Abstract
Carbon dioxide (CO2) sequestration by microalgae has received widespread attention. Growth and biomass quality using flue gas, combined with medium recycling, were evaluated in this study. Results indicated that Spirulina maxima FACHB 438 can use flue gas from biomass power plant as sole carbon source. The final biomass of 26.30, 22.10, and 23.95 g in fresh medium (FM), recycled medium (RM), and recycled medium with activated carbon treatment (RM + AC) was harvested in flat-plate photobioreactors with 10 L working volume after 5 cycles, respectively. The mean specific growth rate and CO2 fixation rate did not differ significantly (p < 0.05), illustrating good growth performance in the three treatments. Activated carbon enhanced growth in RM + AC by 8.4% compared with RM. The quality of biomass in either FM or RM satisfies the Chinese standard for food/feed additives. This manner of mass culture reduced the cost of nutrients by up to 42%. Therefore, combination of CO2 sequestration from biomass power plant and medium recycling is demonstrated to be a new way to enhance the cost-effective Spirulina production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The increase in carbon dioxide (CO2) concentration in the atmosphere, which is the main cause of global warming, is a great concern in the world (Razzak et al. 2017). Many strategies have been proposed to slow the rise in CO2 concentration, such as physical fixation and chemical adsorption, among which, biological mitigation has received wide interest. Microalgae are highlighted due to their ability to convert CO2 to biomass and produce oxygen through photosynthesis (da Rosa et al. 2015). Compared with terrestrial plants, microalgae possess many advantages, such as 10 to 100 times higher growth rate (Hu et al. 2008), ability to use a range of CO2 sources, and being rich in high-value products (Borowitzka 2013). To date, some progress has been made in using microalgae to fix CO2 from flue gas from coal power plants, such as screening of high NOX, SOX, and temperature-tolerant microalgal strains (Radmann et al. 2011; Li et al. 2016; Moheimani 2016), development of highly efficient photobioreactor systems (Richmond et al. 1993; Huang et al. 2015), and algae-based product exploitation (Sawayama et al. 1999; Usui and Ikenouchi 1997). Other studies using microalgae to fix CO2 of simulated flue gas (Zhao et al. 2015) or flue gas from cement plants (Borkenstein et al. 2011) and steel plants (Kao et al. 2014) have also been reported.
While emphasis has been mainly directed to the efficiency of CO2 utilization from flue gas, previous studies also examined the use of biomass, mostly focusing on the production of non-editable products, such as liquid fuels and methanol (Hirano et al. 1998; Sawayama et al. 1999). This is due to the fact that flue gases from traditional fossil fuel generally contain some toxic compounds, thus limiting the application of algal biomass as higher value products like pigments and food or feed additives (Lee and Lee 2003). Therefore, the approach to use microalgae for CO2 sequestration from coal combustion gas has not been applied on a large scale. In contrast, CO2 from cleaner flue gas may be a feasible solution (Lee et al. 2001). Biomass energy, as a renewable energy, is in the vigorous development of the world (BP 2017). For instance, China has vast area of farmland and the Chinese government proposed the goal that the biomass power generation capacity reach 15 million kW by 2020 (China NDRC 2016). It is ordinarily considered that flue gas from biomass power plant has lower NOX, SOX, and toxic matter concentrations compared with coal-fired flue gas, which makes it preferable for the cultivation of microalgae. Furthermore, 3–5% of CO2 concentration in flue gas from biomass power plant, lower than 10–15% in coal-fired flue gas (US DOE 2010), could be better used for the growth of most microalgae (Yadav et al. 2015).
To achieve the goal of cost-effective CO2 sequestration and biomass production, selection and evaluation of optimal microalgae are essential. Spirulina (current recognized name is Arthrospira), a widely cultivated alga for commercial production (Belay et al. 1996; Vonshak et al. 2014), is considered as a candidate for biofixation of CO2, owing to its high growth rate, high protein content, high phycocyanin content, and strong resistance to contamination (Depraetere et al. 2015). In addition, Spirulina has a higher CO2 fixation efficiency under alkaline conditions, since flue gas bubbled into medium reacts with base to form bicarbonate ions, which could be used as carbon source for alkaline-tolerant microalgae (Stewart and Hessami 2005). Maintenance of alkaline condition requires high concentrations of carbonate/bicarbonate. According to our investigation on different Spirulina farms, spanning from north to south of China, approximately 3 kg NaHCO3 and 1–1.5 kg CO2 are needed to produce 1 kg dry Spirulina biomass, accounting for about 30% of cost in Spirulina production. If Spirulina could directly use the flue gas from biomass power plant, cost-effective biomass production coupled with CO2 sequestration could be expected.
