Abstract
The growth and biochemical composition of photoautotrophic and heterotrophic Isochrysis maritima in 50 L of Walne’s medium were compared. Heterotrophic I. maritima fed with 0.02 M glucose had a 4.6-fold higher maximum cell density (38.17 ± 0.23 × 106 cells mL−1) than photoautotrophic cells (8.29 ± 0.70 × 106 cells mL−1). The carbohydrate content was slightly higher in heterotrophic cells at all growth stages (mid-exponential, 40.8%; early stationary, 48.3%; and late stationary, 47.6%), but there was no significant effect on the protein content under either trophic condition. The total saturated fatty acids (SFAs), monounsaturated fatty acids (MUFAs) and polyunsaturated fatty acids (PUFAs) were higher under heterotrophic conditions than those under photoautotrophic conditions. However, because omega-3 PUFAs are the most essential element in feed nutrition, low results for eicosapentaenoic acid (EPA) (0.28 ± 0.06%) and docosahexaenoic acid (DHA) (3.22 ± 0.26%) in the heterotrophic cells compared to the photoautotrophic cells (EPA: 0.44 ± 0.11%; DHA: 8.58 ± 0.73%) plus a low omega-3/6 PUFAs ratio (heterotrophic: 0.16–0.47; photoautotrophic: 2.60–2.88) and high value of (SFA + MUFA)/PUFA (heterotrophic: 5.50–6.81; photoautotrophic: 2.64–3.60) showed that this species is not suitable for aquaculture feed when cultivated under heterotrophic conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The importance of microalgae in aquaculture is not surprising, as microalgae are a natural food source for most aquaculture species. Microalgae are used for either direct consumption, such as for molluscs and penaeid shrimp, or for indirect consumption as food for live prey, such as rotifers and Artemia that feed the small larvae of fish (Brown 2002; Patil et al. 2005; Guedes and Malcata 2012). In addition, the cultivation of microalgae, as the primary source of polyunsaturated fatty acids (PUFAs), has been shown to be a profitable area of biotechnology research in the food and feed industries (Pulz and Gross 2004; Mansour et al. 2005). The inability of aquatic animals to synthesise both eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) makes microalgae the ideal source of PUFAs for the growth and development of these animals (Meireless et al. 2003). The microalgae species most commonly utilised as feed are members of the genera Chlorella, Tetraselmis, Isochrysis, Pavlova, Phaeodactylum, Chaetoceros, Nannochloropsis, Skeletonema and Thalassiosira (Spolaore et al. 2006; Hemaiswarya et al. 2010).
The marine microalgae Isochrysis spp. have received overwhelming research interest due to their ability to provide resources in the form of novel genetic material. These resources contribute to the production of various nutritional and pharmaceutical products, proteins (essential amino acids), energy and other key nutrients, such as vitamins, essential PUFAs and pigments, which are transferred through the food chain (Brown 2002). The most commonly used Isochrysis strain is Isochrysis galbana (note that the T-Iso strain of Isochrysis which has sometimes also been referred to as I. galbana is now known as Tisochrysis lutea—Bendif et al. 2013). Isochrysis strains have been widely used as mariculture feed due to their high content of long-chain PUFAs, especially DHA, and their ease of cultivation (Lin et al. 2007; Nalder et al. 2015). These strains are also able to tolerate cultivation at relatively high temperatures, which can be particularly advantageous under unpredictable tropical conditions (Babuskin and Radhakrishnan 2014). Additionally, they have a high growth rate, lack a cell wall and are robust under outdoor culture conditions (Devos et al. 2006; Lin et al. 2007; Liu et al. 2013), making them a popularly utilised microalgal taxa in aquaculture.
Various studies have attempted to enhance the production of both the cell biomass and biochemical composition of algae. Such attempts have included the manipulation of chemical variables such as nitrogen (Arumugam et al. 2013) and phosphorus concentrations (Sun and Wang 2009), physical variables such as temperature (Durmaz et al. 2009) and salinity (Rao et al. 2007) and cultivation modes including photoautotrophic, heterotrophic and mixotrophic modes (Chojnacka and Noworyta 2004). Microalgae are usually cultivated under photoautotrophic conditions (Gouveia and Oliveira 2009), which involve the use of light as an energy source and inorganic carbon as a carbon source. Although metabolite production is relatively high, these cultivation conditions are frequently associated with a low biomass concentration due to light limitations (Wen and Chen 2001; Chen and Chen 2006). The dependence of photoautotrophic cultivation on light energy prevents the cells from reaching a high density due to self-shading or photoinhibition problems. To eliminate the light requirement, microalgae have begun to be heterotrophically cultivated to possibly increase the cell density (Chen 1996; Huang et al. 2010; Pahl et al. 2010). Heterotrophic cultivation involves the utilisation of organic carbon as the energy and carbon sources for growth in dark conditions (Wen and Chen 2003; Chojnacka and Marquez-Rocha 2004; Chen et al. 2011). Although these conditions are advantageous in terms of microalgal growth, not all microalgae can grow in the dark (Perez-Garcia et al. 2011). The cell density was reportedly enhanced with the elimination of light and the utilisation of an organic carbon source. Thus, this culture mode may be a cost-effective alternative for the mass cultivation of microalgae (Chen 1996; Pahl et al. 2010).
In addition to its ability to improve cell biomass, heterotrophic cultivation also received much attention because of its ability to increase the fatty acid content compared to that obtained with photoautotrophic cultivation (Miao and Wu 2006; Xiong et al. 2008; Liu et al. 2011; Wang et al. 2012). A study by Wen and Chen (2000) on the heterotrophic production of EPA by the diatom Nitzschia laevis indicated that the production of PUFAs, including DHA and EPA, was higher under dark conditions. A study by Liu et al. (2011) on Chlorella zofingiensis also showed an increase in monounsaturated fatty acids (MUFAs) under heterotrophic cultivation (37.4%) compared to under photoautotrophic cultivation (20.1%). Our preliminary investigation showed that Isochrysis maritima isolated from Penang National Park coastal waters was a suitable candidate for aquaculture, meeting all of the general attributes, including being the appropriate size for ingestion and exhibiting rapid growth rates, amenability to mass culture, stability when faced with fluctuation in culture conditions and a good nutrient composition. Thus, the present study aimed to determine the possibility of culturing I. maritima photoautotrophically with different nitrogen sources and heterotrophically with different carbon sources. Subsequently, the best nitrogen and carbon sources were then utilised at a higher cultivation volume under both trophic conditions to evaluate the cell biomass and biochemical compositions of this species at different growth stages for use in aquaculture feed.
