (1959) method. Briefly, 1 g of fresh homogenised algae (crushed using liquid nitrogen) was mixed with chloroform and methanol in the ratio of 1:2. The solution was stirred for 30 min to enhance lipid extraction. Re-extraction was done to ensure complete lipid extraction followed by adding an equal volume of water. By centrifugation, two phases were formed and the lower organic phase was collected. It was then kept in hot air oven overnight to remove the solvent and the total lipid was determined gravimetrically. Then, it was treated with 1% methanolic NaOH at 55 °C for 15 min followed by methanolic HCl at 55 °C for 15 min for methyl ester conversion and analysed for fatty acid composition by GC-MS (QP-2010, Shimadzu, Japan).
Carbohydrate extraction
One hundred-milligram crushed samples were subjected to acid digestion for total carbohydrate extraction. Two hundred fifty microlitres of conc. H2SO4 was added to the homogenised tissue and the solution was kept at room temperature for 30 min. This was followed by addition of 3 mL distilled water to the solution and incubation at 121 °C for 1 h. The solution was then vortexed and neutralised with CaCO3. The supernatants were collected after centrifugation at 9500 rpm for 10 min and the carbohydrate content was estimated spectrophotometrically by the phenol and sulphuric acid method at 490 nm. Galactose was used as standard.
Metabolite profiling
Metabolite profiling was performed by gas chromatography coupled with a mass spectrometer (GC-MS QP-2010, Shimadzu, Japan). A total of 100 mg powder crushed tissue were taken and 0.75 mL of methanol (ice cold) and 50 μL of ribitol (0.2 mg mL−1stock) were added. Extraction was carried out at 70 °C for 15 min followed by centrifugation for 5 min at 10,000 rpm. To the supernatant, 750 μL water and 325 μL chloroform (ice cold) were added. The mixture was then vortexed and centrifuged for 10 min at 5000 rpm. The polar phase (water/methanol) was separated from the non-polar fraction into a new tube. Five hundred microlitre of polar fraction was dried in speed vacuum concentrator. For derivatisation, the dried pellet was reconstituted in 60 μL of 20 mg mL−1 solution of methoxyamine hydrochloride in pyridine and shaken at 37 °C for 120 min. Afterward, 130 μL of N-methyl-trimethylsilyl trifluoroacetamide (MSTFA) was added and shaken at 37 °C for 30 min (Lisec et al. 2015). It was then analysed with the thermal program of 80 °C as initial temperature with 2 min hold, from 10 °C increment to 315 °C with hold for 1 min and final temperature 250 °C with total run time of 40 min. The compounds were identified on their m/z ratio with the help of NIST library. The metabolites were quantified by calculating the relative response ratio of the peak area of individual metabolites to that of internal standard adonitol.
Statistical analysis
In all experiments, a minimum of three biological replicates were taken and all the data are expressed as mean ± standard deviation. Data analysis was carried out by two-way analysis of variance, i.e. phase (tetrasporophyte and female gametophyte) and month of collection (January, February and March) with a significance difference level of p < 0.05 (Table 1). The functional traits were further analysed using Tukey’s post hoc test with the help of software Infostat (Di Rienzo et al. 2018).
Results
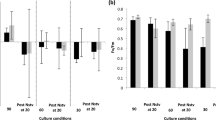
The collections acclimatised well to the culture conditions employed during the study period. DGR for tetrasporophytes over the collection months, i.e. January, February and March was statistically similar and the same was seen for female gametophytes. However, in between the life phases, initial assemblage, i.e. January collection showed 33% higher growth rate for tetrasporophytes than female gametophytes of same collection (F = 6.57, p = 0.024) but in subsequent collections, growth rate was similar for both phases (Fig. 1a).
a Daily growth rate (DGR) over a period of 15 days from acclimatised field material to the laboratory condition of 25 °C temperature, 55 μmol photons m -2s-1 of light intensity, 12 h light period and 35 psu salinity. b Total antioxidant activity equivalent to ascorbic acid (AA) from the field-collected biomass. Different letters above bars depict significant difference between phases (ANOVA, p < 0.05). Data shown are the mean ± SD (n = 3)
The antioxidant potential ranged from 0.51 ± 0.08 to 1.1 ± 0.03 mg ascorbic acid (AA) equivalent g−1 FW. In the female gametophytes, a steady increase was observed in the activity over the subsequent months (from January to March) whereas for tetrasporophytes, February collection showed more activity than January, which again declined in March. Besides, March-collected female gametophytes showed 53.60, 28.40 and 50.40% higher antioxidant potential than the tetrasporophytes of January, February and March, respectively (F = 65.85, p < 0.0001) (Fig. 1b).