Recycling of the medium is a routine procedure commonly applied in the mass cultivation of Spirulina. This procedure maximizes the usage of water and nutrients (Rocha et al. 2015); meanwhile, it also reduces the treatment cost of the vast spent medium. However, there also exist some problems associated with this procedure. It has been reported that organic matter and heavy metals can be accumulated during multiple recycling of the medium. The organic matter may include polysaccharides, proteins, free fatty acids, and cell debris (Bosma et al. 2008; Hulatt and Thomas 2010; Farooq et al. 2015), which may likely to promote bacterial growth and decrease algal growth rate and quality. Accordingly, the influence of medium recycling on algal growth and quality has to be assessed.
In this study, growth of Spirulina in standard Zarrouk medium was compared with that in Zarrouk carbon-free (Zarrouk C-free) medium using flue gas as a carbon source. Then, the performance of Spirulina maxima cultivated in fresh medium and sequentially reused medium was evaluated indoors and outdoors. The aims of this study were (1) to evaluate growth under flue gas supply and medium recycling conditions and (2) to assess the quality of the biomass to estimate whether the manner of cultivation could be used for nutritional purpose. It is hoped that this research will provide an economically and environmentally sustainable approach to combine CO2 mitigation and Spirulina production.
Materials and methods
Site and flue gas for experiments
A pilot experimental base was established to cultivate microalgae by using flue gas from a biomass power plant, located in Huai’an, Jiangsu Province (N33°33′19″, E119°12′16″) (Online Resource 1). The experimental base covered an area over 800 m2 and was equipped with different photobioreactors. The biomass power plant produces roughly 200 million kWh a year by burning biomass mainly from straw. To get rid of the pollutants from the flue gas, a desulfurization tower was set up and kept operating to provide clean flue gas during the experimental process. The treated flue gas was monitored by a portable flue gas analyzer (1600, IMR, Germany) and was composed of O2 (13.68–15.81%), CO2 (3.15–4.09%), SO2 (0.8–11 ppm), and NOX (55–107 ppm).
Algal strain and cultivation conditions
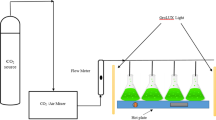
Spirulina (Arthrospira) maxima FACHB 438 was obtained from the Freshwater Algae Culture Collection of Hydrobiology, Chinese Academy of Sciences, Wuhan, China. Spirulina maxima was cultivated in Zarrouk medium (Zarrouk 1966) or Zarrouk C-free medium. The specific components used per liter in the Zarrouk medium were as follows: 16.8 g NaHCO3, 0.5 g K2HPO4, 2.5 g NaNO3, 1 g K2SO4, 1 g NaCl, 0.2 g MgSO4·7H2O, 0.04 g CaCl2, 0.01 g FeSO4·7H2O, 0.08 g Na2EDTA, and 1 mL trace metal solution. One liter of trace metal solution contains the following: 2.86 g H3BO3, 1.81 g MnCl2·4H2O, 0.222 g ZnSO4·7H2O, 0.079 g CuSO4·5H2O, and 0.015 g MoO3. For Zarrouk C-free medium, only NaHCO3 was omitted from the original Zarrouk medium. The pH was adjusted to 9.0 with 1 N NaOH. To verify whether flue gas from biomass power plant could be used as sole carbon source for S. maxima production, a set of batch tests was conducted in flat-plate photobioreactors (70 cm × 10 cm × 60 cm) with 70 L working volume of Zarrouk or Zarrouk C-free medium outdoors, from June 8 to July 2. To test the influence of medium recycling on the growth of S. maxima, the experiments were carried out in flat-plate photobioreactors (30 cm × 10 cm × 50 cm) with a working volume of 10 L both indoors and outdoors. The indoor cultivations were carried out at 28 ± 1 °C and continuously illuminated at approximately 300 μmol photons m−2 s−1 (3415FXSE, Spectrum, USA) under LED lamps, with a cycle of 6 days, while outdoor cultivations were conducted from July 13 to August 6, with a cycle of 5 days. Shading and water cooling were often performed to avoid excessive temperature at noon. The aeration was controlled at the rate of 0.1 vvm (gas volume per working volume per minute). The pH of all the photobioreactors was maintained at the range of 9–10 by regulating flue gas and air. Flue gas was aerated into the medium when pH was above 10, and air was used to adjust pH when it was below 9. At the end of the cultivation, S. maxima were harvested by a nylon mesh (pore size about 50 μm), washed with deionized water three times, and lyophilized for further analysis.
Culture medium recycling treatment
To evaluate the influence of medium recycling on the growth of S. maxima, the medium was recycled four times in sequential cultivation. For the recycled medium treatment (RM), microalgal cells after 1st round cultivation were harvested by a nylon mesh with a pore size of 50 μm, and the filtrate was used as the culture medium for the next cycle, inoculated with 10% of active growing cells. The procedure was repeated three times. For the recycled medium with activated carbon treatment (RM + AC), granular activated carbon (GAC) was added to the filtrate of each cycle to remove the organic matter at a concentration of 6 g L−1 overnight and was filtered with the above mesh. Mixing was not applied to avoid GAC crushing. For consistency of the major nutrients in the recycled medium, macronutrients N and P were re-added to their original concentrations based on the calculation that N and P account for about 10 and 0.8% of algal biomass, respectively (Cornet et al. 1992). For the control (fresh medium, FM), the medium was not recycled during the whole cultivation.
