Abstract
Esophageal squamous cell carcinoma (ESCC) is one of the most malignant tumors in east Asia. However, the molecular mechanism underlying its progression remains unclear. The ubiquitin–proteasome system (UPS) is a central mechanism for protein degradation and turnover. Accumulating evidence showed that more and more deubiquitinases could serve as attractive anti-cancer target. The expression of USP14 and UCH37 in esophagus squamous cell carcinoma tissues were examined by immunohistochemistry and western blot assays. Effect of b-AP15, a USP14 and UCH37 inhibitor, on ESCC cell growth was evaluated by cell viability assay. After cell lines being treated with b-AP15, cell cycle, apoptosis and the expression of related proteins were further explored to investigate the anti-ESCC mechanism of b-AP15. Results showed that deubiquitinating enzymes (DUBs) USP14 and UCH37 expressed at higher levels in ESCC tissues than in adjacent tissues. b-AP15 could inhibit cell proliferation and induce G2/M cell cycle arrest and apoptosis in ESCC cells. Mechanistically, b-AP15 treatment triggered Noxa-dependent apoptosis, which was regulated by c-Myc. Silencing Noxa and c-Myc could reduce b-AP15-induced apoptosis in ESCC cells. Our results revealed a novel mechanism of anti-tumor activity of b-AP15 in ESCC, and b-AP15 could be used as a potential therapeutic agent in ESCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Esophageal cancer is one of the malignant tumors in the digestive system [1, 2], and ranks the sixth most common cancer worldwide [2]. Esophageal squamous cell carcinoma (ESCC) is the major histological form of esophageal cancer in Asian countries, and it ranks the fourth most common cancers in China [3, 4]. The 5-year survival of esophageal cancer is still less than 20% mostly due to the late-stage-diagnosis [5, 6] and limited efficacy of traditionally comprehensive treatment, including surgical resection and standard first-line chemo-radiotherapeutic approaches [5, 7, 8]. These facts underscore the urgent need for early diagnosis and more effective therapeutic strategies, including target therapy and immuno-therapy.
Ubiquitin-specific peptidase 14 (USP14) and Ubiquitin C-terminal hydrolase 37 (UCH37, also known as UCHL5) belong to three proteasome-associated DUBs [9, 10], which bind to the 19S regulatory particle of 26S proteasome [9, 11] and remove ubiquitin from proteasomal substrates, thus regulating the substrates function and participating in various pathophysiology process, including oncogenesis [12]. Recent reports showed that ubiquitin-specific peptidase 14 (USP14) worked as an oncogene and overexpressed in several cancers [13,14,15,16], and it was positively correlated with poor prognosis [15, 17] and elevated cancer recurrence [18]. Genetic and pharmacological inactivation of USP14 could inhibit proliferation [19,20,21], induce cell cycle arrest [19, 21] and trigger apoptosis [15, 19] in cancer cells. Like USP14, UCH37 was also involved in tumor progression. UCH37/UCHL5 was firstly reported overexpressed in cervical carcinoma [22]. Subsequent studies showed that UCH37/UCHL5 expressed higher in ESCC [23], epithelial ovarian cancer [24] and hepatocellular carcinoma [25]. UCH37/UCHL5 could promote tumor progression by regulating several different signaling pathways [26, 27]. These studies suggest that USP14 and UCH37/UCHL5 can be used as potential therapeutic target for cancer.
Several inhibitors targeting DUBs have been discovered as potential therapy strategy in recent years [11, 28,29,30,31]. b-AP15 was first reported by D´Arcy et al. and described as an inhibitor of USP14 and UCH37/UCHL5 [32]. Researches have shown that b-AP15 was wildly used as anticancer reagent [33,34,35,36,37,38]. b-AP15 treatment inhibited tumor progression of colon cancer, Lewis lung carcinoma, breast cancer, prostate cancer and multiple myeloma and corresponding xenografts exhibited prolongs survival [33, 34]. However, the effect and mechanism of b-AP15 in ESCC remains unknown. Here, we used b-AP15 as treatment reagent in four human ESCC cell lines, including EC1, EC109, Kyse510 and Kyse450, to explore its anti-cancer function in ESCC and uncover underlying mechanisms.
Materials and methods
Cell lines and drug sources
Human ESCC cell lines EC1, EC109, Kyse510 and Kyse450 were cultured in DMEM medium (Biological Industries (BI), Biological Industries Israel Beit Haemek LTD., Israel) supplemented with 10% fetal bovine serum (Biological Industries (BI), Israel) at 37 °C in a 5% CO2 incubator. b-AP15 were purchased from MedChemExpress (MCE, Shanghai, China), dissolved in DMSO, and stored at − 80 °C. Diluted working solution was prepared freshly before each experiment.
Immunohistochemical staining
Human ESCC tissue array was purchased from Shanghai Outdo Biotech Co. Ltd (OD-CT-DgEso01-004). The expression of USP14 was detected using immunohistochemistry as previous described [39,40,41]. The expression of USP14 was detected by IHC staining with specific USP14 antibody (1:70, Cat. No. ab137433, Abcam Trading Company Ltd). Briefly, the tissue array was dehydrated, and peroxidase blocked. Antigen retrieval was undergone using 0.01 mol/L citric acid buffer (pH 6.0) and a pressure cooker. Primary antibodies were added and incubated at 4 °C overnight, followed by staining with a Histostain-Plus kits (SP-9000) and 3,3′-Diaminobenzidine tetrahydrochloride (DAB) (ZLI-9032) (ZSGB-BIO, Beijing, China). The slide was counterstained with hematoxylin. The stained slide was observed, and images were acquired by microscopy. Based on staining intensity, samples were classified into 4 groups, from the lowest intensity (–) to the highest (+++) [42]. Images were scanned by Motic digital slide scanning system (Motic (Xiamen) Electric Group Co., Ltd).
