Abstract
Temperature has a range of effects on population demographic rates and physiology of microalgae for potential bioenergy application. The changes in microalgal growth, stoichiometry and cellular lipid contents of 14 different microalgae species with high bioenergy potentials in response to different temperature between 10 and 35 °C were investigated. For most of the chosen microalgae, the specific growth rate increased with temperature over most of the range. On the other hand, the biomass density of most of the selected species declined with temperature at stationary phase. Certain cyanobacteria failed to grow below 20 °C, while eukaryotes had a wider temperature range. Lipid content, by contrast, showed different responses to temperature among the 14 species. Nine of the species showed a positive response with increased temperature, while others showed neural or unimodal response. Cellular C:N and C:P ratios were mostly unimodally related to temperature. Some eukaryotic species showed the lowest total lipid contents but the highest C:N and C:P ratios, the fastest growth, and the highest biomass density at their stationary phases. The results indicated that the responses of the life history and cellular stoichiometry to temperature were more consistent across microalgae than that of total lipid content.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microalgae are regarded as a group of promising organisms with high bioenergy and biofuel (e.g., biodiesel) production potentials without requiring high-quality land (Schenk et al. 2008). Compared to terrestrial plants, algae have the potential for high productivity biofuels used for transportation and bioenergy used for heating/cooling and electricity generation (Srebotnjaka and Hardi 2011), requiring less acreage than traditional feedstock. Moreover, microalgae can even grow on non-arable land using wastewaters (Dismukes et al. 2008; Su et al. 2012). Provided the nutrients are recycled and water loss in evaporation is restricted, lipids accumulated by microalgae for the production of biodiesel are a promising alternative to producing energy without placing competing pressures on food production systems except for fresh water and fertilizers (Borowitzka and Moheimani 2013). In addition, microalgae-based technologies could greatly reduce greenhouse gas emissions (Brune et al. 2009; Tian et al. 2010; Balat and Balat 2011; Demirbas 2011). In spite of advantages previously mentioned, commercialization of microalgae-derived fuel still remains challenging due to various technical and economic constraints. Microalgal species candidates for bio-oil production must show relatively high growth rates and lipid content under a wide range of environmental conditions for the sake of cost-effectively growing outdoors (Markevicius et al. 2010).
Factors both abiotic to biotic affect microalgal growth and biodiesel yield (Courchesne et al. 2009; Bernard 2011; Su et al. 2012). Obviously, a good understanding of the physiology, biochemistry, and ecology of microalgae helps achieve reliable cultures, which is essential to fully exploit the potential of microalgae (Borowitzka 2016). Growth conditions such as temperature, light, pH, salinity, and nutrient status of the culture medium affect microalgal photosynthesis and biomass productivity as well as biochemical composition caused by pattern, pathway, and activity of cellular metabolism (Dean et al. 2008; Cheng et al. 2010; James et al. 2011; Pal et al. 2011; Chaffin et al. 2012; Gonzalez-Fernandez and Ballesteros 2012). Among these factors, temperature influences all metabolic processes and plays an important role in microalgal growth (Raven and Geider 1988) and chemical composition (Falkowski and Raven 2007; Rling et al. 2013). For example, both growth and lipid composition of the marine diatom Odontella aurita were sensitive to culture temperature (Pasquet et al. 2014). It has been reported that high growth temperature can lead to significant decreases in protein content and increases in lipid as well as carbohydrate contents (Renaud et al. 1995; Oliveira et al. 1999). On the contrary, it has been found that the effect of temperature on fatty acids was species-specific and there was no conclusive trend in biochemical composition for different species (Piepho et al. 2012). As a result, the effect of temperature on lipid content of microalgae remains uncertain, especially for different microalgal groups.
