Abstract
Gracilaria fisheri is an important red seaweed on the sea coast of Thailand. Cultivation of this seaweed has brought economic benefits to the farmers in this country. However, its low growth and quality are problematic due to high contamination and epiphyte outbreaks. This study was performed to examine the growth and epiphytic responses of G. fisheri to Ascophyllum seaweed extract (SE). The algal samples were treated with SE at different concentrations (0, 0.1, 0.5, and 1 g SE L–1). Three sets of experiments were conducted in the laboratory under controlled culture conditions of salinity of 30‰, temperature of 25–26°C, and light intensity of 200 μmol photons m–2 s–1. The algal samples were soaked for 30 min in SE alone (Experiment 1), in Provasoli Enriched Seawater (PES)+SE (Experiment 2), and in PES+SE with a 5% CO2 supplement (Experiment 3). The results showed a significant reduction in epiphytes (>90%) in the sample after one week of treatment with 1 g SE L-1. The use of SE significantly stimulated the branching of G. fisheri (p < 0.05). In comparison to the control plant (PES), the growth rate of the samples treated with PES+0.1 g SE L-1 was 3.40 ± 0.51% day-1 in the first week of culture, and this was increased to 3.84 ± 0.63% day-1 in the samples treated with PES+1 g SE L–1. The growth rate was significantly increased to 5.46 ± 1.05% day-1 in the samples treated with PES+1 g SE L-1 with a 5% CO2 supplement. This study suggested that the use of the Ascophyllum seaweed extract could inhibit epiphytic attachment and that supplementation with 5% CO2 resulted in enhanced growth of G. fisheri under controlled culture conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The red seaweed Gracilaria is an important source for the global agar industry, comprising more than 91% of the raw material supply (Porse and Rudolph 2017). The world supply of seaweed is produced mainly in East and Southeast Asia from aquaculture. In 2019, the production of seaweed in Asia constituted 97.4% of the world's production, of which 99.1% was from cultivation (Cai et al. 2021). In Thailand, Gracilaria is a commercially available red seaweed and twenty species have been identified (Chirapart 2008; Muangmai et al. 2014). The species Gracilaria fisheri is commonly used for water treatment in marine aquaculture pond systems. Cultivation is mostly performed in shrimp effluent ponds, which has brought economic benefits to local farmers each year. The total production is approximately 200 t dry weight per year (Lewmanomont and Chirapart 2022). The harvested Gracilaria is mainly sold for human consumption and exported to neighboring countries. However, the growth and production of algae are unstable, especially the quality of the algae. The major problem in Gracilaria farming is the outbreak of epiphytes, particularly in the farming of G. fisheri, in which this seaweed has been reported to be susceptible to epiphytes (Chirapart et al. 2018).
Recently several studies have used seaweed extracts as biostimulants to enhance plant growth and development (Khan et al. 2009; Borlongan et al. 2011; Di Stasio et al. 2017). The beneficial effects of seaweed extracts on cultured plants are well documented (Rayirath et al. 2008; Fan et al. 2011; Mansori et al. 2016; Ali et al. 2019, 2021). The extracts of seaweeds are highly organic; therefore, they are ideal for organic farming and the production of environmentally sensitive crops (Ali et al. 2021). Seaweed extracts have been noted to enhance vegetative propagation and root vigor, increase leaf chlorophyll content and the number of leaves, improve fruit yield, and enhance the flavonoid content of treated plants (van Staden et al. 1995; Blunden et al. 1996; Rayirath et al. 2008; Fan et al. 2011; Mansori et al. 2016). The seaweed liquid extract of Ulva rigida has been used to enhance the antioxidant potential and drought tolerance of a medicinal plant, Salvia officinalis (Mansori et al. 2016). Furthermore, the seaweed extract of the brown alga Ecklonia maxima has been reported to influenc growth, photosynthetic activity, and mineral composition of Brassica rapa L. subsp. sylvestris under nutrient stress conditions (Di Stasio et al. 2017).