Materials and methods
Microalgae strain and growth media
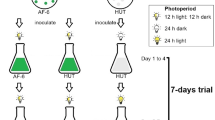
Isochrysis maritima USMAC 19 was isolated from the coastal waters of Teluk Aling, Penang National Park, Malaysia, and identified by Biotech International R & D (BIRD). The strain was purified using mechanical separation to discriminate between the cells and other substances using a vortex mixer (Reax 2000, Heidolph, Germany) at 2000×g for 1 min and centrifugation at 6000×g for 10 min. This procedure was followed by antibiotic treatment involving a mixture of penicillin G, dihydrostreptomycin sulphate and gentamicin sulphate in the ratio of 4:1:1 (Guillard 2005) for 18 to 72 h. The sterility of the culture was examined by inoculating the cells onto a culture plate with sterility test medium, including a mixture of 100 mg of yeast extract and 200 mg of tryptone with 100 mL of 2% agar growth medium. The plates with the algae were incubated for 1 week to observe any bacterial growth. When there was no visible bacterial growth, I. maritima was transferred to a liquid medium and was photoautotrophically maintained in 100 mL of sterilised seawater enriched with Walne’s medium (Walne 1970) at 3‰ salinity. The culture was incubated at a constant temperature of 25 ± 1 °C under a light intensity of 50 μmol photons m−2 s−1, with a 12:12-h light/dark cycle. Agitation of the culture medium was conducted by shaking the flasks twice daily.
Photoautotrophic cultivation
Isochrysis maritima was cultivated in triplicate in 1 L of Walne’s medium with three different nitrogen sources, i.e., nitrate (NaNO3), nitrite (NaNO2) and urea ((NH2)2CO), at a concentration of 4 mg atom N L−1 (Fidalgo et al. 1998). The Walne’s medium was sterilised by autoclaving (HVE-50, Hirayama, Japan) at 121 °C for 20 min. The initial cell density was adjusted to 2.5 × 105 cells mL−1 after the cells were introduced into the culture medium. The cultures were grown in an algae culture room (temperature: 25 ± 1 °C) with a 24-h light duration (light intensity: 50 μmol photons m−2 s−1) and were continuously bubbled with 0.2-μm filtered air at a rate of 1 L min−1. The number of cells was counted daily until the culture reached the late stationary phase. The nitrogen source that produced the highest yields, in terms of cell density, was used to culture this species in 50 L of culture medium. The 50 L of Walne’s medium was sterilised using 50 mL of sodium hypochlorite and was neutralised with 12.5 mL of sodium thiosulphate (250 g L−1) (Kawachi and Noël 2005). The initial density of I. maritima was adjusted to 2.5 × 105 cells mL−1 (Fidalgo et al. 1998). The cultures were grown at a temperature of 28 ± 2 °C under white fluorescent light (50 μmol photons m−2 s−1), with a light duration of 24 h, and they were aerated at 2 L min−1. The number of cells was counted daily until the culture reached the late stationary phase. The microalgae were harvested at three different growth stages (mid-exponential, early stationary and late stationary) by flocculation, and the cell biomass, proteins, carbohydrates and fatty acids were analysed.
Heterotrophic cultivation
Isochrysis maritima was cultivated in triplicate in 1 L of Walne’s medium with urea as the nitrogen source and then sterilised by autoclaving at 121 °C for 20 min. All of the culture vessels were fully wrapped with aluminium foil to ensure no light penetration. The carbon sources, including glucose, sucrose, acetate, lactose and fructose, were added at three different concentrations (0.02, 0.05 and 0.1 M). All of the carbon sources were sterilised by filtration through 0.2-μm membranes before injection into the culture medium. The inocula were taken from the same stock culture at the early stationary growth phase, and the initial densities were adjusted to 2.5 × 105 cells mL−1. All of the cultures were incubated in an algae culture room at 25 ± 1 °C. The cultures were continuously bubbled with 0.2-μm filtered air at a rate of 1 min−1. The cell density was recorded daily until the culture reached the late stationary growth phase. The carbon source and concentration that produced the highest cell growth were used in a subsequent experiment, in which I. maritima was heterotrophically cultivated in larger volumes. This experiment was conducted in triplicate in 50 L of Walne’s medium sterilised using 50 mL of sodium hypochlorite and neutralised with 12.5 mL of sodium thiosulphate (250 g L−1) (Kawachi and Noël 2005) in a batch culture system. The inocula were taken from the same stock culture at the late exponential growth phase, and the initial densities were adjusted to 2.5 × 105 cells mL−1. The cultures were incubated in the closed hatchery laboratory at 28 ± 2 °C and were continuously bubbled with filtered air at a rate of 2 L min−1. Isochrysis maritima was harvested at different growth phases (mid-exponential, early stationary and late stationary), and cell biomass, proteins, carbohydrates and fatty acids were analysed.
Analytical methods
The cell density was counted daily using a haemocytometer (Neubauer-improved haemacytometer, Laboroptik, UK), and the cell biomass was determined using the dry weight and the ash content at the end of the experiment (Liu et al. 2013). The specific growth rate was calculated during the exponential phase, according to the following equation:
where X 2 and X 1 are the cell densities (cells mL−1) at times t 2 and t 1 (day), respectively.
Samples were collected by flocculation at the same time for all growth phases by adjusting the pH to 10–11 using 1 M NaOH. The flocculated cells were centrifuged at 3000×g for 5 min. The pellets were washed with distilled water and centrifuged again at the same speed and for the same amount of time. The cell pellets were freeze dried for total protein, total carbohydrate and fatty acid analyses. The total protein was extracted using a method proposed by Rausch (1981) and was measured using the Lowry assay (Lowry et al. 1951). The carbohydrates were extracted using a method proposed by Chu et al. (1996) and were measured using the phenol–sulphuric assay, according to DuBois et al. (1956).
