Abstract
Choosing the right algae with the relevant properties and optimal harvest time for specific culture conditions and products is essential. As such, biomass, biochemical composition and fatty acid (FA) profile at different growth stages of locally isolated strains, suitable for aquaculture, Chlorella salina and Isochrysis maritima, were determined. Biomass and moisture content of both species were affected by the growth phase. A particular accumulation trend of proximate biochemical compounds was observed in these two strains. Protein content decreased whereby, as culture aged, more carbohydrate and lipid accumulated in C. salina and I. maritima, respectively. Variations in FA profile were exhibited in C. salina where PUFA was the highest, followed by SFA and MUFA throughout the growth phases. I. maritima had the highest SFA content, followed by PUFA and MUFA during the exponential phase. The essential PUFAs in C. salina were linoleic acid (LA) and α-linolenic acid (ALA) with a low occurrence of γ-linolenic acid (GLA; 0.49–0.78%) and docosahexaenoic acid (DHA; 0.21–0.45%). I. maritima recorded relatively high ALA (4.73–6.71%), GLA (5.05–7.80%) and DHA (5.15–7.02%) with minor presence of arachidonic acid (ARA; 0.45–0.59%) and eicosapentaenoic acid (EPA; 0.43–0.58%). Both C. salina and I. maritima are suitable for aquaculture feeds, but I. maritima was more superior by having EPA and higher DHA in their cells. Harvesting regime at a specific phase must be taken into account to achieve maximum yields of a target compound; thus, for feeding purpose, harvesting both strains at stationary phase is recommended as better PUFA compositions were obtained.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microalgae are microscopic photosynthetic organisms that are found in both marine and freshwater environments. As the basis of natural food chains, exploitation of this photosynthetic microorganism has a long history in the aquaculture industry. The nutritional quality of microalgae biomass is correlated to its biochemical composition which plays an essential role in the diet of marine animals, either directly or indirectly (through enrichment of zooplankton). Microalgae lipids have been used as a dietary source for metabolic energy and essential components for aquaculture organisms (Han et al. 2019), with fatty acid content being the central factor in the selection of microalgae species for use as aquafeeds (Huerlimann et al. 2010). Due to their high protein, carbohydrates, lipids and fatty acids content, and richness in minerals and vitamins as well as bioactive compounds, microalgae have received increasing interest as natural source of these valuable compounds (Ljubic et al. 2019; Bhowmick et al. 2020; Shi et al. 2021). Regarding this, recent research has shown that aquafeeds that contain microalgal by-products, for example defatted microalgae biomass generated from high-value microalgal compounds, was reported to improve both the growth and survival of the crustaceans and fish (Gamboa-Delgado et al. 2019).
Microalgae show considerable metabolic flexibility in response to changes in environmental factors (Patil et al. 2021); hence, the microalgae cultivation needs to be controlled and maintained at optimal conditions to ensure maximum growth, high survival rate and high biomass production (Li et al. 2021). Nutrients, light, temperature, salinity and pH are among the key aspects that influence overall biomass productivity and algal biochemical compositions (Renaud et al. 2002; Araujo and Garcia, 2005; Sánchez-García et al. 2020), which may vary during the microalgal growth phase cycle. Chlorella and Isochrysis are among microalgae species commonly utilised as feed due to their high nutritional values, particularly proteins (essential amino acids), long-chain PUFAs and pigments (Matsui et al. 2020). What is more is they are able to be cultivated on a large scale, attaining the proper cellular size with high digestibility and growth rates (Nalder et al. 2015; Safafar et al. 2016).
Malaysia’s aquaculture industry, and particularly fish production, is currently undergoing an expansion, owning increased demand both locally and internationally, which has caused an increase in the demand for microalgae culture for aquafeed. Despite that, research and development on microalgae have received little attention. This is especially true in comparison to research into macroalgae (seaweed), which has been commercialised and has become an economically more important natural source of aquafeed in Malaysia (Phang 1998, 2006; Vairappan et al. 2008; Chan et al. 2013; Eranza et al. 2015; Nor et al. 2017, 2020; Phang et al. 2017, 2019). Microalgae strains used as live feed in Malaysia are usually imported and the biochemical composition has not been characterised in detail. There is increasing demand for products derived from microalgae which relates to the strain’s taxonomic and biochemical compounds (Ljubic et al. 2019; Bhowmick et al. 2020); thus, it is important to choose the right algae with relevant properties for specific culture conditions and products. Since the cultures were maintained in controlled conditions, we assumed that microalgal cellular biochemical attributes will be varied according to the cells’ growth phase and nutrient deficiency in the culture media that occurs as the culture aged. The reaction might be species-specific and could be manipulated to accumulate specific compounds or/and biomass for various commercial industries particularly aquaculture. Economic viability of microalgae for aquaculture feed is largely dependent on their growth and productivity which relies on various biotic and abiotic environmental factors (Yu et al. 2018; Lee et al. 2019). During the course of their growth, microalgae produce compounds with broad range of structural and functional classes essential for their growth, metabolism and other biological processes (Bhowmick et al. 2020). Aging or culture maturation is one of the factors that affect the biochemical composition of algae (Schulze et al. 2019).
To the authors’ knowledge, marine microalgae in Penang coastal areas with the potential for economic importance and benefit have not yet been studied. Chlorella salina and Isochrysis maritima reported in this study are newly identified in Penang coastal waters and are believed to have various attributes conducive to being successfully used in aquaculture. These indigenous tropical microalgae isolates are assumed to be more tolerant to local environmental conditions, and therefore be more suitable for use in Penang hatcheries, whereas by comparison, imported strains may not withstand the local environmental conditions, and thus may produce lower biomass concentration and poorer nutritional values. Moreover, the influence of the growth phase on the biochemical composition of these local strains in relation to productivity of a target compound has not been clearly investigated. In light of this, this study was conducted with the aim of isolating new microalgae strains from Penang coastal waters which are capable of producing essential fatty acids and which possess other attributes suitable for aquaculture.
Materials and methods
Microalgae isolation and experimental design
Chlorella salina USMAC 17 and Isochrysis maritima USMAC 19 were isolated from the Teluk Aling, located at the north western part of Penang coastal waters, Malaysia, and surrounded by fishing activities by local fisherman. Purification of the strains was carried out using mechanical separation to discriminate between the cells and other substances, followed by antibiotic treatment (Mohammad Basri and Wan Maznah 2017). The axenic algal cells were transferred into 100 mL of sterilised seawater enriched with Walne’s medium (Walne 1970) at 30‰ salinity. Algal cultures were maintained photoautotrophically under 12:12-h light/dark cycle with light intensity of 50 μmol photons m−2 s−1 and temperature of 25 ± 2 °C. Flasks containing the algal cells were shaken twice daily to agitate the culture medium. After reaching the late exponential phase, the cultures were transferred into 900 mL of working medium. Aeration was continuously provided through 0.2-µm polytetrafluoroethylene (PTFE) filter to 1 L cultures. Triplicate flasks of 1 L cultures were harvested at three different growth phases of exponential (E), early stationary (ES) and during stationary (S) phases by centrifuging at 3000 rpm for 5 min. The supernatants were discarded, and the pellets obtained were centrifuged again after washing with distilled water. The cell pellets were freeze dried for total protein, total carbohydrate and fatty acid analyses. These batch assays were repeated in independent experiments to support the consistency of the results obtained. Microalgae cultivation was done in hygienic conditions according to culturing procedures and protocols in Kawachi and Noël (2005) to minimise and prevent contamination.