The variability in algal growth and production is influenced by ambient environmental factors, such as light, temperature, and salinity variation (Choi et al. 2010; Gorman et al. 2017). In addition, it is difficult to control growth and epiphytes under non-unialgal culture conditions. Therefore, several studies have attempted to enhance the growth and production in seaweed farming (Bidwell et al. 1985; Ask and Azanza 2002), such as through the cultivation of seaweeds in the sea or in land-based tanks with high densities of Chondrus crispus (Bidwell et al. 1985) and Gracilaria domingensis (Salles et al. 2010). Several studies have applied seaweed extracts in seaweed cultivation to enhance growth and production. The extract of the brown seaweed Ascophyllum nodosum has been used in laboratory and field cultivation of the red seaweeds Kappaphycus alvarezii and K. striatum in the Philippines and Malaysia (Hurtado et al. 2009; Loureiro et al. 2010; Borlongan et al. 2011; Zuldin and Shapawi 2015). However, there have been no reports of the use of seaweed extract for enhancing growth and production in the cultivation of Thai seaweeds. Hence, the current study was initiated to examine the effects of the Ascophyllum seaweed extract on the growth and epiphytes of G. fisheri. The aim of this study was to examine the responses of algal branching, growth and yield and the reduction of epiphytic attachment to the Ascophyllum seaweed extract under controlled culture conditions. Our results will be useful for improving the growth, yield and quality in Gracilaria farming.
Materials and Methods
Sample preparation
Fresh samples of Gracilaria fisheri were gathered from shrimp effluent ponds in Phang Nga and Pattani Provinces, southern Thailand. Samples were cleaned in fresh seawater to remove sand, mud and other contaminants. The algal thalli were cultured using Provasoli Enriched Seawater (PES) medium (20 mL L-1) (Bold and Wynnw 1978). The algal samples were acclimated in the laboratory at a salinity of 30‰, a temperature of 25–26°C and 200 μmol photons m-2 s-1 for approximately 3 months. Then, epiphyte-infected thalli of the algal samples were selected for further experiments.
Three sets of experiments were conducted under controlled culture conditions in the laboratory (outlined above). In each experiment, the pH levels of the treatment combination were examined.
The first experiment was carried out to evaluate the effects of the Ascophyllum seaweed extract (SE) on the epiphytic attachment and growth characteristics of G. fisheri. Two gram each of the epiphyte-infected samples (n=18) was soaked daily in SE at different concentrations of 0, 0.1, 0.5, and 1 g SE L-1 for 30 min. The algal samples were transferred to 300 mL filtered seawater (0.45 μm) in flasks (n=6) to grow the algae without additional PES medium. The culture conditions were controlled at a salinity of 30‰, temperature of 25–26°C and light intensity of 200 μmol photons m-2s-1, with a light:dark period of 12:12 h. The responses of algal branching and epiphyte attachment to SE were observed daily.
The attachment of epiphytes was determined as percent coverage per cm2. The infected algal samples were selected and surface area of the sample was divided into grid area with 10×10 spaces inside. The coverage of epiphytes was estimated (3 replicates) inside the grid spaces under a microscope. The percent cover of epiphytes was calculated as to the following formula:
The branching of the algal sample was determined and the percent of new algal branching was calculated according to the following formula:
The fresh weight of the algal sample was also determined at weekly intervals. The growth rate of the algae was calculated according to the following formula: [μ = [(In(Nt/N0))t-1]×100%], where μ = specific growth rate (% day-1), N0 = initial weight (g fresh weight), Nt = weight after t days (g fresh weight), and t = time (day) (Lobban et al. 1985).
In the second experiment, the growth response of the alga to SE with additional PES medium was examined. Healthy thalli of the algae were selected from the above culture in laboratory. One gram of each algal sample (n=18) was soaked in PES medium (20 ml L-1) mixed with different concentrations of SE (PES+0 g SE L-1 (control), PES+0.1 g SE L-1, PES+0.5 g SE L-1, PES+1 g SE L-1) for 30 min. Then the samples were transferred to 300 mL filtered seawater (0.45 μm) in flasks (n=6) to grow the algae under controlled conditions at a salinity of 30‰, a temperature of 25–26°C, and a light intensity of 200 μmol photons m-2 s-1 with a light:dark period of 12:12 h. The growth rate of the algae was determined and calculated according to the formula described above.
The third experiment examined algal growth under controlled culture conditions with 5% CO2 supplementation. One gram of each healthy sample (n=18) was soaked in a mixed solution of PES and SE for 30 min and then transferred to 300 mL of filtered seawater (0.45 μm) as in the second experiment. The algal samples were cultured under controlled conditions as described above and 5% CO2 was added to the culture for 30 min every day. The concentration of CO2 flowing into the culturing flask was manipulated through an air–CO2 gas mixing system with a flow rate of about 0.4 L L-1 min-1 (vvm). The fresh weight of the algal sample was determined and the growth rate was calculated according to the formula described above. The algal yield was also determined and expressed as the percentage of algal fresh weight increased.