Temperature also affects morphology, stoichiometry, and life history of microalgae. Several studies have shown a decline in average size of cells under warmer conditions (Chen et al. 2009), with a general tendency toward smaller body size in multi-cellular organisms. Almost all species show a general tendency toward unimodal growth response where growth declines sharply once the temperature is above the thermal optimum. Long-term exposure to suboptimal temperature may change the structure and dynamics of the photosynthetic apparatus (Teoh et al. 2004). The optimum growth temperature varies greatly among species (Thomas et al. 2012). Population carrying capacity often declines at higher temperatures (above 30 °C) accompanied with increasing metabolic demands (Savage et al. 2004). Furthermore, temperature also has substantial effects on phytoplankton stoichiometry although Fu et al. (2007) found that there was no change in cellular C:N or C:P of Prochlorococcus or Synechococcus between 20 and 24 °C. The elemental stoichiometric composition of microalgae reflects the integration of the various processes involving nutrient uptake, assimilation, or other environmental factors (Sterner et al. 2002; Ventura 2006). In addition, temperature significantly affects nutrient demands, competitive dynamics, and interactions with consumers (Kalacheva et al. 2002). However, available studies are only based on a single group of microalgae (e.g., Chlorophyta) (Raven and Geider 1988). There is no overall trend in gross biochemical composition for all species under study (Renaud et al. 2002). On the other hand, there has been no systematic investigation of the impact of temperature on different groups of microalgae which have bioenergy potential.
Therefore, in this study, 14 microalgal species belonging to five phyla (Cyanobacteria, Bacillariophyta, Heterokontophyta, Glaucophyta, and Chlorophyta) were chosen to investigate the responses of growth dynamics, C, N, and P, and lipid contents to different temperatures (10, 15, 20, 25, 30, and 35 °C). The study also offers insight for selecting and engineering strains as candidate bioenergy crops, as well as predicting the response of phytoplankton populations and communities to future climate change.
Material and methods
Cultures
The microalgae used in this study were all freshwater microalgae obtained from culture collections and researchers at the San Diego Center for Algal Biotechnology in University of California (San Diego, USA). Species used in the experiment are listed in Table 1.
Pre-culture
The stock cultures of 14 species were maintained in 250-mL Erlenmeyer flasks containing 100 mL WC medium (Guillard and Lorenzen 1972) closed with a cellulose stopper. The cultures were maintained in an incubator at a stable temperature (20 °C) with a light/dark cycle of 12 h/12 h at light intensity of 70 ± 5 μmol photons m−2 s−1 photosynthetically active radiation (PAR). After acclimation, each culture was transferred to clean 50-mL Erlenmeyer flasks with 20 mL of WC medium. The initial concentration in each flask was 1 μg chlorophyll-a L−1 for cyanobacteria, and 3 μg chlorophyll-a L−1 for eukaryotes as the chlorophyll-a concentration is relatively higher in eukaryotic microalgal cells. Flasks were placed in incubators for 15 days at the same light and temperature regimes that would be used during the experiment. Flasks were shaken manually twice every day to homogenize the oxygen, pH, light, temperature, and nutrient gradients.
Culture
Each species was cultured in monoculture in WC at six temperatures including 10, 15, 20, 25, 30, and 35 °C with five replicates. Different light levels were used for prokaryotes vs. eukaryotes because cyanobacteria growth is often inhibited at high light levels (Shurin et al. 2013). The irradiance was 70 ± 5 μmol photons m−2 s−1 PAR for eukaryotes and 20 ± 5 μmol photons m−2 s−1 PAR for cyanobacteria. The experiment was performed in 50-mL Erlenmeyer flasks containing 20 mL WC medium which was renewed every 2 days. Flasks were shaken manually twice every day to homogenize the oxygen, pH, light, temperature, and nutrient gradients. Biomass accumulation was monitored every other day in forms of chlorophyll-a concentration, measured by the in vivo module of a Turner Trilogy fluorometer. The experiments lasted to the stationary phase of algal growth, usually15 to 21 days. Samples were collected at the end of each growth period to analyze N, P, C, and neutral lipid content of the algal biomass.
Analysis and data statistics
C and N contents were measured by filtering 4–8 mL of the culture onto pre-combusted Whatman GF/F filters. Then, they were analyzed by an elemental analyzer at the UC Davis Stable Isotope Facility. P content was measured by the method of Menzel and Corwin (1956). First, a standard curve of the dissolved phosphorus (DP) and total phosphate (TP) was prepared by determining the absorbance of five standard solutions. Then, the standard curves were used to calculate the dissolved phosphorus and total phosphate. Finally, the DP from the TP was subtracted to obtain particulate phosphorus content.
