Abstract
Background
Ubiquitin carboxyl-terminal hydrolase 37 (UCH37), a member of the DUBs, was found to play an important role in oncogenesis through promoting some Proto-oncogenes’ expression and stem cell-like characteristics in the cell in previous research. The aim of this study was to assess the value of UCH37 in predicting tumor recurrence after curative resection in esophageal squamous cell carcinoma (ESCC) patients.
Methods
We analyzed UCH37 protein expression in 111 clinicopathologically characterized ESCC cases, from those who underwent curative resection between 2007 and 2008, by immunohistochemistry. The prognostic significance was assessed using Kaplan–Meier survival estimates and log-rank tests.
Results
We found that UCH37 expression was higher in the cancer tissue than in non-tumorous control tissue at protein level and was overexpressed in tumor tissues of recurrent patients. There was a significant difference of UCH37 expression in patients categorized according to TNM stage (p = 0.038) and lymph nodes metastasis condition (p = 0.009). Univariate analyses revealed that UCH37 was a significant predictor for overall survival and disease-free survival, and multivariate analyses showed that UCH37 was an independent prognostic marker for ESCC recurrence. A prognostic significance of UCH37 was also found in the subgroup of lymph nodes metastasis condition classification. About 90 % of the recurrent patients recurred within 2 years, of which 84.4 % were predicted by UCH37.
Conclusion
UCH37 is associated with outcome and recurrence of ESCC and can be a novel predictor for poor prognosis of ESCC patients after curative resection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Esophageal cancer is the eighth most common cancer worldwide [1]. It is epidemic in many parts of the world, particularly in the developing nations like China [2]. Esophageal squamous cell carcinoma (ESCC) is the major histological type of esophageal cancer and is more common in men [3]. Despite several advances which have been made in staging procedures and therapeutic approaches, the prognosis of ESCC still remains dismal because of its high recurrence and metastasis. Molecular signatures that define the risk of recurrence and metastatic potential of ESCC are crucial but difficult to identify. Such markers would allow appropriate therapeutic regimens to be applied earlier in the disease course. Although several prognostic biomarkers in ESCC have been reported recently [4], there still remains a lack of ideal biomarkers available that can be widely used in clinical settings.
Ubiquitin (Ub), a 76-amino-acid polypeptide, can be covalently conjugated to protein substrates forming an isopeptide bond between the carboxy terminus of Ub and the lysine residues of the substrates. The ubiquitinated substrates are then targeted for the 26S proteasome and degraded into small peptides, releasing free Ub for recycling [5–7]. This process has emerged as a critical regulatory process in virtually all aspects of cell biology. Indeed, the 2004 Nobel Prize in Physiology or Medicine was awarded for the discovery of Ub-mediated proteolysis. Nevertheless, protein ubiquitination is a highly reversible process that can be regulated in the cell. Deubiquitinating enzymes (DUBs), capable of removing Ub from protein substrates, are also involved in numerous biological processes such as transcriptional regulation, growth and differentiation, and oncogenesis [8–10].
Ubiquitin carboxyl-terminal hydrolase 37 (UCH37), a member of the deubiquitinating enzymes which belongs to the UCH family, can suppress protein degradation through disassembling polyubiquitin from the distal subunit of the chain. Unlike the other UCH members, UCH37 is responsible for the Ub isopeptidase activity in the 26S proteasome [11–15]. UCH37 can deubiquitinate the activated type I TGF-β receptor, thereby rescuing it from proteasomal degradation, which raises the TGF-β-dependent gene expression [16–18]. And silencing of UCH37 can efficiently induce apoptosis through activation of caspase-9 and caspase-3; on the other hand, over-expression of UCH37 leads to the opposite effect [19]. These findings indicate a role UCH37 plays in oncogenesis. Rolén et al. [20] have found that the activity of the C-terminal hydrolases UCH37 is up-regulated in the majority of cervical carcinoma tissues compared with the adjacent normal tissues. Fang et al. [21] have shown that the quantity of UCH37 rises in hepatocellular carcinoma (HCC), and have identified glucose-regulated protein 78 (GRP78), essential for cell viability, as one interacting with UCH37. Kapuria et al. [22] have found that WP1130, a partly selective DUB inhibitor which directly inhibits DUB activity of USP9x, USP5, USP14 and UCH37, could mediate inhibition of tumor-activated DUBs resulting in down-regulation of anti-apoptotic and up-regulation of proapoptotic proteins. All of the evidence suggests that the up-regulation of UCH37 may play an important role in oncogenesis through promoting some proto-oncogenes’ expression and stem cell-like characteristics in the cell.