Protein content for all tetrasporophyte collections was statistically similar over the months and the same trend was observed in female gametophytes. However, among the life phases, female gametophytes collected during March showed 23, 19.40 and 19.66% higher protein content than the tetrasporophytes of January, February and March, respectively (F = 25.34, p = 0.0003). For the carbohydrate content within the phases, February-collected female gametophytes showed 37.27% higher carbohydrate content than the tetrasporophytes of January (F = 6.23, p = 0.028). Nevertheless, the lipid content showed decrease over the subsequent collection month considering both life forms. The highest was in January followed by February and March. The maximum value of 0.44% ± 0.06 FW was found in female gametophytes of January while the lowest of 0.18% ± 0.02 FW was observed in tetrasporophytes of March (Fig. 2a–c). Further, the fatty acid composition showed more polyunsaturated fatty acids (PUFA) than saturated fatty acids (SFA) with 3-fold difference. Percentile composition matched in collections of all the months and between the phases, but actual concentration varied. Comparatively, female gametophytes showed higher concentration for SFA (28% in January; 36.75% in February and 62.47% in March), monounsaturated fatty acids (MUFA) (20.25% in January; 44.15% in February and 33.53% in March) and PUFA (28.67% in January; 45.48% in February and 62.73% in March).
Figure 3a–c depicts the pigment contents. The Chl-a content of tetrasporophytes was higher in February compared with the January collection, whereas the March samples showed intermediate values. For the Chl-a content of the female gametophytes, it was statistically similar over the months. Comparing among the phases, March and February female gametophytes had 63.32% and 65.07%, respectively, higher chlorophyll content than the January-collected tetrasporophytes (F = 13.26, p = 0.003). The R-PC content of female gametophytes showed an increasing trend over the months, but it was statistically similar for tetrasporophytes. The March-collected female gametophytes had a 57.80% higher R-PC content than the January terasporophytes (F = 8.88, p = 0.011). Similarly, March-collected female gametophytes had a 65.10% higher R-PE content than January tetrasporophytes (F = 14.26, p = 0.002).
In metabolite profiling, we recorded a total of 61 metabolites incorporating sugars, amino acids, amine derivatives, fatty acids, polyols, organic acids and organooxy compounds (Supplementary material Table 1). A total of 29 metabolites were common in all 3 months, and among these, sugars were the major portion (77.69% in February to 89.38% in January) and amine derivatives (0.30% in January to 0.51% in February) were the least (Supplementary material Fig. 1a, 2a). Within phases, 52 metabolites were common (Supplementary material Fig. 1b, 2b). The sugars were highest and amine derivatives were least. Mannobiose was highest of all, i.e. 4-fold higher in January collection in both the phases compared with February and March collection. Together, 22 metabolites were common between phases and collection months. The concentration of each was subjected to log10 transformation for normalisation and a common pattern of decline in concentration among the subsequent collections was observed (Fig. 4).
Discussion
Gracilaria, being a prominent feedstock for industrial agar production, is cultivated extensively replacing Gelidium and substituting the wild harvest (Porse and Rudolph 2017). This is primarily due to harvest ban from Mediterranean countries coupled with decline in wild populations due to overharvesting. Despite availability of several local agrophytes, G. dura has gained prominence due to its high agarose content (Meena et al. 2007). The cultivation practices have been standardised and large-scale farming has been successfully implemented (Mantri et al. 2020). Genetic improvement studies through breeding, mutation and chromosome manipulations remain inconclusive in Gracilaria (Van der Meer and Bird 1977, Patwary 1987; Zhang and Fei 1990; Patwary and Van der Meer 1992). It is evident from the studies that different cytological processes involved in reproduction (Scrosati and DeWreede 1999) and niche partitioning (Destombe et al. 1989) are responsible for competitive abundance of life cycle stages at given point of time. Thus, strain selection with improved functional trait from wild population is essential for developing and selecting elite germplasm. With this leitmotif, we have studied different functional traits pertaining to biochemical and growth characters in tetrasporophyte and female gametophyte phases during different time intervals.