Algal growth and CO2 fixation analysis
The dry weight of the biomass was measured by filtering 10 mL samples through a pre-weighted glass microfiber filter (GF/C, 47 mm diameter; Whatman, England). The culture was washed twice with de-ionized water, and, then, the filter was dried at 105 °C to constant weight. The differences between the filter were the dry weight of the samples. The CO2 fixation rate (g L−1 d−1) was calculated according to Eq. (1).
where P is defined as biomass productivity (g L−1 d−1), and MCO2 and MC are the molecular weight of CO2 and carbon, respectively. CC is the carbon content of Spirulina cell (%) determined according to Cornet et al. (1992).
The specific growth rate (d−1) was calculated based on Eq. (2).
where X1 and X2 are the biomass concentrations (g L−1) on day T1 and T2, respectively.
Protein and phycocyanin measurement
A total of 400 μL RIPA lysis buffer (Strong) (Beyotime Institute of Biotechnology, Nanjing, China) containing 1× protease inhibitor cocktail (Thermo Scientific, USA) was added to each sample of about 10–20 mg algal powder. The samples were disrupted by the high-speed vibrator (Mini-beadbeater 16, Biospec, USA) and centrifuged at 10,000 rpm for 3 min. The step was repeated until the pellet became colorless. The supernatant was diluted with 0.5 N NaOH to 50 mL. Protein concentration was determined using the BCA Protein Assay Kit (Pierce 23227, Thermo Scientific, USA), which is based on bicinchoninic acid for the colorimetric detection and quantitation of total dissolved protein.
Phycocyanin was extracted with 0.05 M phosphate buffer using the high-speed vibrator. The extraction procedure was the same as that of protein extraction. Phycocyanin concentration was calculated by the absorbance at 615 nm (OD615) and 562 nm (OD562) based on the equation of Patel et al. (2005).
Ash and chlorophyll a determination
The ash content was determined by weighting sample m1 (about 30 mg) into a pre-weighted crucible (m2) and heating the crucible in a muffle furnace at 550 °C for 4 h until white ash remains (Chentir et al. 2017). After cooling in a desiccator, the crucible was re-weighted (m3).
Chlorophyll a (Chl a) was extracted with 99.9% methanol at 45 °C in the dark for 30 min (Pruvost et al. 2011). Samples were centrifuged at 10,000 rpm for 5 min before measurement. Chl a was spectrophotometrically quantified at 666 and 653 nm according to Lichtenthaler and Wellburn (1983) using the following equation:
Heavy metal analysis in biomass
For heavy metal quantification, 100 mg algal powder was first digested by 6 mL ultrapure nitric acid with Anton Paar Microwave sample preparation System (Multiwave 3000, Austria). The microwave-assisted digestion procedure was set as 700 W for 5 min, followed by 1400 W for 25 min, and 0 W for 15 min. After the procedure of acid-driving at 130 °C for about 6.5 h, the resulting clear solution was diluted with 2% HNO3 to a final volume of 10 mL. Total concentrations of heavy metals were analyzed with Inductively Coupled Plasma Mass Spectrometry (ICP-MS, OPTIMA 8000DV, Pekin Elmer, USA) without any further treatment.
Analysis of total organic carbon and heavy metal in culture medium
During indoor cultivation, total organic carbon (TOC) and heavy metals were measured at the end of each cycle. The algal suspension was firstly filtered by 0.22 μm microfilter (SLGP033RB, Millipore, USA). Subsequently, 15 mL samples were analyzed using a TOC-L Series analyzer (Shimadzu, Japan) for TOC quantification, and the remaining 15 mL samples were analyzed using ICP-MS for heavy metal measurement.
Statistical analysis
All experiments were performed in triplicates. The values were expressed as the mean ± standard deviation. The data were analyzed by one-way ANOVA using SPSS (version 19.0). A value of p < 0.05 was considered to denote a statistically significant difference.
Results and discussion
Influence of flue gas on the growth of S. maxima
The cultivation results (Fig. 1a) showed that there is no obvious difference between the two treatments. At the end of the cultivation, the dry weight of S. maxima in Zarrouk and Zarrouk C-free medium reached 2.31 and 2.11 g L−1, respectively. The protein and phycocyanin contents of S. maxima (67.83 and 23.01%, w/w) of Zarrouk C-free medium aerated with flue gas were higher than those in the standard Zarrouk medium (64.41 and 21.85%, w/w) (Fig. 1b). Binaghi et al. (2003) also reported that the growth of Spirulina platensis in C-free medium aerated with CO2 was comparable with that in the complete medium. These results suggest that S. maxima can use CO2 of flue gas from biomass power plant as sole carbon source to obtain optimal growth and protein production.
