Abstract
To evaluate effects of microalga addition in the intensive Litopenaeus vannamei farming system, the amount of Picochlorum sp. in culture water was adjusted to be 1 × 104 cells/mL (KH_1), 2 × 104 cells/mL (KH_2), or 4 × 104 cells/mL (KH_4) respectively by artificial addition, and that without microalgal addition was set as the control. First, it was found that Picochlorum sp. addition was effective to reduce the total ammonia nitrogen (TAN), nitrite, phosphate, and chemical oxygen demand (COD) in culture water, and the water quality of KH_4 was the best among various groups after a 30-day culture, with reduction of 89.85% TAN, 78.17% nitrite, 50.00% phosphate, and 63.28% COD in comparison with the control. Second, addition of Picochlorum sp. had a significant impact on the microalgae community structure of shrimp culture system, and it has become the absolute dominant genus in KH_1, KH_2, and KH_4 group, with relative abundances of 86.57%, 95.48%, and 99.01% respectively, whereas Chaetoceros (51.05%) dominate in the control. Moreover, the growth performance of shrimp in KH_2 and KH_4 groups were better than those in the control (P < 0.05), and the superoxide dismutase (SOD) and lysozyme (LZM) activity in hepatopancreas increased by 31.73–78.05% and 16.93–73.74% for the Litopenaeus vannamei in KH_2 and KH_4. The results of this study indicated that artificial addition of Picochlorum sp. could be effective to improve the water quality and shrimp growth, and be a feasible environmental regulation method in shrimp aquaculture.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Litopenaeus vannamei, a tropical shrimp species highly adaptable to temperature and salinity, is a major farmed species with total production of 4.966 million tonnes, accounting for 52.9% of crustacean production in the world in 2018 (Chen et al. 2020). Along with increasing of shrimp production, eutrophication due to the wastewater discharging in shrimp aquaculture has raised much concerns, and then limits the sustainable development of shrimp aquaculture (Huang et al. 2022; Zhang et al. 2020).
Microalga are effective to absorb nitrogen, phosphorus, and many other harmful substances in wastewater through photosynthesis (Ekasari et al. 2021; Marinho et al. 2017; Pakravan et al. 2018; Wang et al. 2020), and they have already been well used to regulate water quality in aquaculture as well (Marquez et al. 2019; Sandeep et al. 2021, 2023). Chen et al. (2020) discovered that the inclusion of microalgae effectively maintains pH stability and reduces ammonia nitrogen and nitrite levels in aquaculture ponds. Ekasari et al. (2021) demonstrated that supplementing aquaculture water with microalgae not only stimulates the growth of Macrobrachium rosenbergii but also provides a significant amount of essential fatty acids for their development. As a common microalgae in shrimp aquaculture systems, Picochlorum sp. is also a potentially dominant taxon (Krishnan et al. 2021; Dinesh et al. 2016).
Although microalgae play a significant role in regulation of water environment in aquaculture, their optimal concentration in artificial regulation is still not clear and few studies have been reported. The regulatory effect of microalgae is not obvious if their concentration is too low in culture water (Le et al. 2022; Lukwambe et al. 2019). On the other hand, excessive microalgae addition will reduce the microbial diversity of aquaculture water, leading to the instability of the aquaculture system and destroying the balance of bacteria and algae (Shetty & Gulimane 2023; Wang et al. 2023, 2020). Therefore, there is an urgent need to assess the optimal concentration of microalgae and to investigate the appropriate level of microalgae addition in aquaculture system.
In the present study, we investigated the effects of inoculation of Picochlorum sp. at different concentrations on various physicochemical indexes of aquaculture water, the growth characteristics of shrimp, and related enzyme activities. The results of the study could provide a scientific basis for the application of Picochlorum sp. in shrimp farming.
Materials and methods
Shrimp and micoalga
Specific pathogen-free postlarvae (PL10) of L. vannamei, sourced from China, were kindly provided by the Charoen Pokphand (CP) group. The PL were raised in a nursery tank (6 × 6 × 1.5 m) at a stocking density of 2000 PL/m3 for a 7-day acclimation.