The Nile red staining method was used to measure the neutral lipid content in microalgae according to Chen et al. (2009). Firstly, the absorbance of the algal sample was adjusted near to 5 μL of the algal sample (OD750 of 0.06) of known absorbance in a 1.5-mL Eppendorf tube. The 3 μL of Nile red solution was added and mixed with a micropipette. After incubating at room temperature for 10 min, 292 μL of water was added and vortexed for 1 min (120 rpm). One hundred microliters was added to each 384-well plate. The fluorescence of the sample (F s) was measured in a plate reader with an excitation wavelength of 530 nm and an emission wavelength of 580 nm. Three blank replicates (F b) using distilled water were also prepared. The corrected fluorescence intensity (F corr) was obtained by subtracting the fluorescence intensity of the blank from the sample. A standard curve was prepared using different concentrations of triolein (0.5–4 μg mL−1 at 0.5 μg mL−1 intervals). The standard curve was used to calculate the neutral lipid content in the algae.
Growth curves were measured as the change in chlorophyll-a concentration over time and used to estimate asymptotic density at stationary phase (K), specific growth rate (μ), and lag phase duration (λ) for every replicate using the Grofit package in R. This software compares four different models (Logistic, Gompertz, modified Gompertz, and Richards) to fit growth functions and estimates the three parameters from the model with the best fit to the data. Linear models were fitted for all five dependent variables (μ, K, C:N, C:P, lipid content as a percent of dry weight) with species identity and temperature treatment as cross-factored independent variables. We compared fits of first- and second-order models for temperature to test for the possibility of non-linear responses. Individual linear models for response to temperature were then fit to each species, and second-order terms were included whenever they or their interaction with species significantly improved the fit of the overall model including all species.
Correlations among the species-averaged trait values across all temperatures were tested with Pearson correlation coefficients, and a principal components analysis was used to illustrate multivariate patterns of association among them. All analyses were performed in R (Kahm et al. 2010).
Results
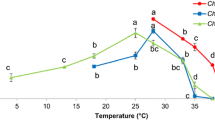
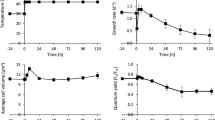
The response of specific growth rate (μ) to different temperatures varied with each species and showed significant non-linearity (Table 2; Fig. 1). Most cyanobacteria failed to grow below 20 °C, while several of the eukaryotic species (e.g., B910, Navicula sp.) also failed to grow well at either high or low temperatures not allowing the estimation of the growth parameters of the logistic equation. Several species (e.g., C. vulgaris) showed declines in growth rate at the highest temperatures while others (e.g., C. reinhardtii) showed no sign of reduced growth due to thermal stress. Carrying capacity (K) most often declined with the increasing temperature (Fig. 2), with evidence for non-linearity in the significance of the second-order term and its interaction with species.
Cellular lipid content varied by both species and temperature and the effect of temperature differed among species (Table 2; Fig. 3). Furthermore, the shape of the relationship varied greatly by species with some showing unimodal responses (e.g., Tetraselmis sp.) while others showing different curvilinear functions (e.g., C. vulgaris).
Stoichiometry of algal cells varied between species and temperature, and the effect of temperature was non-linear and varied for different species (Table 2; Figs. 4 and 5). In addition, cellular C:N ratio responded more strongly to temperature than cellular C:P ratio (calculated from the WC medium). Unimodal relationships were found between temperature and cellular C:N and C:P ratios for most species, with the highest values observed at intermediate temperatures.
The species trait values averaged across all temperatures and showed strong patterns of association (Fig. 6). A principal component analysis found that the first principal axis explained 48% of the variation and was strongly associated with all of the traits except lipid content (Fig. 7). This indicates that μ, K, cellular C:N, and C:P are all positively correlated, as shown in Fig. 6. Cellular lipid content was less strongly correlated with other traits, and mostly showed negative associations, indicating that the species with large size that grew fastest and reached highest asymptotic densities had the lowest cellular N and P contents and the lowest lipid content.
Correlations among mean values across all temperatures for the five traits of all species. Each point indicates the mean trait of a species and the panels above the diagonal show the Pearson correlation coefficient between pairs of traits. Units for μ are in day−1 and K is in loge of chlorophyll-a (μg L−1)
Principal component analysis of the five traits measured in the experiment. The arrows indicate trait values and the points are species with the codes indicated corresponding to the species in Table 1. The direction of the arrow shows the correlation between the trait value and the principal component axis, and arrows that are close together indicate traits that are positively correlated among species
Discussion
Temperature affects the physiology and ecology of algae which are vital to their utility as bioenergy producers. Hence, it would be beneficial for microalgal cultivation to use strains adapted to wide range of temperature at different time of the year (Chaisutyakorn et al. 2017). The results indicate that the responses of different algal traits related to growth, stoichiometry, and lipid content to temperature showed varying levels of consistency among species and taxonomic groups. Higher temperatures were generally associated with faster specific growth rate and lower cell density at stationary phase (K), while cellular C:N and C:P ratios were often unimodal, peaking at intermediate temperatures and declining at either extreme. Most cyanobacteria fared poorly at low temperatures relative to eukaryotes. By contrast, lipid content showed more variable patterns among species.