The adaption of S. maxima to the combustion gas can be explained by the characteristics of flue gas and optimal aeration strategy. Carbon and pH are the two important factors that affect the high production in Spirulina cultivation. Abundant carbon in the culture was obtained, and the suitable pH ranging from 9 to 10 was maintained by the bubbling of flue gas throughout the cultivation due to the relatively low concentrations of CO2, NOX, and SOX in the flue gas. The value of pH is strongly linked to the equilibrium of Ci forms (H2CO3, CO2, HCO3−, and CO32−) in a system (Kalff 2002). In the natural systems, the dissolved CO2 concentrations are far lower than the Km (CO2) for ribulose-1,5-bisphosphate carboxylase/oxygenase (RUBISCO) (Moroney and Somanchi 1999). HCO3− is the dominant form of inorganic C within the range of pH 9 to 10. Cyanobacteria and microalgae have high external carbonic anhydrase (CA) that catalyzes the reversible conversion of HCO3− to CO2 and OH− to provide sufficient CO2 to enter the cell and to serve as the substrate for photosynthesis (Moroney et al. 1985; Husic et al. 1987). It is worth mentioning that 3 to 8 h of flue gas aeration in a day was operated in this study, which was much longer than those in previous reports. For example, when Spirulina sp. LEB 18 was aerated with coal-combustion gas for 10 min every 2 h at 0.05 vvm during the light period, the biomass of 0.63 g L−1 was achieved after 12 days (Vaz et al. 2016). Using the same aeration mode for Spirulina sp., Duarte et al. (2017) achieved a biomass of 0.68 g L−1 after 10 days. It was clearly indicated that the dry weight of the biomass from their study was much less than that in the present study. This difference was apparently due to the more carbon input under this mode; continuous bubbling of flue gas accelerated the gas–liquid mass transfer resulting in more CO2 scrubbed in the alkaline condition. Ultimately, it benefited from the characteristics of the flue gas with low concentrations of CO2 and SOX/NOX. The above-mentioned growth performance appears very promising and indicates that a cheap carbon source could be obtained from flue gas emissions of biomass power plant by simply bubbling into the culture with optimal aeration strategy.
Influence of medium recycling on the growth of S. maxima
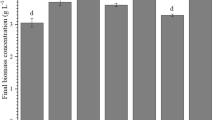
To test the influence of medium recycling on the growth of S. maxima, a set of cultivations was carried out both indoors and outdoors in flat-plate photobioreactors with 10 L working volume. In indoor cultivation, there is no difference in the growth between the fresh medium and the first recycled medium (Fig. 2a). Growth was gradually inhibited with the sequentially repeated use of medium, leading to about 20% reduction in biomass at the end of the 3 cycles. Outdoor cultivations were conducted in summer season, from July to August. The temperature was controlled at below 38 °C with shading and water cooling during daytime, and pH was regulated between 9 and 10 by aeration with flue gas. At the early stage of each cycle, growth in RM was similar to that in FM, but it was lower at the late stage of each cycle (Fig. 2b). A terminal biomass of 22.10 and 26.30 g was harvested from RM and FM, respectively, after 5 cycles. This decrease was likely due to inhibitory factors excreted by algal cells and accumulated within the medium after repeated use (Rodolfi et al. 2003; Farooq et al. 2015). Therefore, to remove possible inhibitors, GAC was added to the recycled medium overnight to test its promotive effect on growth. It was shown that total biomass of 23.95 g was harvested in RM + AC and that the growth was increased by 8.4% compared with RM.
Detailed growth parameters in three different treatments are presented in Table 1. Due to weather reasons, although growth gradually decreased as the cultivation continued, the mean values of maximum dry weight of biomass (Xmax), maximum specific growth rate (μmax), maximum biomass productivity (Pmax), and maximum CO2 fixation rate (Fmax) among FM, RM, and RM + AC did not differ significantly (p > 0.05), indicating that the growth rates of the three treatments were comparable to each other. Da Rosa et al. (2015) studied the cultivation of Spirulina sp. in semi-continuous mode with nutrient recycle for 25 days. Fed with CO2 in 2.0 L vertical tubular photobioreactors, the culture achieved an average specific growth rate of 0.242 days−1, which was much lower than the rate in the present study. Using coal-fired flue gas as carbon source for Spirulina sp. cultivation, the CO2 fixation rate was estimated to be 109.2 mg L−1 day−1 (Vaz et al. 2016), less than half of the values in the current study. Our results showed that the combination of medium recycling and flue gas can support a reasonable growth performance of S. maxima, demonstrating the feasibility of this cultivation mode for Spirulina.