Collection of esophageal cancer tissues and detection of the expression of USP14 and UCH37 in tissues
Fresh primary esophageal cancer tissues and adjacent esophageal tissues were collected from 16 ESCC patients undergoing resection at the Linzhou Cancer Hospital (Linzhou, Henan, China, 2014). Histologic pathological diagnoses were determined in accordance with the American Joint Committee on Cancer manual criteria for esophageal cancer.
Tissues were grinded, and proteins were extracted using RIPA buffer (Cat. No. P0013B, Beyotime Institute of Biotechnology, Jiangsu, China). Protein concentration was determined using BCA kit (Cat. No. P0012, Beyotime, China). 50 μg proteins were loaded per lane and detected using USP14 antibody (1:1000, sc-398009, Santa Cruz Biotechnology, Santa Cruz, CA) and UCH37 antibody (1:5000, ab124931, Abcam Trading Company Ltd, Shanghai, China), respectively. GAPDH (1:1000, AB-P-R 001, Hangzhou Goodhere Biotechnology Co., LTD, Zhejiang, China) was used as loading control.
Cell proliferation and cell colony assays
For cell proliferation assay, EC1, EC109, Kyse510 and Kyse450 cells in log growth phase were seeded in 96-well plates and treated with indicated concentrations of b-AP15 (0.1, 0.2, 0.4, 0.6, 0.8, 1.0 μmol/L) for 72 h, followed by Cell Counting Kit-8 (CCK-8) assay according to the manufacturer’s instructions. CCK-8 reagents (KeyGEN BioTECH, Nanjing, China) were added to each well and the plates were further incubated for 1.5 h at 37 °C. Relative viability was quantified by measuring the absorbance at 450 nm using a microplate reader. Cells treated with DMSO (0.1%) were used as control.
For colony assays, cells were seeded in six-well plates (500 cells per well) in triplicate, treated with DMSO (0.1%) or b-AP15 (0.02, 0.05, 0.1 μmol/L) and cultured for 10 days. The colonies were fixed with 4% paraformaldehyde (Beyotime, China) and stained with crystal violet (Beyotime, China). Colonies comprising at least 50 cells were counted.
Cell cycle analysis
EC1 and Kyse450 cells were treated with DMSO (0.1%) or b-AP15 (0.2, 0.4, 0.6 μmol/L) for 24 h and fixed in 70% ethanol at − 20 °C overnight, then stained with a propidium iodide solution (50 μg/mL; Sigma, St. Louis, MO) containing RNase A (30 μg/mL; Sigma) at 37 °C for 30 min, analyzed for cell-cycle profile using BD Accuri™ C6 (BD Bioscience, USA). Data were analyzed with ModFit LT software (Verity Software House, Topsham, ME).
Detection of apoptosis and caspase3 activity
EC1 and Kyse450 cells were treated with DMSO (0.1%) or b-AP15 (0.4, 0.6, 0.8 μmol/L) for 72 h. Then apoptosis was detected by Annexin V-FITC/PI Apoptosis Detection Kit (KeyGEN BioTECH, Nanjing, China). AnnexinV+ cells were collected as apoptotic cells. Caspase-3 activity was measured by CaspGLOW™ Fluorescein Active Caspase-3 Staining Kit (BioVision, Inc. Milpitas, California) as described in the instruction. Apoptosis and caspase3 activity were analyzed using BD Accuri™ C6 (BD Bioscience, USA).
Mitochondrial membrane potentials assay
JC-1 (Yeasen Inc, Shanghai, China) was employed to measure mitochondrial depolarization in ESCC cells. Briefly, EC1 and Kyse450 cells were seeded into 60-mm dishes and exposed to DMSO (0.1%) or b-AP15 (0.4, 0.6, 0.8 μmol/L) for 48 h. Total cells were collected and incubated with JC-1 for 20 min at 37 °C followed by the procedure. The quantification of fluorescence was detected by flow cytometry (BD Accuri™ C6, BD Bioscience, USA). Cells with intact mitochondria displayed high red fluorescence and appeared in the upper right quadrant of the scatterplots. In contrast, cells that had lost mitochondrial membrane potential (MMP) displayed high green and low red fluorescence and appeared in the lower right quadrant.