Analytical methods
Cell density was measured daily using a haemocytometer (Neubauer-improved haemacytometer, Laboroptik, UK) to estimate cultured population (× 106 cell mL−1). Cell enumeration was subsequently used to estimate the rate of culture augmentation, equivalent to the rate of population increase, often expressed as the rate of cell division. The specific growth rate, SGR (per day), µ, was calculated with differential equation according to Schoen (1988):
where X is the number of cells, µ is the growth rate and t is the time in days. Rearrangement of this equation yields
where X2 and X1 are cell densities at two times, t2 and t1.
Doubling time, Dt, is the time required for cells to double in size and can be calculated from the growth rate:
Dry biomass, AFDW, ash and moisture content
Triplicates of 10 mL algal suspension from exponential, early stationary and stationary of growth were filtered on 0.45 µm Whatman GF/C filter papers with diameter of 47 mm. Filter papers were pre-combusted and weighed. During filtration, the vacuum (Rocker 300) pressure was maintained at 35 to 55 mm Hg. To avoid air exposure of the cells, the vacuum was disconnected during each rinse so that water covered the filter, before suction was then rapidly applied to remove the rinsing solution. Microalgae cultures were washed with 0.5 M ammonium formate (NH4HCO2) to remove salts (Zhu et al. 1997; Hulatt et al. 2012). Dry weight of marine algal samples is profoundly affected by the amount of salts adsorbed on the cell surface and those present in intercellular water (Zhu et al. 1997; Rocha et al. 2003). Thus, washing of cells with suitable washing solution, or buffer, is important to overcome this possible error. Although there are various washing agents for marine samples including distilled water (Goh et al. 2010; Chen et al. 2012), diluted sodium chloride (Tokusoglu and Ünal, 2003) as well as ammonium bicarbonate (Zhu et al. 1997; Liu et al. 2013), ammonium formate was widely used effectively in removing salt particles in seawater media (Hodgson et al. 1991; Chu et al. 1996; Renaud et al. 1999; Brown and Hohmann 2002; Tzovenis et al. 2003; Xu et al. 2006).
To measure dry biomass, filters were dried in an oven at 105 °C for 24 h to a stable constant weight, cooled a desiccator and weighed again (Lee et al. 2019). These filters were then ashed in a muffle furnace at temperature of 540 °C for 4 h, cooled down in a desiccator and weighed to obtain the ash free dry weight (AFDW). Dry weight, AFDW, ash content and moisture content were calculated according to Liu et al. (2013).
Biochemical proximate composition
To get crude protein extract, lyophilised samples were immersed in distilled water for 12 h and the sample pellet was re-extracted by adding 1 mL of 0.1 M Natrium hydroxide (NaOH) according to Barbarino and Lourenço (2005). Total protein was subsequently measured following methods in Bradford (1976). Total lipids were determined by a modified version of the Bligh and Dyer (1959) method as proposed by Ryckebosch et al. (2012). Carbohydrates were extracted according to Chu et al. (1996), where freeze dried sample was first hydrolysed into simple sugar using dilute hydrochloric acid (2 M HCl), and total carbohydrate content was then determined using the phenol–sulphuric assay in Dubois et al. (1956).
Fatty acid analysis was carried out by extracting lyophilised algal biomass using a modified direct transesterification method as described in Abel et al. (1963). Fatty acid methyl esters (FAME) were separated and analysed by a gas chromatography geochemical analyser (GC-2010, Shimadzu) equipped with a flame ionisation detector and a 30 mm × 0.22 mm 70% cyanopropyl polysilphenylene-siloxane (BPX70, SGE) column (Mohammad Basri and Wan Maznah 2017).
Statistical analysis
Data were analysed using SPSS (Statistical Package for the Social Sciences) V20.0 software. Normality of the response variables was tested using a Shapiro–Wilk statistic. A one-way ANOVA was used to test for possible significant differences in the means of dependent variables among the growth stages, followed by post-hoc Tukey’s multiple comparisons test when significant difference was found at p < 0.05. Independent sample t test was performed to evaluate significant differences in dependent variables between Chlorella salina and Isochrysis maritima regardless of the growth phases. All data are presented as mean ± standard error.
Results and Discussion
Microalgal growth
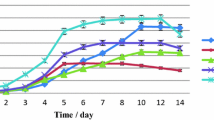
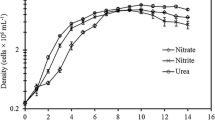
SGR of both strains were not significantly different (Table 1). Growth rate is an important aspect in microalgae cultivation and is used to indicate the health of microalgae cultivation (Sánchez-García et al. 2020). SGR is the best way of expressing the relative ecological adaptation success of a species or strain to the environmental conditions imposed upon it (Cui et al. 2006; Seyfabadi et al. 2010).
C. salina and I. maritima grown in the present study had lower growth rates compared to other species of the same genus in photobioreactor cultivation (Illman et al. 2000; Griffiths et al. 2011) but had higher growth rates compared to outdoor cultivation (Van Bergeijk et al. 2010; Zhao et al. 2011). Automated controlling system in photobioreactor offers better regulation of the culture conditions. Two of these variable parameters, optimum illumination per unit surface area and constant circulation, are key in achieving superior biomass yield and high growth rates (Sánchez-García et al. 2020). Outdoor cultivation on the other hand is illuminated with natural solar light which depend on the prevailing weather conditions and the exposure to bacterial contamination; competition with other microalgae and predation by protozoa are not as easily controlled and can result in lower growth rates (Sánchez-García et al. 2020).
Table 2 shows that the average biomass as dry weight (g) (DW) and ash free dry weight (g) (AFDW) values for both species, C. salina and I. maritima, was affected by the growth phase with one-way ANOVA displayed significant differences between each variable group (p < 0.05). The increases of dry weight throughout the culture period can be explained by the increases in cell concentration (Bresaola et al. 2019), where maximum cellular densities can be attributed to the highest dry weight obtained in stationary phase, most probably due to algal organic matter released by excretion and cell lysis (Cui et al. 2019). Furthermore, the 2.5-fold increase in dry weight was probably due to pronounced growth between the exponential to stationary phases.
Total ash and moisture content were lower in the stationary phase compared with the exponential phase in both species but had no significant differences (p > 0.05), except for the moisture content in I. maritima that was significantly affected by growth stages (p < 0.05) (Fidalgo et al. 1998). The difference between dry weight and AFDW represent the amount of total ash. According to Renaud and Parry (1994), total ash is mainly influenced by salinity. The authors further revealed that increases in cellular inorganic matter (ash) are caused by an adjustment to cellular water stress by high salt content. Total ash content of I. maritima was within the range of other Prymnesiophyta (13–19% ash) reported by Renaud et al. (1999) and Tokusoglu and Ünal (2003) and was significantly lower than C. salina (Table 2). High ash content in microalgae might be a result of their lower digestibility (Gamboa-Delgado et al. 2019).