In this study, we evaluated the prognostic value of UCH37, which contained 111 tumor specimens from ESCC patients who underwent curative resection and whose consistency of immunohistochemistry was maintained steadily. We found that UCH37 is associated with outcome and recurrence of ESCC and can be a novel predictor for poor prognosis of ESCC patients after curative resection.
Materials and Methods
Patients and Specimens
Tumor specimens used in this study were obtained from 111 consecutive male patients with ESCC who underwent curative resection at The Department of Thoracic Surgery, Zhongshan Hospital, Fudan University between 2007 and 2008.
The inclusion and exclusion criteria of the patient cohorts include: (a) having a distinctive pathologic diagnosis of ESCC, (b) having no anticancer treatment before esophageal resection, (c) having curative esophageal resection, and (d) having suitable formalin-fixed and paraffin-embedded tissues.
Curative resection was defined as complete resection of all tumor nodules and the cut surface being free of cancer by histologic examination, i.e. having no cancerous thrombus in the vein under the microscope. Each specimen has a corresponding nontumorous tissue 3 cm away from the tumor. ESCC diagnosis was based on the WHO criteria. Tumor differentiation and staging was defined according to the 7th edition of tumor-node-metastasis (TNM) classification of Unio Internationale Contra Cancrum (UICC). Ethical approval for human subjects was obtained from the research ethics committee of Zhongshan Hospital; informed consent was obtained from each patient.
Follow-Up and Treatment for Tumor Recurrences
Patients were followed up every 3–4 months during the first postoperative year and at least 6 months afterward. The follow-up was finished on January 10, 2012. The median follow-up was 38 months (range 1–48 months). Most patients died from ESCC itself, recurrence, or distal metastasis. Three patients died of unrelated diseases, such as myocardial infarction, cerebral accident, and other unknown reason without recurrence. All patients were monitored prospectively by tumor markers such as CEA, SCC and CA199, and chest X-ray every 3–12 months, according to the postoperative time. A diagnosis of recurrence was based on typical imaging appearance in X-ray, Barium meal, and gastroscope. Patients with confirmed recurrence received further treatment, which was based on tumor size, site, and patients’ condition. Patients with locoregional relapse received the aggressive intervention with curative intent, while those patients with poor physical condition or poor financial situation received the palliation therapy or Chinese traditional medicine instead of chemoradiation therapy, surgery or other options.
Immunohistochemistry
Five-micron thick sections of formalin-fixed and paraffin-embedded tissues were deparaffinized and rehydrated, followed by high-temperature antigen retrieval via microwave in 0.1 M citrate solution (pH 6.0) for 15 min. After blocked in 5 % normal goat serum at room temperature for 30 min, the sections were incubated with the rat anti-UCH37 antibody (Santa Cruz, CA, USA) at 4 °C overnight, then incubated with biotinylated secondary antibody at room temperature for 30 min, and finally immunostained by the avidin–biotin complex (ABC) technique using 3,3′-diaminobenzidine (DAB). Hematoxylin was used as a counterstain.
Evaluation of Immunohistochemical Variables
Immunohistochemical staining was evaluated independently by two pathologists without knowledge of patient characteristics and any discrepancy was resolved by consensus review. The interpretation of immunoreactivity was performed in a semi-quantitative manner by analyzing the extent and intensity of staining positivity of cells. Interpretation scores were as follows: 0, ≤5 % cell positivity or negative staining; +1, 6–20 % cell positivity or mild staining; +2, 21–50 % cell positivity or moderate staining; and +3, ≥50 % cell positivity or intense staining. Total scores were the product of the two. And the final scores, differentials of the tumor total scores and the nontumorous total scores, greater than 3.5 were considered high expression, otherwise they were identified as low expression.
Statistical Analyses
Statistical analyses were done by SPSS 19.0 for Windows (SPSS). The χ2 test, Fisher’s exact probability, and Student’s t test were used for comparison between groups. Cumulative survival time was calculated by the Kaplan–Meier method and analyzed by the log-rank test. Univariate and multivatriate analyses were based on the Cox proportional hazard regression model. Multivariate analysis was done using a Cox regression model with forward stepwise manner. p < 0.05 was considered statistically significant.
Results
Expression of UCH37 in Archival Esophageal Cancer Tissue
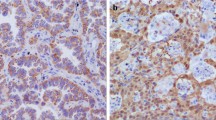
We analyzed the expression and subcellular location of UCH37 by immunohistochemistry. UCH37 protein was detected in all the ESCC samples (111 of 111, 100 %) with moderate to high staining (the average score was 6.98 ± 0.15), and located in both nuclei and cytoplasm. Higher expression of UCH37 was also observed in the invasive front of esophageal carcinoma tissue. However, only weak to moderate UCH37 staining was observed in the basal cells of adjacent normal esophageal epithelium (the average score was 2.34 ± 0.16). All the patients showed higher UCH37 expression in their ESCC samples than in their basal cells of adjacent normal esophageal epithelium (Fig. 1).