Statistical analysis
The data are presented as the means ± standard deviation (SD). The statistical significance of differences was analyzed using the analysis of variance (ANOVA) and post hoc Duncan’s tests at a confidence level of 95%.
Results
The pH levels ranged from 7.80–7.90 for the non-SE treatment, and those of the SE treatment and the mixed solution of PES and SE ranged from 8.00–8.32 and 7.90–8.15, respectively (Table 1).
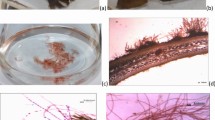
In the first experiment (treatment with SE alone), the Ascophyllum seaweed extract showed significant effects on the attachment of epiphytes and enhanced the growth of G. fisheri. Figure 1a–b shows the epiphytic-infected thalli of G. fisheri before treatment with SE, with many epiphytes attached to the surface of the alga. However, epiphyte attachment was markedly decreased after 1 week of treatment with 0.1, 0.5, and 1 g SE L-1 (Fig. 1c–h). The epiphyte coverage in the non-SE treatment (control) was slightly decreased from week 1 (98.3% cm-2) to week 4 (87.3% cm-2). In contrast, the samples treated with SE showed significant decreases as the concentration increased from 0.1 to 1 g SE L-1. The epiphyte coverage rapidly decreased to 50, 40, and 34% cm-2 after one-week treatments with 0.1, 0.5, and 1 g SE L-1, respectively (Fig. 2). The percent coverage of the epiphytes was significantly decreased to 26.7, 13.3, and 11.7% cm-2 after four weeks of the treatments when compared to the non-SE treatment (p < 0.05).
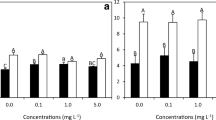
In addition, the alga thalli treated with SE also showed an increase in branching in comparison to the nontreated thalli (Fig. 3). The percent of branching (Fig. 4a) increased to 41.67 ± 2.58%, 46.67 ± 2.58%, and 50.00 ± 0.00% in week 4 when the thalli were treated with 0.1, 0.5, and 1 g SE L-1, respectively, while that of the nontreated thalli was 23.33 ± 2.58%. The branching was significantly different between the non-SE treatment and the SE treatment (p < 0.05). The thalli treated with SE also had a higher growth rate than thalli not treated with SE. The alga had the highest growth rate in week 3, with values of 0.25 ± 0.12, 0.64 ± 0.40, 0.50 ± 0.13, and 0.95 ± 0.11% day-1 in the treatments with 0, 0.1, 0.5, and 1 g SE L-1, respectively (Fig. 4b). The growth rates were significantly different between the non-SE-treated thalli and the 0.1 g SE L-1-treated thalli (p < 0.05), but had no differences with the 0.5 and 1 g SE L-1-treated thalli (p > 0.05) after four weeks of culture.
In the second experiment (PES+SE treatment), G. fisheri showed a significantly higher growth rate (p < 0.05) than in the first experiment (Fig. 5). In week 1 of the treatment without CO2, growth rates of 3.49 ± 0.83% day-1, 3.40 ± 0.51% day-1, and 3.72 ± 1.02% day-1 were obtained from the thalli treated with PES, PES+0.1 g SE L-1, and PES+0.5 g SE L-1, respectively (Fig. 5a). The growth rate was not significantly different among the treatments (p > 0.05). The growth rate increased to 3.84 ± 0.63% day-1 when the thalli were treated with PES+1 g SE L–1, but the algal growth was markedly decreased after week 2 of cultivation in all treatments.
In the PES+SE treatment with 5% CO2 supplementation, growth of the alga increased with increasing concentrations of SE from 0 to 1 g SE L–1. In the first week of cultivation, the growth rate was lower (4.81 ± 1.00% day-1) in the PES without SE treatment (control). However, the growth rates increased to 4.84 ± 1.52% day-1, 5.04 ± 1.48% day-1, and 5.46 ± 1.05% day-1 when the thalli were treated with PES+0.1 g SE L–1, PES+0.5 g SE L–1, and PES+1 g SE L–1, respectively (Fig. 5b). Similar to the treatment without CO2 supplementation (Fig. 5a), algal growth decreased after week 2 of culture in all treatments.