Fatty acid analysis
Isochrysis maritima was extracted by the direct transesterification method as described by Abel et al. (1963), with some modifications. One hundred milligrams of dried cells was weighed and transferred into screw cap bottles, and 2 mL each of methanolic sulphuric acid (15%) and chloroform were added. The mixture was bubbled with nitrogen gas for 15 s and subsequently vortexed for 2 min. After the mixture became visibly homogenous, it was heated using a heating block (HB-48, WiseTherm, Germany) at 80 °C for 30 min. Then, 1 mL of distilled water was added and two layers were formed in the solution. Subsequently, the lower layer of the mixture was transferred to a 2-mL vial, which was then centrifuged at 10,000×g for 5 min. Ten milligram of anhydrous sodium sulphate was added to ensure the complete removal of water from the mixture. Finally, 0.75 μL of the lower layer was transferred into a gas chromatography (GC) vial, which was then sealed and kept at −20 °C until GC analysis.
Fatty acid methyl esters (FAMEs) were separated and quantified by a gas chromatograph (GCMS-2010, Shimadzu, Japan) equipped with a flame ionisation detector and a 30 × 0.22 mm 70% cyanopropyl polysilphenylene–siloxane column (BPX70, SGE). Nitrogen was used as the carrier gas, and the temperature programming was set to increase from 100 to 210 °C at 2 °C min−1 and was then maintained at 210 °C for 30 min. The injector and detector temperatures were set at 250 and 260 °C, respectively. Menhaden oil and Supelco 37 Component FAME Mix were used as the standards for fatty acid identification by the comparison of the peak retention times between the samples and standards at a ratio of 3:1. The concentration of the fatty acids was estimated from the peak area on the chromatogram using 99% methyl enanthate (C7:0) (Sigma Aldrich, USA) as an internal standard.
Statistical analysis
The statistical analyses were conducted using the SPSS v20.0 software. A one-way ANOVA was used to test for possible significant differences in the means of the dependent variables for the microalgae grown with different nitrogen sources, and a 5 × 3 factorial design was used to test for possible significant differences in five carbon sources at three different concentrations. The significant differences between photoautotrophic and heterotrophic cultivations in 50 L of Walne’s medium were analysed using independent sample t test. Multiple comparisons were tested by Duncan’s post hoc analysis, when the main treatment effect was significant at P < 0.05. All of the experiments were conducted in triplicate, and all data are presented as the mean ± standard error.
Results
Effect of different nitrogen sources on photoautotrophic growth
The present study aimed to determine the best nitrogen sources for the maximum growth of the tropical microalga I. maritima in photoautotrophic cultivation. Figure 1 shows the growth curves for I. maritima cultivated with three different nitrogen sources. After 14 days of cultivation, urea showed a significantly higher maximum cell density (11.6 ± 0.27 × 106 cells mL−1) than the other nitrogen sources tested (P < 0.05) (Fig. 1). There was no significant difference in the maximum cell densities between nitrate and nitrite, for which 9.64 ± 0.25 × 106 and 9.38 ± 0.26 × 106 cells mL−1 were recorded, respectively (Fig. 1). The dry weight (DW) analysis and the specific growth rates (SGR) were also significantly greater in urea than in nitrate and nitrite (Table 1). However, the ash-free dry weight (AFDW) was not significantly different (P > 0.05) across all nitrogen sources (Table 1).
Effect of different carbon sources on heterotrophic growth
The effects of various carbon sources at different concentrations on the cell density and specific growth rate of I. maritima are shown in Figs. 2 and 3. Glucose was the most suitable carbon source for culturing this species under heterotrophic conditions. Glucose, at the concentrations of 0.02 and 0.05 M, achieved significantly higher cell densities than the other carbon sources at all concentrations, with maximum cell densities of 41.83 ± 0.36 × 106 and 36.09 ± 0.27 × 106 cells mL−1, respectively, and SGR of 1.30 ± 0.16 and 0.97 ± 0.12 day−1, respectively. Other carbon sources, such as fructose, only demonstrated an average maximum cell density of 5.00–6.00 × 106 cells mL−1, which was also obtained at 0.02 and 0.05 M concentrations, with SGR of 0.54 ± 0.16 and 0.54 ± 0.13 day−1, respectively. The effectiveness of lactose on I. maritima growth was the lowest among the other carbon sources studied, with a maximum cell density of less than 2.00 × 106 cells mL−1 recorded at all concentrations. Isochrysis maritima grown in sucrose and acetate also demonstrated low maximum cell densities in the range of 0.70–3.10 × 106 and 3.10–4.00 × 106 cells mL−1, respectively, at all of the tested concentrations.
Growth, biochemistry and fatty acid composition under photoautotrophic and heterotrophic conditions
Isochrysis maritima photoautotrophically cultured in 50 L of Walne’s medium had a significantly lower maximum cell density (8.29 ± 0.70 × 106 cells mL−1) (Fig. 4) than I. maritima cultured in 8 L of culture medium (11.6 ± 0.27 × 106 cells mL−1, Fig. 1) under the same culture conditions (P < 0.05). Nevertheless, the SGRs were not significantly different between 50 L of Walne’s medium and 8 L of Walne’s medium, (0.98 ± 0.09 and 1.24 ± 0.12 day−1, respectively; P > 0.05). Different results were attained in 50 L of Walne’s medium heterotrophically. The maximum cell density was not significantly different between 50 L of Walne’s medium (38.17 ± 0.23 × 106 cells mL−1, Fig. 4) and 1 L of Walne’s medium (41.83 ± 0.36 × 106 cells mL−1, Fig. 2) (P > 0.05). A similar result was recorded in the SGR for both culture volumes, which were 1.31 ± 0.07 day−1 in 50 L of Walne’s medium and 1.30 ± 0.16 day−1 in 1 L of Walne’s medium from the previous study with the same culture conditions (P > 0.05, Fig. 3).