Proximate biochemical compound
C. salina and I. maritima attained the highest amount of protein in the exponential phase, while carbohydrate and lipid content were less than the protein accumulation (Figs. 1 and 2). At this growth stage, all the dependent variables were not significantly different between these two strains (t test, p > 0.05). The biochemical composition of rapidly growing cells is generally characterised by a high protein and low carbohydrate and/or lipid content (Renaud et al. 1991; Brown et al. 1993, 1997; Zhu et al. 1997; Lourenço et al. 1997; López et al. 2010; Costard et al. 2012; Safafar et al. 2016). Proteins are fundamental during the early stages of microalgae growth because it functions as the building blocks for tissue production, enzyme biosynthesis and metabolic processes of a cell (Mahboob et al. 2012). Thus, here, high protein content most likely corresponds to the increased cellular density observed during the exponential phase in both cultured species.
As cultures shifted to the early stationary phase, the carbohydrate and lipid contents of C. salina increased whereas protein content slightly decreased. A similar tendency occurred during the stationary phase of growth where carbohydrates increases were matched by protein decreases. Lipid content also decreased in the latter phase, although to a lesser degree. On the other hand, although statistical analysis showed all the dependent variables of these strains were not significantly different at early stationary phase, changes in the biochemical compounds of I. maritima were far smaller, with insignificant decreases in total protein and minor, yet significant increases in both carbohydrate and lipid compounds with advancing age. Previous studies had established that with the onset of stationary phase due to nitrate limitation, cultures typically accumulate carbohydrate and/or lipid at the expense of protein (Brown et al. 1993; Přibyl et al. 2012) and at a reduced growth rate (Ha et al. 2019).
As the cultures were maintained under controlled conditions during the experimental cultivation period, changes in biochemical composition might have been an indication of nutrient deficiency in the culture media. The abundance of N is one of the main limiting factors to cell growth over the cultivation period, and thus significantly influence biochemical composition (Xu et al. 2001; Valenzuela-Espinoza et al. 2002; Přibyl et al. 2012; Recht et al. 2012; Huang et al. 2013). Apart from N, autotrophic cultivation of these two strains might increase the metabolic requirement for P (Qu et al. 2008). As cultures grow, algal cells accumulate lipids under P-depleted condition in the medium due to changes in cell biosynthetic pathways; thus, lipids productivity increased in response to this stress (Liang et al. 2013; Chia et al. 2013; Sánchez-García et al. 2020; Arguelles and Martinez-Goss, 2021).
Protein levels in microalgal cells drop synergistically with N levels in the growth media which could be explained by the effect of growth stage–dependent protein drop (Schulze et al. (2019). Most of the cellular N is in proteins; hence, N consumption from the medium can directly affect protein synthesis (Sánchez-García et al. 2020). This statement was further supported by Illman et al. (2000) and Sánchez-García et al. (2020) who indicated a low protein level in C. vulgaris and Scenedesmus obtusiusculus grown in low N concentration medium, conforming the fact that protein content has a positive correlation with N concentration (Safafar et al. 2016). Additionally, a study by Liang et al. (2013) has shown that protein content of Chlorella sp. decreased under N deprivation while no obvious changes were observed under low P conditions, indicating protein synthesis was mainly affected by N rather than P. Thus, higher level of nitrogen in the growth medium is required if protein is the preferred compound in the biomass (Safafar et al. 2016). Although protein decreased significantly as the culture aged, it remained as a major biochemical component in C. salina and I. maritima (Figs. 1 and 2, respectively). High protein content in both species shows their suitability to be applied in aquaculture and other various economic benefits that may be derived from their intracellular metabolites (Ansari et al. 2020).
Higher carbohydrate and lipid yields in phases following the exponential phase were assumed to be associated with biosynthesis and accumulation of metabolic storage (Mourente et al. 1990; Markou et al. 2012; Li et al. 2021). Increased metabolic storage capacity with culture age has been observed in many microalgal taxa, as photosynthetic energy is diverted from lipid and/or carbohydrate production instead of cell division and is often initiated by N limitation (Zhu et al. 1997; Valenzuela-Espinoza et al. 2002; Richmond 2004; Huerlimann et al. 2010).
Although stationary growth phase did not record significant differences of independent variables between the two strains (t test, p > 0.05), Fig. 1 shows that C. salina seemed to favour the production of carbohydrate as storage compound over that of lipid whenever nutrient became insufficient. By contrast, under the same conditions, I. maritima cells produced increased lipids (Fig. 2), most probably by channelling excess light energy and carbon into intercellular compound of lipids and decreased photosynthetic capacity (Sánchez-García et al. 2020). N starvation in microalgae has been shown to significantly increase the lipid fraction in some other species (Converti et al. 2009; Huang et al. 2013; Ördög et al. 2013; Sánchez-García et al. 2020) and causes carbohydrate accumulation in others (Chu et al. 1996; Markou et al. 2012), with the different preferences existing even between strains in the same genus (Richmond 2004). In I. maritima cultivation, when the culture aged, photosynthesis ceased; thus, ATP and NADPH may have been supplied by the cyclic electron flow (CEF) and oxidative pentose phosphate pathway (OPPP), which would increase fatty acid and lipid content (Jeon et al. 2017). Under N starvation, some microalgae switch metabolic pathways to store more carbons into starch and then into lipids (Jeon et al. 2017; Sánchez-García et al. 2020); thus, this could lead to the conclusion that the reaction might be species and culture conditions dependent (Safafar et al. 2016; Schulze et al. 2019; Sánchez-García et al. 2020; Li et al. 2021). Furthermore, N availability has widely been manipulated to induce neutral lipids, carbohydrates and carotenoid production (Sánchez-García et al. 2020).
Fatty acid composition
Fatty acids are structural components of many lipids and are involved in the maintenance of membrane integrity and cellular organisation (Bhowmick et al. 2020; Lu et al. 2021). The type and amount of fatty acids that determine the potential application of lipids accumulated by microalgae vary considerably between species (Gatenby et al. 2003; Lu et al. 2021). In this study, it was found that the amount of fatty acid classes in C. salina and I. maritima changed substantially throughout the growth phase (Figs. 3 and 4). It was discovered that polyunsaturated fatty acids (PUFA) concentration increased with increasing culture age and C. salina recorded significantly higher PUFA than I. maritima. Maximal proportions were achieved during the stationary phase of growth in both strains. Accumulation of PUFA has previously been correlated with the cessation of cellular division at the onset of the stationary phase (Hodgson et al. 1991; Brown et al. 1997; Fidalgo et al. 1998; Huerlimann et al. 2010). Conversely, other studies demonstrated lower amount of unsaturated fatty acid, particularly PUFA, in stationary phase observed in Nannochloropsis oculata (Dunstan et al. 1993; Brown et al. 1997) and Pavlova viridis (Xu et al. 2006), showing that the variations are species-specific depending on culture conditions (Solovchenko et al. 2008) and drying methods (Guldhe et al. 2014; Ljubic et al. 2019).