In comparison, the growth rates of the alga grown for 1–3 weeks were not significantly different in all treatments with or without CO2 supplementation (p > 0.05). However, in week 4, the growth rate was significantly different when the SE concentration increased to 1 g SE L-1 (p < 0.05). This study also showed that the growth of the alga was significantly different in all treatments between the first and the second experiments (p < 0.05).
In addition, under CO2-free conditions, the thalli treated with PES+0 g SE L-1 to 1 g SE L-1 showed the highest yields, ranging from 26.94 ± 4.97% fw to 30.93 ± 6.37% fw in week 1 of culture (Fig. 6a). However, the algal yields of all treatments gradually decreased to the lowest values at week 4 of culture. The yield of the alga was significantly different between the control (PES alone) and the PES+SE-treated alga (p < 0.05).
This study showed the yield of the alga response to 5% CO2 supplementation (Fig. 6b), with an increasing yield from 40.37 ± 10.68% fw in the alga treated with PES alone to 46.94 ± 11.43% fw in the alga treated with PES+1 g SE L-1. Similar to the treatment without CO2, the algal yields of all treatments gradually decreased to the lowest values in week 4 of culture. The yield of algae was significantly different between the treatments with 5% CO2 supplementation (p < 0.05). This study revealed that the treatments with 5% CO2 supplementation had higher yields than those without 5% CO2 supplementation.
Discussion
Seaweed extracts have been reported to have positive effects on plant growth, yield and quality, pest and disease resistance, and environmental stress tolerance (Khan et al. 2009; Fan et al. 2011; Danesh et al. 2012; Lakshmi and Sheeja 2021). In the present study, the use of the Ascophyllum seaweed extract resulted in significant decreases in the percent coverage of the epiphytes in G. fisheri. After four weeks of culture, the occurrence of the epiphytes in the treatment with SE alone at 0.1, 0.5, and 1 g SE L-1 was decreased to 50, 40, and 34% cm-2, respectively which accounted to 3.3, 6.6, and 7.5-fold decreases in comparison to the control treatment, respectively. Similarly, Borlongan et al. (2011) found that the use of Ascophyllum seaweed extract resulted in a decrease in the percent occurrence of epiphytic Neosiphonia in field cultivation of Kappaphycus alvarezii. The authors reported a percent occurrence of Neosiphonia sp. infection on Kappaphycus of 6–50% (with a 0.1 g L-1 AMPEP dipping), which was lower than the percentage of 10–75% in the controls (without AMPEP dipping). Additionally, an extract of A. nodosum (5–30 g L-1) resulted in a decrease in the epiphytic Cladophora and Ulva on the cultivated K. alvarezii after treatment for 2 weeks but not for the treated thalli of Polysiphonia subtilissima which formed small red patches along the thalli (Loureiro et al. 2010). However, cyanobacteria represented the main epiphytes found in the present study, which were mostly found to be in coccoid and filamentous forms and have not been identified to species level. This group of cyanobacteria had significant responses to the seaweed extract at concentrations of 0.1–1 g SE L-1. Thus, it is noted that the effect of seaweed extract on the occurrence of epiphytes depends on the epiphytic organisms. In addition, the laminarin found in the Ascophyllum seaweed extract may result from the defense responses in this seaweed. Laminarin exhibits a wide range of biological activities and has been shown to stimulate natural defense responses in plants (Fritig et al. 1998). Furthermore, it has been reported to be involved in the induction of genes encoding various pathogenesis-related proteins with antimicrobial properties in the algal cells (Fritig et al. 1998; van Loon and van Strien 1999).