The results indicated that female gametophytes differ from tetrasporophytes considerably over the collection months among functional traits studied. The investigations on effect of culture conditions on growth have been well illustrated the but influence of life cycle stage on growth is seldom studied (DeBusk and Ryther 1984; Raikar et al. 2001). The growth rate with respect to life cycle stages has been documented in G. lemaneiformis (Zheng and Gao 2009), G. tenuistipitata [now Agarophyton tenustipitatum] (Skriptsova and Nabivailo 2009), G. arcuata, G. incurvata, G. textorii, G. lichenoides [now Hydropuntia edulis] and G. foliifera (Raikar et al. 2001). In the present study, DGR was higher in tetrasporophytes initially in January but was then similar for both phases in March. It may be noted that high growth in tetrasporophytes of G. caudata [now Crassiphycus caudatus] has been reported (Araújo et al. 2014) together with the report by Barufi et al. (2010) where higher growth rate was observed in tetrasporophytes compared with female gametophytse for G. tenuistipitata in the first week and almost similar in following weeks for both the phases. Furthermore, the differences with respect to growth rates initially can be related to the different nutritional needs, which are advantageous for the survival of the species in heterogeneous environments (Ursi and Plastino 2001), but the inconsistency in growth is also essentially linked to environmental conditions. These variations favour specific life phase under wild conditions to ascertain the maintenance of taxa for a given point of time. But this approach may be successfully exploited for selecting specific life stage for specific farming site that would act as most conducive environment (Araújo et al. 2014).
It is considered that the antioxidant activity of seaweeds is caused not only by phenolic compounds but also by other water-soluble compounds, i.e. phycobiliprotein (Kuda et al. 2006). The sequential extraction performed in this study with both water and methanol was helpful in documenting the higher values than in individual solvent extraction. It may be further noted that significantly high values were observed in gametophytic phase of March collection. This dominance can be explained by the fact that by this time, the alga reaches its reproductive maturity. This period is characterised by high seawater temperature, longer daylength coupled with elevated light intensity which leads to ceased growth and elevated reproductive activities. The pre-dominance of tetrasporophytes over gametophytes in G. dura has been documented (Mantri 2010). The diploid nature of tetrasporophyte was responsible for higher viability in this phase in G. verrucosa [now Gracilariopsis longissima] (Destombe et al. 1989) and G. domingensis (Guimarães et al. 1999). The higher antioxidant response by female gametophytes (March samples) suggests that this life phase struggles to maintain its viability. Therefore, one might need to preferably select sporophytic life phase during summer month for farming.
The proximate content was evident with significant difference in both life phases during different collections. The total carbohydrate content values observed were similar in range as described in Gracilaria spp. as per Hong et al. (2007). The protein content observed in present study was low, but the lipid percentage was similar to the different Gracilaria spp. studied previously (Kumar et al. 2011). Considering fatty acid composition, higher PUFA to SFA ratio is important, which was found to be 2.6 that falls within the prescribed nutritional guidelines. The pigment contents followed a similar trend to that of proximate composition. The March- and February-collected female gametophyte showed higher pigment content than January-collected tetrasporophytes (Chl-a and R-PE). Considering R-PC, the March female gametophytes showed dominance over the January tetrasporophytes and also gradual increase was observed over the months with the intermediate values in February collection. Guillemin et al. (2014) also demonstrated higher pigment content for vegetative stage of female gametophytes in G. chilensis [now Agarophyton chilense]. It may be also noted that the pigments were in a positive correlation with the growth only in the case of female gametophyte. Nevertheless, in the case of tetrasporophyte, growth was not co-related with pigments. Araújo et al. (2014) on the contrary reported that higher pigment content (Chl-a; carotenoinds, R-PE, R-PC and APC) in tetrasporophytes contributed to higher viability of this phase when compared with female gametophyte in G. caudata.