Growth characteristics
It is of interest to determine the ability of specific strains to tolerate extremes that do not allow growth (Radway et al. 1992). In this study, the response of specific growth rate (μ) varied among species and showed significant non-linearity that also varied by species. Most cyanobacteria and several of the eukaryotic species failed to grow below 20 °C, but the absence of growth below 10 °C is not universal among cyanobacteria (Chrismas et al. 2015). As previously reported (Roleda et al. 2013), low to negative growth rates were observed in most species under low temperature. The reason might be that reproduction and/or cell division could be inhibited or even cells die. The variation in response to temperature is also commonly observed, although we found less variation in temperature curves than Thomas et al. (2012) who synthesized such curves across populations growing at all latitudes. The taxa investigated here have grown under lab condition for more than 3 years and were likely acclimatized to growth at room temperature. In addition, we found a decline in density at stationary phase for most species with increasing temperature (Fig. 2), which might indicate a greater resource demand at higher temperatures when metabolism is higher (Thomas et al. 2012). This pattern indicated that phytoplankton growing at elevated temperatures were likely to show higher growth rates but lower standing stock (Shurin et al. 2012). Most of the species such as the Chlorophyte B902, C. minutissima, Tetraselmis sp., and S. dimorphus are good candidates for outdoor cultivation in hot areas, but only a few species such as C. vulgaris are optimal candidates in cold areas. The response of those species to the temperature out of range between 10 and 35 °C still needs to be further studied. Among the 14 species, C. reinhardtii is an ideal one that could adapt to a wide range of temperatures. Meanwhile, it took 15 to 21 days for the species selected in the control experiment condition to reach stationary phase. When they are cultured outdoors, the cultivation time might fluctuate due to hydrology, climate, even species competition, etc.
Cellular neutral lipid content
Microalgae are the basis of aquatic food webs. Temperatures have significant impacts on their adaptation and survival rates. Algae respond to temperature changes by modulating their photosynthetic rates and biochemical composition (Sushchik et al. 2003). Fatty acid unsaturation increases with decreasing temperature (Khotimchenko 2005; Griffiths and Harrison 2009). The ability of microalgae to alter the physical properties and thermal responses of membrane lipids may represent a strategy for enhancing physiological acclimatization over a range of temperatures. Besides, high-temperature stress can enhance biofuel production in oleaginous microalgae during nitrogen deficiency (Xia et al. 2016). Microalgae synthesize mainly membranes or signaling glycolipids. However, under stressful conditions, many species alter their lipid biosynthetic pathways toward the formation and accumulation of neutral lipids. The present study showed that there was a weak negative correlation between the averages of lipid content and growth rate across species (r = − 0.27, P > 0.05), indicating a potential tradeoff between maximizing growth rate and cellular neutral lipid accumulation among different species. The effect of temperature on neutral lipid accumulation was species-specific as reported in various algal species (Sushchik et al. 2003).
Stoichiometry
We found that species varied considerably in cellular C, N, and P contents and that C:N and C:P ratios were most often highest at intermediate temperatures. Although the Redfield ratio characterizes diverse natural microalgal assemblages, the species that make up those communities can vary considerably in elemental composition (Geider and Roche 2002). Actually, stoichiometry is highly plastic within species in response to multiple factors including temperature (Sterner and Elser 2002), which was confirmed by our study. The change in stoichiometry may have been due to the effects of temperature on photosynthetic efficiency and nutrient uptake, as well as activities of carbonic anhydrase, photosynthetic phosphorylase, and other enzymes. Studies have found that carbon to nutrient ratios generally increase with the concentration of dissolved CO2 as well as under high light conditions. In nature, high temperature may reduce nutrient supply by reducing the mixing depth and increasing the stability of stratification.