Microalga Picochlorum sp. was obtained from the Shanghai Whisper Biotechnology Co. The fresh microalga were inoculated into a sterilized BG-11 liquid medium at salinity of 30 and temperature of 28 ± 2℃, and cultivation was conducted under a 12:12-light–dark cycle with a light intensity of 3000 Lux. The composition of BG-11 medium was as follows: sterile seawater (1 L), sodium nitrate (80 mg), potassium dihydrogen phosphate (5 mg), ferric ammonium citrate (0.5 mg), sodium silicate (6 mg), manganese chloride (0.02 mg), vitamin B12 (0.1 μg), vitamin B1 (25 μg), D-biotin (1.0 μg).
Experimental design
The experiment was conducted using 12 tanks with a capacity of 150 L for each. All tanks were filled with 100 L of sterilized seawater and the aeration were provided by air stone. Water temperature maintained at 29.3 ± 0.11 °C, salinity at 30.26 ± 0.09, and dissolved oxygen (DO) at 7.68 ± 0.06 mg/L, and 20% of tank water were replaced every day. Tanks were randomly allotted into four groups, labeled as KH_1, KH_2, KH_4, and in the control respectively, with three replicates for each group. Picochlorum sp. was artificially added into the tanks of KH groups every 3 days to adjust the concentration of Picochlorum sp. at low (1 × 104 cells/mL for KH_1), medium (2 × 104 cells/mL for KH_2), and high (4 × 104 cells/mL for KH_4) level, respectively. The tanks without microalgae addition were set as the control. The concentration of microalgal cells were counted using a hemocytometer. After acclimation, 40 healthy and uniformly sized shrimp were transferred and raised in each tank, they were fed five times a day (6:00, 10:00, 14:00, 18:00, and 22:00) with a commercial pellet feed (41% protein), and the initial feeding rate was set at 4%, and adjustments were made based on the growth and feeding behavior of the shrimp. The experimental period is 30 days.
Water quality monitoring
Water samples were systematically collected and then measured for every 3 days. Dissolved oxygen (DO), pH, temperature, and salinity of water sample were measured with a handheld digital water quality meter (Aqua TROLL 400; In-situ, USA). Total ammonia nitrogen (TAN), nitrite (NO2−-N), orthophosphate (PO43-P), and chemical oxygen demand (COD) were measured following the method reported in the reference (Rice et al. 2012).
Shrimp growth performance
Shrimp in each group were weighed at the beginning and end of the experiment. The growth performance of shrimp was calculated as follows:
Specific growth rate (SGR, %/ day) = 100 × (Ln (final weight) − Ln (initial weight))/culture days.
Survival rate (%) = 100 × final number of shrimps/initial number of shrimps.
Relative growth rate (ADG, %) = 100% × (final weight – initial weight)/ initial weight.
Measurement of enzyme activity
At the end of experiment, ten shrimp were randomly collect in each culture tank, and their hepatopancreas were separated and then pooled as one sample stored at − 80 °C for measurement of enzyme activity. After homogenization with 0.9% saline (volume ratio of 1:9 for SOD or 1:19 for LZM) and centrifugation at 3000 rpm for 10 min. Activity of superoxide dismutase (SOD) or lysozyme (LZM) in the supernatant were measured using a commercial kit purchased from the Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Microalgae community analysis
Sample collection
At the end of experiment, a 500-mL water sample was collected and passed through a 0.22-μm sterile microporous membrane, and the membranes were stored at − 80℃ until the analysis for microalgae communities.
DNA extraction and PCR amplification
DNA in the membranes were extracted using the E.Z.N.A.® soil DNA kit (Omega Bio-tek, Norcross, GA, USA). The primers p23SrVF. (5′-GGACAGAAAGACCCTATGAA-3′) and p23SrVR. (5′-TCAGCCTGTTATCCCTAGAG-3′) were employed for PCR amplification of 23S rRNA gene in sample, and the products underwent detection through 2% agarose gel electrophoresis. The retrieved products were purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, USA). Detection and quantification of the purified PCR products were conducted using the Quantus™ Fluorometer (Promega, USA), and the sequenced products were sent to Meggie Sequencing for PE300 sequencing on the Illumina Miseq platform.
Processing of sequencing data
The raw 23S rRNA gene sequencing reads were demultiplexed, quality-filtered by fastp version 0.20.0 (Chen et al. 2018) and merged by FLASH version 1.2.7 (Magoč & Salzberg 2011) with the following criteria: (i) 300-bp reads were truncated at any site receiving an average quality score of < 20 over a 50-bp sliding window, and the truncated reads shorter than 50 bp were discarded, and reads containing ambiguous characters were also discarded; (ii) only overlapping sequences longer than 10 bp were assembled according to their overlapped sequence. The maximum mismatch ratio of the overlap region is 0.2. Reads that could not be assembled were discarded; (iii) samples were distinguished according to the barcode and primers, and the sequence direction was adjusted, exact barcode matching, two nucleotide mismatches in primer matching.