Gracilaria is a rich source of sulphated polysaccharide; thus, in the metabolic profiling, we observed more number and concentration of sugars which included the precursors of sulphated polysaccharides, i.e. xylose, mannobiose, maltose, galactose and glucose. Polyols were also higher in February as well as in March. They are considered the main storage compounds. The analysis also reported a number of antioxidant organooxy compounds such as gluconoheptolactone that is also a precursor to ascorbic acid biosynthesis (Belghit et al. 2017). The increase in the number of metabolic compounds with the collection intervals in the present investigation might be possibly due to the metabolic switching towards phenological abundance of life cycle stages or seasonal acclimation of alga. There are reports describing the differences in agar yield and viscosity among the life phases of G. bursapastoris and G. dura (Marinho-Soriano et al. 1999; Baghel et al. 2011; Gupta et al. 2011), but to the best of our knowledge, this is the first study describing the subtle differences of proximate content, metabolite profile together with the different life phases of G. dura. It was amply clear from the present investigation that functional trait related to growth (DGR) was more pronounced in tetrasporophytes than in female gametophytes, while those of survival (antioxidant, proximate composition and pigments) were prominent in later-collected female gametophytes. These observations were in accordance with results reported in G. chilensis on fertility potentials and survival of haploid and diploid life cycle stages and could be associated with differentiation on evolution and maintenance of gametophytes and tetrasporophytes life cycles (Vieira et al. 2018).
Gracilaria has played an important role in Indian agar industry since its inception, but unfortunately, feedstock is being harvested from natural resource. Considering potential demand in pharmaceutical as well as processed food industry, there has been consistent effort for the last 3 to 4 years to promote agarophyte farming through various government and non-government agencies. The hydrocolloid from the farmed biomass has been recently validated by leading agar manufactures and they have envisaged interest to procure the farmed biomass. In conclusion, isomorphic gametophytes and tetrasporophytes collected from the same location harboured unique traits and responded differently based on time of collection. The current practise of selecting seed from natural stock without knowing life cycle stage for farming is not apt for sustainable production. The study clearly demonstrated influence of life cycle–based material in selecting different functional traits of commercial importance, the knowledge which dictates essential features for selecting particular life forms as seed for successful farming as well as management practices. Finally, our results reveal that tetrasporophytes were more competitive in terms of growth while the other traits related to accumulation of proximate compounds, antioxidant potentials and concentration of pigments were superior in female gametophyte considering over different collection months. This provides greater opportunity for selection of cultivar-based functional traits of economic interest in red algal farming such as G. dura which is domesticated recently. It may also be noted that detailed bio-ecophysiology of this alga is being undertaken by our group to understand this agarophyte which is highly potential for the industry.
References
Araújo FO, Ursi S, Plastino EM (2014) Intraspecific variation in Gracilaria caudata (Gracilariales, Rhodophyta): growth, pigment content, and photosynthesis. J Appl Phycol 26:849–858
Baghel RS, Kumari P, Bijo AJ, Gupta V, Reddy CRK, Jha B (2011) Genetic analysis and marker assisted identification of life phases of red alga Gracilaria corticata (J. Agardh). Mol Biol Rep 38:4211–4218
Baghel RS, Trivedi N, Gupta V, Neori A, Reddy CRK, Lali A, Jha B (2015) Biorefining of marine macroalgal biomass for production of biofuel and commodity chemicals. Green Chem 17:2436–2443
Barufi JB, De Oliveira EC, Plastino EM, De Oliveira MC (2010) Life history, morphological variability and growth rates of the life phases of Gracilaria tenuistipitata (Rhodophyta: Gracilariales) in vitro. Sci Mar 74:297–303
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Belghit I, Rasinger JD, Heesch S, Biancarosa I, Liland N, Torstensen B, Waagbø R, Lock E-J, Bruckner CG (2017) In-depth metabolic profiling of marine macroalgae confirms strong biochemical differences between brown, red and green algae. Algal Res 26:240–249
DeBusk TA, Ryther JH (1984) Effects of seawater exchange, pH and carbon supply on growth of Gracilaria tikvaiae (Rhodophyceae) in large scale cultures. Bot Mar 27:357–362
Destombe C, Valero M, Vernet P, Couvet D (1989) What controls haploid—diploid ratio in the red alga, Gracilaria verrucosa? J Evol Biol 2:317–338
Di Rienzo J A, Casanoves F, Balzarini M G, Gonzalez L, Tablada M, Robledo CW InfoStat versión (2018). Centro de Transferencia InfoStat, Facultad de Ciencias Agropecuarias, Universidad Nacional de Córdoba, Argentina. URL http://www.infostat.com.ar
FAO (1991). Seaweed (Gracilaria edulis) farming in Vedalai and Chinnapalam. Bay of Bengal Programme Small Scale Fisher Folk Communities. (Eds.) Kalkam I, Rajendran I, Angell CL. Food and Agriculture Organization of United Nations, Rome Publication BOBP/WP/65, 11 pp.