Changes of total organic carbon in the medium and its removal with granular activated carbon
To investigate whether the growth inhibition was associated with organic matter in the culture, TOC concentrations in the medium at the end of each cycle were measured (Fig. 3). In the FM treatment, the TOC concentration remained constant at around 30 mg L−1, while TOC concentration in the RM treatment gradually increased with recycling, finally reaching 85 mg L−1 indoors and 914 mg L−1 outdoors at the end of cultivation. The high TOC concentration was attributed to the organic matter existing in the medium, which may include proteins, carbohydrates, free fatty acids, and other potential growth-inhibiting chemicals (Farooq et al. 2015), thus exerting a negative effect on the growth of microalgal cells. Nevertheless, in the view of multiplying accumulation of TOC and slight growth differences between indoor and outdoor cultivation (Figs. 2 and 3), it was inferred that TOC may be not the only factor responsible for the decrease of growth for the medium recycling.
Increases of TOC also have been observed during the cultivation of other microalgae, i.e., Chlorella (Hadj-Romdhane et al. 2013; Farooq et al. 2015) and Nannochloropsis (Zhang et al. 2016). Algal organic matter (AOM) is released by excretion and cell lysis during growth. Activated carbon seems to be a possible material to remove this organic matter. In this study, TOC concentration in RM + AC was lower than that in RM at the end of each cycle. The limited decrease in TOC concentration may explain the slight increase in biomass yield of the RM + AC treatment (Fig. 2b). In fact, the effect of activated carbon treatment of the used medium varies greatly. Rijstenbil (1989) found that activated carbon cannot remove inhibitory compounds from the filtrate of marine diatom cultures. An organic matter removal efficiency of 92.3% was achieved by using 24.4 mg L−1 activated carbon powder and 20.3 mg L−1 ferric chloride for 20.4 min flocculation and adsorption of Arthrospira used medium (Morocho-Jácome et al. 2016). These data suggest that the removal efficiency of organic matters is concerned with harvesting methods, treatment time, and pore size distribution of the activated carbon, and so on. Advanced and practical medium treatments, such as ozonation and UV, are expected to be combined with activated carbon adsorption for medium recycling to remove harmful materials in future commercial-scale microalgal farms (González-López et al. 2013; Farooq et al. 2015).
Analysis of quality of Spirulina biomass as feed additives
Spirulina is used as health food, food additives, and feed in aquaculture and cosmetics (Spolaore et al. 2006; de la Jara et al. 2018). The choice for application of Spirulina biomass is dependent on its quality. As an alternative protein source for fish meal, Spirulina has gained more and more attention in the feed industry due to its high level of protein (Minjarez-Osorio et al. 2016; Cao et al. 2018). Therefore, protein content is one of the key parameters to be tested. Likewise, heavy metals should also be monitored to ensure the safety of the biomass. As shown in Fig. 4, for the FM group indoors, the protein contents were above 60% and phycocyanin contents were in 14–17%. There were no significant differences between FM and RM in terms of protein content, phycocyanin content, ash content, and Chl a content (p > 0.05). During outdoor cultivation, phycocyanin content reduced, which was probably due to the large fluctuation of the weather during that period (Fig. 5). However, the protein content in RM was higher than 53%, indicating that there was no significant difference between FM and RM (p > 0.05).
Straw is the main burning substrate in the biomass power plant, thus producing a relatively clean flue gas. Although the concentrations of heavy metals in the flue gas are relatively low, they could be enriched with the repeated use of medium. Therefore, the distribution of several heavy metals in the medium was examined at the end of each cycle. As Fig. 6 shows, concentrations of heavy metals (As, Co, Mn, and Zn) decreased with repeated use of medium, which may be attributed to the utilization of microalgal cells as microelements. Concentrations of some heavy metals did not change and were comparable with those in FM which indicated that these heavy metals were not enriched in spite of long bubbling of flue gas for 25 days. Furthermore, heavy metals may accumulate in the biomass of Spirulina and pose a potential threat to product safety if used as feed additives. It has been proposed that heavy metals can accumulate in the microalgal cells by an uptake transport system or chelation by substances excreted by cells, such as phytochelatins (PCs), metallothioneins (MTs), and extracellular polysaccharides (EPS) (García-García et al. 2016). Therefore, to evaluate the risk of heavy metal contamination, the heavy metal content of Spirulina biomass at the end of each cycle indoors (Table 2) and at the last cycle outdoors (Table 3) was determined. Clearly, heavy metals in the biomass did not accumulate during the process of medium recycling.
According to the Chinese feed-grade Spirulina powder standard GB/T 17243-1998, the contents of heavy metals and protein should be less than 0.5 mg kg−1 (cadmium), 6.0 mg kg−1 (lead), or 1.0 mg kg−1 (arsenic), or more than 50% (crude protein), respectively. As shown in Table 3, the maximum concentrations of heavy metals in Spirulina biomass cultivated in recycled medium outdoors were 0.0042 mg kg−1 (cadmium), 0.1424 mg kg−1 (lead), and 0.0288 mg kg−1 (arsenic). All the values met the standard for feed utilization indicating that the biomass cultivated with flue gas combined with medium recycling is safe and suitable for use as feed additives in terms of heavy metals and protein. Similar results of 0.011 mg kg−1 (cadmium), 0.12 mg kg−1 (lead), and 0.015 mg kg−1 (arsenic) were reported by Douskova et al. (2009) who cultivated Chlorella vulgaris using flue gas from a municipal waste incinerator. Unlike this study, however, complex techniques, such as NOX reduction, electrostatic precipitator, quenching, and scrubber, were needed to remove NOX, SOX, dust, HCl, HF, and heavy metals in the flue gas.