Western blot
EC1 and Kyse450 cell lysates treated with DMSO or b-AP15 as indicated concentration were prepared for western blot analysis. 50 μg proteins were loaded per lane and detected using antibodies against p21 (Cat. No. 2947), p27 (Cat. No. 3686), Wee1 (Cat. No. 4936), pWee1 (Cat. No. 4910), pH3 (Cat. No. 3377), cleaved PARP (Cat. No. 5625), cleaved Caspase 3 (Cat. No. 9664), cleaved Caspase 9 (Cat. No.7237), Bax (Cat. No. 5023), Bak (Cat. No. 12105), Bid (Cat. No. 2002), Bim (Cat. No. 2933), c-IAP1 (Cat. No. 7065), Bcl-xl (Cat. No. 2764), Mcl-1 (Cat. No. 5453), XIAP (Cat. No. 2045), Survivin (Cat. No. 2808), ATF4 (Cat. No. 11815), CHOP (Cat. No. 5554) (all diluted into 1:1000, Cell Signaling Technology, Inc., Boston, MA, USA), Noxa (1:1000, Cat. No. OP180, Millipore, Billerica, MA), c-Myc (1:1000, Cat. No. sc-40, Santa Cruz Biotechnology, Santa Cruz, CA), FOXO3a (1:2000, Cat. No. ab53287, Abcam Trading Company Ltd). GAPDH (1:1000, AB-P-R 001, Hangzhou Goodhere Biotechnology Co., LTD, Zhejiang, China) was used as loading control. Secondary antibodies, peroxidase-conjugated goat anti-mouse IgG (Cat. No. ZB.2305) and peroxidase-conjugated goat anti-rabbit IgG (Cat. No. ZB.2301) were purchased from ZGSB.Bio, Inc., (Beijing, China). The membrane was incubated with corresponding secondary antibodies (1:3000) for 2 h at room temperature and detected using an ECL Kit (Cat. No. P0018, Beyotime, China).
Real-time polymerase chain reaction
EC1 and Kyse450 cells were treated with DMSO (0.1%) or b-AP15 (0.6 μmol/L) for 0 h, 12 h, 24 h, and 48 h. Total RNA was isolated using the Trizol reagent and Ultrapure RNA kit (CW Biotech, China) according to the manufacturer’s instructions. RNA (1.0 μg) was purified and reversely transcribed by PrimeScript™ RT reagent Kit with gDNA Eraser (Cat. No. RR047A, Takara, Dalian, China) following the manufacturer’s instructions. The cDNA was quantified by real-time quantitative PCR using QuantiNova™ SYBR Green PCR Kit (Cat. No. 208054, QIAGEN, Germany) and a Real-time PCR system (Applied Biosystems, Foster City, Calif.). For each sample, the mRNA abundance was normalized to the amount of GAPDH. Primers are as follows:
Noxa: forward, 5′- GGAGATGCCTGGGAAGA-3′,
Noxa: reverse, 5′-TTCTGCCGGAAGTTCAGT-3′;
GAPDH: forward, 5′-AAAGGGTCATCATCTCTG-3′,
GAPDH: reverse, 5′-GCTGTTGTCATACTTCTC-3′.
Gene silencing using small interfering RNA (siRNA)
EC1 and Kyse450 cells were transfected using Lipofectamine 2000 (Invitrogen, life technologies) with siRNA mixture targeting Noxa or c-Myc for 48 h and further treated with b-AP15 (0.8 μmol/L) for another 48 h. Then cells were collected for apoptosis and western blot assays. The sequences of the siRNA are as follows:
siNoxa#1: GUAAUUAUUGACACAUUUC
siNoxa#2: GGUGCACGUUUCAUCAAUUUG
sic-Myc#1: AACGUUAGCUUCACCAACA
sic-Myc#2: CGAGCUAAAACGGAGCUUU
siControl: UUCUCCGAACGUGUCACGU
Statistical analysis
The expression of USP14 in IHC assay was analyzed by Mann–Whitney Test using SPSS software. All other statistical tests were performed in GraphPad Prism 5 software. P values less than 0.05 were considered statistically different. *means P < 0.05, **means P < 0.01.
Results
USP14 and UCH37 overexpressed in ESCC tissues
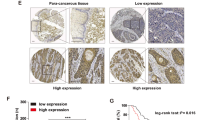
To investigate whether USP14 or UCH37 could be used as anti-cancer target in ESCC, we first examined their expression in tissue microarray with ESCC and adjacent tissues. Results from Immunohistochemistry (IHC) assay showed that the expression of USP14 expressed higher in ESCC tissues than that in adjacent normal tissues (Fig. 1a, b). The expression of UCH37 ESCC and adjacent normal tissues have already been examined by Chen et al. and the results showed that UCH37 also expressed higher in ESCC tissues than that in adjacent normal tissues [23]. Results from western blot assay from 16 paired ESCC and adjacent normal tissues further confirmed these results (P < 0.01) (Fig. 1c, d).
Overexpression of USP14 and UCH37 in ESCC tissues. a Analyze the expression of USP14 by IHC staining of human ESCC tissues array. Samples were divided into four groups according to staining intensity from the weakest (–, group 1) to the strongest (+++, group 4). b Classification and statistical analysis of IHC staining intensity by Mann–Whitney Test (P < 0.01). c Expression of USP14 and UCH37 in 16 pairs of ESCC tissues and corresponding adjacent esophageal tissues by western blot analysis. d Quantification of proteins expression of western blot. The results of a total of 16 pairs of tissues (tumor vs. adjacent normal tissues) were analyzed (P < 0.01). Horizontal lines indicated means ± standard deviations (Error bar = S.D.). A adjacent tissues; T tumor tissues
b-AP15 inhibited the proliferation of ESCC cells
Cell proliferation assay and colony formation assay were used to explore the anti-cancer effect of b-AP15 in ESCC cells. Results showed that ESCC cell growth was inhibited (Fig. 2a) and colony formation ability was compromised after b-AP15 treatment (Fig. 2b). Since similar growth inhibition effect of b-AP15 were shown on Kyse450, Kyse510, EC1 and EC109, we selected Kyse450 and EC1 cell lines in the following study.