The results indicate that temperature also has strong direct effects on stoichiometric ratio. In addition, the effects of climate warming will be non-linear, either increasing or decreasing cellular N and P contents depending on ambient conditions (Jiao et al. 2016). Unimodal relationships between temperature and both cellular C:N and C:P ratios for most species suggest that these species have low N and P requirement for their growth at intermediate temperatures. High C:N and C:P ratios may also decrease the quality of algae as food resources for consumers (Sterner and Elser 2002). Thus, this study also suggests that intermediate temperature may result in lower trophic transfer efficiency as consumers face stronger elemental imbalances with their resources.
Trait correlations
Figs. 6 and 7 indicate that algal traits related to growth and nutrient content might be only weakly associated with lipid content. But the species with the fastest growth and highest biomass had low lipids relative to others in general. It shows a tradeoff which may constrain the selection of potential bioenergy crop taxa between lipid content and growth which are in line with previous studies (Liu et al. 2013; Li et al. 2014).
Conclusions
The results in this study have implications for the potential of candidate bioenergy crops candidate to grow outdoors under a wide range of temperatures. We found that Chlorophyta could grow at a wide range of temperature from 15 to 35 °C, with the optimum temperature of 20 to 30 °C (mainly around 25 °C). Whereas cyanobacteria had a higher tolerance to high temperature than Chlorophyta, the optimum temperature for growth was between 30 and 35 °C. In particular, C. reinhardtii would be an ideal one adapted to a wide range of temperatures. Understanding species-specific thermal stress is essential in understanding how temperature alteration leads to changes in the algal composition (Barati et al. 2017). The optimum growth temperature and the shape of the response to thermal variation varied considerably among taxa, with growth and stoichiometric traits showing more consistent responses than lipids. The patterns observed in this study also lend insight into the anticipated changes in algal populations and communities under ongoing warming of the atmosphere. First, C:N and C:P ratios were unimodally related to temperature in the majority of species examined, indicating that declines in nutrient content may occur with rising temperatures below some critical levels. These declines in nutrient content may affect the quality of algae as food for consumers and therefore the production of higher trophic levels. Second, the increased temperature may lead to higher growth rates but lower standing biomass (density at stationary phase). Shifts in production and composition of phytoplankton with warming may have profound implications for global energy and nutrient cycles and the dynamics and stability of ecosystems.
References
Balat M, Balat H (2011) Progress in biodiesel processing. Appl Energy 7:1815–1835
Barati B, Lim PE, Gan SY, Poong SW, Phang SM, Beardall J (2017) Effect of elevated temperature on the physiological responses of marine Chlorella strains from different latitudes. J Appl Phycol. https://doi.org/10.1007/s10811-017-1198-z
Bernard O (2011) Hurdles and challenges for modeling and control of microalgae for CO2 mitigation and biofuel production. J Process Control 21:1378–1389
Borowitzka MA, Moheimani NR (2013) Sustainable biofuels from algae. Mitig Adapt Strat Global Change 18:13–25
Borowitzka MA (2016) Algal physiology and large-scale outdoor cultures of microalgae. In: Borowitzka MA, Beardall J, Raven JA (eds) The physiology of microalgae. Springer, Dordrecht pp 601–652
Brune DE, Lundquist TJ, Benemann JR (2009) Microalgal biomass for greenhouse gas reductions: potential for replacement of fossil fuels and animal feeds. J Environ Eng-ASCE 135:1136–1144
Chaffin JD, Mishra S, Kuhaneck RM, Heckathorn SA, Bridgeman TB (2012) Environmental controls on growth and lipid content for the freshwater diatom, Fragilaria capucina: a candidate for biofuel production. J Appl Phycol 24:1045–1051