Total PUFA was the main constituent in the fatty acid profile of C. salina, across all growth phases. Of these fatty acids, both linoleic acid (LA) and α-linolenic acid (ALA) were abundant, while γ-linolenic acid (GLA) and docosahexaenoic acid (DHA) were also present but in lower quantities (Table 3). Total MUFA slightly increased and then remained relatively stable showing no significant difference (ANOVA, p > 0.05), whereas total SFA and PUFA were affected by the growth phase and experienced significant increase (ANOVA, p < 0.05). Tukey’s test further exhibited significant difference (p < 0.05) between exponential and stationary phase levels of SFA, while values of PUFA during exponential phase were significantly different (p < 0.05) from early stationary and stationary phases (Fig. 3). However, total SFA and MUFA of C. salina were not significantly different with I. maritima at all growth stages (t test, p > 0.05).
Eicosapentaenoic acid (EPA), 20:5n-3, not found in C. salina in this study, has been found to be a major component of the total fatty acids in some Chlorella spp. (Vazhappilly and Chen 1998; Khoeyi et al. 2011), but values are highly variable across the genus (Petkov and Garcia (2007). Palmitic and oleic acid increased with culture age in response to nitrogen depletion, as has been observed elsewhere (Toledo-Cervantes et al. 2018; Sánchez-García et al. 2020).
I. maritima was found to contain large proportions of polyunsaturated GLA (C18:3n6) and ALA (C18:3n3) and was particularly rich in DHA (C22:6n3), with low occurrence of ARA (C20:4n6) and EPA (C20:5n3) (Table 4). This common trend of high DHA vs low EPA values has been found in other Isochrysis strains by a number of studies (Dunstan et al. 1993; Fernández-Reiriz and Labarta 1996; Liu and Lin 2001; Mansour et al. 2005; Patil et al. 2007), and indeed Isochrysis strains have received increasing interest because of their ability to produce the polyunsaturated DHA which is of high value to the aquatic feed industry (Shi et al. 2012; Liu et al. 2013; Matsui et al. 2020).
In I. maritima, SFA were the predominant (Fig. 4) and consist of myristic acid (C14:0), stearic acid (C18:0) and arachidic acid (C20:0) followed by the PUFA GLA (C18:3n6), ALA (C18:3n3), DHA (C22:6n3) and MUFA of oleic acid (C18:1n9) and nervonic acid (C24:1) during the first two phases of cultivation (Table 4). SFA and PUFA had relatively similar proportions when reached stationary phase. This was due to increased accumulation of PUFA and declining SFA content over time. Although MUFA increased over the culture period, it remained the lowest fraction in I. maritima. According to Zhu et al. (1997), Fidalgo et al. (1998) and Lin et al. (2007), some variation in the fatty acid compositions of Isochrysis spp. between growth phases can be attributed to relative distribution of the lipid bodies in this microalga.
Table 4 shows that EPA, ALA and DHA increased when the culture aged, agreeing with previous studies which reported C20:5n3 in Phaeodactylum tricornutum cultures increased under nutrient starvation (Bai et al. 2016). This contrasts with a study conducted by Chua et al. (2020) who reported a decrease in EPA in Nannochloropsis oceanica under N-deplete condition, showing the response is species-specific. Matsui et al. (2020) recorded an increase of ALA and DHA in T. lutea as the culture aged, with the assumption that there was a reduction of betaine lipids and accumulation of lyso-lipids and free fatty acids when cells reach the stationary phase. ALA and DHA in T. lutea contributed to fatty acid unsaturation in chloroplast membranes and mitochondria, respectively, which has an antioxidant function. Accumulation of ALA and DHA in membrane lipids at the stationary growth phase of I. maritima could protect the cells from oxidative stress which include their tolerance towards deficiency of nutrients (Matsui et al. 2020). In addition to that, it was unveiled that fatty acids from algal cells have antibacterial properties by inhibiting the electron transport chain (ETC) and oxidative phosphorylation that led to bacterial lysis (Bhowmick et al. 2020). Studies have shown the importance of algal fatty acids as antibacterial agents to treat pathogens in aquaculture (Cermak et al. 2015; Ben Hafsa et al. 2017; Bhowmick et al. 2020), rendered their value in aquaculture industrial setup. Ingestion of PUFA-rich microalgae could improve fatty acids profile, promote growth and enhance immunity of aquatic animals (Lu et al. 2021).
The ratios of n3 to n6 PUFA have been normally used as an index of high nutritional value for aquaculture organisms (Renaud et al. 1991; Shamsudin, 1992; Lu et al. 2021). Diets with ratios of either n3/n6 > 2 (Huerlimann et al. 2010) or n6/n3 < 0.5 (Fidalgo et al. 1998) are considered to be optimal for larvae and juvenile oyster. The increment of n3/n6 ratio of C. salina from exponential to early stationary phases (Table 3) was contributed mainly by the n3 family (although overall n6 composition was higher) while in I. maritima, n6 demonstrated significant increase from exponential to stationary phase (Table 4). However, both strains had n3/n6 ratios below what is considered optimal. n3 and n6 fatty acids are vital to the diets of many commercially important marine fish and bivalves, and thus, it is of interest to aquaculturist to maximise these essential PUFA components (Mourente et al. 1990; Patil et al. 2007; Lu et al. 2021). Therefore, harvesting the culture at specific growth phases may enable better yield in PUFA compositions. A recent study conducted by Matsui et al. (2020) using the microalgae Tisochrysis lutea harvested at different growth phases to feed rotifer Brachionus plicatilis recorded varied levels of the highly unsaturated fatty acid (HUFA) in B. plicatilis, showing that harvesting microalgae at a correct timing is crucial to enrich DHA in rotifers intended for marine finfish larviculture.
Conclusion
Indigenous marine microalgae, C. salina (Chlorophyceae) and I. maritima (Prymnesiophyceae), were successfully isolated and grown in a conventional laboratory batch culture system. Variations in biochemical compositions associated with growth phases revealed that the growth stage–dependent variables were more discriminative since other growth factors were closely controlled. Results of this study can provide strategies for these strains’ growth optimisation and their maintenance as aquaculture feed stock. Determination of microalgal optimal harvest time is crucial and is dependent on the nutritional values of the enrichment diet and its effect on the target organisms in aquaculture; thus for this purpose, harvesting both strains at stationary phase is recommended as it yielded better PUFA compositions. C. salina and I. maritima are well suited for use as microalgae feeds for aquaculture organism due to their appropriate small cellular sizes and shapes, but I. maritima was proved to be superior compared to C. salina by accumulating C20:5n3 (EPA) and higher C22:6n3 (DHA). Furthermore, higher ash content in C. salina showed that it has lower digestibility. A combination of these two marine microalgae, or mixture with other species, might provide a better or an optimal balance of nutritional values for use in aquaculture feed.
Data availability
The authors declare that [the/all other] data supporting the findings of this study are available within the article [and its supplementary information files].
Code availability
Not applicable.