b-AP15 inhibits growth and colony formation of esophageal cancer cells. a Effect of b-AP15 on the viability of ESCC cells Kyse450, Kyse510, EC1 and EC109. Cells were treated with DMSO (0.1%) or b-AP15 (0.1, 0.2, 0.4, 0.6, 0.8, 1.0 μmol/L) for 72 h and viability was detected with the CCK-8 kit. b Efficacy of b-AP15 on colony formation of ESCC cells. ESCC cells were treated with b-AP15 at different concentrations for 10 days, and then fixed, stained and counted as described in Materials and Methods
b-AP15 induced G2/M cell cycle arrest
To investigate how b-AP15-induced ESCC cell growth inhibition, we first examined the effect of b-AP15 on cell cycle. Flow cytometry analysis showed that after being treated with b-AP15 for 24 h, EC1 and Kyse450 cells cycles were arrested in G2/M phase (Fig. 3a, b). Expression of cell cycle-related proteins was further examined by western blot assay. The results revealed that b-AP15 treatment increased the expression of G2/M phase related proteins p21, p27 and pWee1 (Fig. 3c). Meanwhile, the M phase marker protein p-H3 [43] also increased in a concentration-dependent manner (Fig. 3c), indicating that b-AP15-treated-cells were arrested at the mitosis phase.
b-AP15 triggers G2/M cell cycle arrest in esophageal cancer cells. a–b b-AP15 induced G2/M cell cycle arrest. EC1 and Kyse450 cells were treated with b-AP15 at different concentration for 24 h, followed by PI staining and FACS analysis for cell cycle profile (upper panel). Distribution was analyzed by Modifit and Graphpad software (lower panel). c Effect of b-AP15 on the expression of cell-cycle-related proteins. EC1 and Kyse450 cells were treated with b-AP15 at indicated concentrations and subjected to western blot subsequently. GAPDH was used as loading control
b-AP15 triggered mitochondrial apoptosis
ESCC cells that being treated with b-AP15 shrank and turned round (data not shown), implying that b-AP15 treatment could trigger apoptosis. This hypothesis was confirmed by Annexin V-FITC/PI assay and caspase3 activity assay, which showed that b-AP15 treatment led to increased Annexin V+ cells (Fig. 4a) and capspase3 activity (Fig. 4b) in a dose-dependent manner. In accordance with the FACS results, b-AP15 treatment also increased the cleavage of caspase9, caspase3 and poly ADP-ribose polymerase (PARP) (Fig. 4c). Meanwhile, b-AP15 induced the loss of mitochondrial membrane potential (ΔΨm), a classical marker of the activation of intrinsic apoptosis (Fig. 5a). These results suggested that b-AP15 could trigger mitochondrial apoptosis in ESCC cells.
b-AP15 induces apoptosis. EC1 and Kyse450 cells were treated with b-AP15 for 72 h. a Apoptosis was determined by FACS analysis using Annexin V-FITC/PI double-staining kit and Annexin V+ cell populations were defined as apoptosis. b CASP3 activity was also analyzed with FACS. All data were representative of at least three independent experiments. c Treatment with b-AP15 increased the expression of cleaved CASP9, CASP3, and PARP. EC1 and Kyse450 were treated with b-AP15 for 72 h and cell lysates were assessed by western blot with primary antibodies against cleaved CASP9, CASP3, or PARP. GAPDH was used as loading control
Effect of b-AP15 on the expression of pro-apoptotic and anti-apoptotic proteins. a b-AP15 induced mitochondrial membrane depolarization. EC1 and Kyse450 cells were treated with b-AP15 as indicated. Cells with intact mitochondria displayed high red fluorescence and appeared in the upper right quadrant of the scatterplots. In contrast, cells that had lost mitochondrial membrane potential (MMP) displayed high green and low red fluorescence and appeared in the lower right quadrant (left panel). Percentage of cells that had lost mitochondrial membrane potential is shown in the right panel. b Effect of b-AP15 on the expression of pro-apoptotic and anti-apoptotic proteins. Cells were treated with b-AP15 with the indicated concentrations for 72 h. Cell extracts were prepared for western blot analysis. GAPDH was used as a loading control
Noxa was transactivated by c-Myc and plays an important role in b-AP15 induced apoptosis
b-AP15 induced the loss of mitochondrial membrane potential (Fig. 5a), implying that b-AP15 induced intrinsic apoptosis. So we examined the effect of b-AP15 treatment on the expression of anti-apoptotic and pro-apoptotic proteins. Results showed that Noxa was upregulated in both EC1 and Kyse450 cells, while other proteins slightly changed (Fig. 5b). Furthermore, we found that the expression of Noxa was upregulated in both mRNA and protein levels in a time-course dependent manner (Fig. 6a). Silencing Noxa rescued b-AP15-induced apoptosis (Fig. 6d). The expression of Noxa-related transcription factor was also investigated. Results showed that polyubiquitin was accumulated after treated with b-AP15 in a concentration-dependent manner (Fig. 6c). Meanwhile, except for up-regulated c-Myc, all the other transcription factors slightly changed (Fig. 6b). Knock-down of c-Myc reduced b-AP15-induced apoptosis (Fig. 6d), down-regulated the expression of Noxa (Fig. 6d) and the cleavage of PARP (Fig. 6d) in both EC1 and Kyse450 cells. These observations indicated that c-Myc-mediated up-regulation of Noxa play a major role in b-AP15 induced apoptosis in ESCC cell lines.