Following the application of default parameters, optimized sequences underwent QC splicing and noise reduction using the DADA2 (Callahan et al. 2016; Yoon et al. 2016) plugin. The resulting sequences, known as ASVs (amplicon sequence variants), were exclude sequences or ASVS annotated with chloroplast and mitochondrial sequences. Furthermore, to mitigate the impact of sequencing depth on subsequent alpha diversity and beta diversity analysis, the counts of all sample sequences were normalized.
The species annotation database was nt_v20230830, and the species annotation method was BLAST best hit. A genus was considered absolutely dominant if it constituted more than 50% of the total relative abundance in the samples.
Statistical analysis
The richness and diversity of microbial communities in the samples were assessed using the alpha diversity index, and the statistical t-test was applied to test for significance. The similarity or difference in the composition of the sample communities was investigated using the PCoA analytical method, and the test of difference between groups was performed by Adonis, with the Bray–Curtis distance algorithm. The samples were analyzed using LEfSe software based on taxonomic composition, and different grouping conditions were subjected to linear discriminant analysis (LDA) to identify the communities or species that had a significant differential effect on the division of the samples. The following software was used for statistical analysis and mapping: mothur 1.30, R language (version 3.3.1), and LEfSe. Results were expressed as mean ± standard deviation (mean ± SD). One-way ANOVA was performed using the SPSS 19.0 for comparative analysis, with a significance threshold set at P < 0.05.
The data from this study have been submitted to the NCBI SRA database under BioProject accession number PRJNA1130669 and will be publicly available on August 31, 2025.
Results
Water quality
The common water quality parameters, including temperature, salinity, pH, and DO, in L. vannamei aquaculture system are shown in Table 1. All parameters except pH in each group maintained stable throughout the experiment, and the pH values in KH_2 and KH_4 were slightly higher than that of the control.
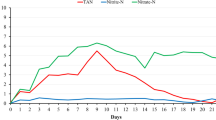
TAN concentration in the control accumulated gradually before day 18, and maintained around a level of 7.70 ± 0.30 mg/L thereafter, whereas the TAN concentrations in KH_1, KH_2, and KH_4 group increased during the early period, and then declined after reaching a peak value of 5.48 ± 0.33 mg/L, 5.16 ± 0.15 mg/L, and 4.41 ± 0.20 mg/L, respectively (Fig. 1a) at day 12. At the end of experiment, TAN concentrations in the control, KH_1, KH_2, and KH_4, were 7.39 ± 0.20 mg/L, 3.30 ± 0.21 mg/L, 2.19 ± 0.02 mg/L, and 0.75 ± 0.18 mg/L, respectively (Fig. 1a). It reduced by 55.35%, 70.37%, and 89.85% in the KH_1, KH_2, and KH_4, respectively, in comparison with the control.
NO2−-N accumulated steadily in all groups, with the control having the fastest accumulation speed while KH_4 having the slowest. At the end of experiment, the NO2−-N concentration in KH_1, KH_2, and KH_4 group were 3.82 ± 0.11 mg/L, 3.30 ± 0.20 mg/L and 1.41 ± 0.02 mg/L, respectively (Fig. 1b), equal to 59.13%, 51.08%, and 21.83% of the control (6.46 ± 0.24 mg/L).
The results on PO43-P concentrations in the experiment showed a similar trend to that of NO2−-N. At the end of the experiment, the PO43-P concentrations in KH_1, KH_2, and KH_4 were 0.94 ± 0.02 mg/L, 0.78 ± 0.05 mg/L, and 0.76 ± 0.03 mg/L, respectively (Fig. 1c), equal to 61.84%, 51.32%, and 50.00% of the control (1.52 ± 0.02 mg/L).
During the initial 18 days of experiment, COD concentration in all groups increased gradually. It maintained stable. At the end of the experiment, the COD concentrations in KH_1, KH_2, and KH_4 were 10.6 ± 0.35 mg/L, 8.4 ± 0.20 mg/L, and 8.2 ± 0.20 mg/L, respectively (Fig. 1d), equal to 47.47%, 37.62%, and 36.72% of the control (22.33 ± 0.42 mg/L). Detailed data are presented in Annex I.