The use of the Ascophyllum seaweed extract has shown a significant increase in the growth rate of K. alvarezii grown at different depths in the Philippines (Borlongan et al. 2011). These authors reported that the growth rates of K. alvarezii var. giant tambalang and var. tungawan dipped in Ascophyllum seaweed extract (AMPEP) were 3.1% day-1 and 4.1% day-1, respectively, which were higher than that of the control. In the current study, although the growth rate of the G. fisheri thalli treated with SE alone was overall not different from that of the control thalli (without SE), there was an increase in the branching of the treated thalli. The seaweed extract significantly enhanced the percent branching of this seaweed species. This was similar to the results of a report on the use of the seaweed extract from A. nodosum in the tissue culture of K. alvarezii, which increased the regeneration of young plants of the alga (Hurtado et al. 2009). The addition of the AMPEP seaweed extract enhanced the growth of K. alvarezii and K. striatum in tank culture (Zuldin and Shapawi 2015). In addition, the use of seaweed extracts from Gracilaria corticata and Grateloupia lithophila enhanced the growth of the microalgae Chlorella vulgaris; this was due to the influence of growth hormones contained in the seaweed (Lakshmi and Sheeja 2021). In this study, the increase in growth and branching of G. fisheri was thought to be influenced by various chemical components (macro- and micronutrients, growth hormones, etc.) of the seaweed extract (Hurtado et al. 2009; Khan et al. 2009; Rafiee et al. 2016; Lakshmi and Sheeja 2021). The seaweed extract from A. nodosum has been reported to contain macro- and microelements and have a total amino acid content of 4.4% (Hurtado et al. 2009), which are essential for plant growth. The positive effects of seaweed extract have been reported in cultivated plants, such as watermelon (Abdel-Mawgoud et al. 2010), cucumber (Danesh et al. 2012), tomato (Ali et al. 2016, 2019), and sweet pepper (Ali et al. 2019). Ascophyllum seaweed extract has been shown to result in a significant increase in tomato plant height and fruit yield compared to the control plants (Ali et al. 2016). Abdel-Mawgoud et al. (2010) stated that the effect of seaweed extract application was positive and correlated with the applied concentrations. Corresponding to the results of the current study, the seaweed extract was found to have a positive effect on algal growth and branching and was correlated with an increase in the concentrations used. There are reports that the use of the brown seaweed extracts resulted to an enhanced number of branches in agricultural crop (Abdel-Mawgoud et al. 2010; Kumar and Sahoo 2011). In addition, the seaweed extract of Ulva rigida enhanced vegetative growth in sage plants under drought stress conditions; the shoot length, total leaf area, and number were significantly reduced under water stress treatment (Mansori et al. 2016). These authors stated that water stress increased the organic osmolyte glycine betaine (GB) content, which was significantly reduced by the applied seaweed extract. This may explain why, in our study, algal growth and branching were higher in the treatment with seaweed extract than in the control treatment.
Although the growth rate of G. fisheri treated with SE was not different from that of the control thalli, the growth rate and yield of the alga were found to be increased when treated with the combination of PES+SE medium and higher when supplemented with 5% CO2. Similar to a previous study, the use of the seaweed extract in combination with a plant growth regulator enhanced the initiation of shoots in tissue culture of Kappaphycus (Hurtado et al. 2009). The seaweed extract may also improve the nutrient uptake of the algal thalli (Rathore et al. 2009). In this study, the increase in the growth rate and yield of G. fisheri was thought to be due to the synergistic activity of SE with PES and CO2, as previously reported by Vernieri et al. (2005). Previous studies reported that elevated CO2 resulted in increased growth in algae (Kang et al. 2017; Liu et al. 2018; Gao 2021). Changes in CO2 concentrations are related to the pH level of the culture medium (Kang et al. 2017). Differences in pH affect the uptake of nutrients, photosynthesis and growth of algae (Nor Salamah et al. 2015; Reidenbach et al. 2017). The highest growth rate of Gracilaria manilaensis has been reported at pH 7.6 (Nor Salamah et al. 2015). In the present study, the PES and SE solutions had a pH of 7.9–8.3, which may affect nutrient absorption and algae growth during the short period used for soaking the algae. The thalli of Gracilaria lemaneiformis grown at a high level of CO2 have been reported to have a lower carbon utilization capacity and a higher nitrogen uptake rate than those grown under ambient CO2 (Xu et al. 2010). Additionally, these authors reported that CO2 enrichment inhibited nitrate uptake by the alga by 28% compared to normal CO2 in culture. The concentration of 5% CO2 used in this study has been reported as the normal concentration used for algal culture (Pooja and Himabindu 2012; Yoshimura et al. 2013; Ruangsomboon and Dimak 2020). The supplementary CO2 after immersion of the algae in PES and SE extracts was able to enhance the yield of the algae and lead to vigorous growth.