Noxa is transactivated by c-Myc and participates in b-AP15-induced apoptosis in ESCC cells. a b-AP15 increased both the mRNA and protein level of Noxa. Cells were treated with b-AP15 (0.6 μmol/L) and collected at the indicated time points. Total RNAs were isolated. qPCR analysis was used to analyze the mRNA level of Noxa (normalized to GAPDH) (top panel). Cell extracts were prepared for western blot analysis of Noxa (bottom panel). b Screen of Noxa-related transcription factors. EC1 and Kyse450 cells were treated with b-AP15 for 72 h and cell lysates were assessed by western blot with specific antibodies. GAPDH was used as a control. c b-AP15 treatment accumulated polyubiquitin in a concentration-dependent manner. EC1 and Kyse450 cells were treated with b-AP15 for 72 h and cell lysates were assessed by western blot with specific antibodies. GAPDH was used as a control. d The expression of c-Myc was responsible for b-AP15-induced apoptosis in ESCC cells. After transfected with the control siRNA, Noxa siRNA mixture or c-Myc siRNA mixture for 48 h, EC1 and Kyse450 cells were further treated with b-AP15 (0.8 μmol/L) for 48 h. Apoptosis was examined by Annexin V-FITC/PI double-staining analysis (top panel). Knockdown efficiency, expression of Noxa and cleaved PARP were assessed by western blot analysis. GAPDH was used as loading control (bottom panel)
Discussion
DUBs have been reported to contribute to tumorigenesis by regulating several different pathways [12]. Targeting DUBs play important and therapeutically exploitable roles in the treatment of several different types of cancers [9, 42, 44, 45]. USP14 and UCH37/UCHL5 -are reported to be over-expressed in several cancers [13,14,15,16,17,18, 22] and DUB inhibitor b-AP15 can selectively induce cell growth inhibition in several different types of tumor cell lines, including colon carcinoma, prostate cancer, leukemia, mantle cell lymphoma (MCL) and multiple myeloma model (MM) [33,34,35,36,37,38]. However, whether b-AP15 has similar effects in ESCC cell lines and the underlying mechanisms remains uninvestigated. Here, we examined the expression of USP14 and UCH37 in ESCC and found that they were both overexpressed in ESCC tissues, which were consistent with most recent reports [17]. Moreover, inactivation of both USP14 and UCH37 with b-AP15 induced effective cell growth inhibition in four different ESCC cell lines. These results indicate that USP14 and UCH37 can serve as anti-ESCC target and b-AP15 have potential to be used as anti-ESCC therapeutics.
b-AP15 was first identified by D´Arcy et al. as an inhibitor for USP14 and UCH37/UCHL5, which could block the deubiquitylating activity of both deubiquitinates without affecting proteolytic activities of the 20S core particle [32]. Reports showed that b-AP15 could effectively inhibit tumor cell growth and metastasis by interfering different pathway. Several studies have shown that b-AP15 induced cell cycle arrest in a tumor-specific manner, while others have shown that b-AP15 triggered apoptosis or regulated proliferation signaling pathway of cancer cells. However, the mechanisms by which b-AP15 inhibit ESCC cell growth are largely unknown.
Recent reports showed that DUBs worked as an integral component of the core cell cycle machinery and cell cycle checkpoints. They regulated cell-cycle-related proteins, such as cyclins, cyclin-dependent kinases and checkpoint proteins, which play an important role by their mis-regulation in cancer. b-AP15 treatment increased the number of hypodiploid cells and induced G2/M arrest in multiple myeloma cell lines [33] and colon cancer cell line HCT116. Mechanistically, b-AP15 treatment could decrease the expression of G2/M phase cell cycle regulatory proteins cdc25c, cdc2, and cyclin B1 [33] and upregulate p21 and p27 [33]. Being consistent with these results, our results found that b-AP15 treatment could lead to G2/M cell cycle arrest by increasing the expression of p21, p27 and pWee1, meanwhile, the M phase marker protein p-H3 [43] also increased in a concentration-dependent manner (Fig. 3c), indicating that b-AP15-treated esophageal cells were arrested at the mitosis phase. However, Cai and his co-workers found that b-AP15 dramatically induced G0/G1 cell cycle arrest in both AR-responsive and AR-irresponsive prostate cancer cells by significantly decreasing the expression of cyclin D1, CDK6, CDK4, and phospho-Rb, while increasing the expression p27 [34]. These results imply that b-AP15 treatment might induce cell cycle arrest in a cell-specific manner.
It was previously reported that apoptosis was an important anti-tumor mechanism employed by b-AP15 [33,34,35, 46]. b-AP15 could enhance DR5 activation-induced apoptosis through stabilizing DR5 [35]. Besides, b-AP15 exhibited cytotoxicity to both androgen receptor-dependent and -independent prostate cancer cells by increasing reactive oxygen species (ROS) generation, endoplasmic reticulum (ER) stress and triggered apoptosis [34]. In view of recent studies showing that b-AP15 induced apoptosis in triple negative breast cancer, natural killer cells and multiple myeloma through mechanisms distinct from extrinsic apoptosis and unfolded protein response [38, 47, 48]. Several reports showed that besides death-reporter pathway, b-AP15 or its analogue could induce mitochondrial apoptotic pathway in different manners [32, 49, 50]. D’Arcy et al. and Brnjic et al. reported that b-AP15 induced BAX and BAK independent apoptosis [32, 50]. b-AP15 and its analogue F6 induces apoptosis in BCL2 overexpressing cells [32, 49]. F6 induced mitochondrial apoptotic pathway accompanied by the upregulation of Noxa [49], which is consistent with our results. Our findings showed that b-AP15 treatment upregulated the expression of Noxa in a time- and concentration-dependent manner. Silencing Noxa rescued the b-AP15 induced apoptosis. Due to previous reports that c-Myc was a prime transcription factors to transactivate Noxa [51, 52], we hypothesized that the accumulation of Noxa would be related to a function of c-Myc after b-AP15 treatment. Our studies confirmed that b-AP15 treatment induced accumulation of c-Myc in EC1 and Kyse450 cells. Knockdown of c-Myc reduced the accumulation of Noxa protein. These results indicated that b-AP15-induced apoptosis was in a tumor-specific manner.