Production performance of shrimp
The average body weight of L. vannamei in KH_1, KH_2 and KH_4 treatment groups were 1.18, 1.37, and 1.36 times of that in the control at the end of experiment. Mean body weight of shrimp in KH_2 and KH_4 was significantly higher than that in the control (P < 0.05). The highest shrimp relative growth rate and specific growth rate were found in the KH_2, and the highest survival rate was found in KH_4 (Table 2).
Enzyme activity
LZM activity
The hepatopancreas LZM activity of L. vannamei was presented in Fig. 2a. The mean LZM activity in each group was as follows: KH_1 (34.47 ± 2.84 U/mg prot), KH_2 (47.17 ± 3.13 U/mg prot), KH_4 (51.22 ± 4.06 U/mg prot), control (29.48 ± 4.45 U/mg prot). The KH_2 and KH_4 groups exhibited a significant increase by 60.01% and 73.74%, respectively, (P < 0.05) relative to the control.
SOD activity
The hepatopancreas SOD activity of L. vannamei was presented in Fig. 2b. The mean SOD activity in each group was as follows: KH_1 (116.58 ± 10.31 U/mg prot), KH_2 (157.57 ± 11.33 U/mg prot), KH_4 (152.23 ± 8.08 U/mg prot), control (88.50 ± 5.46 U/mg prot). The KH_1, KH_2, and KH_4 groups exhibited a significant increase by 31.73%, 78.05%, and 72.01%, respectively, (P < 0.05) relative to the control.
Microalgal community composition and diversity
Alpha diversity
A total of 787,630 sequences were obtained from 12 samples, averaging 65,636 sequences per sample, with an average sequence length of 369 bp. The Shannon and Simpson indices indicate that the microalgal diversity of KH_1 is significantly higher than that of KH_4 (P < 0.05). As shown by sobs, ACE, and chao indices, the richness index of KH_1 is higher than that of in the control, but there is no significant difference (P > 0.05) (Table 3).
Beta diversity
The results of PCoA analysis were shown in Fig. 3. The PCoA analysis plots demonstrated the distribution of microalgae community structure at the ASV level for the control and different concentrations of microalgae treatment groups (KH_1, KH_2, KH_4). The first principal coordinate (PC1) and the second principal coordinate (PC2) explained 64.47% and 16.00% of the total variation, respectively. The community structure of KH_1, KH_2, and KH_4 deviated from that of the control, especially KH_4, indicating that the microalgal community structure of the cultured water body changed significantly after the addition of different concentrations of Picochlorum sp. This result reveals the significant regulatory effect of microalgae concentration on the diversity and structure of microbial communities in the culture environment (P < 0.05).
Microalgae communities’ composition
Microalgal community composition in the KH_1, KH_2, and KH_4 exhibit substantial commonality at the genus level, and their absolute dominant genus were Picochlorum sp., with relative abundances of 86.57%, 95.48%, and 99.01%, respectively (Fig. 4). The absolute dominant genus in the control was Chaetoceros (51.05%). Both unclassified_d__unclassified and unclassified_d__Eukaryota shown in the figure belong to the Isochrysidaceae.
One-way ANOVA was performed for the top three species abundance to test significant differences among groups. Picochlorum sp. exhibited a significant difference between the groups (Fig. 5a). Compared to the control, the relative abundance of Picochlorum sp. in KH_1 was highly significant (P < 0.01), the relative abundances in KH_2 and KH_4 were extremely significant (P < 0.001). No significant differences (P ≥ 0.1) were observed between the relative abundances in KH_1, KH_2, and KH_4 (Fig. 5b).
a Histogram of tests of species differences, b comparison between Picochlorum sp. groups. Differences were examined in abundance of the top three species in abundance across the four groups in panel a, and it was also examined between the proportion of a Picochlorum sp. (genus level) in each group. *0.01 ≤ P ≤ 0.05, **0.001 ≤ P ≤ 0.01, ***P ≤ 0.001
LEfSe analyses revealed significant differences in microalgal community structure between the KH_4 and the control at all taxonomic levels. The addition of high concentrations of Picochlorum sp. significantly increased the abundance of the phylum Trebouxiophyceae, the class Chlorophyceae, and the order Isochrysidales in the KH_4 group (LDA score > 4), whereas in the control, the phylum Haptophyta, the class Bacillariophyceae, the order Chaetocerotales, and the order Bacillariales prevailed (LDA score > 4). At the family and genus levels, the abundance of the family Isochrysidaceae and the genus Picochlorum was significantly higher in the KH_4 (LDA score > 4, P < 0.05), whereas in the control, the families Chaetocerotaceae and Bacillariaceae, and the genera Chaetoceros, Isochrysis, Nitzschia, and Tisochrysis were significantly increased in abundance (LDA score > 4, P < 0.05). These results indicate that the addition of high concentrations of Picochlorum sp. profoundly affected the structure of microalgal communities, increasing the abundance of specific microalgal communities while suppressing others, thus revealing the key role of microalgae in regulating microalgal diversity and ecological balance (Fig. 6).