Chaisutyakorn P, Praiboon J, Kaewsuralikhit C (2017) The effect of temperature on growth and lipid and fatty acid composition on marine microalgae used for biodiesel production. J Appl Phycol. https://doi.org/10.1007/s10811-017-1186-3
Chen WC, Zhang W, Song LR, Sommerfeld M, Hu Q (2009) A high throughput Nile red method for quantitative measurement of neutral lipids in microalgae. J Microbial Meth 77:41–47
Cheng YS, Zheng Y, Labavitch JM, Vander Gheynst JS (2010) The impact of cell wall carbohydrate composition on the chitosan flocculation of Chlorella. Proc Biochem 46:1927–1933
Chrismas NAM, Anesio AM, Sánchez-Baracaldo P (2015) Multiple adaptations to polar and alpine environments within cyanobacteria: a phylogenomic and Bayesian approach. Front Microbiol 6:1070
Courchesne NMD, Parisien A, Wang B, Lan CQ (2009) Enhancement of lipid production using biochemical, genetic and transcription factor engineering approaches. J Biotechnol 141:31–41
Dean AP, Nicholson JM, Sigee DC (2008) Impact of phosphorus quota and growth phase on carbon allocation in Chlamydomonas reinhardtii: an FTIR microspectroscopy study. Eur J Phycol 43:345–354
Demirbas A (2011) Biodiesel from oilgae, biofixation of carbon dioxide by microalgae: a solution to pollution problems. Appl Energy 88:3541–3547
Dismukes GC, Carrieri D, Bennette N, Ananyev GM, Posewitz MC (2008) Aquatic phototrophs: efficient alternatives to land-based crops for biofuels. Curr Opin Biotech 19:235–240
Falkowski PJ, Raven JA (2007) Aquatic photosynthesis. Princeton University Press, New Jersey
Fu FX, Warner ME, Zhang YH (2007) Effects of increased temperature and CO2 on photosynthesis, growth, and elemental rations in marine Synechococcus and Prochloroccus (Cyanobacteria). J Phycol 43:485–496
Gonzalez-Fernandez C, Ballesteros M (2012) Linking microalgae and cyanobacteria culture conditions and key-enzymes. For carbohydrate accumulation. Biotechnol Adv 30:1655–1661
Griffiths MJ, Harrison STL (2009) Lipid productivity as a key characteristic for choosing algal species for biodiesel production. J Appl Phycol 21:493–507
Guillard RR, Lorenzen CJ (1972) Yellow-green algae with chlorophyllide. J Phycol 8:10–14
James GO, Hocart CH, Hillier W, Chen H, Kordbacheh F, Price GD, Djordjevic MA (2011) Fatty acid profiling of Chlamydomonas reinhardtii under nitrogen deprivation. Bioresour Technol 102:3343–3351
Jiao F, Shi XR, Han F, Yuan ZY (2016) Increasing aridity, temperature and soil pH induce soil C-N-P imbalance in grasslands. Sci Rep 6:1–9
Kahm M, Hasenbrink G, Lichtenberg-Fraté H, Ludwig J, Kschischo M (2010) Grofit: fitting biological growth curves with R. JStat Softw 33: 1–21
Kalacheva GS, Zhila NO, Volova TG, Gladyshev MI (2002) The effect of temperature on the lipid composition of the green alga Botryococcus. Microbiology 71:286–293
Khotimchenko SV (2005) Lipids from the marine alga Gracilaria verrucosa. Chem Nat Comp 41:285–288
Li YR, Tsai WT, Hsu YC, Xie MZ, Chen JJ (2014) Comparison of autotrophic and mixotrophic cultivation of green microalgal for biodiesel production. Energy Procedia 52:371–376
Liu JY, Mukherjee J, Hawkes JJ, Wilkinson SJ (2013) Optimization of lipid production for algal biodiesel in nitrogen stressed cells of Dunaliella salina using FTIR analysis. Chem Technol Biotech 10:1807–1814
Markevičius A, Katinas V, Perednis E, Tamašauskienė M (2010) Trends and sustainability criteria of the production and use of liquid biofuels. Renew Sust Energy Rev 14:3226–3231
Menzel DW, Corwin N (1956) The measurement of total phosphorus in sea water based on the liberation of organically bound fraction by per sulfate oxidation. Limnol Oceanogr 10:280–282
de Oliveira MAS, Monteiro MPC, Robbs PG, Leite SGF (1999) Growth and chemical composition of Spirulina maxima and Spirulina platensis biomass at different temperatures. Aquacult Int 7:261–275
Pal D, Khozin-Goldberg I, Cohen Z, Boussiba S (2011) The effect of light, salinity, and nitrogen availability on lipid production by Nannochloropsis sp. Appl Microbiol Biotechnol 90:1429–1441
Pasquet V, Ulmann L, Mimouni V, Guihéneuf F, Jacquette B, Morant-Manceau A, Tremblin G (2014) Fatty acids profile and temperature in the cultured marine diatom Odontella aurita. J Appl Phycol 26:2265–2271