Conclusion
In summary, our studies have identified a novel mechanism of the deubiquitinase inhibitor b-AP15-induced apoptosis in ESCC. This compound requires c-Myc to increase Noxa expression and trigger the mitochondrial apoptotic pathway, which might give an insight into the anti-tumor effect and mechanism of b-AP15 in ESCC.
Data availability
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Abbreviations
- ESCC:
-
Esophageal squamous cell cancer
- UPS:
-
Ubiquitin–proteasome system
- DUBs:
-
Deubiquitinating enzymes
- USP14:
-
Ubiquitin-specific peptidase 14
- UCH37:
-
Ubiquitin C-terminal hydrolase 37
- IHC:
-
Immunohistochemistry
- DAB:
-
3,3′-Diaminobenzidine tetrahydrochloride
- ROS:
-
Reactive oxygen species
- ER:
-
Endoplasmic reticulum
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A (2015) Global cancer statistics, 2012. CA Cancer J Clin 65:87–108
Sohda M, Kuwano H (2017) Current status and future prospects for esophageal cancer treatment. Ann Thorac Cardiovasc Surg 23:1–11
Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J (2016) Cancer statistics in China, 2015. CA 66:115–132
Chen W, Sun K, Zheng R, Zeng H, Zhang S, Xia C, Yang Z, Li H, Zou X, He J (2018) Cancer incidence and mortality in China, 2014. Chin J Cancer Res 30:1–12
Belkhiri A, El-Rifai W (2015) Advances in targeted therapies and new promising targets in esophageal cancer. Oncotarget 6:1348–1358
Codipilly DC, Qin Y, Dawsey SM, Kisiel J, Topazian M, Ahlquist D, Iyer PG (2018) Screening for esophageal squamous cell carcinoma: recent advances. Gastrointest Endosc 88:413–426
Rustgi AK, El-Serag HB (2014) Esophageal carcinoma. N Engl J Med 371:2499–2509
Hall TM, Tetreault MP, Hamilton KE, Whelan KA (2018) Autophagy as a cytoprotective mechanism in esophageal squamous cell carcinoma. Curr Opin Pharmacol 41:12–19
Selvaraju K, Mazurkiewicz M, Wang X, Gullbo J, Linder S, D’Arcy P (2015) Inhibition of proteasome deubiquitinase activity: a strategy to overcome resistance to conventional proteasome inhibitors? Drug Resist Updates 21–22:20–29
de Poot SAH, Tian G, Finley D (2017) Meddling with fate: the proteasomal deubiquitinating enzymes. J Mol Biol 429:3525–3545
D’Arcy P, Wang X, Linder S (2015) Deubiquitinase inhibition as a cancer therapeutic strategy. Pharmacol Ther 147:32–54
Chen YJ, Wu H, Shen XZ (2015) The ubiquitin-proteasome system and its potential application in hepatocellular carcinoma therapy. Cancer Lett 379:245–252
Wang Y, Wang J, Zhong J, Deng Y, Xi Q, He S, Yang S, Jiang L, Huang M, Tang C, Liu R (2015) Ubiquitin-specific protease 14 (USP14) regulates cellular proliferation and apoptosis in epithelial ovarian cancer. Med Oncol 32:379
Zhu L, Yang S, He S, Qiang F, Cai J, Liu R, Gu C, Guo Z, Wang C, Zhang W et al (2016) Downregulation of ubiquitin-specific protease 14 (USP14) inhibits breast cancer cell proliferation and metastasis, but promotes apoptosis. J Mol Histol 47:69–80
Zhu Y, Zhang C, Gu C, Li Q, Wu N (2016) Function of deubiquitinating enzyme USP14 as oncogene in different types of cancer. Cell Physiol Biochem 38:993–1002
Chen X, Wu J, Chen Y, Ye D, Lei H, Xu H, Yang L, Wu Y, Gu W (2016) Ubiquitin-specific protease 14 regulates cell proliferation and apoptosis in oral squamous cell carcinoma. Int J Biochem Cell Biol 79:350–359
Zhang B, Li M, Huang P, Guan XY, Zhu YH (2017) Overexpression of ubiquitin specific peptidase 14 predicts unfavorable prognosis in esophageal squamous cell carcinoma. Thorac Cancer 8:344–349
Vogel RI, Pulver T, Heilmann W, Mooneyham A, Mullany S, Zhao X, Shahi M, Richter J, Klein M, Chen L et al (2016) USP14 is a predictor of recurrence in endometrial cancer and a molecular target for endometrial cancer treatment. Oncotarget 7:30962–30976
Liao Y, Xia X, Liu N, Cai J, Guo Z, Li Y, Jiang L, Dou QP, Tang D, Huang H, Liu J (2018) Growth arrest and apoptosis induction in androgen receptor-positive human breast cancer cells by inhibition of USP14-mediated androgen receptor deubiquitination. Oncogene 37:1896–1910
Zhang J, Zhang D, Sun L (2017) Knockdown of ubiquitin-specific protease 14 (USP14) inhibits the proliferation and tumorigenesis in esophageal squamous cell carcinoma cells. Oncol Res 25:249–257
Liao Y, Liu N, Hua X, Cai J, Xia X, Wang X, Huang H, Liu J (2017) Proteasome-associated deubiquitinase ubiquitin-specific protease 14 regulates prostate cancer proliferation by deubiquitinating and stabilizing androgen receptor. Cell Death Dis 8:e2585