Correlation Between UCH37 Protein Expression and Clinicopathological Features
Table 1 shows the relationship between the expression of UCH37 and clinical characteristics in all 111 ESCC cases. There was no significant correlation between the expression level of UCH37 and age, tumor differentiation, invasive depth, distant metastasis, tumor position and size, while the expression of UCH37 is closely associated with lymph nodes metastasis (p = 0.009) and TNM stage (p = 0.038). Higher staging and lymph nodes metastasis correlated with higher UCH37 expression (Table 1).
Survival Analysis
Kaplan–Meier analysis and the log-rank test were used to evaluate the effect of UCH37 expression on survival. The expression level of UCH37 in esophageal carcinoma was significantly correlated with patients’ overall survival time (OS) and disease-free survival time (DFS) (p = 0.026 and p = 0.016), i.e. the higher level of UCH37 expression was correlated with shorter OS and DFS. The cumulative 3-year OS and DFS rate was 73.5 and 73.5 % in the low UCH37 expression group, whereas it was only 53.2 and 45.5 % in high UCH37 expression group (p = 0.039 and p = 0.006) (Fig. 2).
Univariate analysis showed lymph nodes metastasis, distant metastasis and TNM stage were unfavorable predictors for OS and DFS; UCH37 was associated with OS and DFS; and age, tumor differentiation, position and tumor size had no prognostic significance for OS and DFS (Table 2). We did multivariate survival analysis, which included UCH37 expression level and TNM stage, to determine if UCH37 expression level was an independent prognosticator for OS and DFS. In this analysis, we found that UCH37 was an independent prognosticator for DFS (p = 0.049). But we could not draw a conclusion that UCH37 was an independent prognosticator for OS (p = 0.077) (Table 3).
We also analyzed the prognostic value of UCH37 expression in selective patient subgroups stratified according to the lymph nodes metastasis condition. The 3-year DFS rates of the patients who showed high UCH37 expression were 55.6 %, of which some were reduced compared with UCH37 lower expression patients in the no lymph nodes metastasis subgroup (84.0 %, p = 0.018). Patients with tumors exhibiting high UCH37 expression had shorter DFS compared with patients with low expression of UCH37 when there is no lymph nodes metastasis. But the similar analysis of the lymph nodes metastasis subgroup did not show statistically significant differences between patients with different UCH37 expression level (p = 0.518). Similar conclusions can be made in the OS analysis. Patients with tumors exhibiting high UCH37 expression had significantly shorter OS compared with patients with low expression of UCH37 in the no lymph nodes metastasis subgroup (p = 0.042), while there was no statistical differences between patients with different UCH37 expression in the lymph nodes metastasis subgroup (p = 0.976) (Fig. 3).
Kaplan–Meier analysis of the OS and DFS of esophageal carcinoma patients categorized according to the lymph nodes metastasis condition and UCH37 expression. The statistical difference of UCH37 high-expression and low-expression patients was compared between the no lymph nodes metastasis subgroup in their OS (a) and DFS (b), and lymph nodes metastasis subgroup in their OS (c) and DFS (d). p values were calculated by the log-rank test. The patients who showed higher UCH37 expression had the smaller OS and DFS rates in the no lymph nodes metastasis subgroup (p < 0.05)
About 90 % (45 of 50) of the recurrent patients recurred within 2 years, of which 84.4 % (38 of 45) were predicted by UCH37. Among these patients who recurred within 2 years, 42.2 % (19 of 45) were in TNM stage I or II, of which 78.9 % (15 of 19) were predicted by UCH37. And 57.8 % (26 of 45) of the patients were in TNM stage III or IV, of which 88.5 % (23 of the 26) were predicted by UCH37.
Discussion
Despite improvements in surveillance and clinical treatment strategies, the prognosis of ESCC still remains dismal because of its high recurrence and metastasis. It is critical to classify those patients with a high probability of recurrence and metastasis well in advance to initiate a timely intervention. Current clinicopathologic factors such as TNM stage and tumor size cannot accurately predict the outcome of ESCC patients.