Discussion
Water quality control is critical in L. vannamei farming, and it has become pivotal for the success or failure of shrimp farming (Abreu et al. 2009; Santhana Kumar et al. 2018). Previous studies have shown that addition of Picochlorum sp. is effective to remove nitrogen, phosphorus, and many other nutrient salts; to purify the water; and to improve shrimp survival rate. It was common that N and P accumulate in the aquaculture water during the early stages, and the Picochlorum sp. maintains their own growth through absorbing various nitrogen and carbon chemicals, and COD in water could be degraded in this process (Lee et al. 2017; Angela et al. 2021; Dong et al. 2022; Huang et al. 2022; Lu et al. 2021). Also, ammonia concentrations in water supplemented with Haematococcus pluvialis has been found significantly lower than those in water without H. pluvialis (Wang et al. 2022), and the addition of microalgae Nannochloropsis oculata and Thalassiosira sp. exhibited a superior water purification effect (Zhang et al. 2022). Our present study using Picochlorum sp. showed a positive purification effect on water quality as well, which is consistent with previous study.
It has been found that inoculation of beneficial algae such as Picochlorum sp., Chaetoceros calcitrans, and Phaeodactylum tricornutum Bohlin in culture systems for L. vannamei can significantly enhance shrimp immunity and improve shrimp production (Ekasari et al. 2021; Huang et al. 2022; Marinho et al. 2017). In addition, some of the algae attached to the barrel walls were found to be ingested by the prawns. Addition of Chlorella growth factor to the L. vannamei feed was effective to improve the shrimp survival and growth performance (Pakravan et al. 2018). In the present study, addition of microalgae Picochlorum sp. could also significantly increase shrimp survival and production, and improve the antioxidant and immunity enzyme activity. More significant positive effects may be supposed if Picochlorum sp. concentration increase or algal species change (Abreu et al. 2019; Huang et al. 2022; Marinho et al. 2017).
Microalgae community structure serves as an important indicator of environmental stability of a water body for shrimp farming. In this study, high-throughput sequencing revealed that the number of ASVs in KH_1 was higher than that in the control, whereas KH_2 and KH_4 had lower numbers of ASVs compared to the control. Additionally, there was a decreasing trend in the numbers of ASVs across KH_1, KH_2, and KH_4, indicating that as the concentration of Picochlorum sp. increased, the number of ASVs in the aquaculture water body gradually decreased. It may be due to the fact that appropriate addition of Picochlorum sp. can increase species diversity to a certain extent, but the addition of alga into the culture water at too high concentration will inhibit species diversity (Zhang et al. 2020). Based on species diversity indices (Shannon index, Simpson index) and richness indices (Sobs index, Ace index, Chao index), the KH_1 group had the highest species diversity and species richness of the four treatment groups, and the KH_4 group had the lowest species diversity and species richness. This indicates that in the KH_4 group, where Picochlorum sp. densities are highest, Picochlorum sp. exhibits a greater interspecific competitive advantage, and growing dominance is formed, which can affect other microorganisms in the water environment. This is consistent with the findings of Lu et al. (2021), that different Picochlorum sp. densities have varying effects on the microbial community structure of cultured waters. The absolute dominant population in the control group was Chaetoceros (51.05%). As previously reported, Chaetoceros is also an effective water quality regulator and is one of the most commonly used bait microalgae. As can be seen from Fig. 4, the relative abundance of Chaetoceros in the treatment group with Picochlorum sp. added was significantly lower than the control (P < 0.05). Therefore, synergistic symbiosis with other beneficial algae should be considered in subsequent experiments related to the regulation of microalgae. The artificially added Picochlorum sp. in the KH_1, KH_2, and KH_4 groups all became overwhelmingly dominant populations. This indicates that the growth of harmful algae, such as Cyanobacteria, can be suppressed by artificially controlling the direction of the dominant population in the culture water, allowing beneficial algae to become the dominant population. This provides security for the growth of shrimps.