Fang Y, Shen X (2017) Ubiquitin carboxyl-terminal hydrolases: involvement in cancer progression and clinical implications. Cancer Metastasis Rev 36:669–682
Chen Y, Fu D, Xi J, Ji Z, Liu T, Ma Y, Zhao Y, Dong L, Wang Q, Shen X (2012) Expression and clinical significance of UCH37 in human esophageal squamous cell carcinoma. Dig Dis Sci 57:2310–2317
Wang L, Chen YJ, Xu K, Wang YY, Shen XZ, Tu RQ (2014) High expression of UCH37 is significantly associated with poor prognosis in human epithelial ovarian cancer. Tumour Biol 35:11427–11433
Fang Y, Fu D, Tang W, Cai Y, Ma D, Wang H, Xue R, Liu T, Huang X, Dong L et al (2013) Ubiquitin C-terminal hydrolase 37, a novel predictor for hepatocellular carcinoma recurrence, promotes cell migration and invasion via interacting and deubiquitinating PRP19. Biochim Biophys Acta 1833:559–572
Zhou Z, Yao X, Pang S, Chen P, Jiang W, Shan Z, Zhang Q (2018) The deubiquitinase UCHL5/UCH37 positively regulates Hedgehog signaling by deubiquitinating Smoothened. J Mol Cell Biol 10:243–257
Randles L, Anchoori RK, Roden RB, Walters KJ (2016) The Proteasome ubiquitin receptor hRpn13 and its interacting deubiquitinating enzyme Uch37 are required for proper cell cycle progression. J Biol Chem 291:8773–8783
D’Arcy P, Linder S (2014) Molecular pathways: translational potential of deubiquitinases as drug targets. Clin Cancer Res 20:3908–3914
Popovic D, Vucic D, Dikic I (2014) Ubiquitination in disease pathogenesis and treatment. Nat Med 20:1242–1253
Pal A, Young MA, Donato NJ (2014) Emerging potential of therapeutic targeting of ubiquitin-specific proteases in the treatment of cancer. Cancer Res 74:4955–4966
Crosas B (2014) Deubiquitinating enzyme inhibitors and their potential in cancer therapy. Curr Cancer Drug Targets 14:506–516
D’Arcy P, Brnjic S, Olofsson MH, Fryknäs M, Lindsten K, De Cesare M, Perego P, Sadeghi B, Hassan M, Larsson R, Linder S (2011) Inhibition of proteasome deubiquitinating activity as a new cancer therapy. Nat Med 17:1636–1640
Tian Z, D’Arcy P, Wang X, Ray A, Tai YT, Hu Y, Carrasco RD, Richardson P, Linder S, Chauhan D, Anderson KC (2014) A novel small molecule inhibitor of deubiquitylating enzyme USP14 and UCHL5 induces apoptosis in multiple myeloma and overcomes bortezomib resistance. Blood 123:706–716
Cai J, Xia X, Liao Y, Liu N, Guo Z, Chen J, Yang L, Long H, Yang Q, Zhang X et al (2017) A novel deubiquitinase inhibitor b-AP15 triggers apoptosis in both androgen receptor-dependent and -independent prostate cancers. Oncotarget 8:63232–63246
Oh YT, Deng L, Deng J, Sun SY (2017) The proteasome deubiquitinase inhibitor b-AP15 enhances DR5 activation-induced apoptosis through stabilizing DR5. Sci Rep 7:8027
Kropp KN, Maurer S, Rothfelder K, Schmied BJ, Clar KL, Schmidt M, Strunz B, Kopp HG, Steinle A, Grunebach F et al (2018) The novel deubiquitinase inhibitor b-AP15 induces direct and NK cell-mediated antitumor effects in human mantle cell lymphoma. Cancer Immunol Immunother 67:935–947
Sarhan D, D’Arcy P, Lundqvist A (2014) Regulation of TRAIL-receptor expression by the ubiquitin-proteasome system. Int J Mol Sci 15:18557–18573
Sarhan D, Wennerberg E, D’Arcy P, Gurajada D, Linder S, Lundqvist A (2013) A novel inhibitor of proteasome deubiquitinating activity renders tumor cells sensitive to TRAIL-mediated apoptosis by natural killer cells and T cells. Cancer Immunol Immunother 62:1359–1368
Chen P, Hu T, Liang Y, Li P, Chen X, Zhang J, Ma Y, Hao Q, Wang J, Zhang P et al (2016) Neddylation inhibition activates the extrinsic apoptosis pathway through ATF4-CHOP-DR5 axis in human esophageal cancer cells. Clin Cancer Res 22:4145–4157
Hao Q, Zhao X, Zhang Y, Dong Z, Hu T, Chen P (2017) Targeting overexpressed activating transcription factor 1 (ATF1) inhibits proliferation and migration and enhances sensitivity to paclitaxel in esophageal cancer cells. Med Sci Monit Basic Res 23:304–312
Chen P, Li M, Hao Q, Zhao X, Hu T (2018) Targeting the overexpressed CREB inhibits esophageal squamous cell carcinoma cell growth. Oncol Rep 39:1369–1377
Hu T, Zhang J, Sha B, Li M, Wang L, Zhang Y, Liu X, Dong Z, Liu Z, Li P, Chen P (2018) Targeting the overexpressed USP7 inhibits esophageal squamous cell carcinoma cell growth by inducing NOXA-mediated apoptosis. Mol Carcinog 58(1):42–54