Based on our results it can be concluded that the Ascophyllum seaweed extract is effective in enhancing the growth (in term of branching) and yield of the seaweed G. fisheri. Treatment with SE alone reduced epiphyte attachment 3.3- to 7.5-fold compared to the control. The use of 1 g SE L-1 could significantly reduce epiphytes (>90%) in the alga after 30 min of treatment for one week. The seaweed extract used at a concentration of 0.1–1 g SE L-1 enhanced the branching of this seaweed (~42–50%) compared to the non-treated samples (~23%). The algal growth rates of the SE thalli treatments (except 0.1 g SE L-1 treatment) were not significantly different from those of the non-SE-treated thalli. However, the cultivation of G. fisheri after soaking in the PES medium mixed with the seaweed extract and 5% CO2 supplementation resulted in enhanced algae growth. Further work is required to test the feasibility of the seaweed extract for larger-scale cultivation of Gracilaria.
Data availability
All data generated or analysed during this study are included in this published article. Requests for material should be made to the corresponding authors.
References
Abdel-Mawgoud AMR, Tantaway A, Hafez MM, Habib HAM (2010) Seaweed extract improves growth, yield and quality of different watermelon hybrids. J Agric Biol Sci 6:161–168
Ali N, Farrell A, Ramsubhag A, Jayaraman J (2016) The effect of Ascophyllum nodosum extract on the growth, yield and fruit quality of tomato grown under tropical conditions. J Appl Phycol 28:1353–1362
Ali O, Ramsubhag A, Jayaraman J (2019) Biostimulatory activities of Ascophyllum nodosum extract in tomato and sweet pepper crops in a tropical environment. PLoS One 14:e0216710
Ali O, Ramsubhag A, Jayaraman J (2021) Biostimulant properties of seaweed extracts in plants: Implications towards sustainable crop production. Plants 10:531
Ask EI, Azanza RV (2002) Advances in cultivation technology of commercial eucheumatoid species: a review with suggestions for future research. Aquaculture 206:257–277
Bidwell RGS, McLachlan J, Lloyd NDH (1985) Tank cultivation of Irish Moss, Chondrus crispus. Bot Mar 28:87–97
Blunden G, Jenkins T, Liu YW (1996) Enhanced leaf chlorophyll levels in plants treated with seaweed extract. J Appl Phycol 8:535–543
Bold HC, Wynnw MJ (1978) Introduction to the algae: Structure and reproduction. Prentice-Hall, Engelwood Cliff
Borlongan IAG, Tibubos KR, Yunque DAT, Hurtado AQ, Critchley AT (2011) Impact of AMPEP on the growth and occurrence of epiphytic Neosiphonia infestation on two varieties of commercially cultivated Kappaphycus alvarezii grown at different depths in the Philippines. J Appl Phycol 23:615–621
Cai J, Lovatelli A, Aguilar-Manjarrez J, Cornish L, Dabbadie L, Desrochers A, Diffey S, Garrido Gamarro E, Geehan J, Hurtado A, Lucente D, Mair G, Miao W, Potin P, Przybyla C, Reantaso M, Roubach R, Tauati M, Yuan X (2021) Seaweeds and microalgae: an overview for unlocking their potential in global aquaculture development. FAO Fisheries and Aquaculture Circular No. 1229. FAO, Rome
Chirapart A (2008) A review of the Gracilaria (sensu latu) from Thailand. In: Phang SM, Lewmanomont K, Lim PE (eds) Taxonomy of Southeast Asian Seaweeds. Monograph Series 2. Institute of Ocean and Earth Sciences. University of Malaya, Kuala Lumpur, pp 45–61
Chirapart A, Praiboon J, Boonprab K, Puangsombat P (2018) Epiphytism differences in the commercial species of Gracilaria, G. fisheri, G. tenuistipitata, and G. salicornia, from Thailand. J Appl Phycol 30:3413–3423
Choi TS, Kang EJ, Kim J-H, Kim KY (2010) Effect of salinity on growth and nutrient uptake of Ulva pertusa (Chlorophyta) from an eelgrass bed. Algae 25:17–26
Danesh RK, Bidarigh S, Azarpour E, Moraditochaee M, Bozorgi HR (2012) Study effects of nitrogen fertiliser management and foliar spraying of marine plant Ascophyllum nodosum extract on yield of cucumber (Cucumis sativus L.). Int J Agric Crop Sci 4:1492–1495