References
Abel K, Deschmertzing H, Peterson JI (1963) Classification of microorganisms by analysis of chemical composition. J Bacteriol 85:1039–1044. https://doi.org/10.1128/jb.85.5.1039-1044.1963
Ansari FA, Nasr M, Guldhe A, Gupta SK, Rawat I, Bux F (2020) Techno-economic feasibility of algal aquaculture via fish and biodiesel production pathways: A commercial- scale application. Sci Total Environ 704:135259. https://doi.org/10.1016/j.scitotenv.2019.135259
Araujo SDC, Garcia VMT (2005) Growth and biochemical composition of the diatom Chaetoceros cf. wighamii bright well under different temperature, salinity and carbon dioxide levels. I. Protein, carbohydrates and lipids. Aquaculture 246:405–412. https://doi.org/10.1016/j.aquaculture.2005.02.051
Arguelles EDLR, Martinez-Goss MR (2021) Lipid accumulation and profiling in microalgae Chlorolobion sp. (BIOTECH 4031) and Chlorella sp. (BIOTECH 4026) during nitrogen starvation for biodiesel production. J Appl Phycol 33:1–11. https://doi.org/10.1007/s10811-020-02126-z
Bai X, Song H, Lavoie M, Zhu K, Su Y, Ye H, Chen S, Fu Z, Qian H (2016) Proteomic analyses bring new insights into the effect of a dark stress on lipid biosynthesis in Phaeodactylum tricornutum. Sci Rep 6:25494. https://doi.org/10.1038/srep25494
Barbarino E, Lourenço SO (2005) An evaluation of methods for extraction and quantification of protein from marine macro- and microalgae. J Appl Phycol 17:447–460. https://doi.org/10.1007/s10811-005-1641-4
Ben Hafsa M, Ben Ismail M, Garrab M, Aly R, Gagnon J, Naghmouchi K (2017) Antimicrobial, antioxidant, cytotoxic and anticholinesterase activities of water-soluble polysaccharides extracted from microalgae Isochrysis galbana and Nannochloropsis oculata. J Serb Chem Soc 82(5):509–522. https://doi.org/10.2298/JSC161016036B
Bhowmick S, Mazumdar A, Moulick A, Adam V (2020) Algal metabolites: an inevitable substitute for antibiotics. Biotechnol Adv 43:107571. https://doi.org/10.1016/j.biotechadv.2020.107571
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 8:911–917. https://doi.org/10.1139/o59-099
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Bresaola MD, Morocho-Jácome AL, Matsudo MC, de Carvalho JCM (2019) Semi-continuous process as a promising technique in Ankistrodesmus braunii cultivation in photobioreactor. J Appl Phycol 31:2197–2205. https://doi.org/10.1007/s10811-019-01774-0
Brown MR, Garland CD, Jeffrey SW, Jameson ID, Leroi JM (1993) The gross and amino acid compositions of batch and semi-continuous cultures of Isochrysis sp. (clone T. ISO), Pavlova lutheri and Nannochloropsis oculata. J Appl Phycol 5:285–296. https://doi.org/10.1007/BF02186231
Brown MR, Jeffrey SW, Volkman JK, Dunstan GA (1997) Nutritional properties of microalgae for mariculture. Aquaculture 151:315–331. https://doi.org/10.1016/S0044-8486(96)01501-3
Brown MR, Hohmann S (2002) Effects of irradiance and growth phase on the ascorbic acid content of Isochrysis sp. T. ISO (Prymnesiophyta). J Appl Phycol 14:211–214. https://doi.org/10.1023/A:1019973520381
Cermak L, Prazakova S, Marounek M, Skrivan M, Skrivanova E (2015) Effect of green alga Planktochlorella nurekis on selected bacteria revealed antibacterial activity in vitro. Czech J Anim Sci 60(10): 427-435 https://doi.org/10.17221/8522-CJAS
Chan SW, Mirhosseini H, Taip FS, Ling TC, Tan CP (2013) Comparative study on the physicochemical properties of κ-carrageenan extracted from Kappaphycus alvarezii (doty) doty ex Silva in Tawau, Sabah, Malaysia and commercial κ-carrageenans. Food Hydrocoll 30:581–588. https://doi.org/10.1016/j.foodhyd.2012.07.010
Chen Y, Wang J, Liu T, Gao L (2012) Effects of initial population density (IPD) on the growth and lipid composition of Nannochloropsis sp. J Appl Phycol 24:1623–1627. https://doi.org/10.1007/s10811-012-9825-1
Chia MA, Lombardi AT, Melão MG, Parrish GC (2013) Lipid composition of Chlorella vulgaris (Trebouxiophyceae) as a function of different cadmium and phosphate concentrations. Aquat Toxicol 128:171–182. https://doi.org/10.1016/j.aquatox.2012.12.004
Chu WL, Phang SW, Goh SH (1996) Environmental effects on growth and biochemical composition of Nitzschia inconspicua Grunow. J Appl Phycol 8:389–396. https://doi.org/10.1007/BF02178582
Chua ET, Dal’Molin C, Thomas-Hall S, Netzel ME, Netzel G, Schenk PM (2020) Cold and dark treatments induce omega-3 fatty acid and carotenoid production in Nannochloropsis oceanica. Algal Res 51:102059. https://doi.org/10.1016/j.algal.2020.102059
Converti A, Casazza AA, Ortiz EY, Perego P, Borghi MD (2009) Effects of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chem Eng Process 48:1146–1151. https://doi.org/10.1016/j.cep.2009.03.006
Costard GS, Machado RR, Barbarino E, Martino RC, Lourenço SO (2012) Chemical composition of five marine microalgae that occur on the Brazilian coast. Int J Fish Aquat 4:191–201. https://doi.org/10.5897/IJFA11.092
Cui Y, Zhu J, Wu R (2006) Functional mapping for genetic control of programmed cell death. Physiol Genomics 25:458–469. https://doi.org/10.1152/physiolgenomics.00181.2005
Cui H, Yang Z, Lu Z, Wang Q, Liu J, Song L (2019) Combination of utilization of CO2 from flue gas of biomass power plant and medium recycling to enhance cost-effective Spirulina production. J Appl Phycol 31:2175–2185. https://doi.org/10.1007/s10811-019-1736-y
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356. https://doi.org/10.1021/ac60111a017
Dunstan GA, Volkman JK, Barrett SM, Garland CD (1993) Changes in the lipid composition and maximisation of the polyunsaturated fatty acid content of three microalgae grown in mass culture. J Appl Phycol 5:71–83. https://doi.org/10.1007/BF02182424
Eranza DRD, Bahron A, Alin J (2015) Sustaining seaweed farming in Malaysia. Int J Bus Manag 3(3): 201–205 https://www.researchgate.net/publication/308033563_sustaining_seaweed_farming_in_Malaysia
Fernández-Reiriz MJ, Labarta U (1996) Lipid classes and fatty acid composition of rotifers (Brachionus plicatilis) fed two algal diets. Hydrobiologia 330:73–79. https://doi.org/10.1007/BF00020826
Fidalgo JP, Cid A, Torres E, Sukenik A, Herrero C (1998) Effects of nitrogen source and growth phase on proximate biochemical composition, lipid classes and fatty acid profile of the marine microalga Isochrysis galbana. Aquaculture 166:105–116. https://doi.org/10.1016/S0044-8486(98)00278-6