Lin S, Shang Z, Li S, Gao P, Zhang Y, Hou S, Qin P, Dong Z, Hu T, Chen P (2018) Neddylation inhibitor MLN4924 induces G2 cell cycle arrest, DNA damage and sensitizes esophageal squamous cell carcinoma cells to cisplatin. Oncol Lett 15:2583–2589
Yeasmin Khusbu F, Chen FZ, Chen HC (2018) Targeting ubiquitin specific protease 7 in cancer: a deubiquitinase with great prospects. Cell Biochem Funct 36:244–254
Jin WL, Mao XY, Qiu GZ (2017) Targeting deubiquitinating enzymes in glioblastoma multiforme: expectations and challenges. Med Res Rev 37:627–661
Ding Y, Chen X, Wang B, Yu B, Ge J (2018) Deubiquitinase inhibitor b-AP15 activates endoplasmic reticulum (ER) stress and inhibits Wnt/Notch1 signaling pathway leading to the reduction of cell survival in hepatocellular carcinoma cells. Eur J Pharmacol 825:10–18
Feng X, Holmlund T, Zheng C, Fadeel B (2014) Proapoptotic effects of the novel proteasome inhibitor b-AP15 on multiple myeloma cells and natural killer cells. Exp Hematol 42:172–182
Vogel RI, Coughlin K, Scotti A, Iizuka Y, Anchoori R, Roden RB, Marastoni M, Bazzaro M (2015) Simultaneous inhibition of deubiquitinating enzymes (DUBs) and autophagy synergistically kills breast cancer cells. Oncotarget 6:4159–4170
Aleo E, Henderson CJ, Fontanini A, Solazzo B, Brancolini C (2006) Identification of new compounds that trigger apoptosome-independent caspase activation and apoptosis. Cancer Res 66:9235–9244
Brnjic S, Mazurkiewicz M, Fryknäs M, Sun C, Zhang X, Larsson R, D’Arcy P, Linder S (2014) Induction of tumor cell apoptosis by a proteasome deubiquitinase inhibitor is associated with oxidative stress. Antioxid Redox Signal 21:2271–2285
Knorr KLB, Schneider PA, Meng XW, Dai H, Smith BD, Hess AD, Karp JE, Kaufmann SH (2015) MLN4924 induces Noxa upregulation in acute myelogenous leukemia and synergizes with Bcl-2 inhibitors. Cell Death Differ 22:2133–2142
Wirth M, Stojanovic N, Christian J, Paul MC, Stauber RH, Schmid RM, Hacker G, Kramer OH, Saur D, Schneider G (2014) MYC and EGR1 synergize to trigger tumor cell death by controlling NOXA and BIM transcription upon treatment with the proteasome inhibitor bortezomib. Nucleic Acids Res 42:10433–10447
Acknowledgements
And the authors would also like to thank those who contributed their tissue samples to support scientific research in medical fields.
Funding
This research was funded by the National Natural Science Foundation Grant of China (Grant Nos. 81001102, 81101894, 81672421, U1604189), Natural Science Foundation of Henan Province (Grant No. 162300410302), Outstanding Young Talent Research Fund of Zhengzhou University (Grant Nos. 51999223, 32210449) and Program for Science &Technology Innovation Talents in Universities of Henan Province (Grant No. 18HASTIT046).
Author information
Authors and Affiliations
Contributions
Conceptualization, Tao Hu and Pei Li; Methodology, Beibei Sha, Xiaoyu Chen, Miaomiao Li and Longhao Wang; Validation, Beibei Sha, Xiaoyu Chen, Miaomiao Li, Longhao Wang and Han Wu; Results interpretation, Beibei Sha, Xiaoyu Chen, Miaomiao Li and Xingge Liu; Manuscript preparation: Ping Chen and Jianxiang Shi; Supervision, Tao Hu and Pei Li; Project administration, Tao Hu and Pei Li; Funding acquisition, Ping Chen, Tao Hu and Pei Li.
Corresponding authors
Ethics declarations
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interests.
Ethical approval
All experiments were approved by the Institutional Review Board of Zhengzhou University.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10495_2019_1561_MOESM1_ESM.pdf
Supplementary material 1 (PDF 1176 kb) Supplementary Figure. The effect of c-Myc on the accumulation of polyubiquitin. After transfected with the control siRNA, or c-Myc siRNA for 96 h, cell proteins were collected and knockdown efficiency and the effect of c-Myc on the accumulation of polyubiquitin were assessed by western blot analysis. GAPDH was used as loading control
Rights and permissions
About this article
Cite this article
Sha, B., Chen, X., Wu, H. et al. Deubiquitylatinase inhibitor b-AP15 induces c-Myc-Noxa-mediated apoptosis in esophageal squamous cell carcinoma. Apoptosis 24, 826–836 (2019). https://doi.org/10.1007/s10495-019-01561-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-019-01561-9