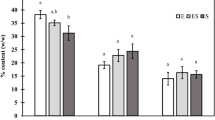
The cell density, DW, ash percentage and AFDW of I. maritima were affected by different trophic conditions, as shown in Table 2. Isochrysis maritima cultivated heterotrophically reached a significantly higher (4.6-fold) maximum cell density than that cultivated in photoautotrophically condition (P < 0.05). The DW, ash percentage and AFDW were also significantly higher in the heterotrophic culture (6.12 ± 0.24 g L−1, 41.0 ± 0.49% and 3.61 ± 0.21 g L−1, respectively) than in the photoautotrophic culture (3.01 ± 0.25 g L−1, 21.9 ± 0.66% and 2.35 ± 0.23 g L−1, respectively). In addition, other than different trophic conditions, the cell density, DW, ash percentage and AFDW were affected by different growth phases (Table 2). The cell density for the photoautotrophic and heterotrophic cultivations increased from 5.62 ± 0.19 × 106 and 20.04 ± 0.19 × 106 cells mL−1 at the mid-exponential phase, respectively, to approximately 8.29 ± 0.70 × 106 and 36.90 ± 0.25 × 106 cells mL−1 at the early stationary phase, respectively. Cellular DW was also increased from 2.14 ± 0.09 and 3.32 ± 0.17 to 2.63 ± 0.07 and 6.12 ± 0.24 g L−1, respectively. Similar results were obtained for the AFDW content, which significantly increased from 1.59 ± 0.07 to 1.91 ± 0.12 g L−1 in the photoautotrophic culture and from 2.1 ± 0.12 to 3.61 ± 0.21 g L−1 in the heterotrophic culture.
The total protein was more affected by the growth phase than the trophic condition (Table 2). The protein content was higher for both trophic conditions at the exponential phase (23.01 ± 3.4 and 26.2 ± 2.10%, respectively) and decreased at the late stationary phase (10.64 ± 3.62 and 18.3 ± 0.60%, respectively). There was a slight increase in the carbohydrate content from the mid-exponential phase to the late stationary phase for both trophic conditions. Meanwhile, there was a significant difference between the photoautotrophic and heterotrophic cultures at all growth phases (P < 0.05). The carbohydrate content was 40.8 ± 3.6% in the heterotrophic culture and 18.8 ± 1.5% in the photoautotrophic culture at the mid-exponential phase, 48.3 ± 1.52 and 21.8 ± 2.4%, respectively, at the early stationary phase and 47.6 ± 2.5 and 26.9 ± 3.4%, respectively, at the late stationary phase.
The fatty acid compositions of I. maritima photoautotrophically and heterotrophically grown at different growth phases are listed in Table 3. The main fatty acids (as % of total fatty acids) were C14: 0 (8.79–14.66%), C16: 0 (11.12–15.7%) and C22: 6 (n-3) (5.68–9.31%) in the photoautotrophic culture and C16: 0 (21.34–36.19%), C18: 0 (7.08–17.38%) and C18: 1 (n-9) (10.32–19.48%) in the heterotrophic culture. The major saturated fatty acid (SFA) for both photoautotrophic and heterotrophic cultures was palmitic acid (C16: 0) (10.7–15.70 and 21.34–36.19%, respectively) (Table 3). Meanwhile, the major monounsaturated fatty acid (MUFAs) for both trophic conditions was oleic acid (C18: 1 (n-9)), which were 3.23–3.67% photoautotrophically and 10.32–19.48% heterotrophically. Docosahexaenoic acid (DHA; C22: 6 (n-3)) was the major polyunsaturated fatty acid (PUFA) in photoautotrophic cells, while linoleic acid (LA; C18: 2 (n-6) was the major PUFA in heterotrophic cells.
The PUFA content significantly increased from the mid-exponential phase to the late stationary phase (P < 0.05). The SFAs increased from the mid-exponential phase towards the late stationary phase for both trophic conditions. There was no difference in the MUFA content in photoautotrophic cells but was slightly decreased from the mid-exponential phase to the late stationary phase in heterotrophic cells. The composition of PUFAs, especially omega-3 and omega-6 fatty acids in the dietary microalgae, determines the suitability of these microalgae as aquaculture feed. In the present study, the highest content of α-linolenic acid (ALA) C18: 3 (n-3), arachidonic acid (AA), C20: 4 (n-6) eicosapentaenoic acid (EPA) C20: 5 (n-3) and docosahexaenoic acid (DHA) C22: 6 (n-3) were all obtained at the late stationary phase in photoautotrophic cells, which were 1.04 ± 0.12, 0.38 ± 0.08, 0.44 ± 0.11 and 8.58 ± 0.73%, respectively. However, the highest content of linoleic acid (LA) C18:2 (n-6) was obtained at the early stationary phase in heterotrophic cells, which was 9.04 ± 0.36%. There were no significant differences in the total omega-3 and total omega-6 PUFAs for all three growth stages in both trophic conditions (P > 0.05). The total (n-3)/(n-6) index represents the quotient between the fatty acids corresponding to the groups (n-3) and (n-6) and also a parameter to estimate the nutritional value of microalgal cells. In this study, the ratio of total (n-3) and total (n-6) PUFAs showed optimal values in photoautotrophic cells (2.60–2.88) compared to heterotrophic cells (0.16–0.47) (Table 3). The ratio of (SFA + MUFA)/PUFA was higher in heterotrophic cells (5.50–6.81) than in photoautotrophic cells (2.64–3.60).
Discussion
Nitrogen is the most commonly reported nutrient-limiting factor in the growth and lipid accumulation of microalgae (Xu et al. 2001; Li et al. 2008; Lin and Lin 2011). Hence, the first experiment aimed to choose the best nitrogen sources for the maximum growth of the tropical microalga I. maritima in photoautotrophic cultivation. The results showed that I. maritima could be cultivated in nitrate, nitrite and urea because the SGR showed no significant difference between them. However, different nitrogen sources did affect the total biomass of the cells. Urea recorded the highest cell density, DW, ash and AFDW compared to the others. Changes in the cell biomass of microalgae due to different nitrogen sources were reported by other researchers, including Fidalgo et al. (1998) on I. galbana, Feng et al. (2011) on Isochrysis zhangjiangensis, González-Garcinuño et al. (2014) on Scenedesmus abundans and Chlorella ellipsoidea, Lin and Lin (2011) on Scenedesmus rubescens, Arumugam et al. (2013) on Scenedesmus bijugatus and Campos et al. (2014) on Nannochloropsis salina. In fact, studies on other haptophytes by Fidalgo et al. (1998) and Feng et al. (2011) also reported that I. galbana and I. zhangjiangensis grown with urea recorded the highest cell biomass compared to other tested nitrogen sources. Most microalgae take up nitrate–nitrogen only if ammonium nitrogen is depleted (Xu et al. 2001), because nitrate–nitrogen must be transformed into ammonium nitrogen before being utilised by algae (Flores et al. 1980). In the present study, this species inclination towards urea might be related to the statement by Sakamoto and Bryant (1998), who reported that urea assimilation to ammonium required less energy than nitrate, because it existed in reduced form. Carvalho et al. (2004) also suggested that the simultaneous actions of urease, which is an enzyme that catalyses the hydrolysis of urea into carbon dioxide and ammonia (Carvajal et al. 1980), and alkaline conditions (Danesi et al. 2002) could have promoted urea hydrolysis to ammonia at a rate consistent with its assimilation by the cell, thereby minimising ammonia inhibition that might occur during the growth phase. On the other hand, compared to other nitrogen sources, urea can easily pass through the plasma membrane (Rashid et al. 2014). Urea as a nitrogen source can provide a slight advantage for commercialization, because urea was among the cheapest nitrogen sources compared to others (Hulatt et al. 2012), and urea may also act as a readily available source of CO2 carbon for photosynthetic organisms (Berman and Chava 1999).