Analysis of cost reduction on current cultivation mode: combination of flue gas and medium recycling
The main restriction to the Spirulina industry is the relatively high cost of cultivation at each step. Taking Spirulina industry in China as example, nutrients account for about 40% of the total cost for Spirulina production, of which 60% of the total nutrient cost comes from carbon (Li et al. 1996; Qiao and Li 2013). Although traditional cultivation is relatively cost-effective, application of flue gas from biomass power plant and medium recycling may be a new mode to expand Spirulina production and facilitate the development of the Spirulina industry. To test the economic feasibility of this cultivation mode, a simple nutrient expenditure analysis was carried out (Table 4). Carbon, nitrogen, and phosphorus are the fundamental elements for algal biomass. In the current study, CO2 from flue gas was bubbled as the sole carbon source. Based on the first part of the work, the growth of S. maxima using flue gas was similar with that using NaHCO3. Taking the flue gas treatment costs into account, including electricity, quicklime, water, and so on, a reduction about 40% in carbon expenditure was estimated (Table 5). To replenish the recycled medium, macro nutrients (NaNO3, K2HPO4·3H2O) were added at the start of each cycle. As a result, a considerable reduction of 66.83% (nitrate) and 73.61% (phosphorus) could be achieved. Other nutrients were just added in the medium at the beginning of the first cycle. Water was not added due to the negligible evaporation of the photobioreactors, which reduced 80% of the requirement. These further reduced the nutrient cost in Spirulina production on the basis of cheap carbon from flue gas. Hence, about 42% of the cost in nutrients was reduced if cultivated with flue gas and medium recycling for four times. Actually, in FM, the majority of the nutrients were not completely assimilated by algal cells, so they had to be discharged into the environment, which would lead to the eutrophication or, in turn, increase the spent medium treatment cost.
Conclusion
This work demonstrates that flue gas from biomass plant can be used as carbon source for Spirulina cultivation. The growth rates of FM, RM, and RM + AC were comparable. GAC (6 g L−1) could enhance growth by 8.4% in comparison with RM. Meanwhile, algal biomass quality was not affected in terms of protein and heavy metal content and met the requirements of the Chinese national standard for feed utilization. By employing this cultivation strategy, a reduction of about 42% in nutrient expenditure was achieved. Thus, this innovative cultivation strategy demonstrates the potential for flue gas biological mitigation and cost-effective Spirulina production.
References
Belay A, Kato T, Ota Y (1996) Spirulina (Arthrospira): potential application as an animal feed supplement. J Appl Phycol 8:303–311
Binaghi L, Del Borghi A, Lodi A, Converti A, Del Borghi M (2003) Batch and fed-batch uptake of carbon dioxide by Spirulina platensis. Process Biochem 38:1341–1346
Borkenstein CG, Knoblechner J, Frühwirth H, Schagerl M (2011) Cultivation of Chlorella emersonii with flue gas derived from a cement plant. J Appl Phycol 23:131–135
Borowitzka MA (2013) High-value products from microalgae—their development and commercialization. J Appl Phycol 25:743–756
Bosma R, Miazek K, Willemsen SM, Vermuё MH, Wijffels RH (2008) Growth inhibition of Monodus subterraneus by free fatty acids. Biotechnol Bioen 101:1108–1114
BP (2017) BP statistical review of world energy. London, England
Cao SP, Zou T, Zhang PY, Han D, Jin JY, Liu HK, Yang YX, Zhu XM, Xie SQ (2018) Effects of dietary fishmeal replacement with Spirulina platensis on the growth, feed utilization, digestion and physiological parameters in juvenile gibel carp (Carassis auratus gibelio var. CAS III). Aquac Res 49:1320–1328
Chentir I, Hamdi M, Doumandji A, HadjSadok A, Ben Ouada H, Nasri M, Jridi M (2017) Enhancement of extracellular polymeric substances (EPS) production in Spirulina (Arthrospira sp.) by two-step cultivation process and partial characterization of their polysaccharidic moiety. Int J Biol Macromol 105:1412–1420
China NDRC (2016) ‘13th Five-year’ planning for renewable energy development. Beijing, China
Cornet JF, Dussap CG, Cluzel P, Dubertret G (1992) A structured model for simulation of cultures of the cyanobacterium Spirulina platensis in photobioreactors: II. Identification of kinetic parameters under light and mineral limitations. Biotechnol Bioeng 40:826–834