Di Stasio E, Rouphael Y, Colla G, Raimondi G, Giordano M, Pannico A, El-Nakhel C, De Pascale S (2017) The influence of Ecklonia maxima seaweed extract on growth, photosynthetic activity and mineral composition of Brassica rapa L. subsp. sylvestris under nutrient stress conditions. Eur J Hortic Sci 82:286–293
Fan D, Hodges DM, Zhang JZ, Kirby CW, Ji XH, Locke SJ, Critchley AT, Prithiviraj B (2011) Commercial extract of the brown seaweed Ascophyllum nodosum enhances phenolic antioxidant content of spinach (Spinacia oleracea L.) which protects Caenorhabditis elegans against oxidative and thermal stress. Food Chem 124:195–202
Fritig B, Heitz T, Legrand M (1998) Antimicrobial proteins in induced plant defense. Curr Opin Immunol 10:16–22
Gao K (2021) Approaches and involved principles to control pH/pCO2 stability in algal cultures. J Appl Phycol 33:3497–3505
Gorman L, Kraemer GP, Yarish C, Boo SM, Kim JK (2017) The effects of temperature on the growth rate and nitrogen content of invasive Gracilaria vermiculophylla and native Gracilaria tikvahiae from Long Island Sound, USA. Algae 32:57–66
Hurtado AQ, Yunque DA, Tibubos K, Critchley AT (2009) Use of Acadian marine plant extract powder from Ascophyllum nodosum in tissue culture of Kappaphycus varieties. J Appl Phycol 21:633–639
Kang JW, Kambey C, Shen Z, Yang Y, Chung IK (2017) The short-term effects of elevated CO2 and ammonium concentrations on physiological responses in Gracilariopsis lemaneiformis (Rhodophyta). Fish Aquat Sci 20:1–8
Khan W, Rayirath UP, Subramanian S, Jithesh MN, Rayorath P, Hodges DM, Critchley AT, Craigie JS, Norrie J, Prithiviraj B (2009) Seaweed extracts as biostimulants of plant growth and development. J Plant Growth Regul 28:386–399
Kumar G, Sahoo D (2011) Effect of seaweed liquid extract on growth and yield of Triticum aestivum var. Pusa Gold. J Appl Phycol 23:251–255
Lakshmi D, Sheeja L (2021) Red seaweed extract as a biostimulant on growth and biochemical parameters of microalgae Chlorella vulgaris. IJMRASC 1:26–37
Lewmanomont K, Chirapart A (2022) Biodiversity, cultivation and utilization of seaweed in Thailand: an overview. In: Rao AR, Ravishankar GA (eds) Sustainable Global Resources of Seaweeds, Bioresources, Cultivation, Trade and Multifarious Applications, vol 1. Springer, Cham, pp 97–107
Liu L, Zou D, Jiang H, Chen B, Zeng X (2018) Effects of increased CO2 and temperature on the growth and photosynthesis in the marine macroalga Gracilaria lemaneiformis from the coastal waters of South China. J Appl Phycol 30:1271–1280
Lobban CS, Harrison PJ, Duncan MJ (1985) The Physiological Ecology of Seaweeds. Cambridge University Press, Cambridge
Loureiro RR, Reis RP, Critchley AT (2010) In vitro cultivation of three Kappaphycus alvarezii (Rhodophyta, Areschougiaceae) variants (green, red and brown) exposed to a commercial extract of the brown alga Ascophyllum nodosum (Fucaceae, Ochrophyta). J Appl Phycol 22:101–104
Mansori M, Chernane H, Latique S, Benaliat A, Hsissou D, El Kaoua M (2016) Effect of seaweed extract (Ulva rigida) on the water deficit tolerance of Salvia officinalis L. J Appl Phycol 28:1363–1370
Muangmai N, Zuccarello GC, Noiraksa T, Lewmanomont K (2014) A new flat Gracilaria: Gracilaria lantaensis sp. nov. (Gracilariales, Rhodophyta) from the Andaman coast of Thailand. Phycologia 53:137–145
Nor Salamah MH, Mohammad-Noor N, Susanti D, Saad S, Mukai Y (2015) The effects of different pH and salinities on growth rate and carrageenan yield of Gracilaria. J Teknol (Sci Eng) 77:1–5
Pooja K, Himabindu V (2012) CO2 removal from industrial flue gas using Botrycoccus branii for simultaneous lipid production. Int J Sci Res 3:366–373
Porse H, Rudolph B (2017) The seaweed hydrocolloid industry: 2016 updates, requirements, and outlook. J Appl Phycol 29:2187–2200