Gamboa-Delgado J, Morales-Navarro YI, Nieto-López MG, Villarreal-Cavazos DA, Cruz-Suárez LE (2019) Assimilation of dietary nitrogen supplied by fish meal and microalgal biomass from Spirulina (Arthrospira platensis) and Nannochloropsis oculata in shrimp Litopenaeus vannamei fed compound diets. J Appl Phycol 31:2379–2389. https://doi.org/10.1007/s10811-019-1732-2
Gatenby CM, Orcutt DM, Kreeger DA, Parker BC, Jones VA, Neves RJ (2003) Biochemical composition of three algal species proposed as food for captive freshwater mussels. J Appl Phycol 15:1–11. https://doi.org/10.1023/A:1022929423011
Goh SH, Yusoff FM, Loh SP (2010) A comparison of the antioxidant properties and total phenolic content in a diatom, Chaetoceros sp. and a green microalgae. Nannochloropsis sp. J Agric Sci 2:123–130https://doi.org/10.5539/jas.v2n3p123
Griffiths MJ, Hille RP, Harrison STL (2011) Lipid productivity, settling potential and fatty acid profile of 11 microalgal species grown under nitrogen replete and limited conditions. J Appl Phycol 24:989–1001. https://doi.org/10.1007/s10811-011-9723-y
Guldhe A, Singh B, Rawat I, Ramluckan K, Bux F (2014) Efficacy of drying and cell disruption techniques on lipid recovery from microalgae for biodiesel production. Fuel 128:46–52. https://doi.org/10.1016/j.fuel.2014.02.059
Ha JS, Lee JW, Seo SH, Ahn CY, Rho GJ, Lee HG, Oh HM (2019) Optimized cryopreservation of Ettlia sp. using short cold acclimation and controlled freezing procedure. J Appl Phycol 31:2277–2287. https://doi.org/10.1007/s10811-019-1743-z
Han P, Lu Q, Fan L, Zhou W (2019) A Review on the Use of Microalgae for Sustainable Aquaculture. Appl Sci 9(11):2377. https://doi.org/10.3390/app9112377
Hodgson PA, Henderson RJ, Sargent JR, Leftley JW (1991) Patterns of variation in the lipid class and fatty acid composition of Nannochloropsis oculata (Eustigmatophyceae) during batch culture I. The Growth Cycle J Appl Phycol 3:169–181. https://doi.org/10.1007/BF00003699
Huang X, Huang Z, Wen W, Yan J (2013) Effects of nitrogen supplementation of the culture medium on the growth, total lipid content and fatty acid profiles of three microalgae (Tetraselmis subcordiformis, Nannochloropsis oculata and Pavlova viridis). J Appl Phycol 25:129–137. https://doi.org/10.1007/s10811-012-9846-9
Huerlimann R, de Nys R, Heimann K (2010) Growth, lipid content, productivity and fatty acid composition of tropical microalgae for scale-up production. Biotechnol Bioeng 107:245–257. https://doi.org/10.1002/bit.22809
Hulatt CJ, Lakaniemi A-M, Puhakka JA, Thomas DN (2012) Energy demands of nitrogen supply in mass cultivation of two commercially important microalgal species, Chlorella vulgaris and Dunaliella tertiolecta. Bioenergy Res 5:669–684. https://doi.org/10.1007/s12155-011-9175-x
Illman AM, Scragg AH, Shales SW (2000) Increase in Chlorella strains calorific values when grown in low nitrogen medium. Enzyme Microb Technol 27:631–635. https://doi.org/10.1016/S0141-0229(00)00266-0
Jeon S, Jeong B, Chang YK 2017 Chemicals and Fuels from Microalgae. In: Lee SY (ed) Consequences of Microbial Interactions with Hydrocarbons, Oils, and Lipids: Production of Fuels and Chemicals. Handbook of Hydrocarbon and Lipid Microbiology, Springer, Cham, pp 1–21
Kawachi M, Noël MH (2005) Sterilization and sterile technique. In: Andersen RA (ed) Algal culturing techniques. Elsevier Academic Press, Burligton, pp 65–82
Khoeyi ZA, Seyfabadi J, Ramezanpour Z (2011) Effect of light intensity and photoperiod on biomass and fatty acid composition of the microalgae, Chlorella vulgaris. Aquac Int 20:41–49. https://doi.org/10.1007/s10499-011-9440-1
Lee JY, Seo SH, Ahn CY, Lee CS, An KG, Srivastava A, Oh HM (2019) Green light as supplementary light for enhancing biomass production of Ettlia sp. and preventing population invasion from other microalgae. J Appl Phycol 31:2207–2215. https://doi.org/10.1007/s10811-019-1737-x
Li X, Slavens S, Crunkleton DW, Johannes TW (2021) Interactive effect of light quality and temperature on Chlamydomonas reinhardtii growth kinetics and lipid synthesis. Algal Res 53:102127. https://doi.org/10.1016/j.algal.2020.102127
Liang K, Zhang Q, Gu M, Cong W (2013) Effect of phosphorus on lipid accumulation in freshwater microalga Chlorella sp. J Appl Phycol 25:311–318. https://doi.org/10.1007/s10811-012-9865-6
Lin YH, Chang FL, Tsao CY, Leu JY (2007) Influence of growth phase and nutrient source on fatty acid composition of Isochrysis galbana CCMP 1324 in a batch photoreactor. Biochem Eng J 37:166–176. https://doi.org/10.1016/j.bej.2007.04.014
Liu J, Sommerfeld M, Hu Q (2013) Screening and characterization of Isochrysis strains and optimization of culture conditions for docosahexaenoic acid production. Appl Microbiol Biotechnol 97:4785–4798. https://doi.org/10.1007/s00253-013-4749-5
Liu CP, Lin LP 2001 Ultrastructural study and lipid formation of Isochrysis sp. CCMP1324. Bot Bull Acad Sin 42: 207–214. https://ejournal.sinica.edu.tw/bbas/content/2001/3/bot423-08.pdf
Ljubic A, Safafar H, Jacobsen C (2019) Recovery of microalgal biomass and metabolites from homogenised, swirl flash-dried microalgae. J Appl Phycol 31:2355–2363. https://doi.org/10.1007/s10811-019-1733-1
López CVG, García MDCC, Fernández FGA, Bustos CS, Chisti Y, Sevilla JMF (2010) Protein measurements of microalgal and cyanobacterial biomass. Bioresour Technol 101:7587–7591. https://doi.org/10.1016/j.biortech.2010.04.077
Lourenço SO, Marquez UML, Mancini-Filho J, Barbarino E, Aidar E (1997) Changes in biochemical profile of Tetraselmis gracilis I. Comparison of Two Culture Media Aquaculture 148:153–168. https://doi.org/10.1016/S0044-8486(96)01416-0
Lu Q, Li H, Xiao Y, Liu H (2021) A state-of-the-art review on the synthetic mechanisms, production technologies, and practical application of polyunsaturated fatty acids from microalgae. Algal Res 55:102281. https://doi.org/10.1016/j.algal.2021.102281
Mahboob S, Rauf A, Ashraf M, Sultana T, Sultana S, Jabeen F, Rajoka MI, Al-Balawi HFA, Al-Ghanim KA (2012) High-density growth and crude protein productivity of a thermotolerant Chlorella vulgaris: production kinetics and thermodynamics. Aquac Int 20:455–466. https://doi.org/10.1007/s10499-011-9477-1