The ability of microalgae to grow in media with organic supplementation depends on the algal species and the sources of organic carbon (Chen and Chen 2006; Alkhamis and Qin 2013). Therefore, the second experiment aimed to choose the best carbon sources at different concentrations for the maximum growth of I. maritima in heterotrophic cultivation. Based on the obtained results, this species only grew well on glucose at 0.02 and 0.05 M concentrations. This finding was not surprising, as glucose is the most commonly used carbon source for the heterotrophic culture of microalgae (Park et al. 2002; Liang et al. 2009; Li et al. 2013; Liu et al. 2014; Gim et al. 2016). A study by Gim et al. (2016) showed that the supplementation of glucose led to a significant improvement in the growth of I. galbana, Nannochloropsis oculata and Dunaliella salina compared to other studied carbon sources. Moreover, C. zofingiensis also demonstrated the highest growth rate, cell biomass, lipid content and lipid yield after being cultivated in glucose as a carbon source (Liu et al. 2010). When glucose was available, a membrane-bound hexose/H+ symport system, which is triggered by the hexose uptake protein gene (hup1) (Sauer and Tanner 1989), was most likely activated. This activated system transports glucose and H+ with a stoichiometry of 1:1 (Komor et al. 1973) into the cytosol and invests one molecule of ATP per molecule of sugar transported (Tanner 2000). The oxidative assimilation of glucose begins with the phosphorylation of hexose to form glucose-6-phosphate, which is an important intermediate product for storage, cell synthesis and respiration (Perez-Garcia et al. 2011; Liu et al. 2014), yielding the reducing equivalents (FADH2 and NADPH) from the ATP synthesis. Hence, all of the produced ATP is used for cell growth and lipid accumulation (Gim et al. 2016). On the contrary, Perez-Garcia et al. (2011) also stated that glucose might be considered a “preferred substrate” for the heterotrophic cultivation of microalgae because the cells grown on other substrates may require a lag period (an acclimation period) to develop the specific transport systems necessary for the uptake of other substrates. Nevertheless, this lag phase may not always occur because of other factors, such as the strain used, the bioreactor configuration and the environmental conditions.
The low maximum cell density at 0.1 M (19.8 g L−1) recorded for all carbon sources was most likely due to an excess of organic substrate, which inhibited the growth of I. maritima (Perez-Garcia et al. 2011; Liu et al. 2014). Ogawa and Aiba (1981) suggested that the initial concentration of glucose should be limited to 10 and 1 g L−1 to promote the cellular growth of Chlorella vulgaris and Scenedesmus acutus, respectively. Meanwhile, Tan and Johns (1991) stated that inhibition occurred for Chlorella saccharophila and Chlorella sorokiniana at glucose concentrations of more than 25 and more than 5 g L−1, respectively. Nitzschia laevis yields also decreased as the substrate concentration increased from 1 to 40 g L−1 (Wen and Chen 2000). However, different species experience carbon inhibition at different concentrations. For example, Chlorella protothecoides was cultivated at carbon concentrations as high as 80 g L−1 to obtain an optimal yield of biomass (Shi et al. 1999). Galdieria sulphuraria was reported to grow under high concentrations of glucose or fructose of up to 166 g L−1, but higher glucose concentrations inhibited its growth (Schmidt et al. 2005).
Based on the results from the first and second experiments, urea was chosen as the nitrogen source for photoautotrophic growth and 0.02 M glucose as the carbon source for the heterotrophic growth to explore the growth and biochemical composition response of I. maritima in both trophic conditions when cultivated in 50 L Walne’s medium. Growth was affected by cultivation in a larger culture volume. The maximum cell density, the DW, the ash content and the AFDW decreased as the culture medium increased from 8 L from the first experiment to 50 L with the same culture conditions. A study by Renaud et al. (1991) on the effect of light intensity on the proximate biochemical and fatty acid composition of Isochrysis sp. and N. oculata for tropical aquaculture also recorded a decrease in microalgal growth as the size of the culture medium increased from 4 to 8000 L. This result was presumably related to the limitation of the physical requirements for microalgae, especially light. This problem occurred when the cell density increased, which led to self-shading phenomena or poor light availability inside the culture (Pruvost et al. 2002; Chen and Chen 2006), even with the supply of aeration. This result was confirmed as I. maritima cultivated heterotrophically in 50 L of Walne’s medium, with urea as the nitrogen source and glucose as the carbon source, showed no significant difference in the cell density compared to that cultivated in 1 L in the second experiment. The elimination of the light requirement in the heterotrophic cultivation gave an advantage to this species to maximise its growth without the light limitation. As a result, photoautotrophic cells recorded lower cell densities than the microalgae grown under heterotrophic conditions, which were 345% higher than those of the photoautotrophic cells.