The addition of high concentrations of Picochlorum sp. significantly improved the production performance of L. vannamei. The study found that L. vannamei in the KH_4 group exhibited higher growth rates and survival, likely due to the nutritional contribution of Picochlorum sp. in the feed and its role as a natural bait. Additionally, microalgae indirectly contributed to the growth and health of L. vannamei by improving water quality. Changes in microalgal community structure may also positively impact the immune system of L. vannamei, thereby enhancing its disease resistance.
Water quality is critical to the health and growth of L. vannamei. In this study, the addition of high concentrations of Picochlorum sp. significantly improved water quality indicators such as dissolved DO, TAN, and NO2−-N levels. The improved water quality provided a superior growth environment for L. vannamei. The increase in DO may have enhanced the metabolic efficiency of L. vannamei, while the decrease in TAN and NO2−-N reduced its toxic stress. These findings suggest that improved water quality is a key mechanism by which microalgae addition enhances the production performance of L. vannamei.
The effect of water quality on microalgal community structure was also significant. The abundance of Trebouxiophyceae and Chlorophyta increased significantly in the KH_4 group, suggesting that the addition of high concentrations of Picochlorum sp. significantly altered the microalgal community structure in the water column. The improved water quality may have provided more favorable conditions for the growth of certain microalgal species, and the ability of different microalgal species to compete for and utilize nutrients may have also led to changes in community structure. Additionally, changes in microalgal community structure may further affect water quality by influencing light and nutrient levels in the water column.
In conclusion, this study revealed the complex and interrelated relationships among shrimp–microalgae, shrimp–water, and water–microalgae. The addition of high concentrations of microalgae not only directly affected microalgal community structure and water quality but also indirectly promoted the growth and health of shrimp by improving environmental conditions. Although it is not yet possible to quantitatively assess the specific contributions of differences in microalgal community structure to water quality and shrimp production, our study highlights the critical role of integrating these relationships in optimizing shrimp aquaculture environments and improving production.
Conclusion
Artificial addition of Picochlorum sp. in intensive aquaculture water for L. vannamei could effectively stabilize water quality and reduce the accumulation of ammonia nitrogen, nitrite, phosphate, and COD in the culture water. The breeding effect of L. vannamei was best when the concentration of Picochlorum sp. in the water reached 4 × 104 cells/mL. Artificial addition of Picochlorum sp. is a feasible environmental regulation method in shrimp farming.
Data availability
No datasets were generated or analysed during the current study.
References
Abreu MH, Varela DA, Henríquez L, Villarroel A, Yarish C, Sousa-Pinto I, Buschmann AH (2009) Traditional vs. integrated multi-trophic aquaculture of Gracilaria chilensis C. J. Bird, J. McLachlan & E. C. Oliveira: Productivity and physiological performance. Aquaculture 293(3):211–220. https://doi.org/10.1016/j.aquaculture.2009.03.043
Angela D, Arbi S, Natrah FMI, Widanarni W, Pande GSJ, Ekasari J (2021) Evaluation of Chlorella sp. and Ankistrodesmus sp. addition on biofloc system performance in giant prawn culture. Aquaculture Res 52(12):6052–6062. https://doi.org/10.1111/are.15466
Callahan BJ, Mcmurdie PJ, Rosen MJ et al (2016) DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods 13(7):581–583. https://doi.org/10.1038/nmeth.3869
Chen S, Zhou Y, Chen Y, Gu J (2018) fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34(17):i884–i890. https://doi.org/10.1093/bioinformatics/bty560