Mansour MP, Frampton DMF, Nichols PD, Volkman JK, Blackburn SI (2005) Lipid and fatty acid yield of nine stationary-phase microalgae: applications and unusual C24–C28 polyunsaturated fatty acids. J Appl Phycol 17:287–300. https://doi.org/10.1007/s10811-005-6625-x
Markou G, Angelidaki I, Georgakakis D (2012) Microalgal carbohydrates: an overview of the factors influencing carbohydrates production, and of main bioconversion technologies for production of biofuels. Appl Microbiol Biotechnol 96:631–645. https://doi.org/10.1007/s00253-012-4398-0
Matsui H, Intoy MMB, Wagalevu V, Ishikawa M, Kotani T (2020) Suitability of Tisochrysis lutea at different growth phases as an enrichment diet for Brachionus plicatilis sp. complex rotifers. J Appl Phycol 32(6):3933–3947. https://doi.org/10.1007/s10811-020-02216-y
Mohammad Basri E, Wan Maznah WO (2017) Differential growth and biochemical composition of photoautotrophic and heterotrophic Isochrysis maritima: evaluation for use as aquaculture feed. J Appl Phycol 29(3):1159–1170. https://doi.org/10.1007/s10811-017-1054-1
Mourente G, Lubián LM, Odriozola JM (1990) Total fatty acid composition as a taxonomic index of some marine microalgae used as food in marine aquaculture. Hydrobiologia 203:147–154. https://doi.org/10.1007/BF00005683
Nalder TD, Miller MR, Packer MA (2015) Changes in lipid class content and composition of Isochrysis sp. (T-Iso) grown in batch culture. Aquac Int 23:1293–1312. https://doi.org/10.1007/s10499-015-9884-9
Nor AM, Gray TS, Caldwell GS, Stead SM (2017) Is a cooperative approach to seaweed farming effectual? An analysis of the seaweed cluster project (SCP), Malaysia. J Appl Phycol 29:2323–2337. https://doi.org/10.1007/s10811-016-1025-y
Nor AM, Gray TS, Caldwell GS, Stead SM (2020) A value chain analysis of Malaysia’s seaweed industry. J Appl Phycol 32:2161–2171. https://doi.org/10.1007/s10811-019-02004-3
Ördög V, Stirk WA, Bálint P, Lovász C, Pulz O, Staden J (2013) Lipid productivity and fatty acid composition in Chlorella and Scenedesmus strains grown in nitrogen-stressed conditions. J Appl Phycol 25:233–243. https://doi.org/10.1007/s10811-012-9857-6
Patil V, Källqvist T, Olsen E, Vogt G, Gislerød HR (2007) Fatty acid composition of 12 microalgae for possible use in aquaculture feed. Aquac Int 15:1–9. https://doi.org/10.1007/s10499-006-9060-3
Patil RR, Kambhar SV, Giriyappanavar BS, Chakraborty S 2021 Algae as Environmental Biotechnological Tool for Monitoring Health of Aquatic Ecosystem. Chapter 2 In: Maddela NR et al. (eds) Advances in the Domain of Environmental Biotechnology. Microbiological Developments in Industries, Wastewater Treatment and Agriculture, Springer Nature, Singapore Pte Ltd, pp 549–563 https://doi.org/10.1007/978-981-15-8999-7
Petkov G, Garcia G (2007) Which are fatty acids of the green alga Chlorella? Biochem Syst Ecol 35:281–285. https://doi.org/10.1016/j.bse.2006.10.017
Phang SM 1998 The Seaweed Resources of Malaysia. In: Critchley AT, Ohno M (eds) Seaweed Resources of the World. Japan International Cooperation Agency, Yokosuka, Japan, pp 79–91
Phang SM (2006) Seaweed resources in Malaysia: current status and future prospects. Aquat Ecosyst Health Manag 9(2):185–202. https://doi.org/10.1080/14634980600710576
Phang SM, Hui-Yin Y, Hussin H, Phaik-Eem L, Hack-Churl Y, Joon Ching J (2017) Techno-economics of seaweed farming along the coasts of Kelantan, east coast peninsular Malaysia. Malays J Sci 36: 85–102 https://doi.org/10.22452/mjs.vol36no2.4
Phang SM, Yeong HY, Lim PE (2019) The seaweed resources of Malaysia. Bot Mar. 62:265–273. https://doi.org/10.1515/bot-2018-0067
Přibyl P, Cepák V, Zachleder V (2012) Production of lipids in 10 strains of Chlorella and Parachlorella, and enhanced lipid productivity in Chlorella vulgaris. Appl Microbiol Biotechnol 94:549–561. https://doi.org/10.1007/s00253-012-3915-5
Qu CB, Wu ZF, Shi XM (2008) Phosphate assimilation by Chlorella and adjustment of phosphate concentration in basal medium for its cultivation. Biotechnol Lett 30:1735–1740. https://doi.org/10.1007/s10529-008-9758-6
Recht L, Zarka A, Boussiba S (2012) Patterns of carbohydrate and fatty acid changes under nitrogen starvation in the microalgae Haematococcus pluvialis and Nannochloropsis sp. Appl Microbiol Biotechnol 94:1495–1503. https://doi.org/10.1007/s00253-012-3940-4
Renaud SM, Parry DL, Kuo C, Padovan A, Sammy N (1991) Effect of light intensity on the proximate biochemical and fatty acid composition of Isochrysis sp. and Nannochloropsis oculata for use in tropical aquaculture. J Appl Phycol 3:43–53. https://doi.org/10.1007/BF00003918
Renaud SM, Parry DL (1994) Microalgae for use in tropical aquaculture II: Effect of salinity on growth, gross chemical composition and fatty acid composition of three species of marine microalgae. J Appl Phycol 6:347–356. https://doi.org/10.1007/BF02181949
Renaud SM, Thinh L, Parry DL (1999) The gross chemical composition, and fatty acid composition of 18 species of tropical Australian microalgae for possible use in mariculture. Aquaculture 170:147–159. https://doi.org/10.1016/S0044-8486(98)00399-8
Renaud SM, Thinh LV, Lambrinidis G, Parry DL (2002) Effect of temperature on growth, chemical composition and fatty acid composition of tropical Australian microalgae grown in batch cultures. Aquaculture 211:195–214. https://doi.org/10.1016/S0044-8486(01)00875-4
Richmond A 2004 Biological principles of mass cultivation. In: Richmond A, (eds) Handbook of microalgae culture: biotechnology and applied phycology. Blackwell Science, Oxford, pp 125–177 https://doi.org/10.1002/9780470995280
Rocha JMS, Garcia JEC, Henriques MHF (2003) Growth aspects of the marine microalga Nannochloropsis gaditana. Biomol Eng 20:237–242. https://doi.org/10.1016/S1389-0344(03)00061-3
Ryckebosch E, Muylaert K, Foubert I (2012) Optimization of an analytical procedure for the extraction of lipids from microalgae. J Am Oil Chem Soc 89:189–198. https://doi.org/10.1007/s11746-011-1903-z
Safafar H, Nørregaard PU, Ljubic A, Møller P, Holdt SL, Jacobsen C (2016) Enhancement of protein and pigment content in two Chlorella species cultivated on industrial process water. J Mar Sci Eng 4(84):2–15. https://doi.org/10.3390/jmse4040084