The cell density, the cell DW, the ash content and the AFDW were also affected by the growth phase for both trophic conditions. The cell density, the cell DW and the AFDW increased as the culture aged. This result was expected because most of the biochemical compounds, except for protein, increased when the culture aged, especially carbohydrate and lipid (Fidalgo et al. 1998; Lin et al. 2007; Chiu et al. 2009). In the present study, the carbohydrate content slightly increased from the mid-exponential phase to the late stationary phase for both trophic conditions. The lipid content was not tested in this study. The protein content showed different results, with the values being higher under both trophic conditions at the mid-exponential phase and decreasing at the late stationary phase. The biochemical composition of photosynthetic microalgae is closely related with their cell metabolism, and protein becomes the main component of the cells harvested during the exponential phase of growth and, consequently, replaced by storage products, such as carbohydrate and lipids, which increase along with the nutrient deficiency (Zhu et al. 1997; Phatarpekar et al. 2000).
As previously described, the objective of this study was to compare the growth and biochemical compositions of I. maritima cultivated in photoautotrophic and heterotrophic conditions for use in aquaculture feed. The obtained results showed that I. maritima grew well heterotrophically compared to photoautotrophic growth. There was no significant difference in the protein content in both trophic conditions. In contrast, the carbohydrate content was significantly higher in the heterotrophic cultures than in the photoautotrophic cultures. However, the content of omega-3, especially EPA and DHA, was low in heterotrophic cells. Because the omega-3 PUFAs are essential in the diets of many commercially important marine fish and shellfish, it is important for an aquaculturist to maximise the content of the omega-3 PUFAs in dietary algae. Therefore, I. maritima cultured heterotrophically was not suitable for use as aquaculture feed. This statement was supported by the low amount of omega-3/6 PUFA ratio obtained in all growth phases (0.16–0.47) and the high value of (SFA + MUFA)/PUFA (5.50–6.81) recorded in heterotrophic cells. The microalgal cells produced in the culture had an acceptable nutritional quality in bivalve larvae production when the omega-3/6 PUFAs quotient was within a range value of 2–5 (Sánchez et al. 2000) and with a lower value of (SFA + MUFA)/PUFA (Lin et al. 2007). In contrast, photoautotrophic cells showed optimal values of omega-3/6 PUFA ratio (2.60–2.88) and a low value of (SFA + MUFA)/PUFA (2.64–3.60). In addition, harvesting the cells at the late stationary growth phase may allow a better yield of its composition because the protein, EPA and DHA contents were highest at this phase. The composition of cells grown mixotrophically also should be examined (Alkhamis and Qin 2015).
In conclusion, I. maritima cells cultivated under photoautotrophic and heterotrophic conditions displayed different growth characteristics and biochemical compositions, especially in terms of PUFAs. Although the heterotrophic algal cells had much higher yields of cell density and total SFAs, MUFAs and PUFAs than the photoautotrophic cells, the high omega-3/6 PUFAs ratio of heterotrophic I. maritima compared to photoautotrophic cells as well as the high value of (SFA + MUFA)/PUFA demonstrated that this species is unsuitable for use as aquaculture feed in the heterotrophic condition.
References
Abel K, Deschmertzing H, Peterson JI (1963) Classification of microorganisms by analysis of chemical composition. J Bacteriol 85:1039–1044
Alkhamis Y, Qin JG (2013) Cultivation of Isochrysis galbana in phototrophic, heterotrophic, and mixotrophic conditions. Biomed Res Int 2013:1–9
Alkhamis Y, Qin JG (2015) Comparison of N and P requirements of Isochrysis galbana under phototrophic and mixotrophic conditions. J Appl Phycol 27:2231–2238
Arumugam M, Agarwal A, Arya MC, Ahmed Z (2013) Influence of nitrogen sources on biomass productivity of microalgae Scenedesmus bijugatus. Bioresour Technol 131:246–249
Babuskin S, Radhakrishnan K (2014) Effect of photoperiod, light intensity and carbon sources on biomass and lipid productivities of Isochrysis galbana. Biotechnol Lett 36:1653–1660
Bendif EM, Probert I, Schroeder DC, Vargas C (2013) On the description of Tisochrysis lutea gen. nov. sp. nov. and Isochrysis nuda sp. nov. in the Isochrysidales, and the transfer of Dicrateria to the Prymnesiales (Haptophyta). J Appl Phycol 25:1763–1776
Berman T, Chava S (1999) Algal growth on organic compounds as nitrogen sources. J Plankton Res 21:1423–1437
Brown MR (2002) Nutritional value of microalgae for aquaculture. In: Cruz-Suarez LE, Ricque-Marie D, Tapia-Salazar M, Gaxiola-Cortes MG, Simoes N (eds) Avances en nutricion acuicola VI. Memorias del VI Symposium Internacional de Nutricion Acuicola. 3–6th September, Cancun, Mexico
Campos H, Boeing WJ, Dungan BN, Schaub T (2014) Cultivating the marine microalga Nannochloropsis salina under various nitrogen sources: effect on biovolume yields, lipid content and composition, and invasive organisms. Biomass Bioenergy 66:301–307
Carvajal N, Fernández M, Rodríguez JP, Donoso M (1980) Urease of Spirulina maxima. Phytochemistry 21:2821–2823
Carvalho JCM, Francisco FR, Almeida KA, Sato S, Converti A (2004) Cultivation of Arthrospira (Spirulina) platensis (Cyanophyceae) by fed-batch addition of ammonium chloride at exponentially increasing feeding rates. J Phycol 40:589–597
Chen F (1996) High cell density culture of microalgae in heterotrophic growth. Tibtech 14:421–426
Chen GQ, Chen F (2006) Growing phototrophic cells without light. Biotechnol Lett 28:607–616