Chen Z, Chang Z, Zhang L, Wang J, Qiao L, Song X, Li J (2020) Effects of carbon source addition on microbial community and water quality in recirculating aquaculture systems for Litopenaeus vannamei. Fish Sci 86(3):507–517. https://doi.org/10.1007/s12562-020-01423-3
De Abreu JL, Brito LO, de Lima PCM, Silva SMBC, Severi W, Gálvez AO (2019) Effects of addition of Navicula sp. (diatom) in different densities to postlarvae of shrimp Litopenaeus vannamei reared in a BFT system: growth, survival, productivity and fatty acid profile. Aquaculture Res 50(8):2231–2239. https://doi.org/10.1111/are.14104
Dinesh KS, Santhanam P, Nandakumar R, Ananth S, Nithya P, Dhanalakshmi B, Kim MK (2016) Bioremediation of shrimp (Litopenaeus vannamei) cultured effluent using copepod (Oithona rigida) and microalgae (Picochlorum maculatam & Amphora sp.)—an integrated approach. Desalin Water Treat 57(54):26257–26266. https://doi.org/10.1080/19443994.2016.1163509
Dong S, Li Y, Huang F, Lin L, Li Z, Li J, Zhang Y, Zheng Y (2022) Enhancing effect of Platymonas addition on water quality, microbial community diversity and shrimp performance in biofloc-based tanks for Penaeus vannamei nursery. Aquaculture 554:738057. https://doi.org/10.1016/j.aquaculture.2022.738057
Ekasari J, Nugroho UA, Fatimah N, Angela D, Hastuti YP, Pande GSJ, Natrah FMI (2021) Improvement of biofloc quality and growth of Macrobrachium rosenbergii in biofloc systems by Chlorella addition. Aquacult Int 29(5):2305–2317. https://doi.org/10.1007/s10499-021-00750-1
Huang C, Luo Y, Zeng G, Zhang P, Peng R, Jiang X, Jiang M (2022) Effect of adding microalgae to whiteleg shrimp culture on water quality, shrimp development and yield. Aquaculture Rep 22:100916. https://doi.org/10.1016/j.aqrep.2021.100916
Krishnan A, Likhogrud M, Cano M, Edmundson S, Melanson J, Huesemann M, McGowen J, Weissman J, Posewitz M (2021) Picochlorum celeri as a model system for robust outdoor algal growth in seawater. Sci Rep 11:11649. https://doi.org/10.1038/s41598-021-91106-5
Lee TH, Jang JK, Kim HW (2017) Optimal temperature and light intensity for improved mixotrophic metabolism of Chlorella sorokiniana treating livestock wastewater. J Microbiol Biotechnol 27(11). https://doi.org/10.4014/jmb.1707.07007
Le VV, Ko S, Kang M, Park C, Lee S, Oh H, Ahn C (2022) The cyanobactericidal bacterium Paucibacter aquatile DH15 caused the decline of Microcystis and aquatic microbial community succession: a mesocosm study. Environ Pollut 311:119849. https://doi.org/10.1016/j.envpol.2022.119849
Lukwambe B, Nicholaus R, Zhang D, Yang W, Zhu J, Zheng Z (2019) Successional changes of microalgae community in response to commercial probiotics in the intensive shrimp (Litopenaeus vannamei Boone) culture systems. Aquaculture 511:734257. https://doi.org/10.1016/j.aquaculture.2019.734257
Lu Q, Xi C, Yuping Q, Limin F, Chao S, Yao Z, Shunlong M, Jiachang C (2021) Effect of Chlorella on the quality and bacterial community structure of GIFT tilapia culture water. Chin Agric Sci Bull 37(12):112–120. https://doi.org/10.11924/j.issn.1000-6850.casb2020-0287 (In Chinese)
Magoč T, Salzberg SL (2011) FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27(21):2957–2963. https://doi.org/10.1093/bioinformatics/btr507
Marinho YF, Brito LO, Silva Campos CVFD, Severi W, Andrade HA, Galvez AO (2017a) Effect of the addition of Chaetoceros calcitrans, Navicula sp. and Phaeodactylum tricornutum (diatoms) on phytoplankton composition and growth of Litopenaeus vannamei (Boone) postlarvae reared in a biofloc system. Aquaculture Res 48(8):4155–4164. https://doi.org/10.1111/are.13235
Marquez A, Lodeiros C, Loor A, Revilla J, Da Costa F, Sonnenholzner S (2019) Microalgae diet for juveniles of Spondylus limbatus. Aquacult Int 27(1):323–335. https://doi.org/10.1007/s10499-018-0327-2
Pakravan S, Akbarzadeh A, Sajjadi MM, Hajimoradloo A, Noori F (2018) Chlorella vulgaris meal improved growth performance, digestive enzyme activities, fatty acid composition and tolerance of hypoxia and ammonia stress in juvenile Pacific white shrimp Litopenaeus vannamei. Aquaculture Nutr 24(1):594–604. https://doi.org/10.1111/anu.12594
Rice EW, Bridgewater L, American Public Health Association (Eds.) (2012) Standard methods for the examination of water and wastewater (Vol. 10). Washington, DC: American public health association. ISBN: 9780875532875