Piepho M, Arts MT, Wacker A (2012) Species-specific variation in fatty acid concentrations of four phytoplankton species: does phosphorus supply influence the effect of light intensity or temperature. J Phycol 48:64–73
Radway JC, Weissman JC, Wilde EW, Benemann JR (1992) Exposure of Fischerella [Mastigocladus] to high and low temperature extremes: strain evaluation for a thermal mitigation process. J Appl Phycol 4:67–77
Raven JA, Geider RJ (1988) Temperature and algal growth. New Phytol 110:441–461
Renaud SM, Zhou HC, Parry DL, Thinh L-V, Woo KC (1995) Effect of temperature on the growth, total lipid content and fatty acid composition of recently isolated tropical microalgae Isochrysis sp., Nitzschia closterium, Nitzschia paleacea, and commercial species Isochrysis sp. (clone T iso). J Appl Phycol 7:595–602
Renaud SM, Thinh L-V, Lambrinidis G, Parry DL (2002) Effect of temperature on growth chemical composition and fatty acid composition of tropical Australian microalgae grown in batch cultures. Aquaculture 211:195–214
Rling M, Eshetu F, Faassen E J (2013) Comparison of cyanobacterial and green algal growth rates at different temperatures. Freshw Biol 58:552–559
Roleda MY, Slocombe SP, Leakey RJG, Day JG, Bell EM, Stanley MS (2013) Effects of temperature and nutrient regimes on biomass and lipid production by six oleaginous microalgae in batch culture employing a two-phase cultivation strategy. Bioresour Technol 129:439–449
Savage VM, Gillooly JF, Brown JH, Charnov EL (2004) Effects of body size and temperature on population growth. Am Nat 3:429–441
Schenk PM, Thomas-Hall S, Stephens E, Marx U, Mussgnug J, Posten C, Kruse O, Hankamer B (2008) Second generation biofuels: high-efficiency microalgae for biodiesel production. Bioenergy Res 1:20–43
Shurin J B, Abbott R L, Deal M S K, Litchman G T, McBride R, Mandal S, Smith V H (2013) Industrial-strength ecology: trade-offs and opportunities in algal biofuel production. Ecol Lett16:1393–1404
Shurin JB, Clasen JL, Greig HS, Kratina P, Thompson PL (2012) Warming shifts top-down and bottom-up control of pond food web structure and function. Phil Trans Roy Soc B 367:3008–3017
Srebotnjaka T, Hardi P (2011) Prospects for sustainable bioenergy production in selected former communist countries. Ecol Indic 11:1009–1019
Sterner RW, Elser JJ (2002) Ecological Stoichiometry. Princeton University Press, Princeton, NJ
Su Y, Mennerich A, Urban B (2012) Comparison of nutrient removal capacity and biomass settleability of four high-potential microalgal species.Biores Technol 124:157–162
Sushchik NN, Kalacheva GS, Zhila NO, Gladyshev MI, Volova TG (2003) A temperature dependence of the intra- and extracellular fatty-acid composition of green algae and cyanobacterium. Russ J Plant Physiol 50:374–380
Teoh ML, Chu WL, Marchant H, Phang SM (2004) Influence of culture temperature on the growth, biochemical composition and fatty acid profiles of six Antarctic microalgae. J Appl Phycol 16:421–430
Thomas Z, Ghazavi R, Merot P, Granier A (2012) Modeling and observation of hedgerow transpiration effect on water balance components at the hillslope scale in Brittany. Hydrol Proc 26:4001–4014
Tian Y, Zhao L, Meng H, Sun L, Yan J (2010) Estimation of unused land potential for biofuels development in (the) People’s Republic of China. Appl Energy 86:77–85
Xia L, Song S, Hu C (2016) High temperature enhances lipid accumulation in nitrogen-deprived Scenedesmus obtusus XJ-15. J Appl Phycol 28:831–837
Ventura M (2006) Linking biochemical and elemental composition of freshwater and marine crustacean zooplankton. Mar Ecol Prog Ser 327:233–246
Funding
The study is supported by Chengdu Technology Bureau (2015-HM01-00013-SF), U.S. Department of Energy Award (DE-EE-0003373), Nature Science Foundation of Sichuan Province (2014JY0224), Science Foundation of China (31100374), and 985 Construction Project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, J., Wang, P., Wang, Y. et al. Thermal constraints on growth, stoichiometry and lipid content of different groups of microalgae with bioenergy potential. J Appl Phycol 30, 1503–1512 (2018). https://doi.org/10.1007/s10811-017-1358-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-017-1358-1