Chen CY, Yeh KL, Aisyah R, Lee DJ, Chang JS (2011) Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: a critical review. Bioresour Technol 102:71–81
Chiu SY, Kao CY, Tsai MT, Ong SC, Chen CH, Lin CS (2009) Lipid accumulation and CO2 utilization of Nannochloropsis oculata in response to CO2 aeration. Bioresour Technol 100:833–838
Chojnacka K, Marquez-Rocha F (2004) Kinetic and stoichiometric relationships of the energy and carbon metabolism in the culture of microalgae. Biotechnology 3:21–34
Chojnacka K, Noworyta A (2004) Evaluation of Spirulina sp. growth in photoautotrophic, heterotrophic and mixotrophic cultures. Enzym Microb Technol 34:461–465
Chu WL, Phang SM, Goh SH (1996) Environmental effects on growth and biochemical composition of Nitzschia inconspicua Grunow. J Appl Phycol 8:389–396
Danesi EDG, Rangel-Yagui CO, Carvalho JCM, Sato S (2002) An investigation of the effect of replacing nitrate by urea in the growth and production of chlorophyll by Spirulina platensis. Biomass Bioenergy 23:261–269
Devos M, Poisson L, Ergan F, Pencreac’h G (2006) Enzymatic hydrolysis of phospholipids from Isochrysis galbana for docosahexaenoic acid enrichment. Enzyme Microb Tech 39:548–554
DuBois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Durmaz Y, Donato M, Monteiro M, Gouveia L, Nunes ML, Gama Pereira T, Gökpinar Ş, Bandarra NM (2009) Effect of temperature on α-tocopherol, fatty acid profile, and pigments of Diacronema vlkianum (Haptophyceae). Aquac Int 17:391–399
Feng D, Chen Z, Xue S, Zhang W (2011) Increased lipid production of the marine oleaginous microalgae Isochrysis zhangjiangensis (Chrysophyta) by nitrogen supplement. Bioresour Technol 102:6710–6716
Fidalgo J, Cid A, Torres E, Sukenik A, Herrero C (1998) Effects of nitrogen source and growth phase on proximate biochemical composition, lipid classes and fatty acid profile of the marine microalga Isochrysis galbana. Aquaculture 166:105–116
Flores E, Guerrero MG, Losadh M (1980) Short term ammonium inhibition of nitrate utilization in Anacystis nidulans and other cyanobateria. ArchMicrobiol 128:137–140
Gim GH, Ryu J, Kim MJ, Kim PI, Kim SW(2016) Effects of carbon source and light intensity on the growth and total lipid production of three microalgae under different culture conditions. J Ind Microbiol Biotechnol 2016:1–12
González-Garcinuño Á, Tabernero A, Sánchez-Álvarez JM, Martin del Valle EM, Galán MA (2014) Effect of nitrogen source on growth and lipid accumulation in Scenedesmus abundans and Chlorella ellipsoidea. Bioresour Technol 173:334–341
Gouveia L, Oliveira AC (2009) Microalgae as a raw material for biofuels production. J Ind Microbiol Biotechnol 36:269–274
Guedes AC, Malcata FX (2012) Nutritional value and uses of microalgae in aquaculture. Aquaculture 10:60–78
Guillard RRL (2005) Purification methods for microalgae. In: Andersen RA (ed) Algal culturing techniques. Elsevier Academic Press, NY, pp 117–132
Hemaiswarya S, Raja R, Ravi Kumar R, Ganesan V, Anbazhagan C (2010) Microalgae: a sustainable feed source for aquaculture. World J Microbiol Biotechnol 27:1737–1746
Huang G, Chen F, Wei D, Zhang X, Chen G (2010) Biodiesel production by microalgal biotechnology. Appl Energy 87:38–46
Hulatt CJ, Lakaniemi AM, Puhakka JA, Thomas DN (2012) Energy demands of nitrogen supply in mass cultivation of two commercially important microalgal species, Chlorella vulgaris and Dunaliella tertiolecta. Bioenergy Res 5:669–684
Kawachi M, Noël MH (2005) Sterilization and sterile technique. In: Andersen RA (ed) Algal culturing techniques. Elsevier Academic Press, NY, pp 65–81
Komor E, Haass D, Komor B, Tanner W (1973) The active hexose-uptake system of Chlorella vulgaris. Km-values for 6-deoxyglucose influx and efflux and their contribution to sugar accumulation. Eur J Biochem 39:193–200
Li Y, Horsman M, Wang B, Wu N, Lan CQ (2008) Effects of nitrogen sources on cell growth and lipid accumulation of green alga Neochloris oleoabundans. Appl Microbiol Biotechnol 81:629–636
Li Y, Mu J, Chen D, Han F, Xu H, Kong F, Xie F, Feng B (2013) Production of biomass and lipid by the microalgae Chlorella protothecoides with heterotrophic-Cu(II) stressed (HCuS) coupling cultivation. Bioresour Technol 148:283–292
Liang Y, Sarkany N, Cui Y (2009) Biomass and lipid productivities of Chlorella vulgaris under autotrophic, heterotrophic and mixotrophic growth conditions. Biotechnol Lett 31:1043–1049
Lin Q, Lin J (2011) Effects of nitrogen source and concentration on biomass and oil production of a Scenedesmus rubescens like microalga. Bioresour Technol 102:1615–1621
Lin YH, Chang FL, Tsao CY, Leu JY (2007) Influence of growth phase and nutrient source on fatty acid composition of Isochrysis galbana CCMP 1324 in a batch photoreactor. Biochem Eng J 37:166–176
Liu J, Huang J, Fan KW, Jiang Y, Zhong Y, Sun Z, Chen F (2010) Production potential of Chlorella zofingienesis as a feedstock for biodiesel. Bioresour Technol 101:8658–8663
Liu J, Huang J, Sun Z, Zhong Y, Jiang Y, Chen F (2011) Differential lipid and fatty acid profiles of photoautotrophic and heterotrophic Chlorella zofingiensis: assessment of algal oils for biodiesel production. Bioresour Technol 102:106–110
Liu J, Sommerfeld M, Hu Q (2013) Screening and characterization of Isochrysis strains and optimization of culture conditions for docosahexaenoic acid production. Appl Microbiol Biotechnol 97:4785–4798
Liu J, Sun Z, Chen F (2014) Heterotrophic production of algal oils. In: Pandey A, Lee DJ, Chisti Y, Soccol CR (eds) Biofuels from algae. Elsevier, San Diego, pp 111–142
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Mansour MP, Frampton DMF, Nichols PD, Volkman JK, Blackburn SI (2005) Lipid and fatty acid yield of nine stationary phase microalgae: applications and unusual C24–C28 polyunsaturated fatty acids. J Appl Phycol 17:287–300