Ganesan M, Eswaran K, Reddy CRK (2017) Farming of agarophytes in India - a long-time sustainability for the industry and preserving wild stocks. J Appl Phycol 29:2239–2248
Ganesan M, Trivedi N, Gupta V, Venu Madhav S, Reddy CRK, Levine I (2019). Seaweed resources in India – current status of diversity and cultivation: prospects and challenges. Bot Mar. https://doi.org/10.1515/bot-2018-0056
Guillemin ML, Valenzuela P, Gaitán-Espitia JD, Destombe C (2014) Evidence of reproductive cost in the triphasic life history of the red alga Gracilaria chilensis (Gracilariales, Rhodophyta). J Appl Phycol 26:569–575
Guimarães M, Plastino EM, Oliveira ECD (1999) Life history, reproduction and growth of Gracilaria domingensis (Gracilariales, Rhodophyta) from Brazil. Bot Mar 42:481–486
Gupta V, Baghel RS, Kumar M, Kumari P, Mantri VA, Reddy CRK, Jha B (2011) Growth and agarose characteristics of isomorphic gametophyte (male and female) and sporophyte of Gracilaria dura and their marker assisted selection. Aquaculture 318:389–396
Hong DD, Hien HM, Son PN (2007) Seaweeds from Vietnam used for functional food, medicine and biofertilizer. J Appl Phycol 19:817–826
Jeffrey ST, Humphrey GF (1975) New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem Physiol Pflanz 167:191–194
Kaladharan P, Vijayakumaran K, Chennubhotla VSK (1996) Optimization of certain physical parameters for the mariculture of Gracilaria edulis (Gmelin) Silva in Minicoy lagoon (Laccadive Archipelago). Aquaculture 139:265–270
Kuda T, Hishi T, Maekawa S (2006) Antioxidant properties of dried product of ‘haba-nori’, an edible brown alga, Petalonia binghamiae (J. Agaradh) Vinogradova. Food Chem 98:545–550
Kumar GR, Reddy CRK, Jha B (2007) Callus induction and thallus regeneration from callus of phycocolloid yielding seaweeds from the Indian coast. J Appl Phycol 19:15–25
Kumar M, Kumari P, Trivedi N, Shukla MK, Gupta V, Reddy CRK, Jha B (2011) Minerals, PUFAs and antioxidant properties of some tropical seaweeds from Saurashtra coast of India. J Appl Phycol 23:797–810
Lisec J, Schauer N, Kopka J, Willmitzer L, Fernie AR (2015) Corrigendum: gas chromatography mass spectrometry-based metabolite profiling in plants. Nat Protoc 10:10–1038
Lowry O, Rosebrough N, Farr A, Randall R (1951) Protein measurement with Folin phenol reagent. J Biol Chem 193:265–275
Mantri VA (2010). Studies on biology of Gracilria dura (C. Agardh) J. Agardh. PhD Thesis, Bhavnagar University, Bhavnagar, India 119 pp.