Ubiquitin can be covalently conjugated to protein substrates, and the ubiquitinated substrates are then targeted for the 26S proteasome and degraded into small peptides [5–7]. This process has emerged as a critical regulatory process in virtually all aspects of cell biology. DUBs could remove Ub from protein substrates to mediate degradation of ubiquitinated protein and promote recycling of Ub, which also is involved in numerous biological processes such as transcriptional regulation, growth and differentiation, and oncogenesis [8–10]. UCH37, a member of the DUBs, can suppress protein degradation through disassembling polyubiquitin from the distal subunit of the chain. It is responsible for the Ub isopeptidase activity in the 26S proteasome [11–15]. Evidence has shown that the up-regulation of UCH37 may play an important role in oncogenesis through promoting some proto-oncogenes’ expression and stem cell-like characteristics in the cell [16–22].
Rolén et al. [20] have found that the activity of the C-terminal hydrolases UCH37 is up-regulated in the majority of cervical carcinoma tissues compared with the adjacent normal tissues, and Fang et al. [21] have showed that the quantity of UCH37 rises in HCC. According to our immunohistochemistry result, all the 111 patients showed higher UCH37 expression in their ESCC samples than in their basal cells of adjacent normal esophageal epithelium. So the expression of UCH37 raised in the tumor cells, which further confirmed the previous research [20, 21, 24]. There was also weak to moderate UCH37 staining observed in the basal cells of adjacent normal esophageal epithelium, mainly because UCH37 is proven to be a protein related to growth and development [23], and the basal cells of normal esophageal epithelium are growing and updating rapidly.
From the clinical data, we found that the expression of UCH37 was related to the lymph nodes metastasis and TNM stage. Higher staining of UCH37 means higher TNM staging. Kaplan–Meier analysis and the log-rank test showed that the expression level of UCH37 in esophageal carcinoma was significantly correlated with patients’ OS and DFS. And multivariate analysis showed UCH37 was an independent prognosticator for DFS (p = 0.049). This finding indicated that UCH37 was suitable to predict early recurrence in ESCC after operation.
The patients who showed higher UCH37 expression had the smaller OS and DFS rates in the no lymph nodes metastasis subgroup. Most of the recurrent patients recurred within 2 years, and about half of them were in TNM stage I or II, of which 78.9 % were predicted by UCH37, and another half were in TNM stages III or IV, of which 88.5 % were predicted by UCH37. Among all the patients who suffered the recurrence within 2 years, 84.4 % can be predicted by UCH37.
Unlike usual predictive markers which are mainly expressed in advanced tumors, we found that the OS and DFS rates of the UCH37+ patients in the no lymph nodes metastasis subgroup were significantly reduced compared with UCH37− patients in the same condition, which suggested that UCH37 may be a promising marker for recurrence and metastasis. But in the lymph nodes metastasis subgroup, there were no statistically significant differences in OS and DFS rate between patients with different UCH37 expression level. There are two possible reasons: (1) the raw materials and space for UCH37 are limited, and it is impossible for the tumor cell to express unlimited UCH37 protein; so the growth rate would slow down or even stop if the tumor deteriorates to some extent; and (2) when an initial patients had lymph node metastasis, it is hard to say recurrence is due to the tumor characteristics or the incompleteness of first-time treatment.
Although our study and previous study have confirmed that UCH37 does relate with the outcome and recurrence of the carcinoma, the mechanisms on the roles of UCH37 on tumorigenesis and metastasis is still unclear. Evidence has shown that the up-regulation of UCH37 may play an important role in oncogenesis through promoting some proto-oncogenes’ expression and stem cell-like characteristics in the cell such as type I TGF-β receptor and GRP78 [16–22]. Hence, the roles of UCH37 on tumorigenesis and metastasis deserve further study.
Conclusion
To our knowledge, this is the first case showing the expression of UCH37 in esophageal tumor tissue, highlighting the clinical significance of UCH37 in esophageal carcinoma. UCH37 may help physicians make informed decisions regarding adjuvant treatment following curative resection. Initiating adjuvant treatment early in high UCH37 expression, ESCC patients may reduce recurrence and prolong life span after surgical procedures. However, further studies are needed to clarify the mechanism by which UCH37 is involved in the development and progression of ESCC, and its exact role in the regulation of carcinogenesis in esophageal carcinoma.
References
Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–2150.
Day NE, Varghese C. Oesophageal cancer. Cancer Surv. 1994;19–20:43–54.
Younes M, Henson DE, Ertan A, Miller CC. Incidence and survival trends of esophageal carcinoma in the United States: racial and gender differences by histological type. Scand J Gastroenterol. 2002;37:1359–1365.
Vallbohmer D, Lenz HJ. Predictive and prognostic molecular markers in outcome of esophageal cancer. Dis Esophagus. 2006;19:425–432.
Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479.
Pickart CM. Mechanisms underlying ubiquitination. Annu Rev Biochem. 2001;70:503–533.
Finley D, Ciechanover A, Varshavsky A. Ubiquitin as a central cellular regulator. Cell. 2004;116:S29–S32.
Sridhar VV, Kapoor A, Zhang K, Zhu J, et al. Control of DNA methylation and heterochromatic silencing by histone H2B deubiquitination. Nature. 2007;447:735–738.
Liu H, Buus R, Clague MJ, Urbe S. Regulation of ErbB2 receptor status by the proteasomal DUB POH1. PLoS ONE. 2009;4:e5544.
Singhal S, Taylor MC, Baker RT. Deubiquitylating enzymes and disease. BMC Biochem. 2008;9:S3.
Yao T, Song L, Xu W, DeMartino GN, et al. Proteasome recruitment and activation of the Uch37 deubiquitinating enzyme by Adrm1. Nat Cell Biol. 2006;8:994–1002.
Schreiner P, Chen X, Husnjak K, Randles L, et al. Ubiquitin docking at the proteasome through a novel pleckstrin-homology domain interaction. Nature. 2008;453:548–552.
Husnjak K, Elsasser S, Zhang N, Chen X, et al. Proteasome subunit Rpn13 is a novel ubiquitin receptor. Nature. 2008;453:481–488.
Hamazaki J, Iemura S, Natsume T, Yashiroda H, et al. A novel proteasome interacting protein recruits the deubiquitinating enzyme UCH37 to 26S proteasomes. EMBO J. 2006;25:4524–4536.
Qiu XB, Ouyang SY, Li CJ, Miao S, et al. hRpn13/ADRM1/GP110 is a novel proteasome subunit that binds the deubiquitinating enzyme, UCH37. EMBO J. 2006;25:5742–5753.
Wicks SJ, Haros K, Maillard M, Song L, et al. The deubiquitinating enzyme UCH37 interacts with Smads and regulates TGF-beta signalling. Oncogene. 2005;24:8080–8084.
Wicks SJ, Grocott T, Haros K, Maillard M, et al. Reversible ubiquitination regulates the Smad/TGF-beta signalling pathway. Biochem Soc Trans. 2006;34:761–763.
Cutts AJ, Soond SM, Powell S, Chantry A. Early phase TGFbeta receptor signalling dynamics stabilised by the deubiquitinase UCH37 promotes cell migratory responses. Int J Biochem Cell Biol. 2011;43:604–612.
Chen Z, Niu X, Li Z, Yu Y, et al. Effect of ubiquitin carboxy-terminal hydrolase 37 on apoptotic in A549 cells. Cell Biochem Funct. 2011;29:142–148.
Rolen U, Kobzeva V, Gasparjan N, Ovaa H, et al. Activity profiling of deubiquitinating enzymes in cervical carcinoma biopsies and cell lines. Mol Carcinog. 2006;45:260–269.
Fang Y, Mu J, Ma Y, Ma D, et al. The interaction between ubiquitin C-terminal hydrolase 37 and glucose-regulated protein 78 in hepatocellular carcinoma. Mol Cell Biochem. 2012;359:59–66.
Kapuria V, Peterson LF, Fang D, Bornmann WG, et al. Deubiquitinase inhibition by small-molecule WP1130 triggers aggresome formation and tumor cell apoptosis. Cancer Res. 2010;70:9265–9276.
Al-Shami A, Jhaver KG, Vogel P, Wilkins C, et al. Regulators of the proteasome pathway, Uch37 and Rpn13, play distinct roles in mouse development. PLoS ONE. 2010;5:e13654.
Fang Y, Fu D, Shen XZ. The potential role of ubiquitin c-terminal hydrolases in oncogenesis. Biochim Biophys Acta. 2010;1806:1–6.
Acknowledgments
The authors would like to express gratitude to the staff of Prof. Xizhong Shen’s laboratory for their critical discussion and reading of the manuscript and the members of the Department of Thoracic Surgery of Zhongshan Hospital for their tumor specimens and follow-up data. This study was supported by the Shanghai Science and Technology Commission (10410709400, 10411950100), National Nature Science Foundation of China (81000968, 81101540, 81101637 and 81172273) and the National Clinical Key Special Subject of China.
Conflict of interest
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Yanjie Chen and Da Fu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Chen, Y., Fu, D., Xi, J. et al. Expression and Clinical Significance of UCH37 in Human Esophageal Squamous Cell Carcinoma. Dig Dis Sci 57, 2310–2317 (2012). https://doi.org/10.1007/s10620-012-2181-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-012-2181-9