Meireless LA, Guedes AC, Malcata FX (2003) Lipid class composition of the microalga Pavlova lutheri: eicosapentaenoic and docosahexaenoic acid. J Agric Food Technol 51:2237–2241
Miao X, Wu Q (2006) Biodiesel production from heterotrophic microalgal oil. Bioresour Technol 97:841–846
Nalder TD, Miller MR, Packer MA (2015) Changes in lipid class content and composition of Isochrysis sp. (T-Iso) grown in batch culture. Aquac Int 23:1293–1312
Ogawa T, Aiba S (1981) Bioenergetic analysis of mixotrophic growth in Chlorella vulgaris and Scenedesmus acutus. Biotechnol Bioeng 23:1121–1132
Pahl SL, Lewis DM, Chen F, King KD (2010) Heterotrophic growth and nutritional aspects of the diatom Cyclotella cryptica (Bacillariophyceae): effect of some environmental factors. J Biosci Bioeng 109:235–239
Park DW, Jo Q, Lim HJ, Véron B (2002) Sterol composition of dark-grown Isochrysis galbana and its implication in the seed production of Pacific oyster, Crassostrea gigas. J Appl Phycol 14:351–355
Patil V, Reitan KI, Knudsen G, Mortensen L, Kallqvist T, Olsen E (2005) Microalgae as source of polyunsaturated fatty acids for aquaculture. Curr Top Plant Biol 6:57–65
Perez-Garcia O, Escalante FME, de-Bashan LE, Bashan Y (2011) Heterotrophic cultures of microalgae: metabolism and potential products. Water Res 45:11–36
Phatarpekar P, Sreepada R, Pednekar C, Achuthankutty C (2000) A comparative study on growth performance and biochemical composition of mixed culture of Isochrysis galbana and Chaetoceros calcitrans with monocultures. Aquaculture 181:141–155
Pruvost J, Legrand J, Legentilhomme P (2002) Simulation of microalgae growth in limiting light conditions: flow effect. AIChE J 48:1109–1120
Pulz O, Gross W (2004) Valuable products from biotechnology of microalgae. Appl Microbiol Biotechnol 65:635–648
Rao AR, Dayananda C, Sarada R, Shamala TR, Ravishankar GA (2007) Effect of salinity on growth of green alga Botryococcus braunii and its constituents. Bioresour Technol 98:560–564
Rashid N, Ur Rehman MS, Sadiq M, Mahmood T, Han J-I (2014) Current status, issues and developments in microalgae derived biodiesel production. Renew Sust Energy Rev 40:760–778
Rausch T (1981) The estimation of micro-algal protein content and its meaning to the evaluation of algal biomass I. Comparison of methods for extracting protein. Hydrobiologia 78:237–251
Renaud SM, Parry DL, Kuo C, Padovanla A, Sammy N (1991) Effect of light intensity on the proximate biochemical and fatty acid composition of Isochrysis sp. and Nannochloropsis oculata for use in tropical aquaculture. J Appl Phycol 3:43–53
Sakamoto T, Bryant DA (1998) Growth at low temperature causes nitrogen limitation in the cyanobacterium Synechococcus sp. PCC 7002. Arch Microbiol 169:10–19
Sánchez S, Martínez ME, Espinola F (2000) Biomass production and biochemical variability of the marine microalga Isochrysis galbana in relation to culture medium. Biochem Eng J 6:13–18
Sauer N, Tanner W (1989) The hexose carrier from Chlorella—cDNA cloning of a eukaryotic H+- cotransporter. FEBS Lett 259:43–46
Schmidt RA, Wiebe MG, Eriksen NT (2005) Heterotrophic high cell-density fed-batch cultures of the phycocyanin-producing red alga Galdieria sulphuraria. Biotechnol Bioeng 9077–9084
Shi XM, Liu HJ, Zhang XW, Chen F (1999) Production of biomass and lutein by Chlorella protothecoides at various glucose concentrations in heterotrophic cultures. Process Biochem 34:341–347
Spolaore P, Joannis-Cassan C, Duran E, Isambert A (2006) Commercial applications of microalgae. J Biosci Bioeng 101:87–96
Sun Y, Wang C (2009) The optimal growth conditions for the biomass production of Isochrysis galbana and the effects that phosphorus, Zn2+, CO2, and light intensity have on the biochemical composition of Isochrysis galbana and the activity of extracellular CA. Biotechnol Bioprocess Eng 14:225–231
Tan CK, Johns MR (1991) Fatty acid production by heterotrophic Chlorella saccharophila. Hydrobiologia 215:13–19
Tanner W (2000) The Chlorella hexose/H+-symporters. Int Rev Cytol 200:101–141
Walne PR (1970) Studies on the food value of nineteen genera of algae to juvenile bivalves of the genera Ostrea, Crassostrea, Mercenaria, and Mytilus. Fish Invest Lond 26:1–62
Wang Y, Chen T, Qin S (2012) Heterotrophic cultivation of Chlorella kessleri for fatty acids production by carbon and nitrogen supplements. Biomass Bioenergy 47:402–409
Wen Z, Chen F (2000) Heterotrophic production of eicosapentaenoid acid by the diatom Nitzschia laevis: effects of silicate and glucose. J Ind Microbiol Biotechnol 25:218–224
Wen ZY, Chen F (2001) Optimization of nitrogen sources for heterotrophic production of eicosapentaenoic acid by the diatom Nitzschia laevis. Enz Microb Technol 29:341–347
Wen ZY, Chen F (2003) Heterotrophic production of eicosapentaenoic acid by microalgae. Biotechnol Adv 21:273–294
Xiong W, Li X, Xiang J, Wu Q (2008) High-density fermentation of microalga Chlorella protothecoides in bioreactor for microbio-diesel production. Appl Microbiol Biotechnol 78:29–36
Xu N, Zhang X, Fan X, Han L, Zeng C (2001) Effects of nitrogen source and concentration on growth rate and fatty acid composition of Ellipsoidion sp. (Eustigmatophyta). J App Phycol 13:463–469
Zhu CJ, Lee YK, Chao TM (1997) Effects of temperature and growth phase on lipid and biochemical composition of Isochrysis galbana TK1. J Appl Phycol 9:451–457
Acknowledgements
This study was supported by the Exploratory Research Grant Scheme (203/PBIOLOGI/6730052) from the Ministry of Higher Education of Malaysia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mohammad Basri, E., Wan Maznah, W.O. Differential growth and biochemical composition of photoautotrophic and heterotrophic Isochrysis maritima: evaluation for use as aquaculture feed. J Appl Phycol 29, 1159–1170 (2017). https://doi.org/10.1007/s10811-017-1054-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-017-1054-1