Mantri VA, Shah Y, Thiruppathi S (2020) Feasibility of farming the agarose-yielding red alga Gracilaria dura using tube-net cultivation in the open sea along the Gujarat coast of NW India. Appl Phycol 1:12–19
Mantri VA, Ganesan M, Gupta V, Krishnan P, Siddhanta AK (2019) An overview on agarophyte trade in India and need for policy interventions. J Appl Phycol 31:3011–3023
Marinho-Soriano E, Bourret E (2005). Polysaccharides from the red seaweed Gracilaria dura (Gracilariales, Rhodophyta). Bioresour Technol 96(3):379–382
Marinho-Soriano E, Bourret E, De Casabianca ML, Maury L (1999) Agar from the reproductive and vegetative stages of Gracilaria bursa-pastoris. Bioresour Technol 67:1–5
Marirajan T, Hoon V, Alicea E (2012) Socio economic monitoring for coastal managers of South Asia: field trials and baseline surveys in Gulf of Mannar region, South Tamilnadu, India: project completion report, NA10NOS4630055
Meena R, Prasad K, Ganesan M, Siddhanta AK (2008) Superior quality agar from Gracilaria species (Gracilariales, Rhodophyta) collected from the Gulf of Mannar, India. J Appl Phycol 20:397–402
Meena R, Siddhanta AK, Prasad K, Ramavat BK, Eswaran K, Thiruppathi S, Rao PS (2007) Preparation, characterization and benchmarking of agarose from Gracilaria dura of Indian waters. Carbohydr Polym 69:179–188
Meena R, Chaudhary JP, Agarwal PK, Maiti P, Chatterjee S, Raval HD, Agarwal P, Siddhanta AK, Prasad K, Ghosh PK (2014) Surfactentinduced coagulation of agarose from aqueous extract of Gracilaria dura seaweed as an energy-efficient alternative to the conventional freeze-thaw process. RSC Adv 4:28093–28098
Mehta GK, Meena R, Prasad K, Ganesan M, Siddhanta AK (2010) Preparation of galactans from Gracilaria debilis and Gracilaria salicornia (Gracilariales, Rhodophyta) of Indian waters. J Appl Phycol 22:623–627
Murano E, Toffanin R, Zanetti F, Knutsen S H, Paoletti S, Rizzo R (1992). Chemical and macromolecular characterisation of agar polymers from Gracilaria dura (C. Agardh) J. Agardh (Gracilariaceae, Rhodophyta). Carbohyd Polym 18(3):171–178
Oza RM (1971) Effect of lAA on the growth of fragment of Gracilaria corticata. J Ag Seaweed Res Util 1:48–49
Oza R M, Tewari A, Rajyaguru M R, Goswamy S (1994). Laboratory and field culture of marine red alga Gracilaria verrucosa (Gracilariaceae, Rhodophyta). Indian J Mar Sci 23:157–161
Oza RM, Zaidi SH (2001) A revised checklist of Indian marine algae. CSMCRI, Bhavnagar, 296
Patwary MU, Van der Meer JP (1992) Genetics and breeding of cultivated seaweeds. Korean J Phycol 7:281–318
Patwary MU (1987) Frond composition and some physical characteristics of agars from female, male and tetrasporophyte clones of Gracilaria tikvhiae mutant MP-40. Bangladesh J Bot 16:211–224
Porse H, Rudolph B (2017) The seaweed hydrocolloid industry: 2016 updates, requirements, and outlook. J Appl Phycol 29:2187–2200
Prieto P, Pineda M, Aguilar M (1999) Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem 269:337–341
Raikar SV, Iima M, Fujita Y (2001) Effect of temperature, salinity and light intensity on the growth of Gracilaria spp. (Gracilariales, Rhodophyta) from Japan, Malaysia and India. Indian J Mar Sci 30:98–104
Raju PV, Thomas PC (1971) Experimental field cultivation of Gracilaria edulis (Gmel.) Silva. Bot Mar 14:71–75
Sampath-Wiley P, Neefus CD (2007) An improved method for estimating R-phycoerythrin and R-phycocyanin contents from crude aqueous extracts of Porphyra (Bangiales, Rhodophyta). J Appl Phycol 19:123–129
Santelices B (1992) Strain selection of clonal seaweeds. Prog Phycol Res 8:85–116