Sánchez-García L, Cabello J, Jiménez-García LF, Revah S, Morales-Ibarría M (2020) Enhancing the lipid content of Scenedesmus obtusiusculus AT-UAM by controlled acidification under indoor and outdoor conditions. Algal Res 51:102024. https://doi.org/10.1016/j.algal.2020.102024
Schoen S (1988) Cell counting. In: Lobban CS, Chapman DJ, Kremer BP (eds) Experimental Phycology: a laboratory manual. Cambridge University Press, New York, pp 16–22
Schulze PSC, Hulatt CJ, Morales-Sánchez D, Wijffels RH, Kiron V (2019) Fatty acids and proteins from marine cold adapted microalgae for biotechnology. Algal Res 42:101604. https://doi.org/10.1016/j.algal.2019.101604
Seyfabadi J, Ramezanpour Z, Khoeyi ZA (2010) Protein, fatty acid, and pigment content of Chlorella vulgaris under different light regimes. J Appl Phycol 23:721–726. https://doi.org/10.1007/s10811-010-9569-8
Shamsudin L (1992) Lipid and fatty acid composition of microalgae used in Malaysian aquaculture as live food for the early stage of penaeid larvae. J Appl Phycol 4:371–378. https://doi.org/10.1007/BF02185795
Shi T, Yu A, Li M, Ou X, Xing L, Li M (2012) Identification of a novel C22-∆4-producing docosahexaenoic acid (DHA) specific polyunsaturated fatty acid desaturase gene from Isochrysis galbana and its expression in Saccharomyces cerevisiae. Biotechnol Lett 34:2265–2274. https://doi.org/10.1007/s10529-012-1028-y
Shi Q, Chen C, Zhang W, Wu P, Sun M, Wu H, Wu H, Fu P, Fan H (2021) Transgenic eukaryotic microalgae as green factories: providing new ideas for the production of biologically active substances. J Appl Phycol 33:705–728. https://doi.org/10.1007/s10811-020-02350-7
Solovchenko AE, Khozin-Goldberg I, Didi-Cohen S, Cohen Z, Merzlyak MN (2008) Effects of light intensity and nitrogen starvation on growth, total fatty acids and arachidonic acid in the green microalga Parietochloris incisa. J Appl Phycol 20:245–251. https://doi.org/10.1007/s10811-007-9233-0
Tokusoglu Ö, Ünal MK (2003) Biomass nutrient profiles of three microalgae: Spirulina platensis, Chlorella vulgaris, and Isochrisis galbana. Food Chem Toxicol 68:1144–1148. https://doi.org/10.1111/j.1365-2621.2003.tb09615.x
Toledo-Cervantes A, Garduño Solórzano G, Campos JE, Martínez-García M, Morales M (2018) Characterization of Scenedesmus obtusiusculus AT-UAM for high-energy molecules accumulation: deeper insight into biotechnological potential of strains of the same species. Biotechnol Reports 17:16–23. https://doi.org/10.1016/j.btre.2017.11.009
Tzovenis I, De Pauw N, Sorgeloos P (2003) Optimisation of T-ISO biomass production rich in essential fatty acids I. Effect of different light regimes on growth and biomass production. Aquaculture 216:203–222. https://doi.org/10.1016/S0044-8486(02)00374-5
Vairappan CS, Chung CS, Hurtado AQ, Soya FE, Lhonneur GB, Critchley A (2008) Distribution and symptoms of epiphyte infection in major carrageenophyte-producing farms. J Appl Phycol 20:477–483. https://doi.org/10.1007/978-1-4020-9619-8_4
Van Bergeijk SA, Salas-Leiton E, Cañavate JP (2010) Low and variable productivity and low efficiency of mass cultures of the haptophyte Isochrysis aff. galbana (T-iso) in outdoor tubular photobioreactors. Aquacult Eng 43:14–23. https://doi.org/10.1016/j.aquaeng.2010.03.001
Valenzuela-Espinoza E, Millán-Núñez R, Núñez-Cebrero F (2002) Protein, carbohydrate, lipid and chlorophyll a content in Isochrysis aff. galbana (clone T-Iso) cultured with a low-cost alternative to the f/2 medium. Aquacult Eng 25:207–216. https://doi.org/10.1016/S0144-8609(01)00084-X
Vazhappilly R, Chen F (1998) Eicosapentaenoic acid and docosahexaenoic acid production potential of microalgae and their heterotrophic growth. J Am Oil Chem Soc 75:393–397. https://doi.org/10.1007/s11746-998-0057-0
Walne PR (1970) Studies on the food value of nineteen genera of algae to juvenile bivalves of the genera Ostrea, Crassostrea, Mercenaria and Mytilus. Fish Invest Lond 26:1–62
Xu N, Zhang X, Fan X, Han L, Zeng C (2001) Effects of nitrogen source and concentration on growth rate and fatty acid composition of Ellipsoidion sp. (Eustigmatophyta). J Appl Phycol 13:463–469. https://doi.org/10.1023/A:1012537219198
Xu H, Miao X, Wu Q (2006) High quality biodiesel production from a microalga Chlorella protothecoides by heterotrophic growth in fermenters. J Biotechnol 126:499–507. https://doi.org/10.1016/j.jbiotec.2006.05.002
Yu J, Wang P, Wang Y, Chang J, Deng S, Wei W (2018) Thermal constraints on growth, stoichiometry and lipid content of different groups of microalgae with bioenergy potential. J Appl Phycology 30:1503–1512. https://doi.org/10.1007/s10811-017-1358-1
Zhao B, Zhang Y, Xiong K, Zhang Z, Hao X, Liu T (2011) Effect of cultivation mode on microalgal growth and CO2 fixation. Chem Eng Res Des 89:1758–1762. https://doi.org/10.1016/j.cherd.2011.02.018
Zhu CJ, Lee YK, Chao TM (1997) Effects of temperature and growth phase on lipid and biochemical composition of Isochrysis galbana TK1. J Appl Phycol 9:451–457. https://doi.org/10.1023/A:1007973319348
Acknowledgements
The author would like to thank all the colleagues involved in this study for the fruitful idea and discussions. This study was supported by the Malaysian government under the Ministry of Science, Technology, and Innovation (MOSTI) and National Oceanographic Directorate (NOD) (304/PBIOLOGI/650422/D111) research grant.
Funding
This study was supported by the Malaysian government under the Ministry of Science, Technology and Innovation (MOSTI) and National Oceanographic Directorate (NOD) (304/PBIOLOGI/650422/D111) research grant.
Author information
Authors and Affiliations
Contributions
Not applicable.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Syazwina, S.S., Basri, E.M. & Maznah, W.O.W. Biomass, proximate biochemical composition and fatty acid profiles associated with the growth phase of Chlorella salina Butcher and Isochrysis maritima Billard and Gayral isolated from the coastal waters of Penang, Malaysia. Aquacult Int 30, 899–918 (2022). https://doi.org/10.1007/s10499-022-00843-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-022-00843-5