Rafiee H, Naghdi Badi H, Mehrafarin A, Qaderi A, Zarinpanjeh N, Sekara A, Zand E (2016) Application of plant biostimulants as new approach to improve the biological responses of medicinal plants- A critical review. J Med Plants 15:6–39
Rathore SS, Chaudhary DR, Boricha GN, Ghosh A, Bhatt BP, Zodape ST, Patolia JS (2009) Effect of seaweed extract on the growth, yield and nutrient uptake of soybean (Glycine max) under rainfed conditions. South Afr J Bot 75:351–355
Rayirath P, Jithesh MN, Farid A, Khan W, Palanisamy R, Hankins SD, Critchley AT, Prithiviraj B (2008) Rapid bioassays to evaluate the plant growth promoting activity of Ascophyllum nodosum (L.) Le Jol. using a model plant, Arabidopsis thaliana (L.) Heynh. J Appl Phycol 20:423–429
Reidenbach LB, Fernandez PA, Leal PP, Noisette F, McGraw CM, Revill AT, Hurd CL, Kübler JE (2017) Growth, ammonium metabolism, and photosynthetic properties of Ulva australis (Chlorophyta) under decreasing pH and ammonium enrichment. PLoS One 12:e0188389
Ruangsomboon S, Dimak J (2020) Optimum carbon dioxide concentrations for enhancing biomass and carbon dioxide biofixation of Scenedesmus dimorphus KMITL. King Mongkut’s Agr J 38:535–544 (in Thai)
Salles JP, Scherner F, Yoshimura CY, Fanganiello M, Bouzon ZL, Horta PA (2010) Cultivation of native seaweed Gracilaria domingensis (Rhodophyta) in Southern Brazil. Braz Arch Biol Technol 53:633–640
van Loon LC, van Strien EA (1999) The families of pathogenesis related proteins, their activities, and comparative analysis of PR-1 type proteins. Physiol Mol Plant Pathol 55:85–97
van Staden J, Becket RP, Rijkenberg MJ (1995) Effect of seaweed concentrate on the growth of the seedlings of three species of Eucalyptus. S Afr J Bot 61:169–172
Vernieri P, Borghesi E, Ferrante A, Magnani G (2005) Application of biostimulants in floating system for improving rocket quality. J Food Agric Environ 3:86–88
Xu Z, Zou D, Gao K (2010) Effects of elevated CO2 and phosphorus supply on growth, photosynthesis and nutrient uptake in the marine macroalga Gracilaria lemaneiformis (Rhodophyta). Bot Mar 53:123–129
Yoshimura T, Okada S, Honda M (2013) Culture of the hydrocarbon producing microalga Botryococcus braunii strain Showa: Optimal CO2, salinity, temperature, and irradiance conditions. Bioresour Technol 133:232–239
Zuldin WH, Shapawi R (2015) Performance of red seaweed (Kappaphycus sp.) cultivated using tank culture system. J Fish Aquat Sci 10:1–12
Acknowledgements
This research was facilitated by the Algal Bioresources Research Center, Department of Fishery Biology, Faculty of Fisheries, Kasetsart University. The authors would like to thank Dr. Alan T. Critchley for his kindly provide the seaweed extract AMPEP (Acadian marine plant extract powder) and for valuable comments. Thanks also to anonymous reviewers whose remarks helped to improve this paper.
Funding
This research was in kind supported (equipment, chemical reagents, etc.) by the Algal Bioresources Research Center, Department of Fishery Biology, Faculty of Fisheries, Kasetsart University.
Author information
Authors and Affiliations
Contributions
A. Chirapart designed all experiment, prepared figures 2 and 4, and wrote the main manuscript text. S. Khreauthong performed experiment and preparing figures 5, 6. J. Praiboon contributed the research work and discussion. S. Rattanasaensri performed experiment and preparing figures 1, 3. R. Ruangchuay provided all materials of Gracilaria fisheri and discussion.
Corresponding author
Ethics declarations
Competing interests
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chirapart, A., Khreauthong, S., Praiboon, J. et al. Growth and epiphytic responses of Gracilaria fisheri to Ascophyllum seaweed extract under controlled culture conditions. J Appl Phycol 34, 3107–3115 (2022). https://doi.org/10.1007/s10811-022-02827-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-022-02827-7