Scrosati R, DeWreede RE (1999) Demographic models to simulate the stable ratio between ecologically similar gametophytes and tetrasporophytes in populations of the Gigartinaceae (Rhodophyta). Phycol Res 47:153–157
Sharma S, Chen C, Khatri K, Rathore MS, Pandey SP (2019) Gracilaria dura extract confers drought tolerance in wheat by modulating abscisic acid homeostasis. Plant Physiol Biochem 136:143–154
Skriptsova AV, Nabivailo YV (2009) Comparison of three gracilarioids: growth rate, agar content and quality. J Appl Phycol 21:443–450
Subbaramaiah K, Thomas PC (1990) Raft cultivation of Gracilaria edulis (Gmel.) Silva. Proc Indian Acad Sci (Plant Sci) 100:123–127
Suto S (1959) Skeletonema no tame no jinkou baiyoueki. Suisan-Zouskoku. 7:17–19 [in Japanese]
Umamaheswara Rao M (1974) On the cultivation of Gracilaria edulis in the near shore areas around Mandapam. Curr Sci 43:660–661
Ursi S, Plastino EM (2001) Crescimento in vitro de linhagens de coloração vermelha e verde clara de Gracilaria sp. (Gracilariales, Rhodophyta) em dois meios de cultura: análise de diferentes estádios reprodutivos. Rev Bras Bot 4:585–592
Van der Meer JP, Bird NL (1977) Genetics of Gracilaria sp. (Rhodophyceae, Gigartinales) I. Mendelian inheritance of two spontaneous green variants. Phycologia 16:159–161
Veeragurunathan V, Eswaran K, Malarvizhi J, Gobalakrishnan M (2015a) Cultivation of Gracilaria dura in the open sea along the southeast coast of India. J Appl Phycol 27:2353–2365
Veeragurunathan V, Eswaran K, Saminathan KR, Mantri VA, Ajay G, Jha B (2015b) Feasibility of Gracilaria dura cultivation in the open sea on the South-eastern coast of India. Aquaculture 438:68–74
Veeragurunathan V, Prasad K, Malarvizhi J, Singh N, Meena R, Mantri VA (2019) Gracilaria debilis cultivation, agar characterization and economics: bringing new species in the ambit of commercial farming in India. J Appl Phycol 31:2609–2621
Vieira VMNCS, Engelen AH, Huanel OR, Guillemin ML (2018) Haploid females in the isomorphic biphasic life-cycle of Gracilaria chilensis excel in survival. BMC Evol Biol 18:174
Zhang XC, Fei XG (1990) Cultivation and hybridization experiments on Gracilaria tenuistipitata (Rhodophyta). In Current topics in marine biotechnology, Proceeding of the 1st International Marine Biotechnology Conference on Sep 4-6 in Tokyo p 203-214
Zheng Y, Gao K (2009) Impacts of solar UV radiation on the photosynthesis, growth, and UV-absorbing compounds in Gracilaria lemaneiformis (Rhodophyta) grown at different nitrate concentrations. J Phycol 45:314–323
Acknowledgements
The first author gratefully acknowledges the University Grant Commission (UGC). We are grateful to the Director, CSIR-CSMCRI for the encouragement. Thanks are also due to Dr. Monica G. Kavale for identifying life cycle phases during sampling and Mr. K. Vijayanand for helping in statistical analysis. Dr. Nikunj Balar, Ms. Tejal Gajaria, Mr. Shahrukh Siddiqui, Mr. Prashant More and Mr. Smit Goswami have rendered support during manuscript preparation.
Funding
This work was carried out by a grant received from Council for Scientific and Industrial Research (CSIR), New Delhi, Government of India.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 413 kb)
Rights and permissions
About this article
Cite this article
Sambhwani, K., Modi, J., Singhala, A. et al. Analysis of functional traits in female gametophytic and tetrasporophytic life phases of industrially important red alga Gracilaria dura (Rhodophyta: Gracilariacae). J Appl Phycol 32, 1961–1969 (2020). https://doi.org/10.1007/s10811-020-02116-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-020-02116-1