Sandeep KP, Avunje S, Dayal JS, Balasubramanian CP, Sawant PB, Chadha NK, Ambasankar K, Vijayan KK (2021) Efficiency of different microalgae as monospecific and bispecific diets in larval rearing of Penaeus indicus with special reference to growth, nutrient composition and antimicrobial activity of microalgae. Aquaculture Res 52(11):5146–5154. https://doi.org/10.1111/are.15382
Sandeep KP, Sivaramakrishnan T, Sudhin S, Raymond JAJ, Sudheer NS, Raja RA, Kumar S, Dayal JS, Balasubramanian CP, Sawant PB, Chadha NK, Ambasankar K (2023) Influence of dietary microalgal concentrates on growth, survival and health status of Penaeus vannamei. Aquacult Int 31(5):2883–2903. https://doi.org/10.1007/s10499-023-01114-7
Santhana Kumar V, Pandey PK, Anand T, Bhuvaneswari GR, Dhinakaran A, Kumar S (2018) Biofloc improves water, effluent quality and growth parameters of Penaeus vannamei in an intensive culture system. J Environ Manag 215:206–215. https://doi.org/10.1016/j.jenvman.2018.03.015
Shetty K, Gulimane K (2023) Application of microalgal diversity in assessing the water quality of freshwater ponds. Environ Monit Assess 195(5):595. https://doi.org/10.1007/s10661-023-11116-w
Wang C, Xu S, Jiang C, Peng X, Zhou X, Sun Q, Zhu L, Xie X, Zhuang X (2023) Improvement of the growth performance, intestinal health, and water quality in juvenile crucian carp (Carassius auratus gibelio) biofortified system with the bacteria-microalgae association. Aquaculture 562:738848. https://doi.org/10.1016/j.aquaculture.2022.738848
Wang H, Hu Z, Chai Z, Deng Y, Zhan Z, Tang YZ (2020) Blooms of Prorocentrum donghaiense reduced the species diversity of dinoflagellate community. Acta Oceanol Sin 39(4):110–119. https://doi.org/10.1007/s13131-020-1585-1
Wang Y, Sun M, Tang Y, Xu A, Tang J, Song Z (2022) Effects of Haematococcus pluvialis on the water quality and performance of Litopenaeus vannamei using artificial substrates and water exchange systems. Aquacult Int 30(4):1779–1797. https://doi.org/10.1007/s10499-022-00872-0
Yoon T, Kang H, Kang C, Lee SH, Ahn D, Park H, Kim H (2016) Development of a cost-effective metabarcoding strategy for analysis of the marine phytoplankton community. PeerJ 4:e2115. https://doi.org/10.7717/peerj.2115
Zhang P, Huang Q, Peng R, Jiang X, Jiang M, Zeng G, Lin J (2022) Environmental factors of rearing water and growth performance of shrimp (Penaeus vannamei) in a microalgal monoculture system. Aquaculture 561. https://doi.org/10.1016/j.aquaculture.2022.738620
Zhang Z, Yang Z, Zheng G, Lin Q, Zhuo X, Zhang G (2020) Effects of addition of sucrose and probiotics on whiteleg shrimp Litopenaeus vannamei postlarvae performance, water quality, and microbial community. N Am J Aquac 82(1):43–53. https://doi.org/10.1002/naaq.10116
Acknowledgements
We would like to thank Qingdao Rui Zi Group company for providing the research base, Shuangyong Zhang, Rui Long Wang, and Chen Tian for their help and support of this study.
Funding
This study was financially supported by the National Key Research and Development Program of China (No. 2023YFD2401002); the China Agriculture Research System (No. CARS-48); the Central Public-interest Scientific Institution Basal Research Fund, CAFS (No. 2023TD50); the Qinhuangdao Science and Technology Research and Development Program (202302B016).
Author information
Authors and Affiliations
Contributions
G.M. wrote the main manuscript text, Y.W. and C.T. provided the key resource data, Z.C. and C.Z. and J.L. reviewed the manuscript. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
All authors agree to participate.
Consent for publication
All authors agree to publish.
Competing interests
The authors declare no competing interests.
Additional information
Handling Editor: Xugan Wu
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Meng, G., Chen, Z., Wang, Y. et al. Effect of Picochlorum sp. addition on water quality, microalgae community composition and production performance in the culture of Litopenaeus vannamei. Aquacult Int (2024). https://doi.org/10.1007/s10499-024-01655-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10499-024-01655-5