da Rosa GM, Moraes L, Cardias BB, de Souza M, Costa JAV (2015) Chemical absorption and CO2 biofixation via the cultivation of Spirulina in semicontinuous mode with nutrient recycle. Bioresour Technol 192:321–327
de la Jara A, Ruano-Rodriguez C, Polifrone M, Assunçao P, Brito-Casillas Y, Wägner AM, Serra-Majem L (2018) Impact of dietary Arthrospira (Spirulina) biomass consumption on human health: main health targets and systematic review. J Appl Phycol 30:2403–2423
Depraetere O, Pierre G, Noppe W, Vandamme D, Foubert I, Michaud P, Muylaert K (2015) Influence of culture medium recycling on the performance of Arthrospira platensis cultures. Algal Res 10:48–54
Douskova I, Doucha J, Livansky K, Machat J, Novak P, Umysova D, Zachleder V, Vitova M (2009) Simultaneous flue gas bioremediation and reduction of microalgal biomass production costs. Appl Microbiol Biotechnol 82:179–185
Duarte JH, de Morais EG, Radmann EM, Vieira Costa JA (2017) Biological CO2 mitigation from coal power plant by Chlorella fusca and Spirulina sp. Bioresour Technol 234:472–475
Farooq W, Suh WI, Park MS, Yang JW (2015) Water use and its recycling in microalgae cultivation for biofuel application. Bioresour Technol 184:73–81
García-García JD, Sánchez-Thomas R, Moreno-Sánchez R (2016) Bio-recovery of non-essential heavy metals by intra- and extracellular mechanisms in free-living microorganisms. Biotechnol Adv 34:859–873
González-López CV, Cerón-García MC, Fernandez-Sevilla JM, González-Céspedes AM, Camacho-Rodriguez J, Molina-Grima E (2013) Medium recycling for Nannochloropsis gaditana cultures for aquaculture. Bioresour Technol 129:430–438
Hadj-Romdhane F, Zheng X, Jaouen P, Pruvost J, Grizeau D, Croué JP, Bourseau P (2013) The culture of Chlorella vulgaris in a recycled supernatant: effects on biomass production and medium quality. Bioresour Technol 132:285–292
Hirano A, Hon-Nami K, Kunito S, Hada M, Ogushi Y (1998) Temperature effect on continuous gasification of microalgal biomass: theoretical yield of methanol production and its energy balance. Catal Today 45:399–404
Hu Q, Sommerfeld M, Jarvis E, Ghirardi M, Posewitz M, Seibert M, Darzins A (2008) Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J 54:621–639
Huang J, Kang S, Wan M, Li Y, Qu X, Feg F, Wang J, Wang W, Shen G, Li W (2015) Numerical and experimental study on the performance of flat-plate photobioreactors with different inner structures for microalgae cultivation. J Appl Phycol 27:49–58
Hulatt CJ, Thomas DN (2010) Dissolved organic matter (DOM) in microalgal photobioreactors: a potential loss in solar energy conversion? Bioresour Technol 101:8690–8697
Husic HD, Moroney JV, Tolbert NE (1987) The role of carbonic anhydrase in the inorganic carbon concentrating system of Chlamydomonas reinhardtii. Progr Photosynth Res 4:317–324
Kalff J (2002) Limnology: inland water ecosystems. San Francisco, United States
Kao C-Y, Chen T-Y, Chang Y-B, Chiu T-W, Lin H-Y, Chen C-D, Chang J-S, Lin C-S (2014) Utilization of carbon dioxide in industrial flue gases for the cultivation of microalga Chlorella sp. Bioresour Technol 166:485–493
Lee JS, Lee JP (2003) Review of advances in biological CO2 mitigation technology. Biotechnol Bioprocess Eng 8:354–359
Lee JS, Kim DK, Lee JP, Park SC, Koh JH, Ohh SJ (2001) CO2 fixation by Chlorella KR-1 using flue gas and its utilization as a feedstuff for chicks. J Microbiol Biotechnol 11:772–775
Li YG, Hu HJ, Zhang LJ, Chen ZX (1996) Studies on CO2 supply technique for Spirulina production. J Wuhan Bot Res 14:349–356
Li TP, Xu G, Rong JF, Chen H, He CL, Giordano M, Wang Q (2016) The acclimation of Chlorella to high-level nitrite for potential application in biological NOx removal from industrial flue gases. J Plant Physiol 195:73–79
Lichtenthaler HK, Wellburn AR (1983) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem Soc Trans 11:591–592
Minjarez-Osorio C, Castillo-Alvarado S, Gatlin DM III, Lizett Gonzalez-Felix M, Perez-Velazquez M, Rossi W Jr (2016) Plant protein sources in the diets of the sciaenids red drum (Sciaenops ocellatus) and shortfin corvina (Cynoscion parvipinnis): a comparative study. Aquaculture 453:122–129
Moheimani NR (2016) Tetraselmis suecica culture for CO2 bioremediation of untreated flue gas from a coal-fired power station. J Appl Phycol 28:2139–2146
Morocho-Jácome AL, Mascioli GF, Sato S, de Carvalho JCM (2016) Evaluation of physicochemical treatment conditions for the reuse of a spent growth medium in Arthrospira platensis cultivation. Algal Res 13:159–166
Moroney JV, Somanchi A (1999) How do algae concentrate CO2 to increase the efficiency of photosynthetic carbon fixation? Plant Physiol 119:9–16
Moroney JV, Husic HD, Tolbert NE (1985) Effect of carbonic anhydrase inhibitors on inorganic carbon accumulation by Chlamydomonas reinhardtii. Plant Physiol 79:177–183
Patel A, Mishra S, Pawar R, Ghosh PK (2005) Purification and characterization of C-phycocyanin from cyanobacterial species of marine and freshwater habitat. Protein Expr Purif 40:248–255
Pruvost J, Van Vooren G, Le Gouic B, Couzinet-Mossion A, Legrand J (2011) Systematic investigation of biomass and lipid productivity by microalgae in photobioreactors for biodiesel application. Bioresour Technol 102:150–158
Qiao C, Li SY (2013) Spirulina (Arthrospira) in alkaline lakes of the Erdos Plateau. Science Press, Beijing (in Chinese)
Radmann EM, Camerini FV, Santos TD, Costa JAV (2011) Isolation and application of SOX and NOX resistant microalgae in biofixation of CO2 from thermoelectricity plants. Energy Convers Manag 52:3132–3136
Razzak SA, Ali SAM, Hossain MM, deLasa H (2017) Biological CO2 fixation with production of microalgae in wastewater—a review. Renew Sust Energ Rev 76:379–390
Richmond A, Boussiba S, Vonshak A, Kopel R (1993) A new tubular reactor for mass-production of microalgae outdoors. J Appl Phycol 5:327–332
Rijstenbil JW (1989) Competitive interaction between Ditylum brightwellii and Skeletonema costatum by toxic metabolites. Neth J Sea Res 23:23–27
Rocha GS, Pinto FHV, Melao MGG, Lombardi AT (2015) Growing Scenedesmus quadricauda in used culture media: is it viable? J Appl Phycol 27:171–178
Rodolfi L, Zittelli GC, Barsanti L, Rosati G, Tredici MR (2003) Growth medium recycling in Nannochloropsis sp mass cultivation. Biomol Eng 20:243–248
Sawayama S, Minowa T, Yokoyama SY (1999) Possibility of renewable energy production and CO2 mitigation by thermochemical liquefaction of microalgae. Biomass Bioenergy 17:33–39
Spolaore P, Joannis-Cassan C, Duran E, Isambert A (2006) Commercial applications of microalgae. J Biosci Bioeng 101:87–96
Stewart C, Hessami MA (2005) A study of methods of carbon dioxide capture and sequestration—the sustainability of a photosynthetic bioreactor approach. Energy Convers Manag 46:403–420
US DOE (2010) National algal biofuels technology roadmap. Maryland, U.S.
Usui N, Ikenouchi M (1997) Biological CO2 fixation and utilization project by RITE. 1. Highly-effective photobioreactor system. Energy Convers Manag 38:S487–S492
Vaz BD, Costa JAV, de Morais MG (2016) CO2 biofixation by the cyanobacterium Spirulina sp LEB 18 and the green alga Chlorella fusca LEB 111 grown using gas effluents and solid residues of thermoelectric origin. Appl Biochem Biotechnol 178:418–429
Vonshak A, Laorawat S, Bunnag B, Tanticharoen M (2014) The effect of light availability on the photosynthetic activity and productivity of outdoor cultures of Arthrospira platensis (Spirulina). J Appl Phycol 26:1309–1315
Yadav G, Karemore A, Dash SK, Sen R (2015) Performance evaluation of a green process for microalgal CO2 sequestration in closed photobioreactor using flue gas generated in-situ. Bioresour Technol 191:399–406
Zarrouk, C (1966) Contribution à l’étude d’une cyanophycée: influence de divers facteurs physiques et chimiques sur la croissance et la photosynthèse de Spirulina maxima Geitler (Ph.D. thesis), University of Paris
Zhang XZ, Lu ZY, Wang YF, Wensel P, Sommerfeld M, Hu Q (2016) Recycling Nannochloropsis oceanica culture media and growth inhibitors characterization. Algal Res 20:282–290
Zhao BT, Su YX, Zhang YX, Cui GM (2015) Carbon dioxide fixation and biomass production from combustion flue gas using energy microalgae. Energy 89:347–357
Acknowledgments
This work was supported by the China Agriculture Research System (CARS-50) and the Chinese Academy of Sciences (ZSSB-006).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Online resource 1
Fig. S1 Outdoor cultivation systems (PDF 273 kb)
Rights and permissions
About this article
Cite this article
Cui, H., Yang, Z., Lu, Z. et al. Combination of utilization of CO2 from flue gas of biomass power plant and medium recycling to enhance cost-effective Spirulina production. J Appl Phycol 31, 2175–2185 (2019). https://doi.org/10.1007/s10811-019-1736-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-019-1736-y